Abstract
Purpose
To assess the efficacy and safety of 5-fluorouracil (5FU) and gemcitabine administered concurrently with radiation in patients with locally advanced, non-metastatic pancreatic cancer.
Patients and Methods
Eligible patients had histologically confirmed pancreatic adenocarcinoma, deemed locally unresectable without evidence of metastatic disease. In addition, all patients underwent laparoscopy or laparotomy prior to study entry to rule out peritoneal carcinomatosis. Patients received radiation therapy (50.4 Gy) with concurrent infusional 5FU (200 mg/m2, 5 days per week) and weekly gemcitabine (200 mg/m2). After a three-week break, patients received weekly gemcitabine at 1000 mg/m2 for 3 of 4 weeks, for four cycles. The primary endpoint of the trial was the proportion of patients surviving nine months from study entry. Secondary endpoints included objective tumor response, CA19-9 response, overall survival (OS) time to progression (TTP) and toxicity.
Results
Between November 2001 and October 2004, 81 patients were enrolled, of whom 78 were eligible for analysis. With a median follow-up of 55.2 months, the median OS was 12.2 months (95% CI, 10.9 – 14.9 months) and median TTP was 10.0 months (95% CI 6.4 – 12.0 months). An objective tumor response was seen in 19 patients (25%) and among 56 patients with an elevated CA19-9 at baseline, 29 (52%) had a sustained CA19-9 response. Overall, 41% of patients had grade 3 or greater treatment-related GI adverse events.
Conclusion
The combination of 5FU, gemcitabine and radiation is well-tolerated. Survival is comparable to the best results of other recent studies of 5FU and radiation or gemcitabine and radiation.
Keywords: pancreatic cancer, gemcitabine, 5-fluorouracil, radiation, combined modality therapy
INTRODUCTION
Pancreatic cancer, the fourth leading cause of cancer death in the United States1, remains one of the most treatment-refractory solid malignancies. Approximately 40% of patients with newly diagnosed pancreatic cancer present with locally advanced, non-metastatic disease.
In two randomized trials by the Gastrointestinal Tumor Study Group, the combination of fluorouracil and external beam radiation was shown to be more effective than either modality alone, with a median overall survival of approximately 42 weeks2–4. An Eastern Cooperative Oncology Group (ECOG) study, however, failed to demonstrate an advantage to combined modality therapy5. The French FFCD-SFRO trial suggested a detriment in survival when combined chemoradiation therapy was compared to gemcitabine alone6. However, in this intensive regimen increased toxicity may have contributed to the poor outcome. Despite these conflicting results, efforts over the past three decades have attempted to improve the efficacy of chemoradiation, largely by incorporating more active systemic therapy.
Among patients with metastatic or locally advanced pancreatic cancer, gemcitabine therapy has led to a superior clinical benefit when compared to 5-fluorouracil (5FU) 7. As gemcitabine has been shown to enhance the sensitivity of human pancreatic cancer cells to radiation8, 9 several investigators have assessed the impact of gemcitabine when combined with radiation. Most investigators combined lower doses of gemcitabine with conventional radiation10–20. An alternative approach has been to combine full-dose gemcitabine with a hypofractionated course of radiation21–23. Several groups have combined radiation and gemcitabine with additional agents, including cisplatin24–29, oxaliplatin30, taxanes31, 32, irinotecan33, mitomycin34 and inhibitors of EGFR and VEGF35–39.
As 5-FU and gemcitabine are both active radiation sensitizing agents and represent the two principal systemic agents for pancreatic cancer, we conducted a phase I/II trial combining 5FU, gemcitabine and radiation in patients with locally advanced disease. The regimen appeared well-tolerated with maximum tolerated doses of 200 mg/m2/day for infusional 5FU and 200 mg/m2/week for gemcitabine with 50.4 Gy of radiation. More recently, Wilkowski reported a similar experience in a group of 32 patients, with a median survival of over 13 months40. In contrast, a similar phase I study of concurrent 5-FU, gemcitabine and radiation led by ECOG reported unacceptable toxicity41. Five of the seven treated subjects experienced dose-limiting toxicities, three of which involved GI bleeding. To clarify the safety and efficacy of this combination, we conducted a multi-institutional phase II trial of 5FU, gemcitabine and external beam radiation therapy in patients with locally advanced pancreatic cancer.
PATIENTS AND METHODS
Patient Eligibility Criteria
Eligible patients had biopsy-proven, localized, unresectable adenocarcinoma of the pancreas and an ECOG performance score of 0 – 2. Criteria for unresectability and eligibility included one or more of the following: a tumor measuring greater than 5 cm, regional lymph nodes greater than 2 cm in size that could be included within the radiation port, involvement of major vessels including the superior mesenteric artery, superior mesenteric vein, portal vein or hepatic artery, and direct extension of tumor to adjacent organs. Staging studies included a chest x-ray and an abdominal/pelvic CT scan; patients underwent a laparotomy or laparoscopy to rule out the presence of occult peritoneal disease. Exclusion criteria included a prior malignancy other than non-melanoma skin cancer or in-situ cervical cancer within the past five years, or other major co-morbidities such as myocardial infarction within six months of study entry. Required laboratory values included a total bilirubin below 2.0 mg/dl, and AST ≤ 3x upper limits of normal, creatinine ≤ 2.0 mg/dl, WBC ≥ 3,000/mm3 and platelets ≥ 100,000/mm3. All patients signed a consent form, and the study was approved by the Human Investigations Committee of participating Cancer and Leukemia Group B (CALGB) institutions.
Study Design and Treatment Plan
This was an open-label, non-randomized phase II study. Cycle one consisted of radiation to 50.4 Gy in 28 fractions over 5.5 weeks, with 5FU given as a continuous infusion from Monday through Friday at 200 mg/m2/day and gemcitabine given weekly at 200 mg/m2, both given throughout the radiation therapy course. Three weeks following the completion of radiation, patients received gemcitabine at a dose of 1000 mg/m2 over 30 minutes weekly for three weeks, followed by a one-week rest, for four 4-week cycles.
