Abstract
Cancer-testis antigens such as NY-ESO-1, MAGE-A1 and MAGE-A3 are immunogenic proteins encoded by genes, which are normally expressed only in male germ cells, but activated by ill-defined epigenetic mechanisms in human tumors including lung cancers. Previously we reported induction of these cancer-testis antigens in cancer cells, but not normal cells, by DNA demethylating agents and histone deacetylase inhibitors using clinically achievable exposure conditions. In the present study, we evaluated chromatin alterations associated with repression/activation of cancer-testis genes in lung cancer cells to further develop gene induction regimens for cancer immunotherapy. Repression of NY-ESO-1, MAGE-A1, and MAGE-A3 coincided with DNA hypermethylation, recruitment and binding of polycomb group proteins, and histone heterochromatin modifications within the promoters of these genes. De-repression coincided with DNA demethylation, dissociation of polycomb proteins, and presence of euchromatin marks within the respective promoters. ShRNAs were used to inhibit several histone methyl transferases (KMTs) and histone demethylases (KDMs) that mediate histone methylation and repress gene expression. Knockdown of KMT6, KDM1 or KDM5B markedly enhanced deoxyazacytidine (DAC)-mediated activation of these cancer-testis genes in lung cancer cells. DZNep, a pharmacologic inhibitor of KMT6 expression, recapitulated the effects of KMT6 knock-down. Following DAC-DZNep exposure, lung cancer cells were specifically recognized and lysed by allogeneic lymphocytes expressing recombinant T cell receptors recognizing NY-ESO-1 and MAGE-A3. Combining DNA demethylating agents with compounds such as DZNep that modulate histone lysine methylation may provide a novel epigenetic strategy to augment cancer-testis gene expression as an adjunct to adoptive cancer immunotherapy.
Keywords: lung cancer, epigenetics, cancer-testis gene, KMT6, KDM1, KDM5B, SirT1, DZNep, immunotherapy
Introduction
Cancer testis antigens (CTA) are encoded by a unique class of genes [cancer-testis (C-T) genes], normally expressed in germ cells or placenta, that are de-repressed by epigenetic mechanisms in various human malignancies (1). Because they are typically expressed only in immune-privileged sites, CTAs induce humoral as well as cell-mediated immune responses when aberrantly expressed in somatic cells; as such, CTAs have emerged as highly attractive targets for cancer immunotherapy (2). Vaccines targeting CTAs such as NY-ESO-1, MAGE-A1, and MAGE-A3 induce anti-tumor immunity, and T cells expressing native or genetically-engineered receptors recognizing these antigens mediate tumor regression in some cancer patients (3-5).
Approximately 50% of C-T genes including NY-ESO-1, MAGE-A1 and MAGE-A3 are located on the X chromosome (6). Cancer-testis-X chromosome (CT-X) genes are normally expressed in spermatogonia, and typically comprise extended families associated with inverted DNA repeats (7). Relative to autosomal C-T genes, CT-X genes are more frequently activated in cancer cells, and particular gene families appear to be de-repressed in a tumor-specific manner. Although believed to be activated as a result of global DNA demethylation, the epigenetic mechanisms mediating coordinate de-repression of C-T genes during multi-step carcinogenesis have not been fully elucidated (7-9).
Whereas NY-ESO-1, MAGE-A1, and MAGE-A3 are expressed in 25-40% of non-small cell lung cancers (NSCLC) (10),immune responses to these CTAs are uncommon in lung cancer patients (11, 12), due in part to levels of antigen expression, which are below the threshold for immune recognition. Conceivably, up-regulation of CTA expression by chromatin remodeling agents can enhance immunogenicity of lung cancer cells, facilitating their eradication by endogenous immune mechanisms, or adoptively transferred T cells. Previously, we demonstrated that the DNA demethylating agent, 5-aza-2′ deoxycytidine (Decitabine; DAC) and the HDAC inhibitor depsipeptide (romidepsin; DP) mediate synergistic activation of CT-X gene expression in cultured lung cancer cells, but not normal epithelia or lymphoid cells (8). In addition, we reported that following DAC or sequential DAC/DP exposure, lung cancer cells can be recognized by cytolytic T lymphocytes (CTL) expressing receptors specific for NY-ESO-1 or MAGE-A3 (13-15). Furthermore, we have demonstrated up-regulation of NY-ESO-1 as well as MAGE-A3 expression in primary lung cancers in patients receiving 72h continuous Decitabine infusions (steady-state plasma concentrations ~ 50-100nM) (16)(Schrump et al., manuscript in preparation). Lastly, we have shown that a CTA induced in tumor cells in vivo by systemic DAC administration can be effectively targeted by adoptively-transferred CTL in immunocompetent mice (17). The present study was undertaken to comprehensively examine mechanisms regulating NY-ESO-1, MAGE-A1 and MAGE-A3 expression in lung cancer cells to further develop epigenetic strategies for human cancer immunotherapy.
Materials and Methods
Cell lines and drug treatment conditions
All lung cancer lines were obtained from American Type Culture Collection (Manassas, VA), and were characterized and authenticated at the repository by methods including mycoplasma testing, DNA profiling, and cytogenetic analysis; these lines were used within six months of purchase for this study, validated in our laboratory by periodic HLA typing, and cultured as described (8). Primary normal human bronchial epithelial cells (NHBE), small airway epithelial cells (SAEC) and normal human dermal fibroblasts (NHDF) were purchased from Lonza, Inc (Walkersville, MD), and cultured per vendor instructions. Immortalized human bronchial epithelial cells (HBEC) were generously provided by John D. Minna (U-T Southwestern, Dallas, TX), and cultured as described (18). 5-aza-2′-deoxycytidine (DAC) and trichostatin A (TSA) were purchased from Sigma Chemical Co. (St. Louis, MO). DZNep was provided by the Chemical Biology Laboratory, NCI. Depsipeptide (romidepsin; DP) was obtained from the Developmental Therapeutics Program, NCI. The effects of DAC and DZNep treatment on CT-X gene expression were determined after exposure to 0.1 μM DAC or 0.5-5μM DZNep for 72 hours or concurrent DAC(0.1μM)–DZNep (0.5μM) for 72 hrs followed by normal media for 18-24 hours. DAC/DP and DAC/TSA treatments were performed as described (8).
Real time RT-PCR analysis
RNA was isolated using RNeasy mini kit (Qiagen, Valencia, CA). cDNAs were made using reverse transcription kit (Bio-Rad; Hercules, CA). qRT-PCR primers for CT-X genes and β-actin expression are listed in Supplementary Table 1.
Immunoblot analysis
Total cell proteins were extracted and immunoblotting was performed as described (19) with minor modifications, using primary antibodies listed in Supplementary Table 2, appropriate horseradish peroxidase–conjugated secondary antibodies, and SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL). Testis lysate (Abcam) was used as positive control for NY-ESO-1 and MAGE-A1. Lysates from HEK293 cells constitutively expressing MAGE-A3 were used as a positive control for this CTA (15).
