Abstract
Background
Newcastle disease virus (NDV) is a paramyxovirus that is pathogenic in birds, but only causes mild flu-like symptoms in humans. NDV(F3aa)-GFP is a genetically modified, fusogenic NDV. We assessed the utility of NDV(F3aa)-GFP in treating head and neck squamous cell carcinoma.
Methods and Results
At a multiplicity of infection (MOI) of 1, NDV(F3aa)-GFP infection of three cell lines supported strong GFP expression by 36 hours. Four cell lines were highly sensitivity to viral cytotoxicity, with >75% of cells lysed by day 6 at MOI 0.1, and two other cell lines were partially susceptible. Murine SCC25 flank tumors exhibited robust GFP expression after a single intratumoral viral injection, and showed near complete tumor regression over 34 days. There were no adverse effects attributable to therapy.
Conclusions
We demonstrate that a fusogenic NDV exerts potent oncolytic effects against human head and neck cancer, and supports its continued investigation for clinical application.
Introduction
Approximately 650,000 cases of head and neck cancer are newly diagnosed annually, and this disease is estimated to cause approximately 350,000 deaths yearly worldwide (1). In the United States, about 36,000 new cases of squamous cell carcinoma involving the oral cavity and pharynx were diagnosed in 2009 (2). Despite progress in advancing surgical techniques and improvement in the delivery of radiotherapy and chemotherapy, the mortality of these patients remains significant, particularly for those with advanced malignancies failing conventional therapy. The development of therapeutic agents with novel mechanisms of activity is needed to improve outcomes for these patients (3).
A variety of viruses have been shown to possess oncolytic, anti-tumoral activity against cancer, including herpes simplex virus type 1, vaccinia virus, and adenovirus among others (4). The first report of the utility of Newcastle disease virus (NDV) as a form of treatment for human cancer was published in 1964 (5). Naturally occurring NDV has been shown to be able to directly lyse human cancer cells (6). NDV has also been used as a form of immunotherapy for neuroblastoma (7), melanoma (8–9), and other malignancies. As a therapeutic agent, NDV possesses several favorable attributes. First, NDV is a paramyxovirus that causes disease in birds, but in humans only causes mild flu-like symptoms. Second, NDV is a single-stranded RNA virus that replicates in the cytoplasm of infected cells without affecting host DNA, reducing the risk of undesired recombination events. Third, genetically modified oncolytic NDV viruses may be engineered, allowing for novel strategies to be incorporated in future vector design to facilitate cancer cell lysis.
A non-pathogenic, chicken vaccine strain of NDV, Hitchner B1 (NDV/B1), was modified using an established reverse genetic system to express a modified F protein that contains a multibasic cleavage and activation site; this modified virus was termed NDV(F3aa)-GFP (10). NDV(F3aa)-GFP is a highly fusogenic virus and is able to form syncytia, which enhanced its oncolytic potential against a murine colon carcinoma cell line (11). In the current study, we evaluated the oncolytic efficacy of NDV(F3aa)-GFP against a panel of human head and neck squamous cell carcinomas in vitro and in a flank tumor model of squamous cell carcinoma in vivo. We found that NDV(F3aa)-GFP possesses potent oncolytic effects in vitro against head and neck cancers. A single intratumoral injection of NDV(F3aa)-GFP into murine flank tumors of SCC25 was able to induce nearly complete tumor regression without any toxicity attributable to viral administration.
Materials and Methods
Cell lines
Six human head and neck squamous cell carcinoma cell lines were studied: MSK 922, MSK QLL1, MDA 1386, MDA 1586, SCC 15 and SCC 25. All cell lines were grown in MEM containing 10% FCS and 1% penicillin and streptomycin (P&S). All cells were grown in a humidified incubator at 5% CO2 and 37°C.
Virus
The NDV mutant viruses with modified F cleavage site (NDV(F3aa)-GFP) were previously described (10). Briefly, the attenuated NDV of Hitchner B1 strain (NDV-B1) was modified using a reverse-genetics system, and the cleavage site of the F protein was replaced with three amino acid changes to create NDV(F3aa)-GFP. To generate NDV(F3aa)-GFP virus expressing green fluorescent protein (GFP), a DNA fragment of GFP flanked by the appropriate NDV-specific RNA transcriptional signals was inserted into the XbaI site created between the P and M genes of pT7NDV/F3aa. Viruses were rescued from cDNA using methods described previously and sequenced by reverse transcription-PCR for insert fidelity (10).
