Abstract
Background
Whether the method of local anesthetic administration for continuous femoral nerve blocks —basal infusion versus repeated hourly bolus doses —influences block effects remains unknown.
Methods
Bilateral femoral perineural catheters were inserted in volunteers (n = 11). Ropivacaine 0.1% was administered through both catheters concurrently: a 6-h continuous 5 ml/h basal infusion on one side and 6 hourly bolus doses on the contralateral side. The primary endpoint was the maximum voluntary isometric contraction (MVIC) of the quadriceps femoris muscle at Hour 6. Secondary end points included quadriceps MVIC at other time points, hip adductor MVIC, and cutaneous sensation 2 cm medial to the distal quadriceps tendon in the 22 h following local anesthetic administration initiation.
Results
Quadriceps MVIC for limbs receiving 0.1% ropivacaine as a basal infusion declined by a mean (SD) of 84% (19) compared with 83% (24) for limbs receiving 0.1% ropivacaine as repeated bolus doses between baseline and Hour 6 (paired t test P = 0.91). Intrasubject comparisons (left vs. right) reflected a lack of difference as well: the mean basal-bolus difference in quadriceps MVIC at Hour 6 was −1.1% (95% CI −22.0 to 19.8%). The similarity did not reach our a priori threshold for concluding equivalence, which was the 95% CI falling within ± 20%. There were similar minimal differences in the secondary endpoints during local anesthetic administration.
Conclusions
This study did not find evidence to support the hypothesis that varying the method of local anesthetic administration —basal infusion versus repeated bolus doses —influences continuous femoral nerve block effects to a clinically significant degree.
A continuous femoral nerve block (cFNB) —also termed “perineural local anesthetic infusion” —is frequently used to provide analgesia following major knee surgery.1 Although inhibition of pain fibers is the primary goal of cFNB, currently-available local anesthetics approved for clinical use also influence other afferent (e.g., nonpain-related sensory and proprioception) and efferent (e.g., motor) nerve fibers as well.2 Consequently, one well-recognized side effect of perineural infusion is muscular weakness,3 particularly undesirable during cFNB because the femoral nerve innervates the quadriceps femoris muscle required for ambulation, in itself a critical component of postknee surgery rehabilitation. Recently-published data suggests that perineural infusion involving the femoral nerve increases the risk of falling.4 Considering perineural infusions are often provided for analgesia following major surgical procedures in elderly patients, and a fall in this patient population may prove catastrophic,5,6 it is imperative that any risks be minimized. The potential gravity of the issue is suggested in the over 500,000 knee arthroplasties performed every year in the United States alone, with that number expected to grow to 3.5-million annually within the next 20 yr.7
Because quadriceps femoris weakness is associated with significant functional disability8 and an increased risk of falls in elderly patients,9 it is postulated that any nerve block-induced muscular weakness is best minimized during perineural local anesthetic infusion.4–6 Towards this end, various femoral perineural infusion strategies have failed to decrease muscle weakness while still providing adequate analgesia. For example, lowering the local anesthetic dose results in decreased muscle weakness, yet also reduces analgesia.10 Alternatively, decreasing the concentration of local anesthetic and increasing the delivered volume—to retain total delivered dose—has demonstrated little effect on either sensory or motor block.11 However, one recent study involving continuous popliteal-sciatic nerve blocks following hallux valgus repair reported increased analgesia without a concurrent increase in motor block by administering local anesthetic in repeated 5 ml hourly boluses compared with a 5 ml/h continuous infusion.12 These results raise the exciting possibility of decreasing the fall risk during cFNB by decreasing muscle weakness while maintaining analgesia.
We therefore tested the hypothesis that differing the local anesthetic delivery method (continuous basal infusion vs. repeated bolus doses)—while providing an equal total local anesthetic dose—produces relatively equivalent effects when used for cFNB. The primary endpoint was the difference in maximum voluntary isometric contraction (MVIC) of the quadriceps femoris muscle expressed as a percentage of the baseline measurement 6 h following initiation of local anesthetic administration. Secondary end points included quadriceps femoris MVIC at other time points, hip adductor MVIC, and cutaneous sensation 2 cm medial to the distal quadriceps tendon in the 22 h following initiation of local anesthetic administration.
Materials and Methods
Enrollment
Our local Institutional Review Board (University of California San Diego, San Diego, CA) approved all study procedures. The trial was prospectively registered at clinicaltrials.gov (NCT01144559). Enrollment included a convenience sample of relatively healthy adult (≥18 yr) volunteers of both sexes. Exclusion criteria included current daily analgesic use; any opioid use within the previous 4 weeks; any neuro-muscular deficit of either femoral nerves and/or thigh musculature; body mass index more than 35 kg/m2; pregnancy; and incarceration. Of note, any individual (e.g., medical trainees, study coordinators, etc.) whose nonstudy performance was potentially evaluated by the Principal Investigator (B.I.) was considered part of a “vulnerable population” and excluded from volunteering as a study subject as mandated by current United States ethical guidelines.13 All participants provided written, informed consent before any study procedures. This study was undertaken in a Clinical and Translational Research Institute (University of California, San Diego, California, United States).
Perineural catheter insertion
In the supine position, subjects had an intravenous line placed in the upper extremity, standard American Society of Anesthesiologists-recommended external monitors applied, and oxygen administered by nasal cannula (3 l/min). Intravenous midazolam (1 mg) and fentanyl (50 μg) were administered, while ensuring that patients remained responsive to verbal cues. Any hair in the area that would be subsequently covered by the catheter dressings was removed with a surgical hair clipper. After sterile preparation (chlorhexidinegluconate and isopropyl alcohol) and draping, bilateral femoral perineural catheters were inserted using the identical insertion protocol. The dominant side (right vs. left) was always inserted first.