Immunofluorescence analysis
NY-ESO-1, MAGE-A1 and MAGE-A3 expression in cultured cells was detected by immunofluorescence techniques using primary antibodies recognizing these CTAs (Supplementary Table 2) and visualized using FITC-labeled secondary antibodies (Supplementary Table 2) as described (20). Nuclei were counterstained with DAPI.
Pyrosequencing analysis
CpG islands within the NY-ESO-1, MAGE-A1 and MAGE-A3 promoters were identified using an online CpG island search engine (21). Genomic DNA was isolated from drug treated or control cells using the Qiagen DNeasy kit. Bisulfite modification of DNA was done using the Qiagen Epifect kit. Pyrosequencing was performed as described (18), using primers listed in Supplementary Table 1.
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed as described (22) with minor modifications. Briefly, DNA-protein complexes were cross-linked with formaldehyde at a final concentration of 1% for 15 min. Immune complexes were formed with either nonspecific IgG or ChIP-grade antibodies listed in Supplementary Table 2. DNA was eluted and purified from complexes, followed by PCR amplification of the NY-ESO-1, MAGE-A1,or MAGE- promoters as previously described (8), using primers listed in Supplementary Table 1.
Generation of KMT6, KDM1, KDM5B or SirT1 knockdown cells and KMT6 overexpressing stable cells
H841 cells were transduced with lentiviral shRNA vectors targeting KMT6, KDM1, KDM5B and SirT1 or sham sequences (Sigma), or transfected with pCMV6-AC-GFP or pCMV6-AC-GFP-KMT6 (Origene, Rockville, MD). Target gene knockdown or overexpression was confirmed by RT-PCR and immunoblot. Stable transfectants (four independent clones for each knockdown or overexpression) were isolated and expanded under puromycin (knockdowns) or G418 selection (over-expressors). Following re-confirmation of target gene knockdown or overexpression, individual clones were pooled for subsequent experiments.
Retroviral transduction of tumor cell lines with HLA-A*0201 and peripheral blood lymphocytes with T cell receptor (TCR) genes against NY-ESO- 1 or MAGE-A3
H1299 or H841 lung cancer cells, SAEC, HBEC or NHDF were transduced with a retroviral vector expressing cDNA of HLA-A*0201 (23). H1299 and H841 cell lines stably expressing HLA-A*0201 were expanded under G418 selection. PBL expressing HLA-A*0201-restricted TCRs recognizing NY-ESO-1 or MAGE-A3 were generated as described (14, 15).
Cytokine release assays
Drug treated or control tumor cells with or without HLA-A*0201 expression were co-cultured with untransduced or MAGE-A3 or NY-ESO-1 TCR transduced lymphocytes; IFN-γ secretion in supernatants was measured by ELISA as described (14, 15).
Chromium release assays
The ability of NY-ESO-1 or MAGE-A3 specific TCR transduced PBL to lyse HLA-A*0201+ lung cancer or normal lung cell targets was measured using 51Cr release assays. Briefly, after DAC, DZNep, DAC-DZNep exposure, H2087, and H841-A*0201 or H1299-A*0201 and their respective controls were co-cultured with effector cells, with subsequent analysis of 51Cr release as described (15, 23).
Results
Expression Profiles of CT-X Genes in Lung Cancer Cells
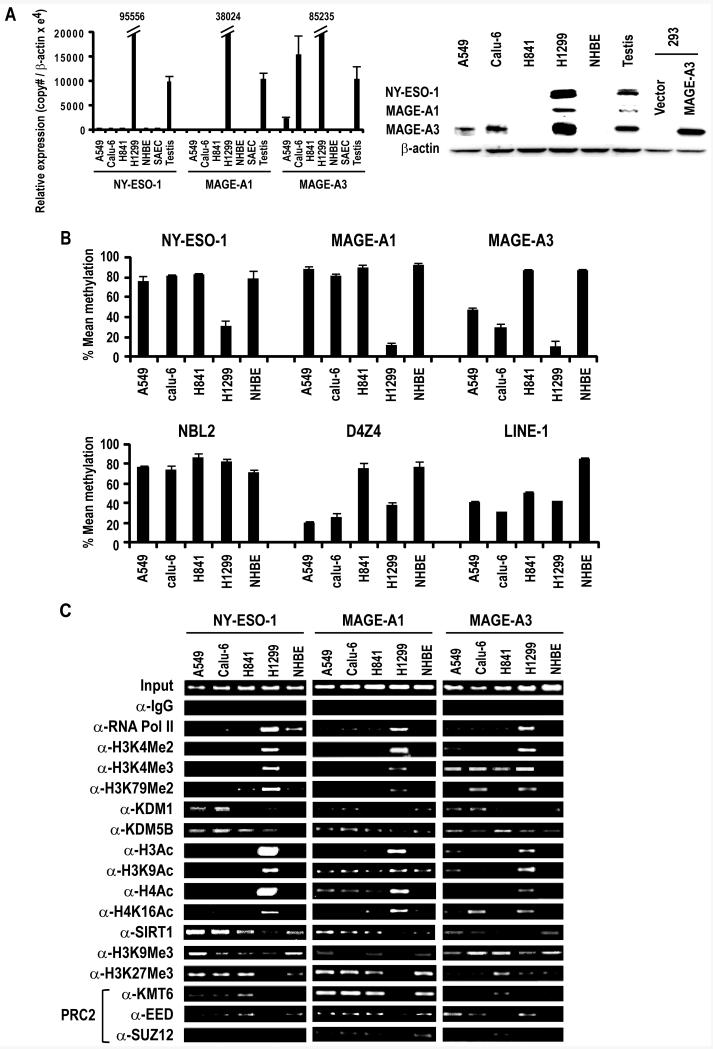
Preliminary qRT-PCR experiments were performed to examine CT-X gene expression in cultured lung cancer cells as well as normal or immortalized respiratory epithelial cells (Supplementary Table 3). This analysis, which revealed heterogeneous CT-X gene expression in non-small cell lung cancer (NSCLC) as well as small cell lung cancer (SCLC) cells, but not normal respiratory epithelia, allowed us to choose several cell lines for further study (Figure 1A; left panel). Relative to control testis, H1299 cells exhibit high level expression of NY-ESO-1, MAGE-A1, and MAGE-A3. In contrast, H841 cells do not express NY-ESO-1, MAGE-A1, or MAGE-A3. A549 and Calu-6 cells exhibit moderate levels of MAGE-A3, but do not express NY-ESO-1 or MAGE-A1. NHBE and SAEC do not express any CT-X genes. Immunoblot analysis (Figure 1A; right panel) confirmed results of these qRT-PCR experiments.
Figure 1.
A. Left Panel: qRT-PCR analysis of NY-ESO-1, MAGE-A1 and MAGE-A3 expression in cultured lung cancer cells, NHBE, SAEC and control testis. Results are expressed as Mean ±SD of three independent experiments. Right Panel: Immunoblot analysis depicting expression of NY-ESO-1, MAGE-A1 and MAGE-A3 in A549, Calu-6, H841 and H1299 lung cancer cells and NHBE cells. Testis protein lysate was used as positive control for NY-ESO-1 and MAGE-A1. For MAGE-A3, protein lysate from HEK293 overexpressing MAGE-A3 was used as a positive control, since the MAGE-A3 antibody exhibits low-level cross-reactivity with other related MAGE A proteins.
