SUMMARY
OBJECTIVE
To investigate whether mammalian target of rapamycin (mTOR) inhibition by rapamycin is therapeutically efficacious in combination with cisplatin for bladder cancer.
MATERIALS AND METHODS
Using a panel of human urothelial carcinoma cell lines, we determined the effect of rapamycin on cell viability, cell-cycle progression, signalling and apoptosis.
The effect of mTOR inhibition on chemosensitivity was investigated by treating cells with rapamycin, alone, or with cisplatin.
The effect of rapamycin or cisplatin treatment was assessed in xenograft mice inoculated with urothelial carcinoma cells.
Expression of p-mTOR in human bladder cancer specimens was assessed using a tissue microarray.
RESULTS
Treatment with rapamycin significantly decreased cell viability in UMUC3 (P=0.004) and 253J (P<0.001) cells. It induced arrest in the G0–G1 phase and decreased activation of p-mTOR and its downstream effector, p-S6K, in both cell lines.
Treatment with rapamycin increased the ability of cisplatin to inhibit cell viability in UMUC3 (P=0.002) and 253J (P=0.03) cells. No evidence for apoptosis induction was noted after treatment with rapamycin alone.
Mouse xenografts of UMUC3 cells revealed that rapamycin significantly prolonged survival and enhanced the therapeutic efficacy of cisplatin.
In patient urothelial carcinoma specimens, p-mTOR expression was increased in cancer vs. non-tumour bladder tissue in 65/203 (32.0%) tumours.
CONCLUSION
mTOR blockade inhibits urothelial carcinoma cell proliferation and enhances the effectiveness of cisplatin.
Suppression of the mTOR pathway has the potential to be a therapeutic target in bladder cancer for selected patients.
Keywords: bladder cancer, mTOR, chemotherapy, rapamycin, radical cystectomy
INTRODUCTION
Urothelial carcinoma (UC) of the bladder is the second most common malignancy of the genitourinary tract, and the second most common cause of death among genitourinary tumours, with 70,980 new cases and 14,330 deaths projected from 2009 [1]. Approximately 25% of new bladder cancers are invasive at the time of diagnosis, and the standard treatment has been radical cystectomy with urinary diversion. However, despite definitive surgery, approximately half of these patients subsequently develop disease recurrence [2].
Currently, the most effective systemic treatment for metastatic bladder cancer consists of cisplatin-based combination chemotherapy. Unfortunately, although UC has been shown to be cisplatin-sensitive [3], responses are not typically durable, and the majority of patients experience subsequent disease progression [3,4]. As a result, survival for patients with advanced tumours has not significantly improved over time, such that the 5-year survival for pT3 UC was 33% before 1985, and has been noted to be 35% since 1985 [5]. Thus, the existing systemic treatment options for bladder cancer are in need of improvement, and the development of novel targeted therapies remains an important goal.
Increasingly, attention has turned to investigating the molecular pathogenesis of UC in order to elucidate the pathways involved in the development and progression of the disease. Understanding these mechanisms may help to identify selective targets for therapy. One particular subject of recent investigation for its relevance to bladder cancer has been the phosphatidylinositol 3-kinase (PI3K)/AKT pathway [6], which regulates key cellular processes, including survival, angiogenesis and invasion [7]. Mammalian target of rapamycin (mTOR), a serine/threonine-specific kinase downstream of PI3K/AKT which functions through its effectors to mediate protein synthesis and cell-cycle progression [8,9], represents a potential specific target of interest within this pathway. Indeed, elevated mTOR activity has been noted to be important in a variety of human tumours [8,10–16], including UC [17–23]. Interestingly, mTOR inhibition has been found to sensitize certain cancer cells to cisplatin therapy [11,13,24].
In the present study, we evaluated the efficacy of mTOR inhibition with rapamycin using in vitro and in vivo models of bladder cancer, and investigated whether rapamycin accentuates the response of bladder cancer cells to cisplatin. In addition, we assessed expression of the mTOR pathway in UC patient tissue specimens.
MATERIALS AND METHODS
Cell culture and reagents
The human UC cell lines UMUC3, TCC-SUP, T24, and 1365 were purchased from American Type Culture Collection (Manassas, VA, USA), and the human UC cell line 253J was obtained from Dr. William See (Medical College of Wisconsin, Milwaukee, WI, USA). These lines were derived from a spectrum of non-invasive to invasive bladder tumours. Cells were cultured in DMEM medium, supplemented with 10% foetal bovine serum and 100 units/mL penicillin/streptomycin. All cultures were maintained in a humidified incubator at 37°C in 5% CO2.
Rapamycin was purchased from BioMol (Plymouth Meeting, PA, USA), and cisplatin was obtained from Sigma (St Louis, MO, USA). Antibodies were purchased from different manufacturers: mTOR, p-mTOR (Ser 2448), S6K, p-S6K (Ser 235/236), ERK, p-ERK (Thr 202/Tyr204), p-AKT (Thr 308), and poly(ADP-ribose)polymerase (PARP) were obtained from Cell Signaling (Beverly, MA, USA), while AKT was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), Ki-67 from DAKO (Carpinteria, CA, USA), and β-actin from Sigma.
Western blot analysis
Cells were washed with ice-cold PBS and trypsinized, and lysis buffer was pipetted onto the cells. The resulting lysates were then boiled and stored at −20°C until analysis. Equal amounts of protein were loaded onto Readygels (4–20% Tris-HCL; Biorad, Hercules, CA, USA), and electrophoresis was done according to the manufacturer’s instructions. Proteins were blotted onto PVDF membranes (Millipore, Bedford, MA, USA). Blots were reprobed with antibodies against β-actin to evaluate potential differences in protein loading.