With a 13–6 MHz 38 mm linear array transducer (HFL 38x, SonoSite M-Turbo, Bothell, WA) in a sterile sleeve, the femoral nerve was identified in a transverse cross-sectional (short axis) view at the inguinal crease. A local anesthetic skin wheal was raised lateral to the US transducer. An uninsulated, 8.9 cm, 17 gauge, Tuohy-tip needle (FlexTip, Arrow International, Reading, PA) was inserted through the skin wheal and directed medially in-plane beneath the US transducer toward the femoral nerve with an anterior bevel direction. Normal saline (5 ml) was injected as the needle tip approached the lateral edge of the femoral nerve to open the space between the nerve and underlying muscle to avoid needle-nerve contact. A 19 g flexible epidural-type catheter (FlexTip) was then placed through the length of the needle and advanced 0.5 cm beyond the needle tip just posterior to the nerve. With the catheter tip remaining visualized by ultrasound to ensure that its position remained fixed, the needle was withdrawn over the catheter until the needle tip was superficial to the iliac fascia. The needle was then held in place and 2 cm of catheter inserted above the iliac fascia while ensuring that the catheter tip remained stationary; and the needle withdrawn over the remaining catheter. The injection port was attached to the catheter and the catheter secured with sterile liquid adhesive, an occlusive dressing, and an anchoring device.
Randomization
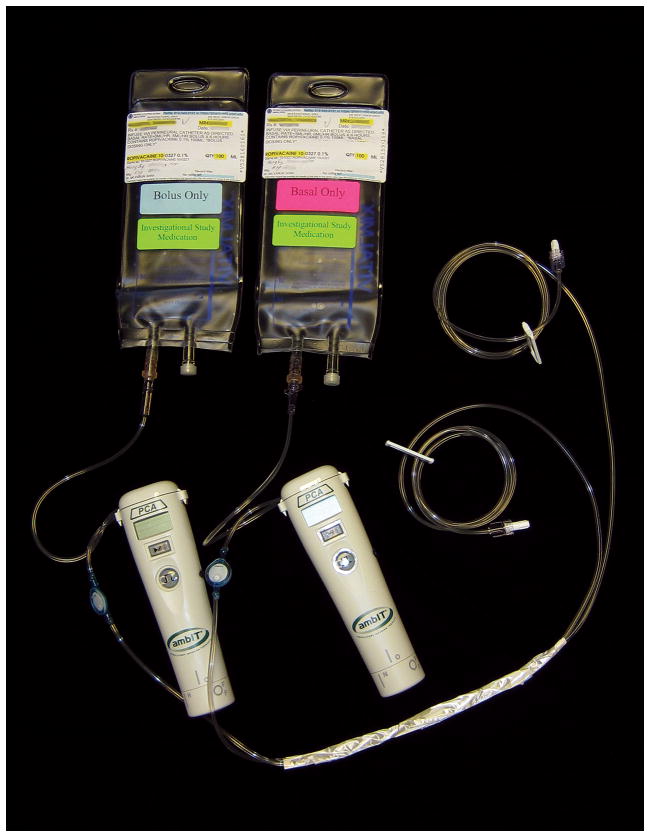
The dominant side (right or left) was randomized to one of two treatment groups: ropivacaine 0.1% administered as either a basal-only infusion (5 ml/h) or bolus-only doses (5 ml administered once hourly). The nondominant, contralateral side received the other possible treatment. In this way, subjects acted as their own controls. Randomization was based on computer-generated codes in blocks of two, stratified by sex. The Investigational Drug Service prepared the randomization list and all ropivacaine infusions. Two 100 ml sterile bags were filled with ropivacaine 0.1% and connected to disposable infusion pump cassettes and integrated tubing (fig. 1). One bag was labeled “Basal” and the opposite end of its tubing labeled either “dominant” or “other,” depending upon the randomization for each subject. The other bag was labeled “Bolus” and the opposite end of its tubing labeled either “dominant” or “other,” as well. The two pieces of tubing were then gently wound at least 5 rotations and covered with opaque tape, masking from all but the Investigational Drug Service pharmacists which tubing delivered the basal and bolus dosing (ropivacaine is clear, so the flow through the clear tubing from the tape to the perineural catheters was not visually distinguishable). The Investigational Drug Service delivered this apparatus to the investigators.
Fig. 1.
Infusion apparatus allowing randomized, double-masked local anesthetic administration.
The investigators programmed two electronic, portable infusion pumps (ambIT PCA, Summit Medical, West Jordan, UT), one delivering a continuous basal infusion (5 ml/h), and the other on-demand bolus doses (5 ml). These infusion pumps were attached to the appropriate cassettes connected to the ropivacaine reservoirs labeled either “Basal” or “Bolus” by the Investigational Drug Service. The remaining tubing ends were connected to the femoral catheters, as they were labeled either “Dominant” or “Other.” In this manner, all investigators, nursing staff, and subjects remained masked to treatment group assignment: all subjects received both a basal-only infusion and repeated bolus doses, but which femoral catheter received which treatment remained unknown.
Perineural infusion
At Hour 0, both infusion pumps were turned on. The infusion pump providing a basal infusion of 5 ml/h remained active for 6 h (30 ml = 30 mg). The infusion pump providing bolus doses of 5 ml was activated by one of the investigators at Hour 0, and every hour following for a total of 6 delivered bolus doses (30 ml = 30 mg), the final being at Hour 5. Infusion pumps were turned off at Hour 6, and the perineural catheters removed.