B. Methylation status of NY-ESO-1, MAGE-A1, MAGE-A3, NBL2, D4Z4 and LINE-1 sequences in A549, Calu-6, H841 and H1299 lung cancer and NHBE cells as measured by pyrosequencing. Results are expressed as Mean ±SD of three independent experiments.
C. ChIP analysis of the NY-ESO-1, MAGE-A1 and MAGE-A3 promoters in A549, Calu-6, H841, and H1299 lung cancer cells and NHBE cells. Presence of activation (euchromatin) marks and decreased repressive marks within the NY-ESO-1, MAGE-A1 and MAGE-A3 promoters coincided with activation of these CT-X genes. See text for further details.
Chromatin Structure Relative to CT-X Gene Expression in Lung Cancer Cells
Pyrosequencing and chromatin immunoprecipitation (ChIP) experiments were undertaken to examine DNA methylation and a variety of histone marks in lung cancer cells exhibiting differential CT-X gene expression. Pyrosequencing experiments (Figure 1B) revealed that the NY-ESO-1 and MAGE-A1 promoters were hypermethylated in A549, Calu-6, and H841 lung cancer cells as well as normal human bronchial epithelial (NHBE) cells; these promoters were hypomethylated in H1299 cells. In contrast, the MAGE-A3 promoter was hypermethylated in H841 and NHBE cells, partially methylated in A549 and Calu-6, and demethylated in H1299 cells. These findings were consistent with results of aforementioned qRT-PCR and immunoblot experiments. Although demethylation of MAGE-A3 appeared to coincide with demethylation of D4Z4, no consistent relationship was evident regarding NY-ESO-1, MAGE-A1 or MAGE-A3 promoter demethylation and global DNA demethylation assessed by pyrosequencing of NBL2, D4Z4 and LINE-1 repetitive DNA sequences, possibly due to incomplete analysis of these regions by pyrosequencing methods.
ChIP experiments were performed to further investigate epigenetic phenomena associated with repression/activation of NY-ESO-1, MAGE-A1 and MAGE-A3 in lung cancer cells. As shown in Figure 1C, the NY-ESO-1, MAGE-A1 and MAGE-A3 promoters in H1299 cells exhibited increased occupancy of RNA polymerase II (pol II), enrichment of euchromatin/activation marks such as H3K4Me2, H3K4Me3, H3K79Me2, total H3Ac, H3K9Ac, total H4Ac and H4K16Ac, with decreased occupancy of SirT1 as well as polycomb repressor complex (PRC)-2 components (KMT6, EED and SUZ12), and the associated PRC-2 mediated repression mark, H3K27Me3. In contrast, RNA Pol II and histone activation marks were markedly diminished, whereas SirT1, PRC-2 components, and H3K27Me3 levels were considerably higher within the NY-ESO-1 and MAGE-A1 promoters in A549, Calu-6, H841, and NHBE cells, which do not express these CT-X genes. Variable levels of RNA Pol II, as well as activation/repression marks were present within the MAGE-A3 promoter in A549, Calu-6, H841, and NHBE cells, consistent with levels of expression of this CT-X gene in these cells. No consistent relationship was observed between activation/repression of these CT-X genes and H3K9Me3, previously considered to be a mark of stable silenced heterochromatin (24), but more recently shown to coincide with RNA Pol II-mediated gene activation (25). Densitometry results of these ChIP experiments are summarized in Supplementary Table 4. Collectively, these experiments established that differential repression of NY-ESO-1, MAGE-A1, and MAGE-A3 in lung cancer cells is attributable to persistence of apparently normal heterochromatin structure within the promoters of these CT-X genes. Furthermore, levels of euchromatin marks, particularly H3K79Me2 appear to coincide with magnitude of CT-X gene de-repression in lung cancer cells.
Effects of Histone Lysine Methylation on CT-X Gene Expression
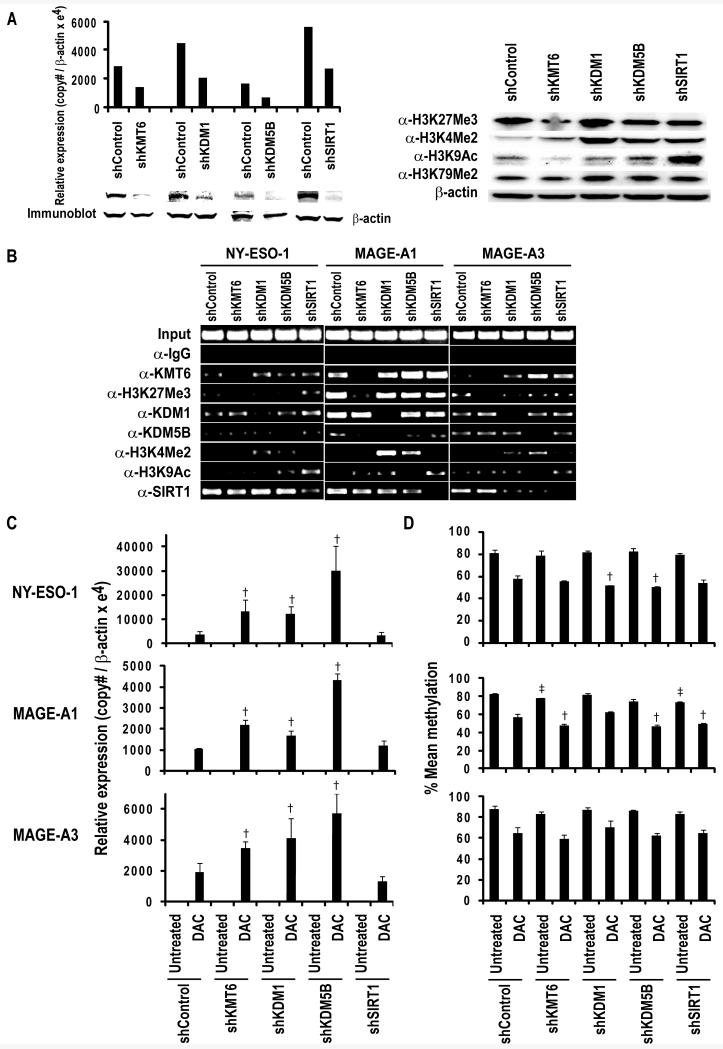
Our previous studies have demonstrated that pharmacologic inhibition of histone lysine deacetylation enhances CT-X gene activation by DNA demethylating agents (10). Therefore, additional experiments were performed to ascertain if modulation of histone lysine methylation alters CT-X gene expression in lung cancer cells. Briefly, lentiviral shRNA transduction techniques were used to knockdown LSD-1(KDM1) and JARID1B (KDM5B) that mediate demethylation of mono-, di-, and trimethylated H3K4 (26, 27), or the histone lysine methyltransferase KMT6 that mediates trimethylation of H3K27 (28) in H841 cells; these cells were chosen for analysis since they do not express NY-ESO-1, MAGE-A1 or MAGE-A3. Preliminary qRT-PCR and immunoblot experiments demonstrated ~50-70% reduction in target gene expression by the respective shRNAs relative to controls (Figure 2A; left panel). Immunoblot analysis (Figure 2A; right panel) demonstrated decreased global H3K27Me3, and increased global H3K9Ac in KMT6 and SirT1 knockdowns, respectively, relative to control cells. Increased global levels of H3K4Me2 were evident in KDM1 and KDM5B knockdowns relative to control cells; interestingly, an increase in this activation mark was also observed in SirT1 knockdown cells. ChIP experiments revealed that global changes in these activation and repression marks tended to coincide with similar alterations and decreased occupancy of the respective histone modifiers in the NY-ESO-1, MAGE-A1 and MAGE-A3 promoters in knockdowns relative to control cells (Figure 2B). Subsequent qRT-PCR experiments revealed that knockdown of KMT6, KDM1 or KDM5B alone was insufficient to activate NY-ESO-1, MAGE-A1, or MAGE-A3 in H841 lung cancer cells. However, knockdown of KMT6, KDM1, or KDM5B enhanced DAC-mediated induction of these CT-X genes approximately 3-11 fold in these cells (Figure 2C); knockdown of KDM5B appeared to have the most effect regarding potentiation of DAC-mediated CT-X gene activation in lung cancer cells. The effects of targeted modulation of histone lysine methylation appeared more pronounced than those observed following knockdown of the class III histone deacetylase, SirT1.