Cell viability assay
UMUC3 and 253J cells were seeded in 96-well tissue culture plates at a density of 1.5×103 cells/well and were treated with 5 nM rapamycin, alone or in combination with 5 µM cisplatin. After 24, 48 and 72 h of treatment, the relative number of viable cells were measured using the fluorimetrical, resazurin-based Cell Titer Blue assay (Promega, Mannheim, Germany) according to the manufacturer’s instructions. ANOVA testing was used to identify statistically significant differences in cell viability between the treatment groups.
Cell-cycle analysis
Urothelial carcinoma cells were plated at 6×104 cells/well in 6-well plates. Cells were treated 24 h after seeding and, 48 h after treatment, cells were collected and resuspended in 0.5 mL staining solution. Cells were analysed by flow cytometry on a FACscan station using Cell Quest Software (Becton Dickinson, Franklin Lakes, NJ, USA), collecting 10,000 events. Cell-cycle distribution was compared between treatments using the binomial test of proportions.
Subcutaneous xenograft model
All procedures involving animal care were approved by the Animal Care and Usage Committee of Fox Chase Cancer Center. UMUC3 cells (106 cells/mouse) were injected s.c. into the flank of 5-week-old severe combined immunodeficiency mice. Once tumour volumes reached 100 mm3, the mice were randomized into four groups (10 mice per group). Group 1 received rapamycin (5mg/kg) 5 days per week. Group 2 received cisplatin (3 mg/kg) once every 2 weeks. Group 3 received a combination of both rapamycin and cisplatin. Group 4 (control) received diluent only. Drugs were freshly prepared weekly and were injected i.p. Mice were treated for 6 weeks or until their tumours reached a size over 10% of their body weight, at which point they were killed. At this point, tumours were fixed in 4% paraformaldehyde and paraffin-embedded for immunohistochemical staining. Survival was recorded from the first day of treatment until death as previously described [11]. Survival rates were reported using the Kaplan–Meier method, and compared among the treatment groups using the log-rank test.
Human bladder cancer tissue microarray (TMA) construction and analysis
After approval from our Institutional Review Board, 205 patients treated with radical cystectomy for UC of the bladder were identified from the Fox Chase Tumor Registry. TMA slides were constructed from 1-mm cores (two per sample) and, whenever possible, contained tumour and adjacent, non-tumour urothelium. For immunohistochemistry, sections (4 µm) were incubated with the primary antibody (anti-p-mTOR, diluted 1:1000), followed by a peroxidase-conjugated secondary antibody. Negative control tumour sections were treated identically to other sections except that the primary antibody was not included in the staining. Breast, skin and kidney tissue from archival specimens were used as positive controls for p-mTOR expression. Staining was quantified using a DAKO ACIS III image analyser as an intensity score (scale 0–255). Differences in staining were assessed between pathological subgroups using the Wilcoxon rank-sum and Kruskal–Wallis tests as appropriate.
RESULTS
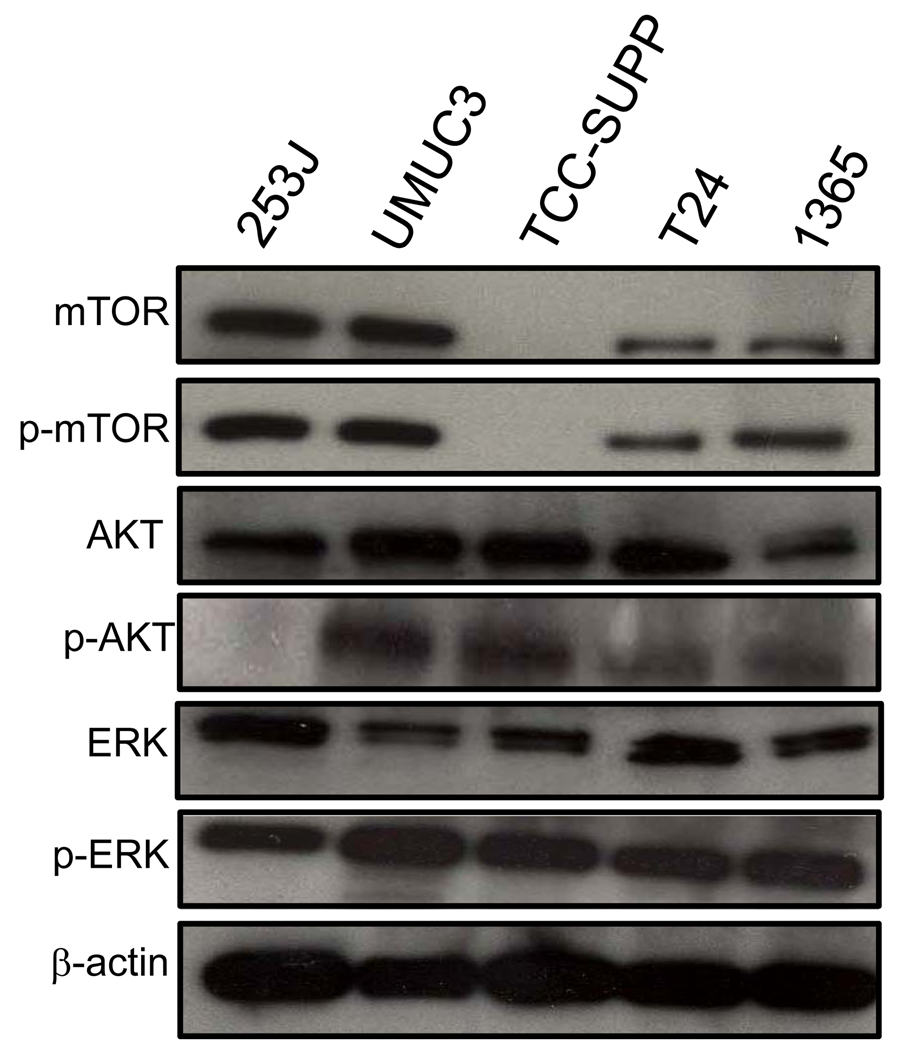
We assessed mTOR expression in a panel of five human bladder cancer cell lines by immunoblotting whole cell lysates from cultured cells. As shown in Fig. 1, mTOR, as well as p-mTOR, the activated form of the protein, were detected in 4/5 cell lines. Since mTOR has been shown to be a substrate of the PI3K/AKT signalling pathway [25], we evaluated AKT activity in these UC cells, and found highly active AKT in all lines tested, although 253J cells, a line derived from an invasive tumour, did not demonstrate detectable levels of p-AKT. We also noted high activity of ERK, another upstream regulator of mTOR, and p-ERK, in all cell lines tested.