In vitro GFP expression
Cells were plated at 2×104 per well in 96-well plates in 100 µl of media per well. After 6 hours of incubation, NDV(F3aa)-GFP in 50 µl of media was added to each well at a multiplicity of infection (MOI) of 1. At time points up to 72 hours post-infection, cells were examined with an inverted fluorescence microscope (Nikon Eclipse TE300; Nikon, Tokyo, Japan) for GFP expression and photographs were taken. All samples were analyzed in triplicate.
Cytotoxicity assays
Each cell line was plated at 2×104 cells per well in 12-well plates in 1 ml media. After incubation for 6 hours, NDV-F3aa in 100 µl media was added to each well at MOI’s of 0.1, 1, and 3. Viral induced cytotoxicity was measured at daily intervals for one week. After the day 3 measurements, an additional 1 mL of fresh media was added to remaining wells. Cells were washed with phosphate buffered saline (PBS) and lysed with Triton X (1.35%, Sigma) to release intracellular lactate dehydrogenase (LDH), which was quantified with a Cytotox 96 kit (Promega, Madison, WI). LDH was detected by the conversion of a tetrazolium salt into a red formazan product that was measured by spectrophotometry (EL321e, Bio-Tek Instruments, Winooski, Vi) at 450nm. Results are expressed as the percentage of surviving cells determined from comparing the LDH of each test sample to untreated samples that were considered 100% viable. The assay was performed on three replicate samples for each condition, and mean values and standard errors were plotted.
Murine flank tumor therapy
All animal procedures were performed with the approval of the Memorial Sloan-Kettering Institutional Animal Care and Use Committee. Six-week-old female athymic nude mice (National Cancer Institute) were anesthetized with inhalational isoflurane (Baxter International). 5×106 SCC 25 tumor cells were injected in 100 µl of PBS into the subcutaneous flanks of mice to establish flank tumors. When tumor diameter reached approximately 3–5 mm, eight mice were treated by intratumoral injections of a single dose of NDV(F3aa)-GFP at 1×107 plaque forming units (pfu) in 50 µl PBS, and four mice were treated with 50 µl PBS as a control. Mean starting volumes were identical for the two groups. A representative animal was imaged with a Maestro in vivo fluorescence imaging system (Cambridge Research & Instrumentation, Woburn, MA) at day 3 after injection of NDV(F3aa)-GFP. Tumor dimensions were serially measured every two days and tumor volumes calculated by the formula: volume = length×width2×π/6. Animals were monitored for body weights and signs of toxicity. Photographs were taken of representative animals. Experiments involving live NDV(F3aa)-GFP viruses were completed before July 2009.
Results
In vitro GFP expression
NDV(F3aa)-GFP gene expression in six SCC cell lines was monitored by GFP expression after infection at MOI 1.0. Very few cells for any cell line expressed visible GFP at 18 hours. MDA 1386, SCC 25, and MDA 1586 supported the greatest GFP expression by 36 hours, followed by MSK 922, and SCC 15. MSK QLL1 showed the lowest levels of GFP expression (Figure 1). GFP expression appeared to peak at 36–48 hours for MSK 922, SCC 25, and MDA 1586, while the peak was later at 72 hours for MDA 1386.
Figure 1.