Figure 2.
A. Left Panel: qRT-PCR (top panel) and immunoblot analysis (lower panel) of KMT6, KDM1, KDM5B and SirT1 in H841 cells transfected with shRNAs against respective targets or sham control sequences, demonstrating target gene knockdown. Right Panel: Immunoblot analysis demonstrating that knockdown of KMT6 and SirT1 leads to decreased levels of H3K27Me3 and H3K9Ac respectively, whereas knockdown of KDM1 and KDM5B results in increased global H3K4Me2 levels. As expected, no changes in global H3K79Me2 levels were seen in these knockdowns.
B. ChIP analysis of the NY-ESO-1, MAGE-A1 and MAGE-A3 promoters in knockdown of KMT6, KDM1, KDM5B and SirT1 in H841 cells. Knockdown of KMT6 and SirT1 was associated with decreased occupancy of these histone modifiers, with corresponding changes in H3K27Me3 and H3K9Ac, respectively; knockdown of KDM1 and KDM5B decreased occupancy of these HMTs with a corresponding increase in H3K4Me2 within the NY-ESO-1, MAGE-A1 and MAGE-A3 promoters.
C. qRT- PCR analysis demonstrating that KMT6, KDM1 and KDM5B knockdown significantly enhances DAC-mediated activation of NY-ESO-1, MAGE-A1 and MAGE-A3 in H841 cells. Effects of SirT1 knockdown were considerably less pronounced in these cells. The bars represent Mean ±SD of triplicate experiments. † P<0.05 vs DAC-shControl.
D. Pyrosequencing analysis of NY-ESO-1, MAGE-A1 and MAGE-A3 promoter methylation in H841 cells with or without knockdown of KMT6, KDM1, KDM5B or SirT1 exposed to normal media or DAC. Knockdown of these histone modifiers had variable and relatively modest effects on methylation status of these CT-X promoters. † P<0.05 vs DAC-shControl; ‡ P<0.05 vs untreated-shControl.
Additional pyrosequencing experiments were performed to ascertain if modulation of histone lysine methylation affected DNA methylation status of NY-ESO-1, MAGE-A1, and MAGE-A3 in DAC-treated and control lung cancer cells. Results of this analysis are depicted in Figure 2D. The effects of histone methylation changes varied somewhat among the three CT-X genes. In general, the effects of KMT6, KDM1 or KDM5B knockdown on NY-ESO-1, MAGE-A1 or MAGE-A3 promoter methylation were modest, and did not directly coincide with magnitude of enhancement of DAC-mediated activation of these CT-X genes. A similar phenomenon was observed following knockdown of SirT1 in H841 cells.
Effects of DZNep on CT-X Gene Expression
Additional experiments were performed to ascertain if pharmacologic agents in preclinical development could recapitulate the aforementioned effects of histone lysine methylation on DAC-mediated activation of CT-X genes. Our studies focused on DZNep, a novel inhibitor of PRC-2 expression (29). Briefly, lung cancer cells were cultured for 72h in normal media with or without DAC (0.1μM), DZNep (0.5 or 5μM) or concurrent DAC(0.1μM)-DZNep(0.5μM) followed by analysis 24h later. Preliminary immunoblot experiments demonstrated that DZNep mediated dose-dependent depletion of KMT6, EED and SUZ12 with concomitant reduction in global H3K27Me3 levels in H841 cells (Figure 3A; left panel). QRT-PCR experiments revealed that DZNep mediated modest dose-dependent reductions in KMT6 and SUZ12 but not EED mRNA levels (Figure 3A; right panel). Additional experiments demonstrated that low dose DZNep (0.5μM-approximately one log lower than the cytotoxic dose of this agent in cancer cells) mediated very modest activation of NY-ESO-1, MAGE-A1 and MAGE-A3 in H841 cells; in contrast, DZNep significantly enhanced DAC-mediated CT-X gene activation in these cells (Figure 3B). Immunofluorescence experiments confirmed that DZNep enhanced DAC-mediated expression of NY-ESO-1, MAGE-A1 and MAGE-A3 in H841 cells (Figure 3C). This phenomenon extended to other CT-X genes such as MAGE-A12 (Figure 3B), and was observed in other lung cancer lines (Supplementary Table 5). The magnitude of enhancement of DAC-mediated de-repression of CT-X genes in cancer cells by DZNep was markedly higher than that observed in SAEC (Figure 3B) or NHBE (data not shown). Relative to normal SAEC, immortalized HBEC appeared more responsive to DAC and DZNep; however DZNep did not appear to augment DAC-mediated CT-X gene activation in these cells. The magnitude of DAC-DZNep mediated CT-X gene induction in lung cancer cells approximated or exceeded that observed following sequential DAC-DP or DAC-TSA treatment; addition of TSA or DP did not consistently improve CT-X gene activation mediated by low-dose DAC-DZNep (Supplementary Table 5).
Figure 3.
A. Left Panel: Immunoblot analysis demonstrating dose-dependent depletion of KMT6, EED and SUZ12 and reduced global H3K27Me3 levels in H841 cells mediated by DZNep. Right Panel: qRT-PCR analysis of KMT6, EED and SUZ12 levels in H841 cells following 72h DZNep exposure. * P<0.05, DZNep vs untreated.
B. qRT-PCR analysis of NY-ESO-1, MAGE-A1 and MAGE-A3 expression in cultured cells following DAC, DZNep or DAC-DZNep treatment. Modest CT-X gene activation was seen in immortalized HBEC cells, but not in SAEC cells. The bars represent the Mean ±SD of triplicate experiments. * P<0.05, DAC vs untreated; † P<0.05, DAC-DZNep vs DAC; ‡ P<0.05, DZNep vs untreated.