Radiation was delivered on a linear accelerator with a minimum energy of 6 MV. Patients were simulated on a machine that reproduced the geometry of the treatment machine; multi-field techniques were mandatory. Doses were specified to isocenter. Patients were treated to 4500 cGy in 25 fractions to an initial tumor / nodal field, followed by a boost field for an additional 540 cGy in 3 fractions. The gross tumor volume (GTV) included the pancreatic mass and any lymph nodes measuring > 1.5 cm as visualized on CT scan. The clinical target volume (CTV) was defined by expanding the GTV for 1 to 1.5 cm, including the porta hepatic and pancreaticoduodenal nodes for head lesions, and the celiac axis for tumors of both the head and body/tail. The planning target volume (PTV) was based on a 1 cm expansion of the CTV. The boost volume consisted of the GTV with a 1 cm expansion for the boost PTV. Normal tissue constraints included no portion of the spinal cord receiving above 4500 cGy, no more than 50% of the combined renal volume receiving above 2000 cGy and no more than 1/3 of the total liver volume receiving above 3000 cGy. All treatment plans were reviewed by the Quality Assurance Review Center (QARC) in Providence, RI, and by the study chair.
Dose Modifications for Adverse Events
Adverse events were scored using version 2.5 of the Common Terminology Criteria for Adverse Events. During cycle 1, a decrease in platelets to between 50,000–99,999/mm3 and/or a decrease in the ANC to 500–999/mm3 resulted in a 75% dose-reduction for gemcitabine and a 50% dose-reduction for 5FU. If the ANC decreased below 500/mm3 or the platelets decreased below 50,000/mm3, then chemotherapy and radiation were held until the ANC was greater than or equal to 1,000/mm3 and the platelet count was above 100,000/mm3. If treatment was held for greater than three weeks, the patient was removed from protocol treatment.
For non-hematologic events, both gemcitabine and 5FU were reduced to 75% of the dose for grade 3 toxicity and to 50% of the dose for grade 4 toxicities. Reduced doses of chemotherapy were not re-escalated. Owing to concerns about GI bleeding raised by ECOG 229742 stopping rules dictated study termination if more than 10 cases of grade 3 or higher GI bleeding were observed among the first 35 patients. The incidence of bleeding was followed in cohorts of seven patients, with plans to stop accrual if any of the following proportions of patients had grade 3 or higher GI bleeds: 5 of 7, 6 of 14, 7 of 21, 9 of 28 or 11 of 35.
During cycles 2–5 (gemcitabine alone), the dose of gemcitabine was reduced by 25% for an ANC between 500 and 999/mm3 and held for an ANC below 500/mm3. For grade 3 non-hematologic toxicities gemcitabine was held and re-started at a 25% dose reduction when the toxicity had resolved. For grade 4 non-hematologic adverse events, the gemcitabine was held and re-started at a 50% dose reduction when the toxicity had resolved. Dose reductions were continued through all subsequent cycles of gemcitabine.
Patient Monitoring
Patients were assessed weekly by history and physical examination. Laboratory studies, including blood counts, BUN, creatinine, bilirubin, AST, alkaline phosphatase and CA19-9 were obtained weekly during combined chemoradiation and prior to each subsequent cycle of gemcitabine. Blood counts were assessed prior to each weekly administration of gemcitabine.
Statistical Considerations
The primary endpoint was the proportion of patients surviving nine months. Secondary endpoints included overall survival (OS), measured from study entry to death from any cause, time-to-tumor progression (TTP) measured from study entry to documented progression of disease or death from any cause, CA19-9 response defined as ≥ 75% decrease from the baseline maintained for two consecutive measurements at least four weeks apart among patients with an elevated baseline CA19-9, and toxicity. The prior protocol for patients with locally advanced pancreatic cancer, CALGB 89805, studied concurrent gemcitabine (40 mg/m2 twice weekly) and radiation therapy in a similar patient population. Based on the median survival of 8 months in that study17, it was determined that a median survival of 9 months or less in the current protocol would be considered unworthy of further investigation. An accrual goal of 78 evaluable patients to be followed for a minimum of 9 months was targeted resulting in 80% power to distinguish between median survival rates of 9 and 13 months. If the 90% lower confidence bound estimate (90% LCB) for the proportion of patients surviving 9 months were to exceed 0.5, the regimen would be considered for further investigation.
Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. All analyses were based on the study database frozen on March 3, 2009 and performed by CALGB statisticians using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Eighty-one patients from 15 U.S. institutions were accrued between November 2001 and October 2004, of whom 78 were eligible for analysis. Three patients who canceled their registration prior to starting any protocol treatment were excluded from the analysis. Pre-treatment characteristics of the eligible patients are shown in Table 1. Thirty-six percent of patients underwent palliative bypass surgery prior to starting treatment, 47% underwent the placement of a biliary stent. The majority of patients had T4 tumors with vascular involvement.
Table 1.