Subjects remained in the Clinical and Translational Research Institute for 23 h, or until their quadriceps femoris strength had returned to baseline levels, whichever was longer.
Outcome measurements
We selected measures that have established reliability and validity,9,14–17 and minimal interrater discordance.16 Measurements were performed at Hour 0 (baseline), and on the hour until Hour 14, as well as the following morning at approximately Hour 22. In addition, measurements were performed every 10 min from Hour 0–1 and 5–6. In all cases, measurements were taken in the seated position with the dominant side measured first, followed by the nondominant side. Initially, quadriceps femoris strength was evaluated; followed by hip adductor strength; and, lastly, tolerance of transcutaneous electrical stimulation slightly medial to the distal quadriceps tendon.
Muscle strength
We evaluated muscle strength with an isometric force electromechanical dynamometer (MicroFET2, Lafayette Instrument Company, Lafayette, IN) to measure the force produced during a MVIC in a seated position with the knees flexed at 90°.16 For quadriceps femoris evaluation, the dynamometer was placed on the ipsilateral anterior tibia perpendicular to the tibial crest just proximal to the medial malleolus.15–17 For hip adductor evaluation, the femoral shaft was held at 30° off midline and the dynamometer placed over the medial femoral epicondyle (adductor tubercle). For all measurements, subjects were asked to take 2 s to come to maximum effort contracting the target muscle(s), maintain this effort for 5 s, and then relax.9,17 The measurements immediately before perineural ropivacaine administration were designated baseline measurements, and all subsequent measurements are expressed as a percentage of the preinfusion baseline.9
Sensory effect
We evaluated tolerance of transcutaneous electrical stimulation with the same quantitative procedure as one described previously.11,14 Electrocardiogram pads were placed 2 cm medial to the distal quadriceps tendon and attached to a nerve stimulator (EZstimII, Model ES400; Life-Tech, Stafford, TX). The current was increased from 0 mA until subjects described mild discomfort, at which time the current was recorded as the tolerated level and the nerve stimulator turned off. All sensory measurements are expressed as a percentage of each patient’s preinfusion baseline.
Statistical Analysis
Sample size calculations were based on our primary hypothesis that differing the method of perineural local anesthetic administration (continuous basal infusion vs. repeated bolus doses)—while providing an equal total dose—produces comparable effects when used in continuous femoral nerve blocks. To this end, the primary endpoint was the quadriceps femoris MVIC expressed as a percentage of the baseline MVIC at Hour 6. We consider a difference of 20% points to be clinically relevant because a 10% side-to-side strength difference is common, yet functionally unnoticeable in healthy individuals.18,19 We used the method described by Armitage and colleagues,20 whereby we would conclude equivalence if the 95% confidence interval for the difference falls within a prespecified tolerated interval (−20% to 20%). Based on previously published data, we assumed the SD of percent change from baseline in MVIC would be 16%.14 Assuming a within-subject correlation of percent change from baseline in MVIC of 0.4, yields a SD of the treatment difference of 17.5% = [16%2×2× (1−0.4)]1/2. Under these assumptions, a trial with n = 11 subjects would correctly conclude there is no treatment difference with probability 85% (“power”), and incorrectly conclude equivalence when there is a difference of 18% with probability 5% (“α”). Subjects were deemed “nonresponders” if one or both extremities did not exhibit more than 20% change from baseline in quadriceps femoris MVIC or sensory effect at Hour 4. “Nonresponders” were not included in the primary analyses, but were included in post hoc intent-to-treat analyses.
We applied the same analysis of percent change from baseline at hour 6 to the secondary outcome measures. In addition, we examined the time profiles of the responses over time with spaghetti and mean plots. Further secondary analyses included mixed-effects modeling of the repeated hourly measures to confirm the analysis of percent change at Hour 6. These models accounted for the hierarchical correlation of paired measures from each subject over time. We adjusted the P values for each of the 25 time points considered using Holm’s method.21 These analyses were executed using R version 2.12 (2010).† Additional analyses included the Mann–Whitney U for nonparametric comparisons and Chi Square for categorical variables (GraphPadInStat, GraphPad Software, San Diego, CA).
Results
Fifteen subjects enrolled during a 3-month period beginning July 2010. All had bilateral femoral perineural catheters successfully inserted per protocol. Each subject’s dominant side was randomized to either one of the two ropivacaine 0.1% treatments—a continuous 5 ml/h basal infusion for 6 h or 6 hourly 5 ml bolus doses—and the nondominant side received the opposite treatment. Four subjects failed to exhibit at least a 20% change from baseline in either their quadriceps MVIC or sensory effects (table 1). While only the remaining 11 subjects were included in the primary analyses, all 15 subjects were included in the intent-to-treat analyses (table 1).
Table 1.