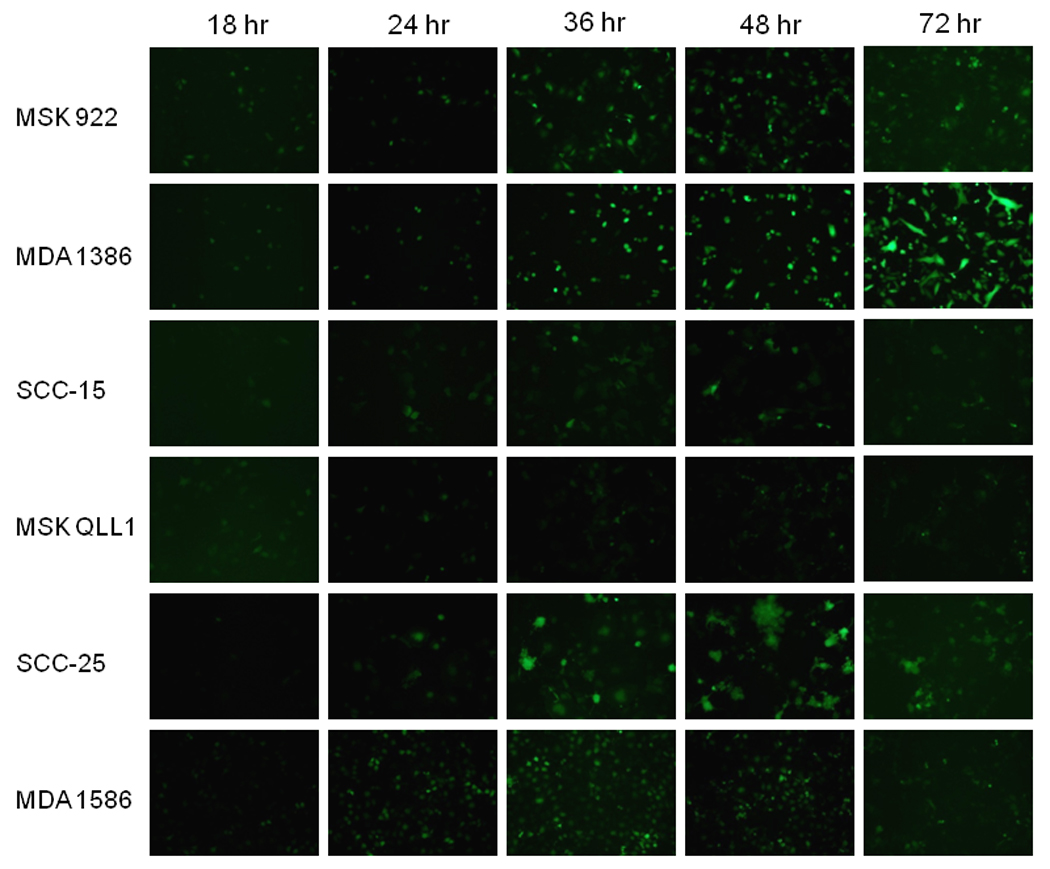
A panel of human head and neck squamous cell carcinoma cell lines was exposed to NDV-F3aa at a multiplicity of infection (MOI) of 1. At varying times post-infection, cells were examined with an inverted fluorescence microscope for GFP expression and representative photographs were taken.
Cytotoxicity of NDV in head and neck cancer cells in vitro
Six human squamous cell carcinoma cell lines (MSK 922, MSK QLL1, MDA 1386, MDA 1586, SCC 15 and SCC 25) were infected with NDV(F3aa)-GFP at varying multiplicities of infection (MOI 0.1, 1, 3). Over a 7-day period, four of the cell lines (SCC 15, MSK QLL1, SCC 25, MDA 1586) demonstrated high sensitivity to NDV-F3aa induced cytotoxicity at all of the MOI’s tested. Even at a relatively low MOI of just 0.1, >75% of cells were lysed by the virus by day 6 for all four of these cell lines (Figure 2). The two remaining cell lines (MSK 922, MDA 1386), though less sensitive, still demonstrated partial susceptibility to NDV(F3aa)-GFP at an MOI of 3, with 39% and 50% cells lysed by day 6, respectively.
Figure 2.