Patient characteristics
| Gender | M | 39(50%) |
| F | 39(50%) | |
| Race | White | 70(90%) |
| Hispanic American | 1(1%) | |
| African American or Black | 6(7%) | |
| Asian | 1(1%) | |
| Age | Mean (Std. Dev.) | 62.3(9.8) |
| Performance Status | 0 | 27(34%) |
| 1 | 4 (58%) | |
| 2 | 6(8%) | |
| Size (cm) | Mean (Std. Dev.) | 3.7(1.7) |
| Median (Range) | 3.8(0 – 8.8) | |
| Grade | Well differentiated | 8(10%) |
| Moderately differentiated | 19(24%) | |
| Poorly differentiated | 17(22%) | |
| Undifferentiated | 2(3%) | |
| Unknown | 32(41%) | |
| T | 2 | 13(17%) |
| 3 | 14(18%) | |
| 4 | 51(65%) | |
| N | 0 | 50(64%) |
| 1 | 21(27%) | |
| X | 7(9%) | |
| Location | Head | 67(87%) |
| Body | 16(21%) | |
| Tail | 5(7%) | |
| Vessel involvement | 69(89%) | |
| Baseline CA19-9 | Mean(Std. Dev.) | 1288(2130) |
| Median(Range) | 402(3,10854) |
Efficacy
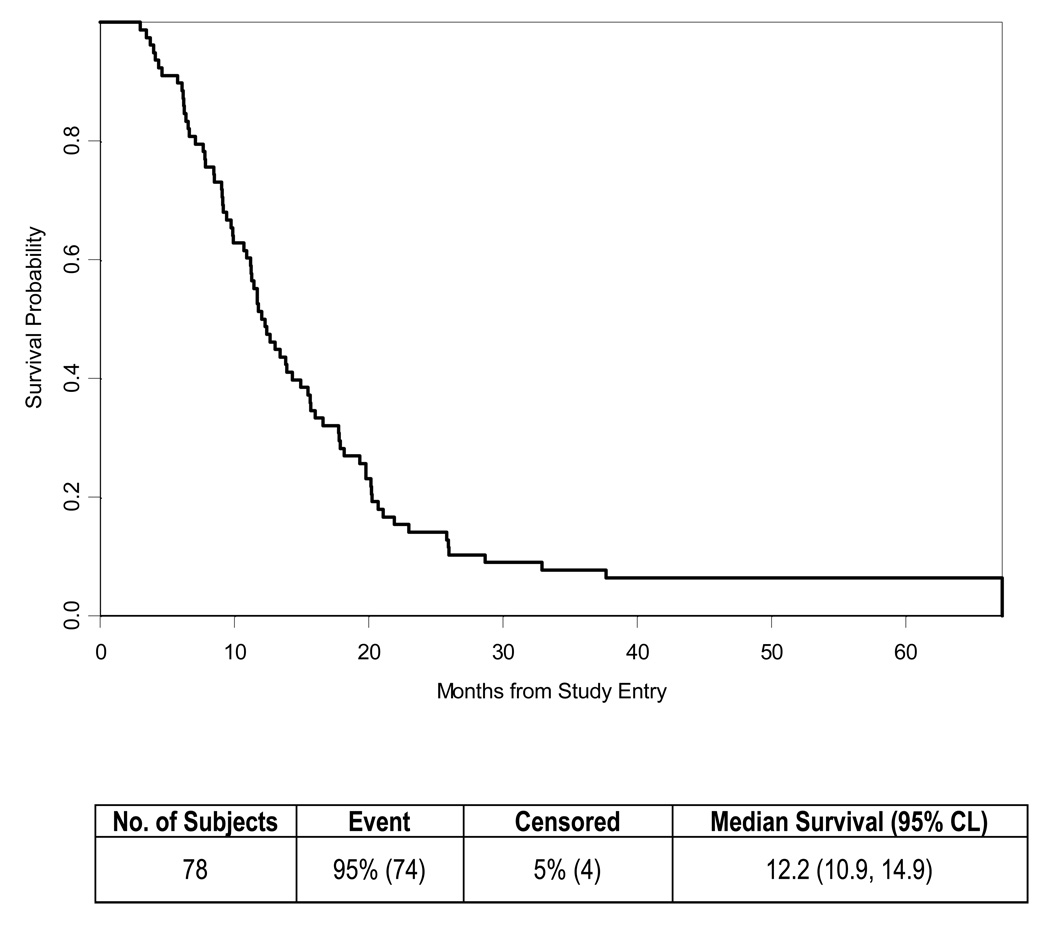
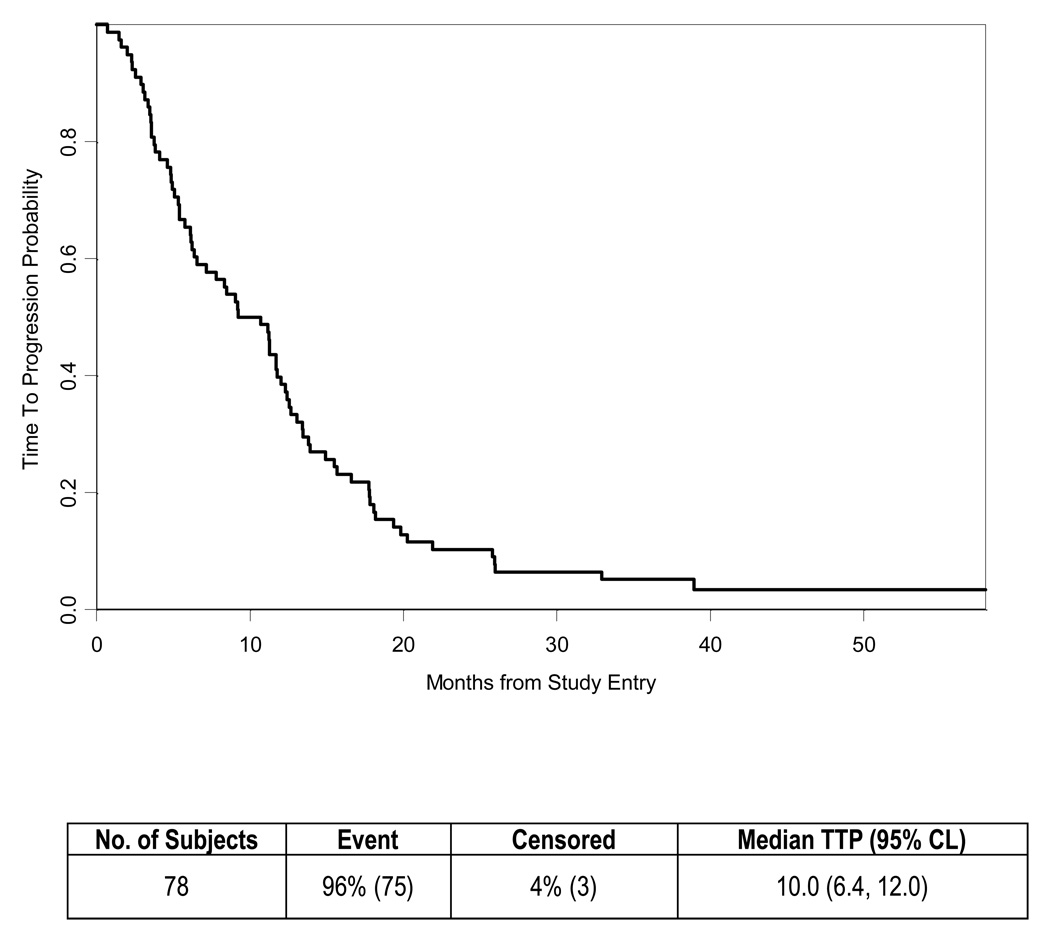
The median survival of the 78 eligible patients was 12.2 months (Table 2, Fig. 1) with four patients reported alive at 39, 52, 58 and 60 months of follow-up. The estimated one-year survival was 51% (95% CI 0.4 – 0.62). The 90% lower confidence bound for the proportion of patients surviving 9 months was 0.64. The Kaplan-Meier survival estimate at 9 months was 73% (95% CI 0.62 – 0.82). The probability of being progression free (Table 2, Fig. 2) was 0.54 (95% CI 0.42 – 0.64) at 9 months and 0.40 (95% CI 0.29 – 0.50) at 1 year. Median TTP was 10 months (95% CI 6.4 – 12.0).