FIG. 1.
mTOR expression and pathway activation in UC cell lines. Cell extracts from five UC cell lines were subjected to immunoblotting using antibodies directed against mTOR, p-mTOR, AKT, p-AKT, ERK, and p-ERK. Protein loading was normalized using an antibody recognizing β-actin. Blots representative of three separate experiments are shown.
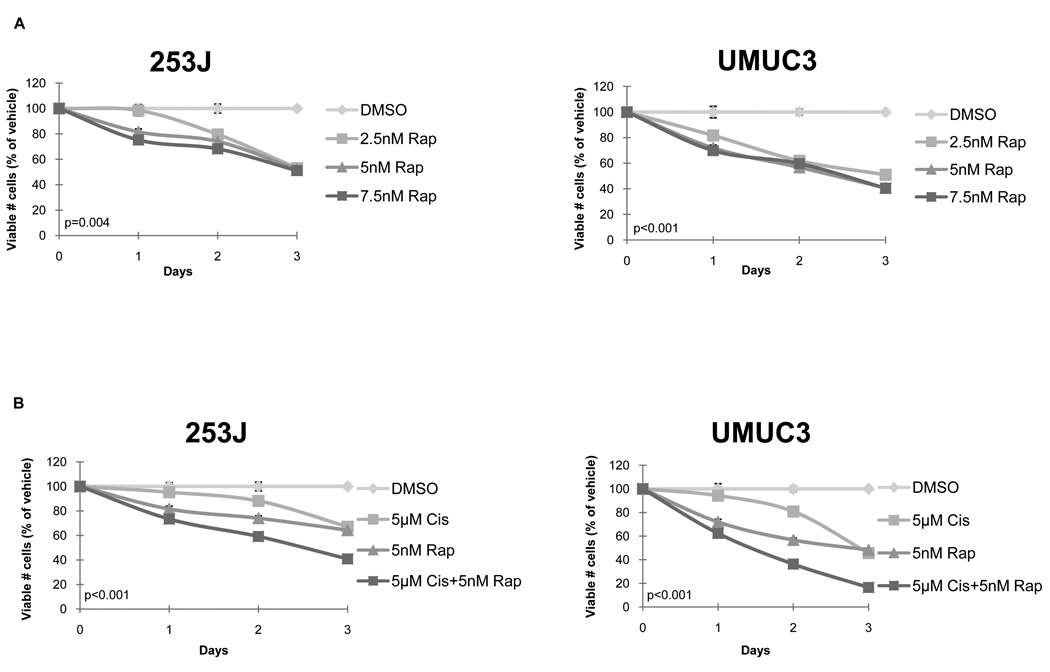
We next examined the effect of rapamycin, a known mTOR inhibitor [8], on UC cell viability. Because one of the objectives of our study was to assess whether mTOR inhibition might be a useful addition to the current chemotherapeutic regimens for advanced bladder cancer, we chose two lines, 253J and UMUC3, that showed high levels of mTOR expression (Fig. 1) and that were derived from invasive tumours, for the remainder of the investigations. We found that rapamycin significantly reduced the number of viable cells in both 253J (P= 0.004) and UMUC3 (P<0.001) cells at each of the concentrations tested (Fig. 2A).
FIG. 2.
(A) Rapamycin inhibits UC cell viability in vitro. 24 h after seeding, cells grown in their regular medium were treated with rapamycin (Rap). Cell viability was measured 24, 48 and 72 h after treatment using the resazurin-based Cell Titer Blue fluorescence assay. Values from three wells were measured per treatment group for each time point. Viable cell numbers are expressed as a percentage of vehicle, dimethyl sulphoxide (DMSO), control. Results were obtained from three separate experiments and are expressed as mean ± SD. (B. Additive impact of rapamycin + cisplatin on UC cell viability. 253J and UMUC3 cells were treated with rapamycin (Rap), cisplatin (cis), or the combination of both agents. Values from three wells were measured per treatment group for each time point. Viable cell numbers are expressed as a percentage of vehicle (DMSO) control. Results were obtained from three separate experiments and are expressed as mean± SD.