C. Immunofluorescence analysis of NY-ESO-1, MAGE-A1 and MAGE-A3 expression in H841 cells following DAC, DZNep or DAC-DZNep exposure. H1299 used as a positive control.
Effects of DZNep on DNA Methylation and H3K27Me3 within CT-X Gene Promoters
Pyrosequencing and ChIP analyses were performed to further examine the mechanisms by which DZNep modulates CT-X gene expression in lung cancer cells. Results of these experiments are depicted in Figure 4. NHBE and H1299 were used as positive and negative methylation controls, respectively. As anticipated, DAC-mediated activation of NY-ESO-1, MAGE-A1 and MAGE-A3 coincided with significant demethylation of the respective promoters. In contrast to what was observed following histone lysine methyltransferase knockdown (Figure 2D), DZNep alone mediated a modest, but significant demethylation of all three CT-X gene promoters (Figure 4A); in combination with DAC, DZNep exhibited an additive demethylation effect in the NY-ESO-1 and MAGE-A3 promoters (Figure 4A). The effects of DAC, DZNep or combined DAC-DZNep on NY-ESO-1, MAGE-A1 and MAGE-A3 promoters coincided with similar effects on global DNA methylation assessed by pyrosequencing analysis of NBL2, D4Z4 and LINE-1 sequences (Figure 4B). Subsequent ChIP experiments confirmed that DZNep decreased KMT6 and H3K27Me3 levels within the NY-ESO-1, MAGE-A1 and MAGE-A3 promoters (Figure 4C); the magnitude of decrease in KMT6 and H3K27Me3 levels appeared to coincide with extent of demethylation and de-repression of these promoters.
Figure 4.
A/B. Pyrosequencing analysis of NY-ESO-1, MAGE-A1 and MAGE-A3 promoters and NBL2, D4Z4 and LINE-1 methylation in H841 cells following treatment with DAC, DZNep, or DAC-DZNep. Relative to treatment with either agent alone, DAC-DZNep appeared to exert a modest additive effect on demethylation of these sequences. CT-X promoter demethylation appeared to coincide with global DNA demethylation mediated by the treatment regimens. † P<0.05, DAC+DZNep vs DAC; ‡ P<0.05, DZNep vs untreated.
C. ChIP analysis demonstrating reduced KMT6 and H3K27Me3 levels within the NY-ESO-1, MAGE-A1 and MAGE-A3 promoters in H841 cells following treatment with DAC, DZNep, or DAC-DZNep. Concurrent DAC-DZNep treatment appeared to markedly decrease KMT6 and H3K27Me3 levels within these promoters.
Effects of KMT6 Overexpression on CT-X Gene Activation Mediated by DZNep
Additional experiments were undertaken to specifically examine if the effects of DZNep on DAC-mediated activation of CT-X genes were attributable, at least in part, to depletion of KMT6. Briefly, H841 cells stably expressing KMT6 were treated with DAC, DZNep, or combined DAC-DZNep as previously described. Immunoblot analysis (Figure 5A; left panel) demonstrated increased global levels of KMT6 and H3K27Me3 in KMT6-transfected H841 cells relative to vector controls. DZNep markedly depleted KMT6 and H3K27Me3 levels in KMT6 overexpressors. QRT-PCR experiments demonstrated a modest, but statistically insignificant diminution of KMT6 expression by DZNep (Figure 5A; right panel). Additional qRT-PCR experiments revealed that overexpression of KMT6 significantly attenuated the enhancement effect of DZNep on DAC-mediated induction of NY-ESO-1, MAGE-A1 or MAGE-A3 (Figure 5B).
Figure 5.
A. Left Panel: Immunoblot analysis showing DZNep significantly reduces KMT6 and H3K27Me3 protein levels in H841 cells overexpressing KMT6 (H841-KMT6) as well as vector controls. Right Panel: qRT-PCR analysis of KMT6 in H841-KMT6 cells.
B. qRT-PCR analysis revealing significant attenuation of DAC-DZNep mediated activation of NY-ESO-1, MAGE-A1 and MAGE-A3 expression in H841-KMT6 cells relative to vector controls following treatment with DAC-DZNep. The bars represent Mean ±SD of triplicate experiments. * P<0.05, ** P<0.005.
C. Epigenetic modification patterns of CT-X genes in normal and cancer cells. In NHBE and H841 lung cancer cells, which do not express NY-ESO-1, MAGE-A1, or MAGE-A3, respective promoters exhibit hypermethylated DNA, occupancy of PRC-2-SIRT1 complex, and repressive heterochromatin marks such as H3K9Me3 and H3K27Me3. H1299 lung cancer cells expressing these CT-X genes exhibit hypomethylated DNA, dissociation of PRC-2-SIRT1, decreased heterochromatin marks, and presence of euchromatin marks such as H3K4Me2/3 and H3K9Ac within the respective promoters. DAC/DZNep treatment activates CT-X gene promoters by inhibiting DNMT and PRC-2 activity.
Recognition of Lung Cancer Cells by NY-ESO-1 and MAGE-A3-Specific TCR-Engineered T cells following DZNep Exposure
Additional experiments were performed to examine if DZNep enhances immunogenicity of lung cancer cells. Briefly, H2087 lung cancer cells, which endogenously express HLA-A*0201, as well as SAEC (chosen because they proliferate faster than NHBE) and H841 cells transduced with HLA A*0201 (SAEC-A2 and H841-A2, respectively) were exposed to NM, DAC, DZNep, or DAC-DZNep as previously described, and subsequently co-cultured with TCR-engineered PBL recognizing NY-ESO-1 or MAGE-A3 in the context of HLA-A*0201. Representative results from two independent experiments performed using PBL from two different patients are depicted in Figure 6. For these experiments, H1299 and H1299-A2 cells served as negative and positive controls, respectively (Figure 6A). As shown in Figure 6B, increased interferon-γ release was observed following co-culture of NY-ESO-1 and MAGE-A3 effector cells with H2087 as well as H841-A2 cells previously exposed to DAC, which was significantly augmented by concomitant exposure to DZNep (0.5μM). Very low level cytokine release was observed following co-culture of effector cells with DZNep-treated H2087 and H841-A2 targets. The magnitude of enhancement of DAC-mediated cytokine release by DZNep was more pronounced for MAGE-A3 relative to NY-ESO-1 effector cells; these results were consistent with qRT-PCR analysis of CT-X gene expression in target cells following drug treatment. Background levels of interferon-γ release were observed following co-culture of effector cells with parental untreated H1299 cells, or drug-treated H841 cells lacking HLA A*0201 expression. Effector cells did not recognize drug treated HLA-A*0201-transduced SAEC (Figure 6B), nor HBEC or NHDF (data not shown), presumably due to very low levels of NY-ESO-1 and MAGE-A3 induction in these cells by the treatment regimen (Supplementary Table 6).
Figure 6.