Anthropometric subject characteristics
| Included in Primary Analyses (n=11) | Excluded from Primary Analyses (n=4) | P-Value | |
|---|---|---|---|
| Age (yr) | 26 (4) | 26 (3) | 0.85 |
| Sex (female/male) | 4/7 | 3/1 | 0.19 |
| Height (cm) | 175 (8) | 171 (14) | 0.57 |
| Weight (kg) | 69 (11) | 66 (13) | 0.64 |
| Body mass index (kg/m2) | 22 (2) | 22 (2) | 0.91 |
| Dominant side (left) | 0 | 0 |
Values are reported as mean (SD) or number of subjects
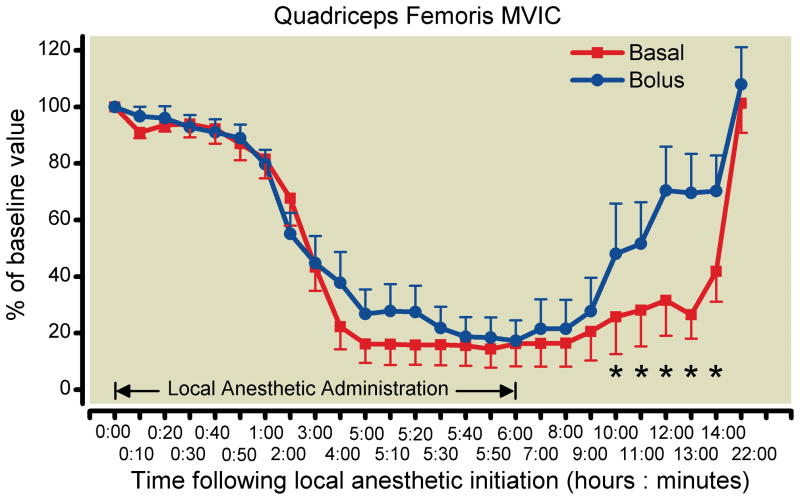
Quadriceps femoris MVIC for limbs receiving 0.1% ropivacaine as a basal infusion declined by a mean (SD) of 84%19 compared with 83%24 for limbs receiving 0.1% ropivacaine as repeated bolus doses between baseline and Hour 6 (paired t test P = 0.91). Intrasubject comparisons (left vs. right) reflected this lack of difference as well: the mean basal-bolus difference in quadriceps MVIC at Hour 6 was −1.1% (95% CI −22.0 to 19.8%). The similarity did not reach our a priori threshold for concluding equivalence, which was the 95% CI falling within ± 20%. The mixed-effects model of the repeated measures of basal-bolus differences over time resulted in a tighter confidence interval for quadriceps femoris MVIC at 6 h (95% CI −20.8 to 18.6%), but the confidence intervals were still not within −20 to 20% at any observed time point. On the contrary, at Hours 10 through 14, the basal-bolus difference in MVIC was statistically significant (p less than 0.05), and the difference at Hours 12 and 13 retained significance (p less than 0.01) after the Holm adjustment.
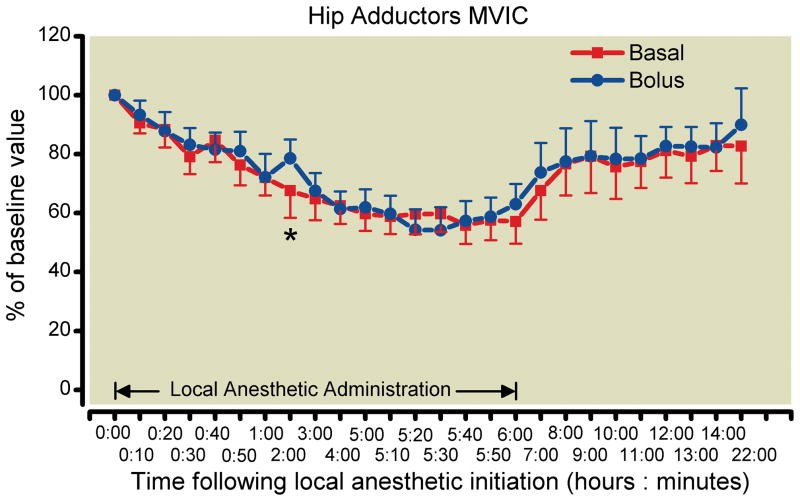
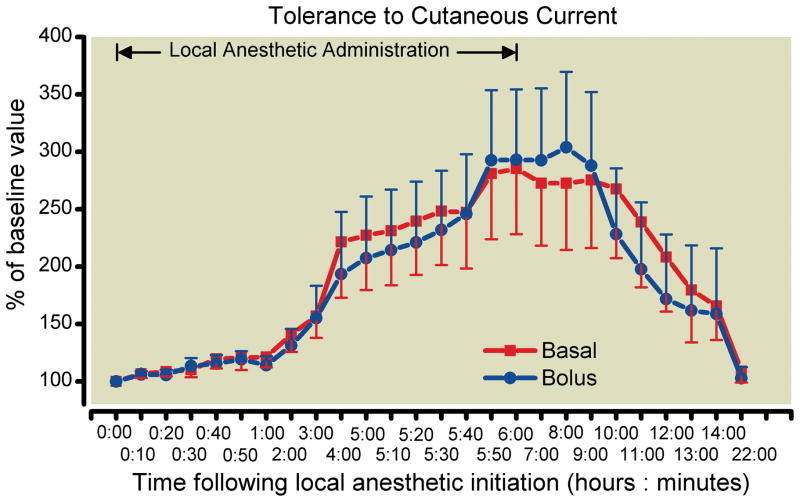
There were similar minimal differences between treatment groups in the secondary endpoints during local anesthetic administration (fig. 2). The estimated difference in hip adductor strength at hour 6 was −6% (95% CI −14 to 2%), which allows a formal conclusion of equivalence (fig. 3). There was a great degree of variability in the measurement of sensory effect resulting in very wide confidence intervals. The mean difference in sensory effect at Hour 6 was −7% (95% CI −127 to 112%; fig. 4).
Fig. 2.
Effects of femoral perineural local anesthetic administration method (basal-only vs. repeated boluses) on quadriceps femoris strength (mean [SE] illustrated). Statistically significant differences between treatments at 95% confidence are designated with an asterisk.
Fig. 3.
Effects of femoral perineural local anesthetic administration method (basal-only vs. repeated boluses) on hip adductor strength (mean [SE] illustrated). The one statistically significant difference between treatments at 95% confidence is designated with an asterisk.
Fig. 4.