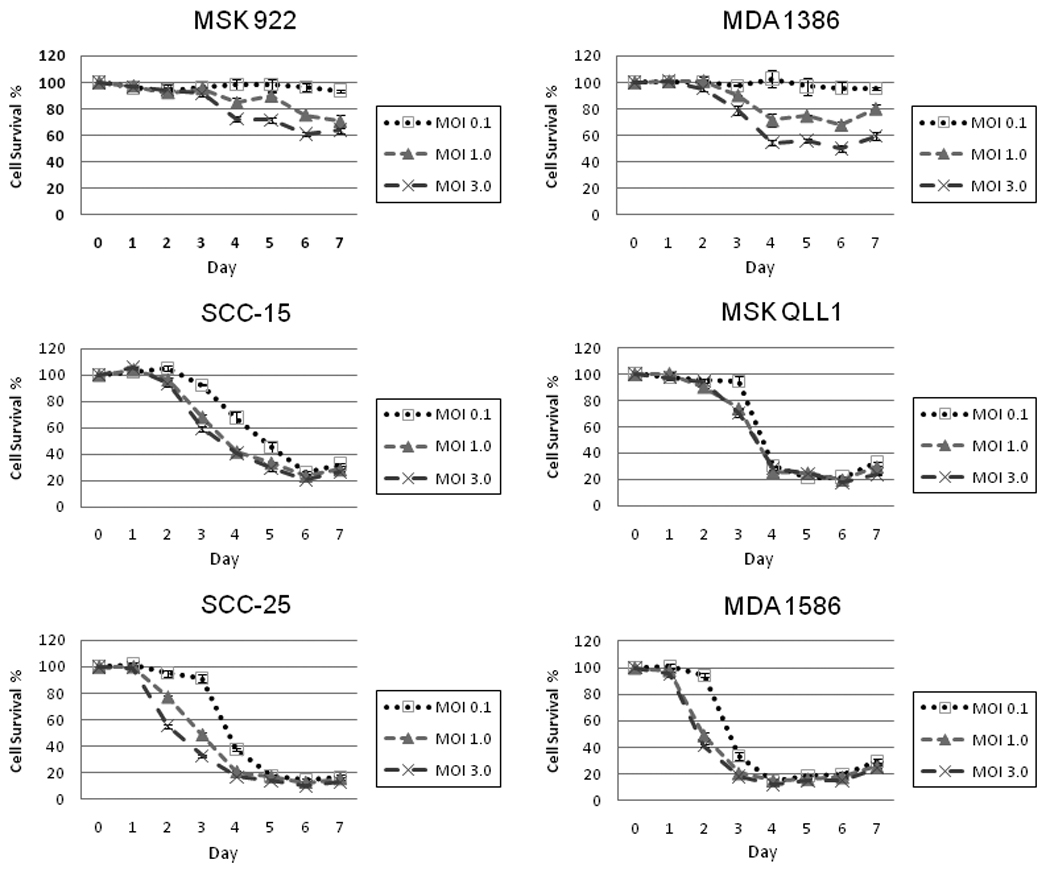
A panel of human head and neck squamous cell carcinoma cell lines was exposed to NDV-F3aa at MOI 0.1, 1, and 3. Viral induced cytotoxicity was measured at daily intervals for one week by lactate dehydrogenase assay. Results are expressed as the percentage of surviving cells determined from comparing the LDH of each test sample to untreated samples that were considered 100% viable. The assay was performed on three replicate samples for each condition, and mean values and standard errors were plotted.
Murine flank tumor therapy of head and neck cancer in vivo
Athymic nude mice were inoculated with SCC 25 cells to establish flank tumors. When flank tumors reached a mean volume of 45 mm3, a single dose of NDV(F3aa)-GFP at 1×107 pfu or PBS as control was injected into each tumor. Three days after injection, one animal injected with NDV(F3aa)-GFP was imaged with the Maestro in vivo fluorescence imaging system and demonstrated robust GFP expression within the injected tumor (Figure 3).
Figure 3.