Table 2.
Survival and time to progression
| Proportion Surviving 9 Months | ||
| Proportion | 0.731 | |
| 90% Lower Confidence Bound | 0.64 | |
| Median Follow-up Time | ||
| N alive | 4 | |
| Median (range) | 55.2(38, 60) months | |
| Overall Survival | ||
| N | 78 | |
| Number Censored (%) | 4 (5.1%) | |
| Kaplan-Meier Estimate, 9-months (95% CI)9- month Survival Estimate (95% CI) |
0.73(0.62, 0.82) | |
| Kaplan-Meier Estimate, 12-months (95% CI)12-month Survival Estimate (95% CI) |
0.51(0.40, 0.62) | |
| Median Follow-up Time | ||
| N alive | 4 | |
| Median | 55.2 months | |
| Time to Progression | ||
| N | 78 | |
| Number Censored (%) | 3 (3.8%) | |
| 3-month Survival Estimate (95%CI) | 0.90(0.81, 0.95) | |
| Kaplan-Meier Estimate, 9-months Survival Estimate (95% CI) |
0.54(0.42, 0.64) | |
| Kaplan-Meier Estimate, 12-month Survival Estimates (95% CI) |
0.40(0.29, 0.50) |
Figure 1.
Overall Survival for all patients
Figure 2.
Time to Progression for all patients
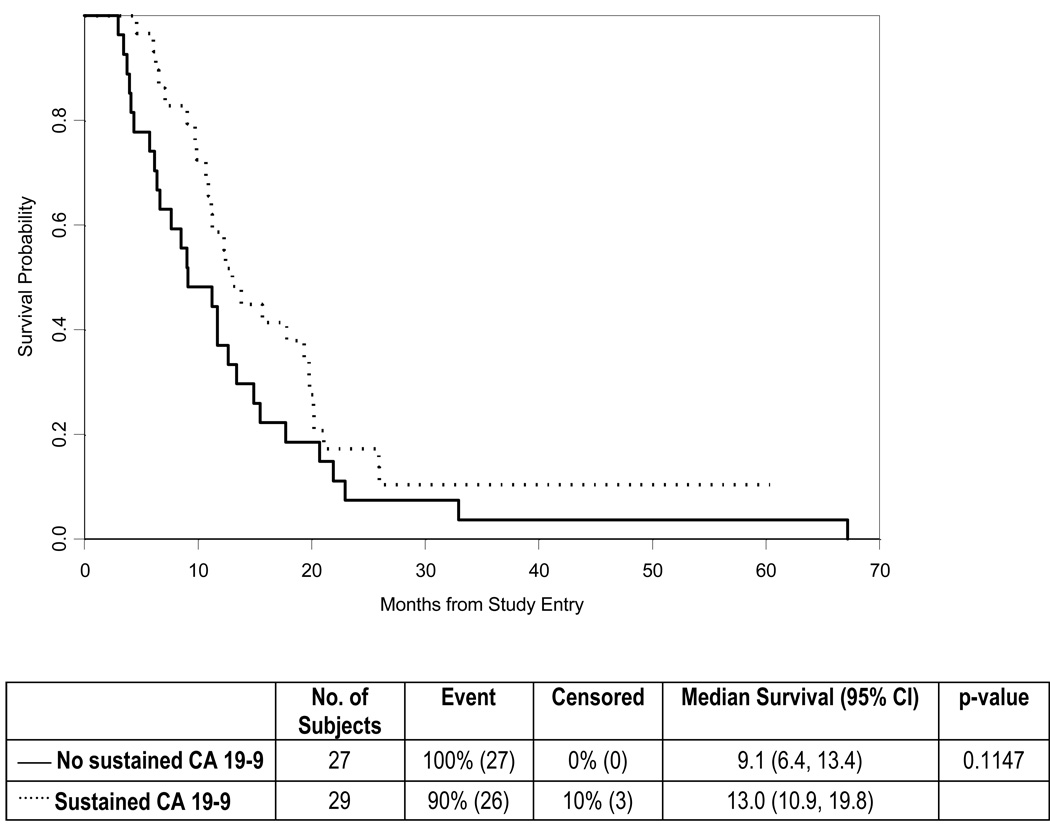
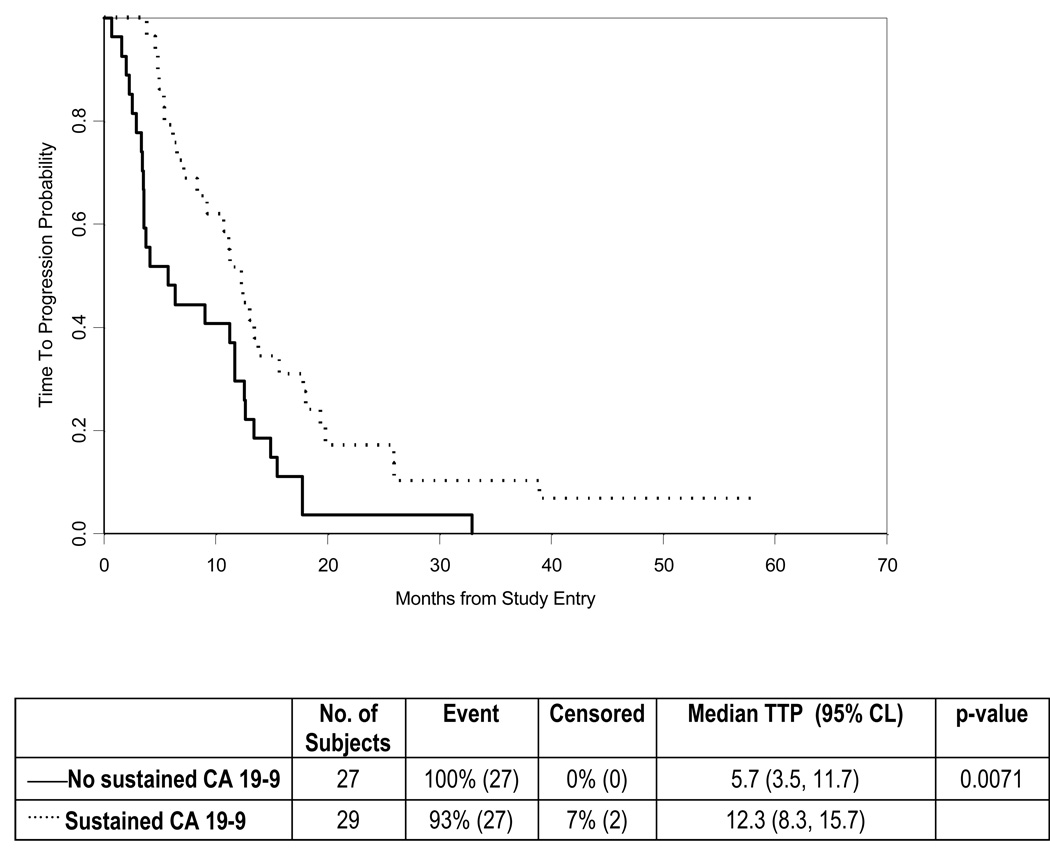
Of the 56 patients with an elevated serum CA19-9 level at study baseline, 29 (52%) experienced a sustained CA19-9 response, defined as a greater than 75% decrease lasting for at least two measurements more than four weeks apart. When compared to patients who did not experience a sustained CA19-9 response, subjects with a sustained response exhibited a trend toward improved OS (median, 13 vs. 9 months; P = 0.11) and a statistically significant improvement in TTP (median, 12.3 vs. 5.7 months, P = 0.007) (Figs. 3–4). Based on RECIST criteria, 19 patients (24%) experienced an objective response, of whom 5 (6%) had a complete response and 14 (18%) had a partial response. The median response duration was 5.5 months, with a minimum and maximum duration of 1 and 52.2 months, respectively (including 12 patients with progressive disease and 4 patients who died without documented progression). In addition, 41 (53%) had stable disease as their best response, 14 (18%) had progressive disease and 4 patients (5%) were unevaluable. The median time to objective response was 3.6 months (range 1.7 – 9.0).
Figure 3.
Overall Survival analyzed according to CA19-9 response
Figure 4.
Time to Progression analyzed according to CA19-9 response
Patterns of Failure
Twenty-eight (36%) of the patients had local progression in the pancreas as the first site of failure. The next most common site of disease progression was in the liver, which occurred in 23 (30%) of patients, followed by the lung (10%) and peritoneum (9%).
Treatment Modifications
Twenty-seven patients (35%) completed all protocol therapy. The most common reason for stopping treatment was progressive disease, which occurred in 21 (27%) patients. Nine (12%) discontinued treatment because of adverse events. Other common reasons for discontinuing protocol treatment included being switched to non-protocol therapy (9%) and patient refusal (9%).