Effects of femoral perineural local anesthetic administration method (basal-only vs. repeated boluses) on tolerance of transcutaneous electrical stimulation in the cutaneous distribution of the femoral nerve (mean [SE] illustrated). There were no statistically significant differences between treatments at 95% confidence.
Results were similar in the intent-to-treat population. The Hour 6 basal-bolus difference in quadriceps MVIC was −0.6% (95% CI −24.7 to 23.5%). For hip adductor strength, the basal-bolus was equivalent: −6.0% (95% CI −13.7 to 1.7%). The difference in sensory effect was again highly variable with a mean of 15.2% (95% CI −99.2 to 68.7%).
Discussion
This randomized, double-masked, controlled investigation did not find evidence to support the hypothesis that varying the method of local anesthetic administration—basal infusion versus repeated bolus doses—influences continuous femoral nerve block effects to a clinically significant degree. However, due to a larger SD than anticipated, the 95% confidence interval at Hour 6 (−22.0 to 19.8%) did not fall within our prespecified tolerance interval of −20 to 20%. To conclude equivalence would require a larger independent cohort. Conversely, we detected little difference between treatments at any time point during local anesthetic administration (fig. 2–4). This might be viewed as a positive finding if the sensory block—and, therefore, possibly analgesia—was greatly increased in the bolus-only group, in line with previous reports of continuous popliteal-sciatic nerve blocks.12,22 Unfortunately, this was not the case: tolerance to cutaneous electrical current was virtually indistinguishable at Hour 6 between the two groups (fig. 4).
Previously-published evidence
The differences between our findings for femoral infusion and the previous reports for popliteal-sciatic infusion may be explained in several ways. The first is in catheter-insertion methodology: the previous studies used nerve stimulation to guide needle placement, but then “blindly” advanced nonstimulating catheters 4–5 cm past the needle tip.12,22 As the previous investigators noted, “the final location of the catheter tip… may have actually been located distant from the targeted nerve… This may render it difficult, or even impossible, for the local anesthetic to traverse the distance between the tip and the two sciatic nerve trunks when a slow continuous infusion with an overall small volume is used… In contrast, the use of an intermittent bolus is accompanied by higher pressure and more volume per time, which may increase the spread of local anesthetic…”12 In contrast, our current study used ultrasound to allow positioning of the catheter tips at a precise (and bilaterally equivalent) location immediately posterolateral to the femoral nerve, resulting in very accurate perineural local anesthetic deposition and possibly improved nerve-anesthetic contact.
A second possible explanation for the differing results among studies is the anatomic location of the catheters: local anesthetic deposited in the popliteal fossa in previous studies may have been absorbed by perineural tissue, including blood vessels and the lymphatic system.12,22 Conversely, in the femoral region, the local anesthetic may better pool between the iliac fascia and underlying muscle, providing a conduit and/or reservoir keeping the medication in contact with the femoral nerve.
Regardless of which—or either—supposition is accurate, the results of the current study suggest that the “novel” repeated-bolus application will not be clinically useful to decrease the motor-induced weakness (and possible increased risk of falling) of cFNB following knee surgery. Our intention is not to suggest that repeated boluses are unhelpful for all continuous peripheral nerve blocks: there is high-quality evidence they improve analgesia for continuous popliteal-sciatic blocks.12,22 However, the current study suggests that the increased analgesia previously reported with repeated boluses may result not from a more-favorable motor-sensory block ratio—as suggested recently22—but rather simply from additional local anesthetic reaching the target nerves, increasing both the motor and sensory blocks concurrently.
If this latter supposition is accurate, then previous investigations involving the sciatic nerve in the popliteal fossa should have detected an increased motor block in the bolus-only group accompanying the improved analgesia.12,22 While the investigators did not describe the technique used to evaluate motor block,12,22 they did find in their second publication that 20% of the bolus-only group experienced “motor block” (undefined) at 6 h, compared with only 8% of patients receiving a basal-only infusion (specific P value not provided, but reported as more than 0.05).22 Given that the study was powered for postoperative pain, it is very possible—even probable—that a true difference in motor block was not detected in this secondary endpoint that appears to have been evaluated without a sensitive instrument as a binary variable (“motor block” vs. “no motor block”). Therefore, the repeat-bolus administration method may be superior to an infusion-only method for catheter locations in which increased motor weakness is acceptable such ambulating with crutches following foot surgery; but, it does not appear a promising technique to decrease the risk of quadriceps weakness and possibly falls during cFNB.
Of note is that a statistically significant difference between treatments was detected by mixed-effects models of the repeated measures 4–8 h following catheter removal: those in the bolus-only group experienced a faster resolution of quadriceps femoris weakness compared with patients receiving solely a basal infusion (fig. 2). The clinical relevance of this finding is unclear. It is also noteworthy that for continuous fascia iliaca blocks—very similar to cFNB—bolus-only administration of 10 ml ropivacaine 0.2% (60 min lockout) resulted in similar analgesia to a continuous 10 ml/h infusion of the same medication.23 However, in this investigation, the bolus doses were not repeated hourly; but rather patient-controlled, resulting in a decreased consumption of local anesthetic in the bolus-only group. This study reported a lack of motor block in all patients of both treatment groups, but the method(s) of evaluation were not reported, and it is therefore impossible to compare and contrast their results with the current study.