A. Interferon-γ release by HLA-restricted CTLs recognizing NY-ESO-1 and MAGE-A3 in the context of HLA *A0201 after overnight incubation with H1299 or H1299-A2 targets (negative and positive controls, respectively).
B. Interferon-γ release by HLA-restricted CTLs specific for NY-ESO-1 and MAGE-A3 after overnight incubation with H2087, as well as H841-A2 and SAEC-A2. Targets represent untreated controls, or cells exposed to DAC (0.1μM), DZNep (0.5 μM) or DAC (0.1μM) –DZNep (0.5 μM). Data are representative of two independent experiments performed with two different PBL donors. * P<0.05, DAC vs untreated; † P<0.05, DAC-DZNep vs DAC; ‡ P<0.05 vs DZNep vs untreated.
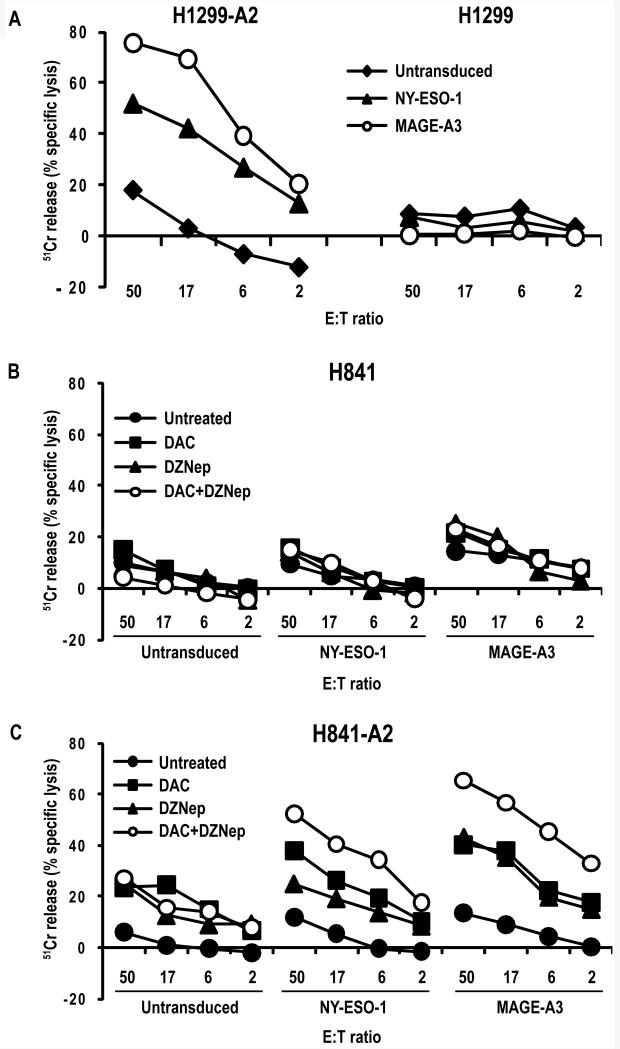
Chromium release experiments were performed to evaluate lysis of H841-A2 cells by MAGE-A3 or NY-ESO-1 specific effector cells. Representative results from two independent experiments performed using PBL from two different donors are depicted in Figure 7. H1299-A2 and parental H1299 cells served as positive and negative controls, respectively (Figure 7A). Low-level lysis was observed following co-culture of untransduced effector cells with H841 targets possibly due to non-specific alloreactivity, recognition of tumor targets by endogenous T cell receptors, presence of NK cells, as well as mild toxicity of the drug treatment regimens (Figure 7B). Compared to untreated controls, DAC-treated H841-A2 cells were more efficiently lysed by the effector cells (Figure 7C). Interestingly, DZNep treatment also lead to increased lysis of H841-A2 cells; whereas percent specific lysis of H841-A2 cells treated with DAC exceeded that observed following treatment of target cells with DZNep for NY-ESO-1 effector cells, percent lysis following exposure of tumor targets to DZNep was comparable to that observed following treatment with DAC when tumor targets were co-cultured with MAGE-A3 effector cells. Concurrent DAC-DZNep treatment of target cells markedly enhanced percent specific lysis mediated by NY-ESO-1 or MAGE-A3 effector cells. The magnitude of lysis of DAC, DZNep, or DAC-DZNep-treated H841-A2 cells by MAGE-A3 effector cells exceeded that observed for NY-ESO-1 effector cells, possibly due to simultaneous up-regulation of other MAGE-A genes such as MAGE-A12 encoding HLA-A*0201-restricted epitopes recognized by the genetically engineered MAGE-A3 TCR(15). Specific lysis of H841-A2 cells by NY-ESO-1 and MAGE-A3 effector cells corresponded with mRNA copy numbers (Supplementary Table 6) and interferon-γ release observed in co-culture assays.
Figure 7.
A. 51Cr release assay depicting lysis of H1299 and H1299-A2 targets by NY-ESO-1 and MAGE-A3 TCR-transduced PBLs. Minimal lysis was observed against HLA-A2 negative cells.
B/C. Lysis of H841 and H842-A2 cells by NY-ESO-1 or MAGE-A3 or control vector – transduced PBLs following pretreatment of tumor targets with DAC, DZNep, or DAC-DZNep. Both NY-ESO-1 and MAGE-A3 effector cells mediated lysis of untreated H1299-A2 and H841-A2 targets following DAC, DZNep or DAC-DZNep treatment. Pretreatment of tumor targets with DAC, DZNep, or DAC-DZNep markedly enhanced specific lysis mediated by NY-ESO-1 and MAGE-A3 effector cells.
Discussion
DNA methylation is the major epigenetic mechanism silencing CT-X genes in normal somatic cells (30, 31). Whereas CT-X gene expression can be induced in cancer cells by DNA demethylating agents (10), or simultaneous knockdown of DNMT1 and DNMT3b (32, 33), de-repression of CT-X genes during malignant transformation cannot be attributed solely to global DNA demethylation. Transfected methylated MAGE-A1 transgenes do not undergo promoter demethylation, and unmethylated MAGE-A1 transgenes become methylated except for the 5′ region in cancer cells (34). Complex chromatin architecture including formation of double cruciform DNA(35) that potentially affects access of methyl binding proteins, DNMTs, and transcription factors such as CTCF, BORIS and SP1(8, 32, 36) may contribute to coordinated repression/activation of CT-X genes within large inverted repeats (7).
In the present study, we sought to examine the feasibility of modulating histone lysine methylation as a strategy to enhance CT-X gene activation by DNA demethylating agents under conditions potentially achievable in clinical settings (16). Our experiments demonstrated that knockdown of KMT6, KDM1 and KDM5B significantly enhances DAC-mediated activation of NY-ESO-1 and several MAGE-A genes in lung cancer cells. Whereas knockdown of KMT6, KDM1 and KDM5B coincided with decreased occupancy of these histone lysine methyltransferases and their respective marks within the NY-ESO-1, MAGE-A1 and MAGE-A3 promoters, our data do not exclude the possibility that depletion of these histone modifiers facilitates CT-X gene activation via mechanisms independent of inhibition of methyltransferase activity (37).