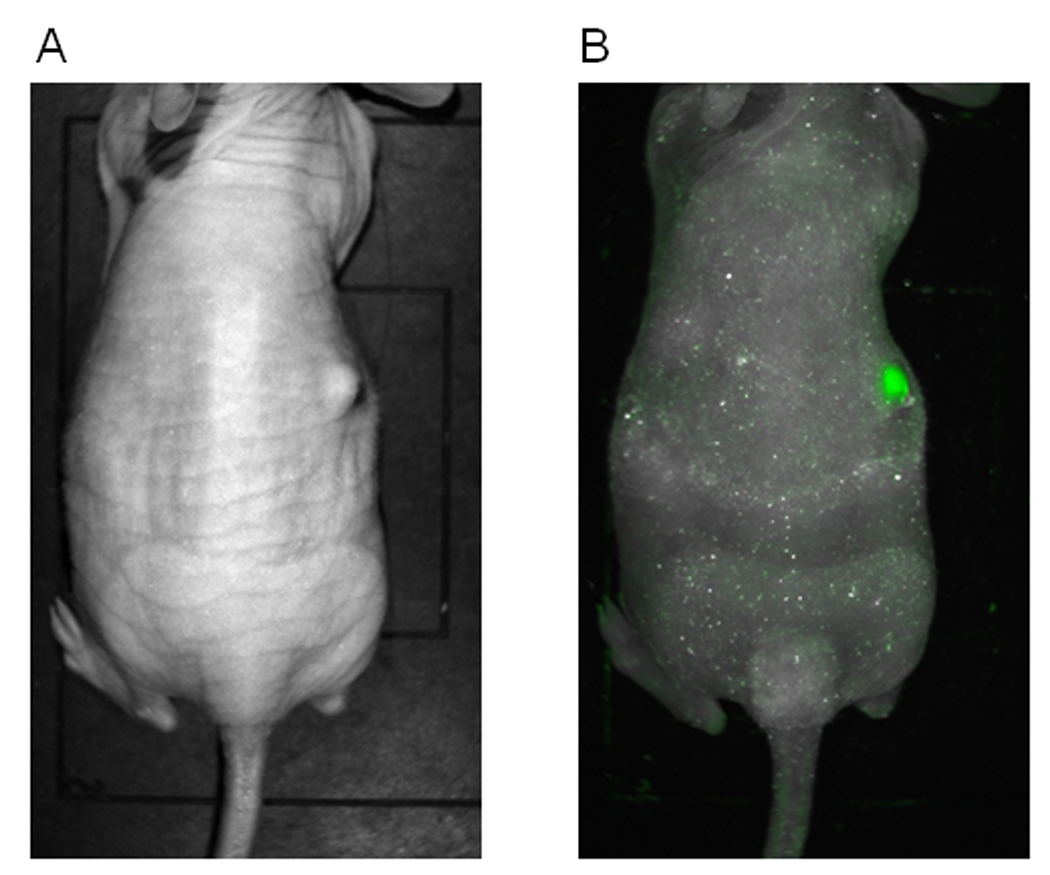
SCC 25 cells were injected into the subcutaneous flanks of mice to establish tumors. A single dose of NDV-F3aa at 1×107 plaque forming units (pfu) was injected, and three days later viral green fluorescent protein (GFP) expression imaged using a Maestro in vivo imaging system to demonstrate successful tumor infection. A representative photograph was taken (A) with GFP imaging overlay (B).
All animals were followed clinically for 34 days. NDV(F3aa)-GFP treated tumors demonstrated near complete tumor volume regression over a month period. By day 34, the mean tumor volume was just 4.7 ± 2.8 mm3 (standard error, Figures 4, 5a). In contrast, all of the control tumors progressively increased in size, and by day 34, the mean tumor volume of control tumors was 87.7 ± 23.6 mm3 (p<0.001, t-test, 2-tailed). There were significant differences between control and NDV(F3aa)-GFP treated tumors at all time points from day 8 and later (p<0.01, 2-tailed t-test for all comparisons).
Figure 4.