A total of 64 patients (82%) had at least one treatment modification during the course of therapy at a mean of 22 days after starting protocol treatment. Twenty-two patients had a single dose modification. The maximum number of dose adjustments was 5 in two patients.
Radiotherapy Quality Assurance
All of the radiation therapy treatment plans were reviewed by QARC (Quality Assurance in Radiation Oncology) in Providence, RI, and by the study chair. Major deviations were defined as field borders that transected the gross tumor (GTV) or potentially tumor bearing areas (CTV). In addition, a dose discrepancy of more than 10% above or below the recommended dose at the prescription point, or exceeding the recommended dose to adjacent critical organs was considered a major deviation. Of the 78 patients who started radiation therapy, 65 cases were scored as appropriate, three had major deviations and seven had minor deviations. The remaining 3 cases were unevaluable due to insufficient data provided. Sixty-eight patients (87%) completed the planned 28 fractions to a total dose of 5040 cGy. Ten patients discontinued radiation, one each after 3, 4, 13, 21, 22, and 24 fractions, two each after 25 and 26 fractions.
Adverse Events
Among the 78 patients, 55 (71%) experienced grade 3 or higher non-hematological adverse events possibly attributed to treatment and 50 (64%) patients experienced grade 3 or higher related hematologic adverse events (Table 3). The most common non-hematologic events were gastrointestinal in 32 (41%) subjects, and constitutional and metabolic/laboratory each in 19 (24%).
Table 3.
Commonly observed treatment related adverse events, grade 3 and higher
| Category | Grade 3 N (%) |
Grade 4 N (%) |
Grade 5 N (%) |
|---|---|---|---|
| Blood / bone marrow | 40(51) | 10(13) | 0 |
| Hemoglobin | 6(8) | 1(1) | 0 |
| Total WBC | 30(38) | 6(8) | 0 |
| Lymphopenia | 20(26) | 0 | 0 |
| Neutropenia | 16(21) | 7(9) | 0 |
| Thrombocytopenia | 11(14) | 1 (1) | 0 |
| Transfusion: platelets | 1(1) | 0 | 0 |
| Transfusion: pRBCs | 4(5) | 0 | 0 |
| Non-hematological | 47(60) | 21(27) | 2(3) |
| Cardiovascular (general) | 3(4) | 1(1) | 0 |
| Constitutional symptoms | 17(22) | 2(3) | 0 |
| Gastrointestinal | 29 (37) | 3(4) | 0 |
| Hemorrhage | 2(3) | 2(3) | 1(1) |
| Hepatic | 7(9) | 0 | 0 |
| Infection / febrile neutropenia | 8(10) | 0 | 1(1) |
| Metabolic / laboratory | 17(22) | 2(3) | 0 |
| Neurology | 5(6) | 0 | 0 |
| Pain | 8(10) | 1(1) | 0 |
Without regard to attribution, 11 patients (14%) had at least one episode of gastrointestinal bleeding during the study or follow-up period, of which five were considered grade 3 or higher and possibly related to treatment. Neither a palliative surgical bypass nor placement of a stent correlated with an increased risk of GI bleeding. Five of these 11 gastrointestinal hemorrhages were localized within the radiation field.