Safety of large bolus doses
Because quadriceps femoris weakness is associated with significant functional disability8 and an increased risk of falls in elderly patients,9 perineural infusion induces motor block,3 and cFNB is associated with postarthroplasty falls,4 it is postulated that any local anesthetic-induced muscular weakness is best minimized during cFNB.4–6 It is thus disappointing that we did not detect clinically-relevant muscle-sparing by varying the technique of local anesthetic administration (basal vs. bolus). However, our results may be viewed as reassuring when providing relatively large-volume patient-controlled bolus doses during cFNB. Of previous concern was the scenario of a patient having recent knee surgery standing in preparation for therapy, experiencing pain, self-administering a local anesthetic bolus, ambulating, and 15–20 min subsequently having abrupt-onset bolus-induced quadriceps weakness leading to a high risk of falling.1,4,24 It is for this reason that we evaluated quadriceps MVIC every ten minutes during two one-hour periods: from Hour 0–1 to observe the initial affect of a bolus; and from Hour 5–6 to observe the affect of a bolus following 5 previous hours of administration (figs. 2–4). While progressive infusion effects were observed at all time periods, as anticipated, no subject exhibited dramatic bolus-induced abrupt-onset quadriceps weakness. Our results suggest that a relatively large bolus dose retains an acceptable margin of safety.25
Adductor strength
Multiple investigations have suggested that local anesthetic deposition near the femoral nerve at the level of the inguinal crease results in a low (less than 20%) incidence of obturator nerve block.26–30 Given that the obturator nerve innervates the majority of the hip adductor muscles, it may appear confusing that our study found a greater-than 40% decrease in adductor MVIC after 6 h of local anesthetic administration. However, we do not believe that our results contrast with previous reports.26–28 Rather, the pectineus muscle is both innervated by the femoral nerve and adducts the femur.31 Therefore, the adduction weakness found in our study most-likely reflects a block of the femoral—as opposed to obturator—nerve, with resulting motor block in the pectineus muscle and, consequently, weakness in hip adduction.
Study Limitations
The current findings regarding cFNB with 0.1% ropivacaine may not be applicable to other anatomic catheter locations,12,22,32–34 local anesthetics,3 infusion durations, or ropivacaine concentrations/dosages.10,11 Previous findings,11 along with the current study, suggest that local anesthetic dose (mass) is the primary determinant of perineural infusion effects; however, that is not to suggest that in clinical use, delivery method,12,22 concentration, and volume are irrelevant.10 Related to this issue, our study measured cutaneous sensation just medial to the distal quadriceps tendon in nonsurgical volunteers. Whether cutaneous sensation correlates well with postoperative pain following various knee procedures remains unknown, as the anterior branch of the femoral nerve contains afferent nerve fibers providing cutaneous sensation; while the posterior branch contains both afferent (muscle and bone innervation) as well as efferent (muscle innervation) nerve fibers. Answering this question will require a clinical trial involving patients following multiple types of surgical procedures. Regardless, our study did not find evidence to support the hypothesis that varying the method of local anesthetic administration—basal infusion versus repeated bolus doses—influences cFNB effects to a clinically significant degree. However, due to a larger SD than anticipated, the 95% confidence interval at Hour 6 did not fall within our prespecified tolerance interval of −20 to 20%. To conclude equivalence would require a larger independent cohort. Lastly, because each comparison dilutes all other P values, findings in secondary outcomes should be viewed as suggestive, requiring confirmation in a future trial before considering them as definitive.
Study model
By including only nonsurgical volunteers, we were able to exclude postoperative pain as a confounding variable, allowing isolation of the variable local anesthetic administration method affects on muscle strength and cutaneous sensation. For example, in postsurgical patients, inadequate analgesia is often (best?) treated with patient-controlled bolus doses, adding a confounding variable to the study design.22,23 And, by having each subject concurrently receive both study treatments (basal and bolus) and analyzing the intrasubject differences, the study model provided equivalent power with fewer subjects as compared to a parallel-group design. Of course, the inclusion of nonsurgical volunteers also makes extrapolation to clinical practice more difficult.
However, there is evidence that perineural catheter tip location must be equivalent bilaterally for the study model to retain the desired power to detect either equivalency or treatment differences.14 Therefore, we used ultrasound to precisely place the tip of each catheter immediately posterolateral relative to the femoral nerve, confirmed by sonographic visualization. This required that no catheter “slack” be placed between where the catheter penetrated the iliac fascia and the catheter tip itself; resulting in what we had theorized might be a high risk of catheter tip retraction above the iliac fascia. Given this was not an outcomes trial—but rather a pharmacodynamics study—we elected to exclude from the primary analyses subjects in whom catheter tip dislodgement was a probability (as opposed to intention-to-treat analysis optimally used in outcomes trials). We defined probable dislodgement as exhibiting less than a 20% change from baseline in quadriceps femoris MVIC or sensory effect at Hour 4. Using this definition, we may have excluded subjects who were simply very resistant to local anesthetic effects, as opposed to subjects who had a dislodged catheter tip. Of 15 total subjects, four failed to exhibit at least a 20% change from baseline: one limb receiving bolus doses in one subject; one limb receiving basal doses in two different subjects; and complete lack of changes in one subject bilaterally. Of note, there were no differences in subject characteristics between included and excluded subjects (table 1).
In summary, this study did not find evidence to support the hypothesis that varying the method of local anesthetic administration—basal infusion versus repeated bolus doses—influences cFNB effects to a clinically significant degree. Thus, it is doubtful that when using a continuous femoral nerve block, varying the method of local anesthetic administration will provide an increased sensory-to-motor block ratio and minimize motor block and the risk of falling while optimizing cutaneous analgesia. However, additional study within a surgical population is warranted due to limitations of the volunteer subject model of the present investigation.
Acknowledgments
The authors gratefully acknowledge the invaluable assistance of the nursing staff of the University of California San Diego Clinical and Translational Research Institute; and University of California San Diego Investigational Drug Service (San Diego, California).