Next, we sought to determine whether combination therapy with rapamycin might improve the efficacy of cisplatin in bladder cancer. We found that the addition of rapamycin (5 nM) to relatively low-dose cisplatin (5 µM) was associated with a significant reduction in the number of viable cells compared with treatment with either of the agents alone in both UC cell lines (P< 0.001 for both; Fig. 2B).
Given the effects observed after treatment with rapamycin on the number of viable UC cells, we sought to determine whether these findings were attributable to changes in cell proliferation or apoptosis. Specifically, we evaluated the impact of treatment on UC cell-cycle progression using flow cytometry. We found that, for both cell lines, 48 h of treatment with 5 nM rapamycin resulted in a modest but significant increase in the proportion of cells in the G0/G1 phase, and a corresponding decrease in the number of cells in the S phase (Table 1). These results are consistent with those that have been noted in other cell systems as well, where mTOR inhibition has been shown to decrease G1 transit through the cell cycle [14,20,26]. On the other hand, 5 µM cisplatin treatment was associated with a decrease in the proportion of cells in G0/G1 and a marked increase in the number of cells in G2/M, consistent with the described mechanism of G2 cell-cycle arrest for cisplatin [27]. Interestingly, when the agents were combined in treatment, the effect of cisplatin appeared to predominate, with a decreased number of cells noted in G0/G1, and an increased number in G2/M. Importantly, the absence of a peak in the sub-G0 phase of the cell cycle (data not shown) suggested that rapamycin at these dosages was cytostatic but not cytotoxic for UC cells.
TABLE 1.
Urothelial carcinoma cell-cycle distribution 48 h after treatment with 5 nM rapamycin, alone or in combination with 5 µM cisplatin.
| Cell line | ||||||||
|---|---|---|---|---|---|---|---|---|
| 253J | UMUC3 | |||||||
| Stage | Control, % |
Rapamycin, %* |
Cisplatin, %* |
Rapamycin + cisplatin, %* |
Control , % |
Rapamycin ,%* |
Cisplatin, %* |
Rapamycin + cisplatin, %* |
| G0/G1 | 46.9 | 61.7 | 22.7 | 28.2 | 53.5 | 62.0 | 16.1 | 12.6 |
| S | 15.6 | 8.7 | 8.7 | 12.7 | 22.8 | 17.3 | 32.2 | 15.6 |
| G2/M | 33.7 | 30.2 | 69.9 | 59.7 | 20.6 | 20.7 | 52.4 | 65.8 |
P<0.001 vs. control.
Control cells were treated with DMSO only.
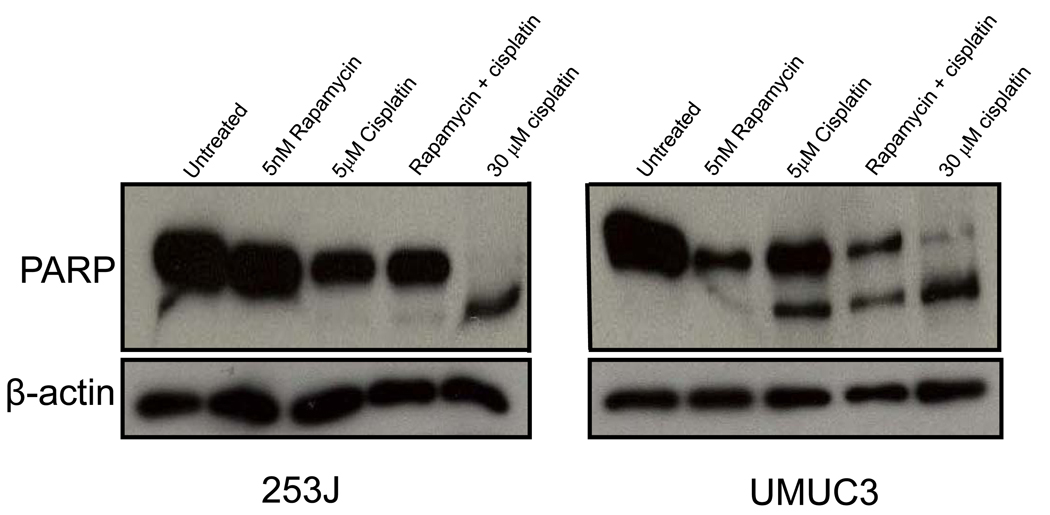
In order to further assess for evidence of apoptosis, cell lysates obtained from 253J and UMUC3 cells treated with rapamycin, cisplatin, or both agents were also analysed for expression of PARP cleavage. As shown in Fig. 3, rapamycin alone did not generate a cleaved PARP fragment. UMUC3 cells did show some evidence of cisplatin-induced apoptosis, but no evidence for an additive effect with combination treatment was noted.
FIG. 3.
The antiproliferative effect of rapamycin in UC cells is not attributable to the induction of apoptosis. Cell lysates were obtained from 253J and UMUC3 cells treated for 48 h with 5 nM rapamycin, 5 µM cisplatin, or both agents. Western blotting for expression of the cleavage fragment of PARP was performed. Cell lysates obtained from 253J and UMUC3 cells treated with high-dose (30 µM) cisplatin for 48 h were evaluated as a positive control. Protein loading was normalized with an antibody recognizing β-actin.