Sixty-five (88%) of the deaths in the study population were secondary to disease progression. Two deaths (2%) were considered to be treatment-related, resulting from gastrointestinal bleeding in one subject and sepsis without neutropenia in the second. Three deaths resulted from causes other than treatment or disease.
DISCUSSION
The benefit for treating locally advanced, non-metastatic pancreatic cancer remains limited, with median survival ranging from eight to ten months43. In the current study of 78 patients, a combination of gemcitabine and 5FU with radiation therapy conferred a median OS of 12.2 months and a median TTP of 10 months. In a previous phase I/II trial of gemcitabine, 5-FU and concurrent radiation therapy, we similarly observed a median survival of 12 months in patients with locally advanced disease. These results compare favorably to prior studies of 5-FU with radiation, which reported a median OS of nine months and a median TTP of eight months or less.
Following the initial studies of 5-FU and radiation therapy in locally advanced pancreatic cancer, subsequent studies have also yielded median survivals well above the historical level of nine months. This gradual improvement in outcome over the past several years is apparent for both studies of radiation with gemcitabine10, 11, 14, 15, 17–23, 44 and for other chemoradiation combinations3, 27, 39, 40, 45–50. The reasons for this improvement are speculative, but may include slightly improved treatment regimens, improved staging which excluded patients with not easily apparent metastatic disease, improved supportive care, and improved salvage chemotherapy.
The current trial found the combination of 5FU and gemcitabine with concurrent radiation to be tolerable, though toxicity was moderate. Twelve percent of patients discontinued protocol therapy due to adverse events and 82% had at least one treatment modification. Our incidence of adverse events was comparable to other recent cooperative group trials in this patient population including a study of radiation with concurrent capecitabine and bevacizumab39 and a trial of radiation with concurrent gemcitabine and cisplatin29. In contrast to a prior ECOG trial51, the incidence and severity of gastrointestinal bleeding was manageable.
Our trial met its target of 50% of patients surviving for at least 9 months; 9-month survival was 73% and median overall survival was 12.2 months. A similarly designed trial of 32 patients combining higher doses of weekly gemcitabine and infusional 5-FU with radiation, followed by gemcitabine and cisplatin, observed a median OS of 13.6 months, although toxicity in that trial was considerable40.
The merits of combined chemoradiation as initial treatment for locally advanced pancreatic cancer have been questioned by the results of a recent randomized trial that demonstrated a superior outcome for patients receiving gemcitabine alone compared to chemoradiation followed by gemcitabine6. An alternative approach has been examined in which patients receive initial chemotherapy, with chemoradiation offered only to those patients without disease progression. In one retrospective study of 188 patients who had received three months of initial chemotherapy, 128 patients who did not demonstrate progressive disease received either further chemotherapy or combined chemoradiation. Although such non-randomized data must be interpreted with caution, the median progression free survival and OS for the patients receiving chemoradiation were 10.8 and 15 months, respectively, compared to 7.4 and 11 months for those treated with chemotherapy alone52. Other investigations have similarly suggested a benefit to selecting patients for combined chemoradiation following induction chemotherapy53–55. Such an approach potentially avoids radiation in patients who are destined to manifest metastatic disease, limiting local therapy to those who are most likely to derive a benefit. One promising strategy for future studies may be to build on this schedule of induction chemotherapy followed by chemoradiation with the addition of targeted agents emerging from an improved understanding of pancreatic cancer biology56–59.
We have demonstrated the feasibility of combining both 5-FU and gemcitabine with radiation, with several long term survivors and a superior overall survival compared to historical levels of eight to ten months. Although this regimen has achieved the pre-defined goals of improving median survival beyond nine months with acceptable morbidity, the observed median survival of 12 months is similar to the results of other recent phase II trials in patients with locally advanced pancreatic cancer. Given the multiple other treatment regimens that similarly appear to confer a 12-month median survival, we do not recommend further study of this treatment combination in future trials.
Acknowledgments
Research Support:
The research for CALGB 80003 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601).
The research was also supported, in part, by a grant from Eli Lilly and Company.
Footnotes
Financial Disclosures:
Richard Goldberg: Sanofi Aventis, Amgen, Astra Zeneca
William Blackstock: Sanofi Aventis, Eli Lilly Oncology, Sicel Technologies
Charles Fuchs: Astra-Zeneca, Imclone, Amgen, Pozen, Genentech, Roche, Genomic Health, Alnylam, Merck
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.A multi-institutional comparative trial of radiation therapy alone and in combination with 5-fluorouracil for locally unresectable pancreatic carcinoma. The Gastrointestinal Tumor Study Group. Ann Surg. 1979;189(2):205–208. [PMC free article] [PubMed] [Google Scholar]
- 3.Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48(8):1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 4.GTSG. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80(10):751–755. [PubMed] [Google Scholar]
- 5.Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3(3):373–378. doi: 10.1200/JCO.1985.3.3.373. [DOI] [PubMed] [Google Scholar]