Funding: Funding for this project provided by the National Institutes of Health grant GM077026 from the National Institute of General Medical Sciences (Bethesda, Maryland; Principal Investigator: Dr. Ilfeld); the Department of Anesthesiology, University of California San Diego (San Diego, California); the Clinical and Translational Research Institute, University of California, San Diego, with funding provided by the National Institutes of Health National Center for Research Resources grant UL1RR031980; and Summit Medical (West Jordan, Utah) provided both an unrestricted research grant and the infusion pumps used in this investigation. This company had no input into any aspect of study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funding entities. Dr. Mariano is currently affiliated with Stanford University School of Medicine (Palo Alto, California).
Footnotes
Conflict of Interest: None.
R Software Environment for Statistical Computing, R Foundation for Statistical Computing (version 2.12), Vienna, Austria. Available at: http://www.r-project.org. Accessed October 15, 2010.
References
- 1.Ilfeld BM, Mariano ER, Girard PJ, Loland VJ, Meyer RS, Donovan JF, Pugh GA, Le LT, Sessler DI, Shuster JJ, Theriaque DW, Ball ST. A multicenter, randomized, triple-masked, placebo-controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge-readiness following total knee arthroplasty in patients on general orthopaedic wards. Pain. 2010;150:477–84. doi: 10.1016/j.pain.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilfeld BM, Yaksh TL. The end of postoperative pain–a fast-approaching possibility? And, if so, will we be ready? Reg Anesth Pain Med. 2009;34:85–7. doi: 10.1097/AAP.0b013e3181962547. [DOI] [PubMed] [Google Scholar]
- 3.Borgeat A, Kalberer F, Jacob H, Ruetsch YA, Gerber C. Patient-controlled interscalene analgesia with ropivacaine 0.2% versus bupivacaine 0.15% after major open shoulder surgery: The effects on hand motor function. AnesthAnalg. 2001;92:218–23. doi: 10.1097/00000539-200101000-00042. [DOI] [PubMed] [Google Scholar]
- 4.Ilfeld BM, Duke BK, Donohue MC. Association between lower extremity continuous peripheral nerve blocks and patient falls following knee and hip arthroplasty. Anesth Analg. 2010;111:1552–4. doi: 10.1213/ANE.0b013e3181fb9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandasami M, Kinninmonth AW, Sarungi M, Baines J, Scott NB. Femoral nerve block for total knee replacement - A word of caution. Knee. 2009;16:98–100. doi: 10.1016/j.knee.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Feibel RJ, Dervin GF, Kim PR, Beaule PE. Major complications associated with femoral nerve catheters for knee arthroplasty: A word of caution. J Arthroplasty. 2009;24:132–7. doi: 10.1016/j.arth.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint SurgAm. 2007;89:780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 8.Mizner RL, Snyder-Mackler L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J OrthopRes. 2005;23:1083–90. doi: 10.1016/j.orthres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Stevens JE, Mizner RL, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J OrthopRes. 2003;21:775–9. doi: 10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 10.Brodner G, Buerkle H, Van Aken H, Lambert R, Schweppe-Hartenauer ML, Wempe C, Gogarten W. Postoperative analgesia after knee surgery: A comparison of three different concentrations of ropivacaine for continuous femoral nerve blockade. Anesth Analg. 2007;105:256–62. doi: 10.1213/01.ane.0000265552.43299.2b. [DOI] [PubMed] [Google Scholar]
- 11.Ilfeld BM, Moeller LK, Mariano ER, Loland VJ, Stevens-Lapsley JE, Fleisher AS, Girard PJ, Donohue MC, Ferguson EJ, Ball ST. Continuous Peripheral Nerve Blocks: Is Local Anesthetic Dose the Only Factor, or Do Concentration and Volume Influence Infusion Effects as Well? Anesthesiology. 2010;112:347–54. doi: 10.1097/ALN.0b013e3181ca4e5d. [DOI] [PubMed] [Google Scholar]
- 12.Taboada M, Rodriguez J, Bermudez M, Valino C, Ulloa B, Aneiros F, Gude F, Cortes J, Alvarez J, Atanassoff PG. A “new” automated bolus technique for continuous popliteal block: A prospective, randomized comparison with a continuous infusion technique. Anesth Analg. 2008;107:1433–7. doi: 10.1213/ane.0b013e3181824164. [DOI] [PubMed] [Google Scholar]
- 13.The Common Rule, Title 45 (Public Welfare), Code of Federal Regulations, Part 46 (Protection of Human Subjects) U.S. Department of Health and Human Services, National Institutes of Health, and Office for Human Research Protections; 2001. pp. 1–18. [PubMed] [Google Scholar]
- 14.Salinas FV, Neal JM, Sueda LA, Kopacz DJ, Liu SS. Prospective comparison of continuous femoral nerve block with nonstimulating catheter placement versus stimulating catheter-guided perineural placement in volunteers. Reg Anesth Pain Med. 2004;29:212–20. doi: 10.1016/j.rapm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Kwoh CK, Petrick MA, Munin MC. Inter-rater reliability for function and strength measurements in the acute care hospital after elective hip and knee arthroplasty. Arthritis Care Res. 1997;10:128–34. doi: 10.1002/art.1790100208. [DOI] [PubMed] [Google Scholar]