Originally developed as an anti-viral agent (38),DZNep has been shown to deplete KMT6, EED and SUZ12 primarily via proteolytic mechanisms leading to growth arrest, differentiation or apoptosis in cancer cells depending on histology and genotype (29, 39-43). Of particular interest, tumor initiating cells appear exquisitely sensitive to DZNep, due to the critical role of polycomb proteins in maintenance of cancer stem cells (44). In addition to decreasing global H3K27Me3 levels, DZNep diminishes numerous other repressive as well as activation histone lysine methylation marks such as H3K9Me2, and H3K4Me3, respectively (45). DZNep reactivates genes silenced by polycomb mechanisms; however, despite the fact that DZNep exhibits mild DNA demethylating effects, this agent is insufficient to de-repress hypermethylated genes (45). Our analysis revealed that low dose DZNep alone did not activate NY-ESO-1, MAGE-A1 or MAGE-A3 in lung cancer cells, but significantly enhanced DAC-mediated induction of these CT-X genes. Although our experiments suggested that enhancement of DAC-mediated CT-X gene induction by DZNep is attributable in part to depletion of KMT6, the precise mechanisms underlying this phenomenon have not been fully defined, and are a focus of ongoing experiments.
Deciphering the mechanisms mediating de-repression of CT-X genes in cancer cells may provide fundamental insights regarding malignant transformation, and facilitate development of novel strategies for epigenetic therapy for cancer. Our observations that DZNep enhances DAC-mediated up-regulation of NY-ESO-1 and MAGE-A family members, and markedly augments recognition and lysis of lung cancer cells by T cells specific for these CTAs, have direct translational implications regarding the development of gene-induction regimens for cancer immunotherapy. Our findings pertaining to the lack of CT-X gene induction in normal cells following DAC-DZNep exposure are consistent with our previously published data demonstrating negligible activation of CT-X genes in SAEC or NHBE by DAC/DP (13, 14)or normal tissues from lung cancer patients receiving these agents (16, 46). The fact that the magnitude of DAC-DZNep (as well as DAC/DP)-mediated CT-X gene induction is more pronounced in HBEC relative to SAEC, NHBE, or NHDF, but less than lung cancer cells with similar proliferation rates, suggests that global methylation changes associated with malignant transformation (18) contribute in part to the relative sensitivity of cancer cells to epigenetic treatment regimens. Whereas the mechanisms underlying this intriguing phenomenon remain elusive and are a focus of ongoing investigation, our data support further development of DZNep and other inhibitors of histone lysine methylation for cancer immunotherapy.
Supplementary Material
Acknowledgements
The authors express our gratitude to Ms. Jan Pappas for assistance with preparation of this manuscript.
References
- (1).Almeida LG, Sakabe NJ, deOliveira AR, et al. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 2009;37:D816–D819. doi: 10.1093/nar/gkn673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100:2014–21. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Karbach J, Gnjatic S, Bender A, et al. Tumor-reactive CD8+ T-cell responses after vaccination with NY-ESO-1 peptide, CpG 7909 and Montanide ISA-51: association with survival. Int J Cancer. 2010;126:909–18. doi: 10.1002/ijc.24850. [DOI] [PubMed] [Google Scholar]
- (4).Carrasco J, Van PA, Neyns B, et al. Vaccination of a melanoma patient with mature dendritic cells pulsed with MAGE-3 peptides triggers the activity of nonvaccine anti-tumor cells. J Immunol. 2008;180:3585–93. doi: 10.4049/jimmunol.180.5.3585. [DOI] [PubMed] [Google Scholar]
- (5).Robbins PF, Morgan RA, Feldman SA, et al. Tumor Regression in Patients With Metastatic Synovial Cell Sarcoma and Melanoma Using Genetically Engineered Lymphocytes Reactive With NY-ESO-1. J Clin Oncol. 2011;29:917–24. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Stevenson BJ, Iseli C, Panji S, et al. Rapid evolution of cancer/testis genes on the X chromosome. BMC Genomics. 2007;8:129. doi: 10.1186/1471-2164-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bredenbeck A, Hollstein VM, Trefzer U, Sterry W, Walden P, Losch FO. Coordinated expression of clustered cancer/testis genes encoded in a large inverted repeat DNA structure. Gene. 2008;415:68–73. doi: 10.1016/j.gene.2008.02.019. [DOI] [PubMed] [Google Scholar]
- (8).Hong JA, Kang Y, Abdullaev Z, et al. Reciprocal binding of CTCF and BORIS to the NY-ESO-1 promoter coincides with derepression of this cancer-testis gene in lung cancer cells. Cancer Res. 2005;65:7763–74. doi: 10.1158/0008-5472.CAN-05-0823. [DOI] [PubMed] [Google Scholar]
- (9).Vatolin S, Abdullaev Z, Pack SD, et al. Conditional expression of the CTCF-paralogous transcriptional factor BORIS in normal cells results in demethylation and derepression of MAGE-A1 and reactivation of other cancer-testis genes. Cancer Res. 2005;65:7751–62. doi: 10.1158/0008-5472.CAN-05-0858. [DOI] [PubMed] [Google Scholar]
- (10).Schrump DS, Nguyen DM. Targeting the epigenome for the treatment and prevention of lung cancer. Semin Oncol. 2005;32:488–502. doi: 10.1053/j.seminoncol.2005.07.007. [DOI] [PubMed] [Google Scholar]
- (11).Stockert E, Jager E, Chen YT, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–54. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Groeper C, Gambazzi F, Zajac P, et al. Cancer/testis antigen expression and specific cytotoxic T lymphocyte responses in non small cell lung cancer. Int J Cancer. 2007;120:337–43. doi: 10.1002/ijc.22309. [DOI] [PubMed] [Google Scholar]
- (13).Weiser TS, Guo ZS, Ohnmacht GA, et al. Sequential 5-Aza-2 deoxycytidine-depsipeptide FR901228 treatment induces apoptosis preferentially in cancer cells and facilitates their recognition by cytolytic T lymphocytes specific for NY-ESO-1. J Immunother. 2001;24:151–61. doi: 10.1097/00002371-200103000-00010. [DOI] [PubMed] [Google Scholar]
- (14).Wargo JA, Robbins PF, Li Y, et al. Recognition of NY-ESO-1+ tumor cells by engineered lymphocytes is enhanced by improved vector design and epigenetic modulation of tumor antigen expression. Cancer Immunol Immunother. 2009;58:383–94. doi: 10.1007/s00262-008-0562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Chinnasamy N, Wargo JA, Yu Z, et al. A TCR Targeting the HLA-A*0201-Restricted Epitope of MAGE-A3 Recognizes Multiple Epitopes of the MAGE-A Antigen Superfamily in Several Types of Cancer. J Immunol. 2011;186:685–96. doi: 10.4049/jimmunol.1001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Schrump DS, Fischette MR, Nguyen DM, et al. Phase I study of decitabine-mediated gene expression in patients with cancers involving the lungs, esophagus, or pleura. Clin Cancer Res. 2006;12:5777–85. doi: 10.1158/1078-0432.CCR-06-0669. [DOI] [PubMed] [Google Scholar]
- (17).Guo ZS, Hong JA, Irvine KR, et al. De novo induction of a cancer/testis antigen by 5-aza-2′-deoxycytidine augments adoptive immunotherapy in a murine tumor model. Cancer Res. 2006;66:1105–13. doi: 10.1158/0008-5472.CAN-05-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Liu F, Killian JK, Yang M, et al. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene. 2010;29:3650–64. doi: 10.1038/onc.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Xi S, Yang M, Tao Y, et al. Cigarette smoke induces C/EBP-beta-mediated activation of miR-31 in normal human respiratory epithelia and lung cancer cells. PLoS One. 2010;5:e13764. doi: 10.1371/journal.pone.0013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Liu W, Cheng S, Asa SL, Ezzat S. The melanoma-associated antigen A3 mediates fibronectin-controlled cancer progression and metastasis. Cancer Res. 2008;68:8104–12. doi: 10.1158/0008-5472.CAN-08-2132. [DOI] [PubMed] [Google Scholar]
- (21).http://cpgislands.use.edu/cpg.aspx.