- 6.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol. 2008;19(9):1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 7.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first- line therapy for patients with advanced pancreas cancer: a randomized trial [see comments] J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence TS, Chang EY, Hahn TM, Hertel LW, Shewach DS. Radiosensitization of pancreatic cancer cells by 2',2'-difluoro-2'-deoxycytidine. Int J Radiat Oncol Biol Phys. 1996;34(4):867–872. doi: 10.1016/0360-3016(95)02134-5. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence TS, Eisbruch A, McGinn CJ, Fields MT, Shewach DS. Radiosensitization by gemcitabine. Oncology (Huntingt) 1999;13(10 Suppl 5):55–60. [PubMed] [Google Scholar]
- 10.Crane CH, Janjan NA, Evans DB, et al. Toxicity and efficacy of concurrent gemcitabine and radiotherapy for locally advanced pancreatic cancer. Int J Pancreatol. 2001;29(1):9–18. doi: 10.1385/IJGC:29:1:09. [DOI] [PubMed] [Google Scholar]
- 11.de Lange SM, van Groeningen CJ, Meijer OW, et al. Gemcitabine-radiotherapy in patients with locally advanced pancreatic cancer. Eur J Cancer. 2002;38(9):1212–1217. doi: 10.1016/s0959-8049(02)00076-x. [DOI] [PubMed] [Google Scholar]
- 12.Epelbaum R, Rosenblatt E, Nasrallah S, et al. Phase II study of gemcitabine combined with radiation therapy in patients with localized, unresectable pancreatic cancer. J Surg Oncol. 2002;81(3):138–143. doi: 10.1002/jso.10159. [DOI] [PubMed] [Google Scholar]
- 13.Ammori JB, Colletti LM, Zalupski MM, et al. Surgical resection following radiation therapy with concurrent gemcitabine in patients with previously unresectable adenocarcinoma of the pancreas. J Gastrointest Surg. 2003;7(6):766–772. doi: 10.1016/s1091-255x(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 14.Okusaka T, Ito Y, Ueno H, et al. Phase II study of radiotherapy combined with gemcitabine for locally advanced pancreatic cancer. Br J Cancer. 2004;91(4):673–677. doi: 10.1038/sj.bjc.6602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnino A, Gatti M, Massucco P, et al. Phase II trial of primary radiation therapy and concurrent chemotherapy for patients with locally advanced pancreatic cancer. Oncology. 2005;68(4–6):493–499. doi: 10.1159/000086993. [DOI] [PubMed] [Google Scholar]
- 16.Blackstock AW, Bernard SA, Richards F, et al. Phase I trial of twice-weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer [see comments] J Clin Oncol. 1999;17(7):2208–2212. doi: 10.1200/JCO.1999.17.7.2208. [DOI] [PubMed] [Google Scholar]
- 17.Blackstock AW, Tepper JE, Niedwiecki D, Hollis DR, Mayer RJ, Tempero MA. Cancer and leukemia group B (CALGB) 89805: phase II chemoradiation trial using gemcitabine in patients with locoregional adenocarcinoma of the pancreas. Int J Gastrointest Cancer. 2003;34(2–3):107–116. doi: 10.1385/ijgc:34:2-3:107. [DOI] [PubMed] [Google Scholar]
- 18.Cengiz M, Zorlu F, Yalcin S, Gurkaynak M, Atahan IL, Gullu IH. Concurrent gemcitabine and radiotherapy for locally advanced pancreatic cancer. Med Oncol. 2007;24(2):239–243. doi: 10.1007/BF02698046. [DOI] [PubMed] [Google Scholar]
- 19.Huang PI, Chao Y, Li CP, et al. Efficacy and factors affecting outcome of gemcitabine concurrent chemoradiotherapy in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2009;73(1):159–165. doi: 10.1016/j.ijrobp.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Maximous DW, Abdel-Wanis ME, El-Sayed MI, Abd-Elsayed AA. Preoperative gemcitabine based chemo-radiotherapy in locally advanced non metastatic pancreatic adenocarcinoma. Int Arch Med. 2009;2(1):7. doi: 10.1186/1755-7682-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy JD, Adusumilli S, Griffith KA, et al. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68(3):801–808. doi: 10.1016/j.ijrobp.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 22.Small W, Jr, Berlin J, Freedman GM, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2008;26(6):942–947. doi: 10.1200/JCO.2007.13.9014. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki H, Nishiyama K, Koizumi M, et al. Concurrent chemoradiotherapy for advanced pancreatic cancer: 1,000 mg/m2 gemcitabine can be administered using limited-field radiotherapy. Strahlenther Onkol. 2007;183(6):301–306. doi: 10.1007/s00066-007-1641-1. [DOI] [PubMed] [Google Scholar]
- 24.Brunner TB, Grabenbauer GG, Klein P, et al. Phase I trial of strictly time-scheduled gemcitabine and cisplatin with concurrent radiotherapy in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2003;55(1):144–153. doi: 10.1016/s0360-3016(02)03818-x. [DOI] [PubMed] [Google Scholar]
- 25.Muler JH, McGinn CJ, Normolle D, et al. Phase I trial using a time-to-event continual reassessment strategy for dose escalation of cisplatin combined with gemcitabine and radiation therapy in pancreatic cancer. J Clin Oncol. 2004;22(2):238–243. doi: 10.1200/JCO.2004.03.129. [DOI] [PubMed] [Google Scholar]
- 26.Symon Z, Davis M, McGinn CJ, Zalupski MM, Lawrence TS. Concurrent chemoradiotherapy with gemcitabine and cisplatin for pancreatic cancer: from the laboratory to the clinic. Int J Radiat Oncol Biol Phys. 2002;53(1):140–145. doi: 10.1016/s0360-3016(01)02790-0. [DOI] [PubMed] [Google Scholar]
- 27.Hong SP, Park JY, Jeon TJ, et al. Weekly full-dose gemcitabine and single-dose cisplatin with concurrent radiotherapy in patients with locally advanced pancreatic cancer. Br J Cancer. 2008;98(5):881–887. doi: 10.1038/sj.bjc.6604247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkowski R, Thoma M, Schauer R, Wagner A, Heinemann V. Effect of chemoradiotherapy with gemcitabine and cisplatin on locoregional control in patients with primary inoperable pancreatic cancer. World J Surg. 2004;28(10):1011–1018. doi: 10.1007/s00268-004-7338-z. [DOI] [PubMed] [Google Scholar]
- 29.Haddock MG, Swaminathan R, Foster NR, et al. Gemcitabine, cisplatin, and radiotherapy for patients with locally advanced pancreatic adenocarcinoma: results of the North Central Cancer Treatment Group Phase II Study N9942. J Clin Oncol. 2007;25(18):2567–2572. doi: 10.1200/JCO.2006.10.2111. [DOI] [PubMed] [Google Scholar]
- 30.Desai SP, Ben-Josef E, Normolle DP, et al. Phase I study of oxaliplatin, full-dose gemcitabine, and concurrent radiation therapy in pancreatic cancer. J Clin Oncol. 2007;25(29):4587–4592. doi: 10.1200/JCO.2007.12.0592. [DOI] [PubMed] [Google Scholar]
- 31.Allendorf JD, Lauerman M, Bill A, et al. Neoadjuvant chemotherapy and radiation for patients with locally unresectable pancreatic adenocarcinoma: feasibility, efficacy, and survival. J Gastrointest Surg. 2008;12(1):91–100. doi: 10.1007/s11605-007-0296-7. [DOI] [PubMed] [Google Scholar]