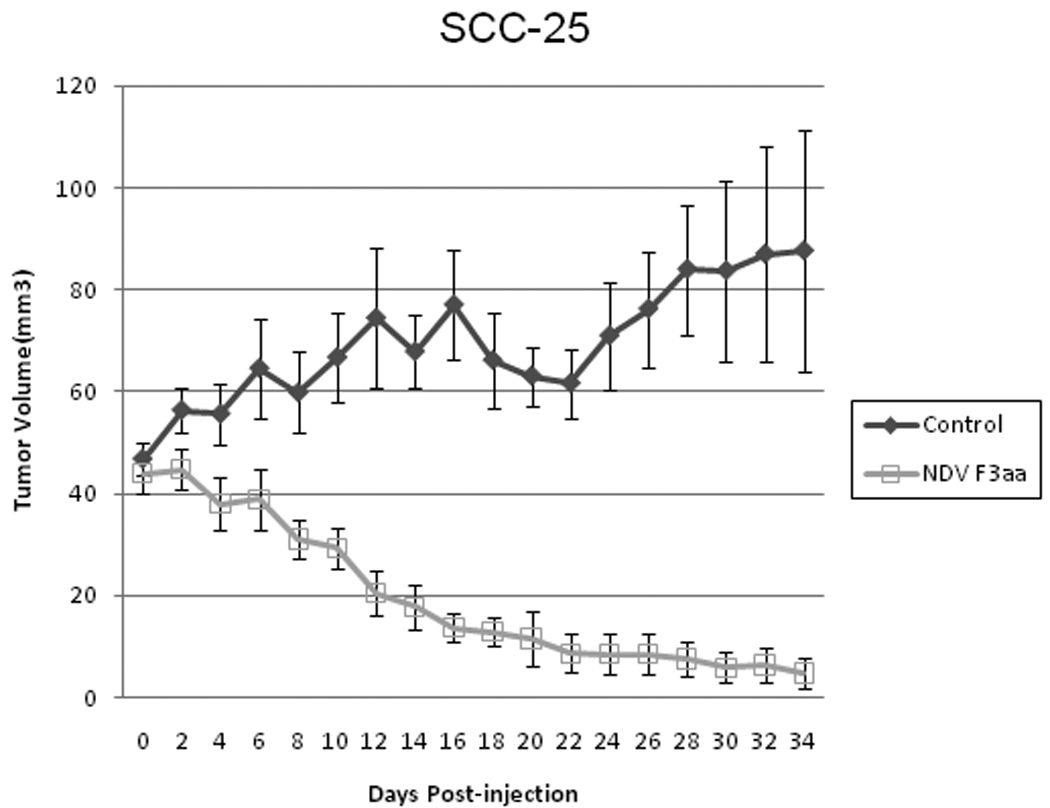
SCC 25 flank tumors were treated with a single dose of NDV-F3aa (1×107 pfu) or saline as control. Tumor dimensions were measured and mean tumor volumes with standard errors calculated over a 34 day course. There were significant differences between control and NDV-F3aa treated tumors at all time points from day 8 and later (p<0.01, 2-tailed, t-test, for all comparisons).
Figure 5.
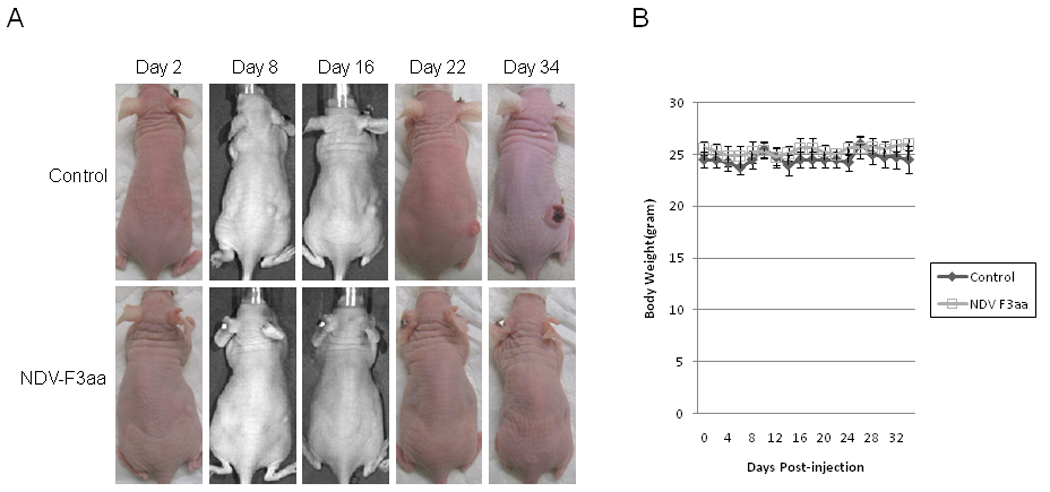
SCC 25 flank tumors were treated with a single dose of NDV-F3aa (1×107 pfu) or saline as control. No animals demonstrated any evidence of toxicity. (A) Representative photographs were taken of animals over a 34 day course. (B) Mean body weights with standard errors were recorded.