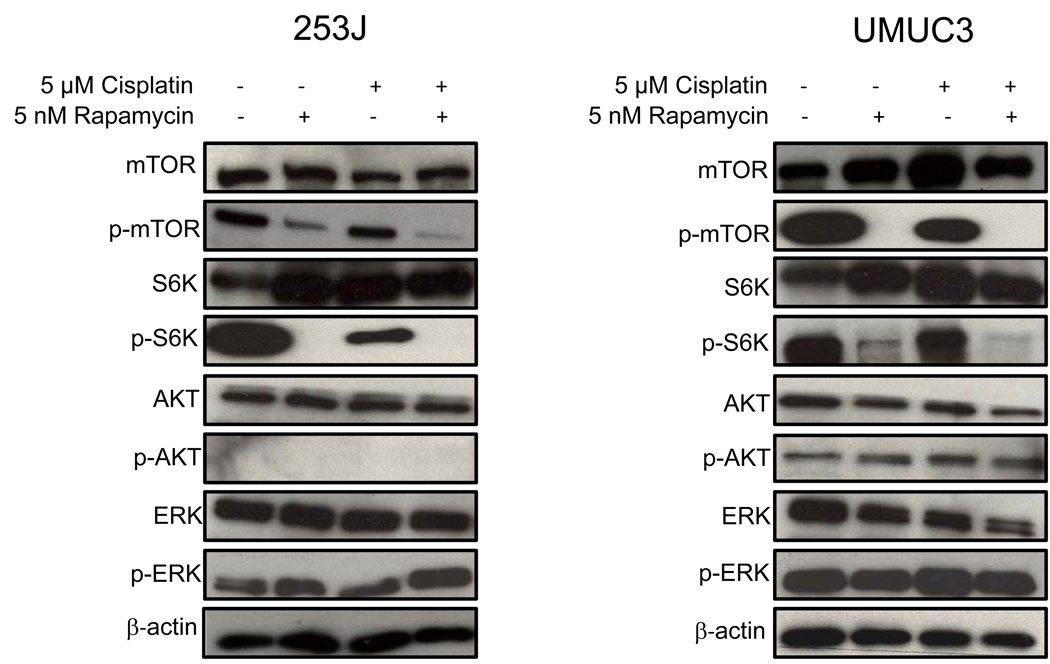
To investigate whether the antiproliferative effects noted with rapamycin were associated with inhibition of the mTOR signalling pathway, we examined expression of p-mTOR and its downstream effector, p-S6K, after rapamycin treatment. As shown in Fig. 4, treatment with 5 nM rapamycin for 48 h decreased expression of both p-mTOR and p-S6K. Treatment with rapamycin, alone or in combination with cisplatin, did not, however, alter levels of p-AKT, AKT, p-ERK, or ERK in either cell line, again with a noted absence of p-AKT activation in 253J cells.
FIG. 4.
Rapamycin inhibits mTOR signalling in UC cells. 253J and UMUC3 cells were seeded in their regular culture medium for 24 h and then treated with 5 nM rapamycin, 5 µM cisplatin, or both. Control cells received vehicle (DMSO) only. Whole-cell lysates, collected after 48 h of treatment, were subjected to Western blotting using antibodies directed against mTOR, p-mTOR, S6K, p-S6K, AKT, p-AKT, ERK, and p-ERK. Protein loading was normalized with an antibody recognizing β-actin. Blots representative of three separate experiments are shown.
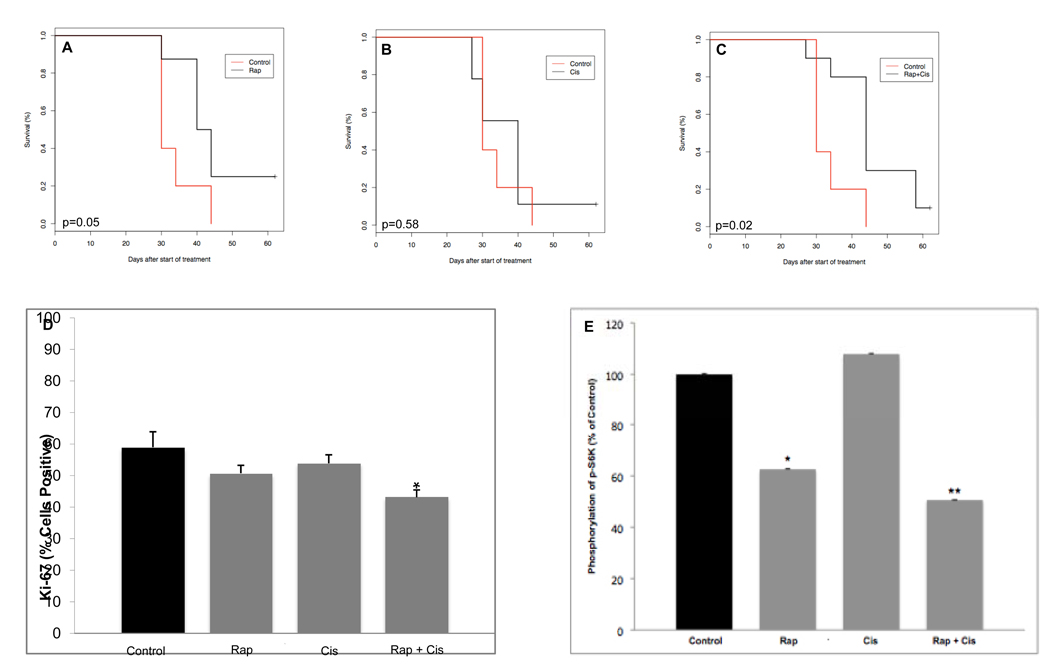
We examined the impact of rapamycin treatment, alone and with low-dose cisplatin, on bladder cancer cells in vivo using a xenograft model as described. Drug treatment was well tolerated, with no apparent toxicity throughout the study. As shown in Fig. 5, we found that rapamycin treatment was associated with an improvement in overall survival of the xenografts, such that the median survival in mice treated with rapamycin was 42 days, vs. 30 days in the control animals (P=0.05). Moreover, the median overall survival for mice treated with rapamycin + cisplatin was 44 days, again representing a significant improvement compared with controls (P=0.02), and suggesting a potential, albeit modest, benefit to combined therapy.