- 32.Safran H, Dipetrillo T, Iannitti D, et al. Gemcitabine, paclitaxel, and radiation for locally advanced pancreatic cancer: a Phase I trial. Int J Radiat Oncol Biol Phys. 2002;54(1):137–141. doi: 10.1016/s0360-3016(02)02902-4. [DOI] [PubMed] [Google Scholar]
- 33.Mishra G, Butler J, Ho C, et al. Phase II trial of induction gemcitabine/CPT-11 followed by a twice-weekly infusion of gemcitabine and concurrent external beam radiation for the treatment of locally advanced pancreatic cancer. Am J Clin Oncol. 2005;28(4):345–350. doi: 10.1097/01.coc.0000159559.42311.c5. [DOI] [PubMed] [Google Scholar]
- 34.Kornek GV, Potter R, Selzer E, et al. Combined radiochemotherapy of locally advanced unresectable pancreatic adenocarcinoma with mitomycin C plus 24-hour continuous infusional gemcitabine. Int J Radiat Oncol Biol Phys. 2001;49(3):665–671. doi: 10.1016/s0360-3016(00)01388-2. [DOI] [PubMed] [Google Scholar]
- 35.Bianco C, Giovannetti E, Ciardiello F, et al. Synergistic antitumor activity of ZD6474, an inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling, with gemcitabine and ionizing radiation against pancreatic cancer. Clin Cancer Res. 2006;12(23):7099–7107. doi: 10.1158/1078-0432.CCR-06-0833. [DOI] [PubMed] [Google Scholar]
- 36.Duffy A, Kortmansky J, Schwartz GK, et al. A phase I study of erlotinib in combination with gemcitabine and radiation in locally advanced, non-operable pancreatic adenocarcinoma. Ann Oncol. 2008;19(1):86–91. doi: 10.1093/annonc/mdm441. [DOI] [PubMed] [Google Scholar]
- 37.Krempien R, Muenter MW, Huber PE, et al. Randomized phase II--study evaluating EGFR targeting therapy with cetuximab in combination with radiotherapy and chemotherapy for patients with locally advanced pancreatic cancer--PARC: study protocol [ISRCTN56652283] BMC Cancer. 2005;5:131. doi: 10.1186/1471-2407-5-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan MA, Parsels LA, Kollar LE, Normolle DP, Maybaum J, Lawrence TS. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res. 2008;14(16):5142–5149. doi: 10.1158/1078-0432.CCR-07-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crane CH, Winter K, Regine WF, et al. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol. 2009;27(25):4096–4102. doi: 10.1200/JCO.2009.21.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkowski R, Thoma M, Bruns C, Wagner A, Heinemann V. Chemoradiotherapy with gemcitabine and continuous 5-FU in patients with primary inoperable pancreatic cancer. Jop. 2006;7(4):349–360. [PubMed] [Google Scholar]
- 41.Talamonti MS, Catalano PJ, Vaughn DJ, et al. Eastern cooperative oncology group phase I trial of protracted venous infusion fluorouracil plus weekly gemcitabine with concurrent radiation therapy in patients with locally advanced pancreas cancer: A regimen with unexpected early toxicity. J Clin Oncol. 2000;18(19):3384–3389. doi: 10.1200/JCO.2000.18.19.3384. [DOI] [PubMed] [Google Scholar]
- 42.Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB., 3rd Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20(15):3270–3275. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 43.Philip PA, Mooney M, Jaffe D, et al. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol. 2009;27(33):5660–5669. doi: 10.1200/JCO.2009.21.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loehrer P, Powell M, Cardenes H, et al. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer: E4201. J Clin Oncol. 2008;26(214S) doi: 10.1097/COC.0b013e3181e9c103. (suppl: abstr 4506) [DOI] [PubMed] [Google Scholar]
- 45.GITSG. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80(10):751–755. [PubMed] [Google Scholar]
- 46.Shinchi H, Takao S, Noma H, et al. Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2002;53(1):146–150. doi: 10.1016/s0360-3016(01)02806-1. [DOI] [PubMed] [Google Scholar]
- 47.Li CP, Chao Y, Chi KH, et al. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. Int J Radiat Oncol Biol Phys. 2003;57(1):98–104. doi: 10.1016/s0360-3016(03)00435-8. [DOI] [PubMed] [Google Scholar]
- 48.Rich T, Harris J, Abrams R, et al. Phase II study of external irradiation and weekly paclitaxel for nonmetastatic, unresectable pancreatic cancer: RTOG-98-12. Am J Clin Oncol. 2004;27(1):51–56. doi: 10.1097/01.coc.0000046300.88847.bf. [DOI] [PubMed] [Google Scholar]
- 49.Cohen SJ, Dobelbower R, Jr, Lipsitz S, et al. A randomized phase III study of radiotherapy alone or with 5-fluorouracil and mitomycin-C in patients with locally advanced adenocarcinoma of the pancreas: Eastern Cooperative Oncology Group study E8282. Int J Radiat Oncol Biol Phys. 2005;62(5):1345–1350. doi: 10.1016/j.ijrobp.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 50.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23(15):3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 51.Talamonti MS, Catalano PJ, Vaughn DJ, et al. Eastern Cooperative Oncology Group Phase I trial of protracted venous infusion fluorouracil plus weekly gemcitabine with concurrent radiation therapy in patients with locally advanced pancreas cancer: a regimen with unexpected early toxicity. J Clin Oncol. 2000;18(19):3384–3389. doi: 10.1200/JCO.2000.18.19.3384. [DOI] [PubMed] [Google Scholar]
- 52.Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25(3):326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 53.Ko AH, Quivey JM, Venook AP, et al. A phase II study of fixed-dose rate gemcitabine plus low-dose cisplatin followed by consolidative chemoradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68(3):809–816. doi: 10.1016/j.ijrobp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110(1):47–55. doi: 10.1002/cncr.22735. [DOI] [PubMed] [Google Scholar]
- 55.Reni M, Cereda S, Balzano G, et al. Outcome of upfront combination chemotherapy followed by chemoradiation for locally advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2009;64(6):1253–1259. doi: 10.1007/s00280-009-0995-8. [DOI] [PubMed] [Google Scholar]
- 56.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324(5924):217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong HH, Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nat Rev Gastroenterol Hepatol. 2009;6(7):412–422. doi: 10.1038/nrgastro.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]