Two of the NDV(F3aa)-GFP treated mice were cured by day 22. Mean body weights remained stable for both control and NDV(F3aa)-GFP groups with no significant differences (Figure 5b). There was no morbidity and no side effects attributable to NDV(F3aa)-GFP viral therapy during the entire experiment. The experiment was concluded at day 34 due to ulceration of the control tumors.
Discussion
Oncolytic viral therapy is an approach to cancer therapy that harnesses a virus’ natural ability to infect, replicate within, and lyse a host cell as part of its natural life cycle. Although a variety of viruses have demonstrated significant oncolytic properties, a major concern when considering their clinical application as a therapeutic agent is their potential for exhibiting harmful, pathogenic behavior. Attenuation strategies have been employed to genetically weaken these viruses and enhance their safety for clinical use. However, the replication-competent abilities of these viruses, and their origins as human pathogens, still remain a concern when designing clinical trials. Newcastle disease virus is a paramyxovirus with pathogenic effects in birds. However, unlike herpes simplex type 1 or adenovirus, NDV does not have significant adverse effects in humans, with mild flu-like symptoms or conjunctivitis as its worst reported symptoms. The relatively innocuous behavior of NDV in humans makes it attractive as a potential therapeutic biologic agent.
A genetically modified NDV, NDV(F3aa)-GFP, was originally designed with a series of other viral constructs in an attempt to develop a vaccine (10). NDV(F3aa)-GFP was noted to have a highly fusogenic phenotype, a quality that enables the formation of syncytia that may facilitate oncolytic potential (11). NDV(F3aa)-GFP previously exhibited effective oncolytic ability in a murine model of colon cancer. The purpose of the present study was to examine the potential of NDV(F3aa)-GFP to treat a panel of human head and neck squamous cell carcinomas, and determine its efficacy in vivo.
Our study demonstrates that NDV(F3aa)-GFP is able to infect and lyse four of the six squamous cell carcinoma cell lines with high efficiency, with a relatively low MOI of 0.1 resulting in >75% cell lysis by day 6. The remaining two cell lines demonstrated partial sensitivity at an MOI of 3, the highest dose tested, and could potentially be more susceptible to higher concentrations of NDV(F3aa)-GFP. Interestingly, we did not see a clear correlation between early NDV(F3aa)-GFP gene expression (GFP) and susceptibility to cytotoxicity, suggesting that there are other factors following initial viral infection that may determine a cancer’s ultimate response to NDV therapy.
A single intratumoral injection of NDV(F3aa)-GFP at a modest dose of 1×107 pfu resulted in dramatic tumor regression over a one month period, with two of eight animals treated exhibiting complete tumor regression. This in vivo viral dose is similar to the doses that our group has previously employed against head and neck cancer flank tumors using genetically modified herpes simplex (12) and vaccinia (13) viruses. Importantly, none of the animals treated with NDV(F3aa)-GFP demonstrated any evidence of viral-induced toxicity, and their body weights remained stable throughout the entire study.
It should be noted that the present model (athymic nude mice) does not allow for the induction of a protective/therapeutic immune response as a result of the infection of the tumor cells. The modulation of the host immune response may significantly increase the efficacy of this approach in humans. Other variants of oncolytic NDV have recently been constructed that incorporate additional therapeutic strategies that may complement direct oncolysis. NDV engineered to express IL-2 enhanced therapeutic potential in a murine model of melanoma (14). Interestingly, NDV expressing the influenza virus protein NS1, an interferon antagonist, may also enhance therapy against murine melanoma by suppressing host anti-viral immune responses, yet still allowing for the development of an anti-tumoral cytotoxic T lymphocyte response (15). These recent studies demonstrate that oncolytic NDV may serve as a platform for the design of second generation vectors to apply additional therapeutic strategies to enhance viral oncolysis.