FIG. 5.
Rapamycin, alone and with cisplatin, improves overall survival of mouse xenografts after s.c. flank implantation of UMUC3 cells. Curves represent survival of animals treated with (A)rapamycin,(B) cisplatin or (C)rapamycin + cisplatin, vs. control. (D) Rapamycin + cisplatin treatment decreases cell proliferation in UC xenograft tumours as measured by Ki-67 expression. Columns and vertical bars represent mean±SD from animals in each treatment group. *P=0.02 vs. control, P=0.03 vs. rapamycin alone, P=0.03 vs. cisplatin alone. (E) Treatment with rapamycin results in downregulation of p-S6K in xenograft UC tumours vs. control. Columns and vertical bars represent mean±SD from animals in each treatment group. *P=0.03 vs. control; **P=0.001 vs. control.
As our cell line results demonstrated that rapamycin altered cell-cycle progression, we then evaluated expression of Ki-67, a marker for cell proliferation, in the xenograft tumour tissue (Fig. 5D). We found a marked decrease in Ki-67 expression from the tumours of mice treated with rapamycin in combination with cisplatin relative to the controls (P=0.02). Interestingly, we also noted that treatment with rapamycin and cisplatin was associated with a significantly decreased expression of Ki-67 compared with treatment with rapamycin alone (P=0.03) or cisplatin alone (P=0.03). Together, these data further suggest that inhibition of the mTOR pathway results in altered cell-cycle progression, and that combination treatment may offer an additive advantage.
We next investigated the mechanism by which rapamycin prolongs survival in vivo by performing immunohistochemistry on tumour tissue harvested from the animals after treatment. Consistent with our cell line findings, we determined that rapamycin significantly decreased p-S6K expression in UMUC3 tumours from rapamycin-treated and rapamycin/cisplatin-treated mice compared with placebo (P=0.03 and P=0.001, respectively; Fig. 5E).
In order to investigate the clinical relevance of the mTOR pathway in human UC, we evaluated the expression of the activated form of the protein, p-mTOR, in patient tissue specimens. Specifically, we constructed a TMA from the specimens of 205 patients who underwent radical cystectomy for UC at our institution between 2000 and 2008, including 121 patients who also had adjacent, non-neoplastic urothelium present. Median (range) patient age at surgery was 70 (37–90) years. Other clinicopathological demographics are shown in Table 2.
TABLE 2.
Clinicopathological demographics for patients in TMA analysis (N=205)
| Variable | n (%) |
|---|---|
| Gender | |
| Male | 152 (74.1) |
| Female | 53 (25.9) |
| Race | |
| White | 181 (88.3) |
| African-American | 17 (8.3) |
| Other | 7 (3.4) |
| Pathological tumour stage (n=202) | |
| Ta/Tis | 19 (9.4) |
| T1 | 14 (6.9) |
| T2 | 32 (15.9) |
| T3 | 85 (42.1) |
| T4 | 52 (25.7) |
| Lymph node status (n=203) | |
| N0 | 114 (56.2) |
| N+ | 73 (36.0) |
| Nx | 16 (7.8) |
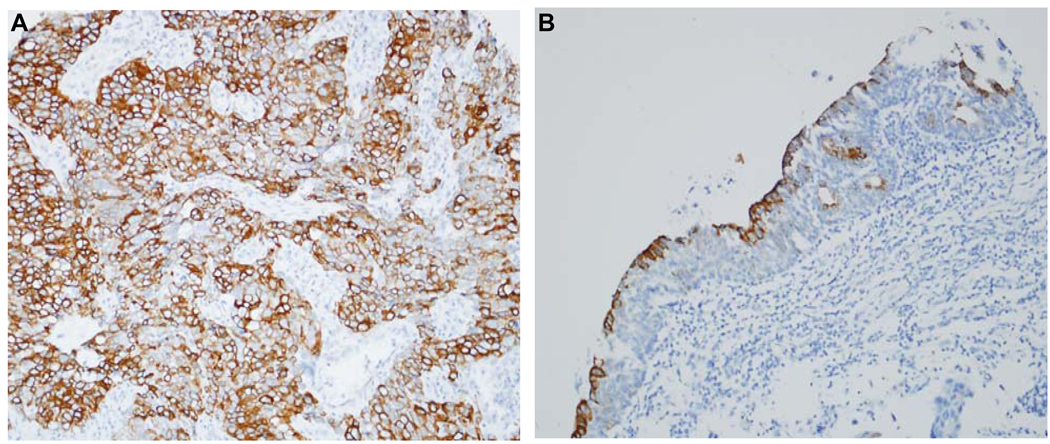
A total of 328 specimens, including tumour and non-tumour bladder tissues, were included in the TMA. The overall median intensity score as determined by the DAKO image analyser for these samples was 86 (range 27.5–131.5). Interestingly, we found p-mTOR upregulation in only a subset of bladder tumours, such that 65/203 (32.0%) UC specimens demonstrated increased staining compared with the median expression detected in non-tumour bladder specimens (Fig. 6). No significant difference in p-mTOR expression was noted across different tumour stages or according to lymph node status (data not shown).