- 16.Roy MA, Doherty TJ. Reliability of hand-held dynamometry in assessment of knee extensor strength after hip fracture. Am J Phys Med Rehabil. 2004;83:813–8. doi: 10.1097/01.phm.0000143405.17932.78. [DOI] [PubMed] [Google Scholar]
- 17.Bohannon RW. Measuring knee extensor muscle strength. Am J Phys Med Rehabil. 2001;80:13–8. doi: 10.1097/00002060-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan C, Williams GN. Evoked tetanic torque and activation level explain strength differences by side. Eur J Appl Physiol. 2009;106:769–74. doi: 10.1007/s00421-009-1057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostenberg A, Roos E, Ekdahl C, Roos H. Isokinetic knee extensor strength and functional performance in healthy female soccer players. Scand J Med Sci Sports. 1998;8:257–64. doi: 10.1111/j.1600-0838.1998.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 20.Armitage P, Berry G, Matthews JNS. Statistical methods in medical research. Oxford: Blackwell Science; 2002. [Google Scholar]
- 21.Holm S. A simple sequentially rejective multiple test procedure. Scand J Statistics. 1979;6:65–70. [Google Scholar]
- 22.Taboada M, Rodriguez J, Bermudez M, Amor M, Ulloa B, Aneiros F, Sebate S, Cortes J, Alvarez J, Atanassoff PG. Comparison of continuous infusion versus automated bolus for postoperative patient-controlled analgesia with popliteal sciatic nerve catheters. Anesthesiology. 2009;110:150–4. doi: 10.1097/ALN.0b013e318191693a. [DOI] [PubMed] [Google Scholar]
- 23.Eledjam JJ, Cuvillon P, Capdevila X, Macaire P, Serri S, Gaertner E, Jochum D. Postoperative analgesia by femoral nerve block with ropivacaine 0.2% after major knee surgery: Continuous versus patient-controlled techniques. Reg Anesth Pain Med. 2002;27:604–11. doi: 10.1053/rapm.2002.36454. [DOI] [PubMed] [Google Scholar]
- 24.Ilfeld BM, Le LT, Meyer RS, Mariano ER, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Berry LF, Spadoni EH, Gearen PF. Ambulatory continuous femoral nerve blocks decrease time to discharge readiness after tricompartment total knee arthroplasty: A randomized, triple-masked, placebo-controlled study. Anesthesiology. 2008;108:703–13. doi: 10.1097/ALN.0b013e318167af46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fredrickson MJ, Danesh-Clough TK. Ambulatory continuous femoral analgesia for major knee surgery: A randomised study of ultrasound-guided femoral catheter placement. AnaesthIntensive Care. 2009;37:758–66. doi: 10.1177/0310057X0903700514. [DOI] [PubMed] [Google Scholar]
- 26.Bouaziz H, Vial F, Jochum D, Macalou D, Heck M, Meuret P, Braun M, Laxenaire MC. An evaluation of the cutaneous distribution after obturator nerve block. Anesth Analg. 2002;94:445–9. doi: 10.1097/00000539-200202000-00041. [DOI] [PubMed] [Google Scholar]
- 27.Lang SA, Yip RW, Chang PC, Gerard MA. The femoral 3-in-1 block revisited. J ClinAnesth. 1993;5:292–6. doi: 10.1016/0952-8180(93)90121-t. [DOI] [PubMed] [Google Scholar]
- 28.Atanassoff PG, Weiss BM, Brull SJ, Horst A, Kulling D, Stein R, Theiler I. Electromyographic comparison of obturator nerve block to three-in-one block. Anesth Analg. 1995;81:529–33. doi: 10.1097/00000539-199509000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Cappelleri G, Aldegheri G, Ruggieri F, Carnelli F, Fanelli A, Casati A. Effects of using the posterior or anterior approaches to the lumbar plexus on the minimum effective anesthetic concentration (MEAC) of mepivacaine required to block the femoral nerve: A prospective, randomized, up-and-down study. Reg Anesth Pain Med. 2008;33:10–6. doi: 10.1016/j.rapm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Marhofer P, Nasel C, Sitzwohl C, Kapral S. Magnetic resonance imaging of the distribution of local anesthetic during the three-in-one block. AnesthAnalg. 2000;90:119–24. doi: 10.1097/00000539-200001000-00027. [DOI] [PubMed] [Google Scholar]
- 31.Snaith R, Dolan J. Ultrasound-guided interfascial injection for peripheral obturator nerve block in the thigh. Reg Anesth Pain Med. 2010;35:314–5. doi: 10.1097/AAP.0b013e3181c6f25c. [DOI] [PubMed] [Google Scholar]
- 32.Le LT, Loland VJ, Mariano ER, Gerancher JC, Wadhwa AN, Renehan EM, Sessler DI, Shuster JJ, Theriaque DW, Maldonado RC, Ilfeld BM. Effects of local anesthetic concentration and dose on continuous interscalene nerve blocks: A dual-center, randomized, observer-masked, controlled study. Reg Anesth Pain Med. 2008;33:518–25. doi: 10.1016/j.rapm.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilfeld BM, Le LT, Ramjohn J, Loland VJ, Wadhwa AN, Gerancher JC, Renehan EM, Sessler DI, Shuster JJ, Theriaque DW, Maldonado RC, Mariano ER. The effects of local anesthetic concentration and dose on continuous infraclavicular nerve blocks: Amulticenter, randomized, observer-masked, controlled study. Anesth Analg. 2009;108:345–50. doi: 10.1213/ane.0b013e31818c7da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ilfeld BM, Loland VJ, Gerancher JC, Wadhwa AN, Renehan EM, Sessler DI, Shuster JJ, Theriaque DW, Maldonado RC, Mariano ER. The effects of varying local anesthetic concentration and volume on continuous popliteal sciatic nerve blocks: A dual-center, randomized, controlled study. Anesth Analg. 2008;107:701–7. doi: 10.1213/ane.0b013e3181770eda. [DOI] [PMC free article] [PubMed] [Google Scholar]