- (22).Hussain M, Rao M, Humphries AE, et al. Tobacco smoke induces polycomb-mediated repression of Dickkopf-1 in lung cancer cells. Cancer Res. 2009;69:3570–8. doi: 10.1158/0008-5472.CAN-08-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–23. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).McGarvey KM, Fahrner JA, Greene E, Martens J, Jenuwein T, Baylin SB. Silenced tumor suppressor genes reactivated by DNA demethylation do not return to a fully euchromatic chromatin state. Cancer Res. 2006;66:3541–9. doi: 10.1158/0008-5472.CAN-05-2481. [DOI] [PubMed] [Google Scholar]
- (25).Wiencke JK, Zheng S, Morrison Z, Yeh RF. Differentially expressed genes are marked by histone 3 lysine 9 trimethylation in human cancer cells. Oncogene. 2008;27:2412–21. doi: 10.1038/sj.onc.1210895. [DOI] [PubMed] [Google Scholar]
- (26).Shi Y, Lan F, Matson C, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–53. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- (27).Xiang Y, Zhu Z, Han G, et al. JARID1B is a histone H3 lysine 4 demethylase up-regulated in prostate cancer. Proc Natl Acad Sci U S A. 2007;104:19226–31. doi: 10.1073/pnas.0700735104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Cao R, Wang L, Wang H, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- (29).Tan J, Yang X, Zhuang L, et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–63. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).De Smet C, Lurquin C, Lethe B, Martelange V, Boon T. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol. 1999;19:7327–35. doi: 10.1128/mcb.19.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Link PA, Gangisetty O, James SR, et al. Distinct roles for histone methyltransferases G9a and GLP in cancer germ-line antigen gene regulation in human cancer cells and murine embryonic stem cells. Mol Cancer Res. 2009;7:851–62. doi: 10.1158/1541-7786.MCR-08-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Kang Y, Hong JA, Chen GA, Nguyen DM, Schrump DS. Dynamic transcriptional regulatory complexes including BORIS, CTCF and Sp1 modulate NY-ESO-1 expression in lung cancer cells. Oncogene. 2007;26:4394–403. doi: 10.1038/sj.onc.1210218. [DOI] [PubMed] [Google Scholar]
- (33).James SR, Link PA, Karpf AR. Epigenetic regulation of X-linked cancer/germline antigen genes by DNMT1 and DNMT3b. Oncogene. 2006;25:6975–85. doi: 10.1038/sj.onc.1209678. [DOI] [PubMed] [Google Scholar]
- (34).De Smet C, Loriot A, Boon T. Promoter-dependent mechanism leading to selective hypomethylation within the 5′ region of gene MAGE-A1 in tumor cells. Mol Cell Biol. 2004;24:4781–90. doi: 10.1128/MCB.24.11.4781-4790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Losch FO, Bredenbeck A, Hollstein VM, Walden P, Wrede P. Evidence for a large double-cruciform DNA structure on the X chromosome of human and chimpanzee. Hum Genet. 2007;122:337–43. doi: 10.1007/s00439-007-0405-4. [DOI] [PubMed] [Google Scholar]
- (36).Wischnewski F, Friese O, Pantel K, Schwarzenbach H. Methyl-CpG binding domain proteins and their involvement in the regulation of the MAGE-A1, MAGE-A2, MAGE-A3, and MAGE-A12 gene promoters. Mol Cancer Res. 2007;5:749–59. doi: 10.1158/1541-7786.MCR-06-0364. [DOI] [PubMed] [Google Scholar]
- (37).Yang Z, Jiang J, Stewart DM, et al. AOF1 is a histone H3K4 demethylase possessing demethylase activity-independent repression function. Cell Res. 2010;20:276–87. doi: 10.1038/cr.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Mayers DL, Mikovits JA, Joshi B, et al. Anti-human immunodeficiency virus 1 (HIV-1) activities of 3-deazaadenosine analogs: increased potency against 3′-azido-3′-deoxythymidine-resistant HIV-1 strains. Proc Natl Acad Sci U S A. 1995;92:215–9. doi: 10.1073/pnas.92.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Puppe J, Drost R, Liu X, et al. BRCA1-deficient mammary tumor cells are dependent on EZH2 expression and sensitive to Polycomb Repressive Complex 2-inhibitor 3-deazaneplanocin A. Breast Cancer Res. 2009;11:R63. doi: 10.1186/bcr2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Fiskus W, Wang Y, Sreekumar A, et al. Combined epigenetic therapy with the histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A and the histone deacetylase inhibitor panobinostat against human AML cells. Blood. 2009;114:2733–43. doi: 10.1182/blood-2009-03-213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Musch T, Oz Y, Lyko F, Breiling A. Nucleoside drugs induce cellular differentiation by caspase-dependent degradation of stem cell factors. PLoS One. 2010;5:e10726. doi: 10.1371/journal.pone.0010726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Hayden A, Johnson PW, Packham G, Crabb SJ. S-adenosylhomocysteine hydrolase inhibition by 3-deazaneplanocin A analogues induces anti-cancer effects in breast cancer cell lines and synergy with both histone deacetylase and HER2 inhibition. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-0982-0. [DOI] [PubMed] [Google Scholar]
- (43).Kemp CL, Rao M, Xi S, et al. Polycomb repressor complex-2 is a novel target for mesothelioma therapy. 2011 doi: 10.1158/1078-0432.CCR-11-0962. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Suva ML, Riggi N, Janiszewska M, et al. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res. 2009;69:9211–8. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- (45).Miranda TB, Cortez CC, Yoo CB, et al. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol Cancer Ther. 2009;8:1579–88. doi: 10.1158/1535-7163.MCT-09-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Schrump DS, Fischette MR, Nguyen DM, et al. Clinical and molecular responses in lung cancer patients receiving Romidepsin. Clin Cancer Res. 2008;14:188–98. doi: 10.1158/1078-0432.CCR-07-0135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.