FIG. 6.
p-mTOR is upregulated in a subset of human bladder cancers relative to non-tumour bladder tissue. Representative images from bladder TMA demonstrating diffuse, intense p-mTOR expression in an invasive UC (A) vs. limited expression in adjacent, non-tumour bladder tissue (B). Images represent original magnification ×20.
DISCUSSION
While response rates of approximately 50% have been demonstrated for cisplatin-based regimens in advanced bladder cancer, the median survival for patients with metastatic disease has plateaued at 14–15 months for nearly two decades [3]. Continued investigation of the molecular pathways involved in UC development and progression are, therefore, required to identify novel therapeutic opportunities. As activation of PI3K/AKT has been associated with disturbances in the control of cell growth and survival, which ultimately lead to metastatic competence and treatment resistance [28], this pathway may be particularly relevant for investigation. mTOR, a downstream effector of PI3K/AKT, mediates protein synthesis and cell-cycle progression and thereby represents a specific target of interest [28].
In the present study, we report that mTOR inhibition with rapamycin significantly decreased bladder cancer cell proliferation in UC cell lines and mouse xenografts. Rapamycin induced G0–G1 cell-cycle arrest without stimulating apoptosis. The efficacy of rapamycin for inhibiting mTOR signalling in vitro and in vivo was evident from the marked decrease in expression of p-S6K in treated cells relative to controls. The present study provides, to our knowledge, the first report of the impact of mTOR inhibition on bladder cancer sensitivity to cisplatin, the most effective systemic therapy for advanced UC identified to date. Treatment with rapamycin increased the ability of cisplatin to inhibit cell viability in vitro. Moreover, we found that in UC xenografts survival was improved with the combination therapy of rapamycin and cisplatin. Tissue analysis from treated animals confirmed a decrease in cell proliferation with combined treatment.
These results extend previous preclinical studies which have investigated the role of the mTOR signalling pathway in UC [18,20,21]. Indeed, rapamycin and its derivative, RAD001 (Everolimus) have been noted to inhibit bladder cancer cell proliferation [18,20,21] and induce G0–G1 cell-cycle arrest without apoptosis in bladder cancer cell lines [20]. The efficacy of mTOR inhibition on UC proliferation and tumour growth has also been shown previously in mouse xenografts [20].
The optimal role for mTOR pathway inhibition in cancer therapies continues to evolve. With regard to bladder cancer, mTOR inhibition has been found to decrease the development of chemically [22] and genetically [19] induced bladder tumours in mice, and may be effective when delivered intravesically [19]. Furthermore, a series of 15 renal transplant patients with UC reported using rapamycin in the immunosuppressive regimen and noted no tumour recurrences in 9/15 patients over 2 years of follow-up [17]. However, whether mTOR inhibitors will be used alone in the treatment of bladder cancer, or whether mTOR inhibition might be integrated into multi-agent regimens, for example as a means to sensitize tumours to additional, cytotoxic agents [8,13], remains to be determined. Our results support data from other cell systems which have shown that inhibition of mTOR increases the susceptibility of cancer cells to cisplatin [11,13,24]. It is notable that efforts to enhance the activity of cisplatin by combining treatment with sunitinib, a targeted therapy against vascular endothelial growth factor, have also shown promising preclinical results in bladder cancer [29]. Nevertheless, the precise mechanism of rapamycin efficacy and the optimal regimen for delivery remain to be established.
Because the clinical efficacy of molecularly targeted therapies is likely to depend, perhaps more than conventional chemotherapies, on the relevant signalling pathway being deregulated, identifying patients who have tumours in which these pathways are disrupted might be an important means of identifying appropriate candidates for targeted agents [15]. For example, establishing the status of mTOR pathway expression in a tumour may suggest the relevance of that pathway for an individual patient, and may thereby help guide the judicious application of targeted therapy. As such, we constructed a TMA to evaluate p-mTOR expression in patient UC specimens. We found that p-mTOR expression was increased in 32% of bladder cancers compared with non-tumour bladder tissue. These findings are consistent with the data from Puzio-Kutzer et al. [23], which demonstrated activation of p-S6K, the downstream effector of p-mTOR, in 25% of invasive UC tumours.
In summary, our data support a functional relevance for the mTOR signalling pathway in UC. Moreover, we provide evidence to suggest the potential for mTOR inhibitors to enhance the current cisplatin-based chemotherapy regimens. As p-mTOR was detected in only a subset of human bladder cancer tissue samples, however, future studies to develop markers of mTOR pathway dependency, for example by evaluating tumour biopsy specimens, will be necessary to identify patients who might benefit most from such a treatment approach. In addition, as new rapamycin analogues which display increased specificity and/or affinity for distinct intracellular mTOR complexes continue to be developed [30], the role of these agents in UC will need to be established.
ACKNOWLEDGEMENTS
This research was supported by National Institutes of Health R01 CA58836 (JC) and P30 CA006927, the Fox Chase Cancer Center Kidney Keystone Program, and by an appropriation from the state of Pennsylvania.