Conventional treatment for head and neck cancer consists of surgery, radiation therapy, and chemotherapy, applied singularly or in combination, depending upon the scenario. Despite refinements in the application of these therapies, the survival rates for patients with head and neck cancers have not significantly improved for decades. Novel therapies with novel mechanisms of activity are necessary to improve stagnant outcomes for these patients, particularly for those with advanced or recurrent head and neck cancer. NDV may represent one such novel therapy. NDV are safe in humans, and exhibit natural oncolytic behavior. We demonstrate in this study that a fusogenic variant of a non-pathogenic chicken vaccine strain, NDV(F3aa)-GFP, may infect and induce significant oncolysis against a panel of human head and neck squamous carcinoma cell lines. Importantly, a single intratumoral injection of NDV(F3aa)-GFP led to dramatic tumor regression without any appreciable viral toxicity. These findings support the continued investigation of NDV as a therapeutic agent for head and neck cancer, and merit consideration for application in future clinical trials.
Acknowledgments
RJW is supported by a Clinical Innovator Award from the Flight Attendant Medical Research Institute, and NIH grant R21DE019015. This work was also supported by the Northeastern Biodefense Grant U54AII057158 (PP).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 4.Mullen JT, Tanabe KK. Viral oncolysis. Oncologist. 2002;7:106–119. doi: 10.1634/theoncologist.7-2-106. [DOI] [PubMed] [Google Scholar]
- 5.Wheelock EF, Dingle JH. Observations on the repeated administration of viruses to a patient with acute leukemia. A preliminary report. N Engl J Med. 1964;271:645–651. doi: 10.1056/NEJM196409242711302. [DOI] [PubMed] [Google Scholar]
- 6.Reichard KW, Lorence RM, Cascino CJ, et al. Newcastle disease virus selectively kills human tumor cells. J Surg Res. 1992;52:448–453. doi: 10.1016/0022-4804(92)90310-v. [DOI] [PubMed] [Google Scholar]
- 7.Lorence RM, Reichard KW, Katubig BB, et al. Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy. J Natl Cancer Inst. 1994;86:1228–1233. doi: 10.1093/jnci/86.16.1228. [DOI] [PubMed] [Google Scholar]
- 8.Cassel WA, Murray DR. Treatment of stage II malignant melanoma patients with a Newcastle disease virus oncolysate. Nat Immun Cell Growth Reg. 1988;7:351–352. [PubMed] [Google Scholar]
- 9.Wallack MK, Sivanandham M, Balch CM, et al. Surgical adjuvant active specific immunotherapy for patients with stage III melanoma: the final analysis of data from a phase III, randomized, double-blind, multicenter vaccinia melanoma oncolysate trial. J Am Coll Surg. 1998;187:69–77. doi: 10.1016/s1072-7515(98)00097-0. [DOI] [PubMed] [Google Scholar]
- 10.Park MS, Steel J, Garcia-Sastre A, Swayne D, Palese P. Engineered viral vaccine constructs with dual specificity: avian influenza and Newcastle disease. Proc Natl Acad Sci USA. 2006;103:8203–8208. doi: 10.1073/pnas.0602566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vigil A, Park MS, Martinez O, et al. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007;67:8285–8292. doi: 10.1158/0008-5472.CAN-07-1025. [DOI] [PubMed] [Google Scholar]
- 12.Wong RJ, Kim SH, Joe JK, Shah JP, Johnson PA, Fong Y. Effective treatment of head and neck squamous cell carcinoma by an oncolytic herpes simplex virus. J Am Coll Surg. 2001;193:12–21. doi: 10.1016/s1072-7515(01)00866-3. [DOI] [PubMed] [Google Scholar]
- 13.Yu Z, Li S, Brader P, Chen N, et al. Oncolytic vaccinia therapy of squamous cell carcinoma. Mol Cancer. 2009;8:45. doi: 10.1186/1476-4598-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamarin D, Vigil A, Kelly K, Garcia-Sastre A, Fong Y. Genetically engineered Newcastle disease virus for malignant melanoma therapy. Gene Ther. 2009;16:796–804. doi: 10.1038/gt.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamarin D, Martinez-Sobrido L, Kelly K, et al. Enhancement of oncolytic properties of recombinant Newcastle disease virus through antagonism of cellular innate immune responses. Mol Ther. 2009;17:697–706. doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]