Abbreviations
- mTOR
mammalian target of rapamycin
- UC
urothelial carcinoma
- PI3K
phosphatidylinositol 3-kinase
- PARP
poly(ADP-ribose)polymerase
- TMA
tissue microarray
- DMSO
dimethyl sulphoxide
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 3.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher DJ, Milowsky MI, Bajorin DF. Advanced bladder cancer: status of first-line chemotherapy and the search for active agents in the second-line setting. Cancer. 2008;113:1284–1293. doi: 10.1002/cncr.23692. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW, Dotan Z, Donat SM, Bajorin DF. Defining optimal therapy for muscle invasive bladder cancer. J Urol. 2007;177:437–443. doi: 10.1016/j.juro.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Cassidy A, Gu J, et al. Genetic variations in PI3K-AKT-mTOR pathway and bladder cancer risk. Carcinogenesis. 2009;30:2047–2052. doi: 10.1093/carcin/bgp258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 8.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 9.Garcia JA, Danielpour D. Mammalian target of rapamycin inhibition as a therapeutic strategy in the management of urologic malignancies. Mol Cancer Ther. 2008;7:1347–1354. doi: 10.1158/1535-7163.MCT-07-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–2287. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mabuchi S, Altomare DA, Cheung M, et al. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13:4261–4270. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- 12.Robb VA, Karbowniczek M, Klein-Szanto AJ, Henske EP. Activation of the mTOR signaling pathway in renal clear cell carcinoma. J Urol. 2007;177:346–352. doi: 10.1016/j.juro.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 13.Faried LS, Faried A, Kanuma T, et al. Expression of an activated mammalian target of rapamycin in adenocarcinoma of the cervix: a potential biomarker and molecular target therapy. Mol Carcinog. 2008;47:446–457. doi: 10.1002/mc.20402. [DOI] [PubMed] [Google Scholar]
- 14.Ackler S, Xiao Y, Mitten MJ, et al. ABT-263 and rapamycin act cooperatively to kill lymphoma cells in vitro and in vivo. Mol Cancer Ther. 2008;7:3265–3274. doi: 10.1158/1535-7163.MCT-08-0268. [DOI] [PubMed] [Google Scholar]
- 15.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, et al. Targeting AKT-mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnsen JI, Segerström L, Orrego A, et al. Inhibitors of mammalian target of rapamycin downregulate MYCN protein expression and inhibit neuroblastoma growth in vitro and in vivo. Oncogene. 2008;27:2910–2922. doi: 10.1038/sj.onc.1210938. [DOI] [PubMed] [Google Scholar]
- 17.Xiao-peng H, Lin-lin M, Yong W, et al. Rapamycin instead of mycophenolate mofetil or azathioprine in treatment of post-renal transplantation urothelial carcinoma. Chin Med J (Engl) 2008;122:35–38. [PubMed] [Google Scholar]
- 18.Fechner G, Classen K, Schmidt D, Hauser S, Müller SC. Rapamycin inhibits in vitro growth and release of angiogenic factors in human bladder cancer. Urology. 2009;73:665–669. doi: 10.1016/j.urology.2008.09.070. [DOI] [PubMed] [Google Scholar]
- 19.Seager CM, Puzio-Kuter AM, Patel T, et al. Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer. Cancer Prev Res (Phila) 2009;2:1008–1014. doi: 10.1158/1940-6207.CAPR-09-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansure JJ, Nassim R, Chevalier S, Rocha J, Scarlata E, Kassouf W. Inhibition of mammalian target of rapamycin as a therapeutic strategy in the management of bladder cancer. Cancer Biol Ther. 2009;8:2339–2347. doi: 10.4161/cbt.8.24.9987. [DOI] [PubMed] [Google Scholar]
- 21.Pinto-Leite R, Botelho P, Ribeiro E, Oliveira PA, Santos L. Effect of sirolimus on urinary bladder cancer T24 cell line. J Exp Clin Cancer Res. 2009;28:3. doi: 10.1186/1756-9966-28-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira PA, Arantes-Rodrigues R, Sousa-Diniz C, et al. The effects of sirolimus on urothelial lesions chemically induced in ICR mice by BBN. Anticancer Res. 2009;29:3221–3226. [PubMed] [Google Scholar]
- 23.Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, et al. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009;23:675–680. doi: 10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geoerger B, Kerr K, Tang CB, et al. Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res. 2001;61:1527–1532. [PubMed] [Google Scholar]
- 25.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 26.Pene F, Claessens YE, Muller O, et al. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene. 2002;21:6587–6597. doi: 10.1038/sj.onc.1205923. [DOI] [PubMed] [Google Scholar]
- 27.Chu G. Cellular responses to cisplatin. The roles of DNA-binding proteins and DNA repair. J Biol Chem. 1994;269:787–790. [PubMed] [Google Scholar]
- 28.Porta C, Figlin RA. Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney cancer, and the therapeutic potential of phosphatidylinositol-3-kinase/Akt inhibitors. J Urol. 2009;182:2569–2577. doi: 10.1016/j.juro.2009.08.085. [DOI] [PubMed] [Google Scholar]
- 29.Sonpavde G, Jian W, Liu H, Wu MF, Shen SS, Lerner SP. Sunitinib malate is active against human urothelial carcinoma and enhances the activity of cisplatin in a preclinical model. Urol Oncol. 2009;27:391–399. doi: 10.1016/j.urolonc.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]