Abstract
Heterogeneous molecular species of epidermal ceramide (Cer) play critical roles in forming a competent permeability barrier of lamellar membrane structures in the stratum corneum, which is a prerequisite to preventing excess water loss for terrestrial mammals. Lipids containing very long chain lengths of fatty acids (VLCFA) (hydrocarbon chain lengths over 28) have been found in selected tissues, including epidermis. In particular, ω-hydroxy (ω-OH) VLCFA as well as Cer containing ω-OH VLCFA and ω-O-acylCer (acylCer) are unique to epidermis. The fatty acid elongation system that generates VLCFA, which requires four enzymatic steps, has been characterized, while recent studies using transgenic animals have further revealed the importance of ω-OH Cer species for barrier formation and have also elucidated the synthetic pathway of these essential Cer species in conjunction with VLCFA metabolism. This review article discusses the generation of VLCFA and unique epidermal Cer species containing VLFCA in the relation to their roles in epidermis.
Key words: ceramide, epidermal barrier, fatty acid, fatty acid elongation, stratum corneum, very long chain fatty acid
Introduction
Epidermis, the outermost layer of skin, deploys an epidermal permeability barrier to prevent excess water loss1 and ingress of pathogens, allergens and xenotoxic agents.2 The presence of this barrier is necessary for survival of mammalian species in a terrestrial environment. More precisely, lipid lamellar membrane structures in the extracellular domain of the stratum corneum (SC) are responsible for barrier function. Heterogeneous molecular species of ceramide (Cer) are key constituents that form lamellar structures. The most unique and essential Cer species contain very long omega (ω)-hydroxy fatty acids (ω-OH VLCFA) (hydrocarbon chain length ranging from 28–34). Such very long chain lengths of fatty acids (VLCFA) have been found in selected tissues, such as retina,3 brain,4 testis,5 spermatozoa6 and epidermis (reviewed in ref. 7), while ω-OH VLCFA as well as Cer and their glucosylated counterparts containing ω-OH VLCFA have been found only in differentiated layers of the epidermis of terrestrial mammals. This review article describes the generation and roles of VLCFA in epidermis.
Note.
FA are generally classified into Short chain fatty acid (SCFA), containing fewer than six chain lengths of hydrocarbons, Medium chain fatty acid (MCFA) with 6–12 hydrocarbons, Long chain fatty acid (LCFA) with longer than 12 hydrocarbons, and VLCFA with longer than 22. In this review article, VLCFA indicates FA containing longer than 28 hydrocarbons.
VLCFA Biosynthesis
Lipids are divided into neutral lipids and complex lipids. Fatty acids (FA) are constituents of both of these lipid species. Neutral lipids are generated from alcohol and FA, i.e., acylglyceride (glycerol + FA), wax (primary alcohol + long chain FA), Cer (long chain amino alcohol + FA) and sterol esters (sterol + FA). Complex lipids include phospholipids and glycolipids, which contain either a glycerol or sphingoid base (long chain amino alcohol) backbone structure, respectively. FA serve as a major source of energy as well as structural components of membranes as both free forms and esterified forms, and also act as nuclear hormone ligands to regulate cellular functions.
FA with carbon chain lengths up to C16 are synthesized by the FA synthase complex localized in cytosolic fraction (FAS-I) and mitochondria (FAS-II)8 (c.f. see in the review of this series by Dr. Denis Khnykin). Synthesized FA or FA supplied from dietary sources are then elongated by membrane-associated FA chain-elongation system at the endoplasmic reticulum (ER).8 Similar to the FA synthase complex, the FA chain-elongation system uses four metabolically-linked enzymes for chain elongation: 3-keto-acyl-CoA synthase (Condensation), 3-keto-acyl-CoA reductase (Reduction), 3-hydroxyacyl-CoA hydrase (Dehydration) and 2,3 enoyl-CoA reductase (Reduction). Chain extension occurs via two carbons per cycle. While the fatty acid synthase (FAS-I) is a single, homodimeric, multifunctional protein, both the FA elongation complex and FAS-II consist of four different classes of enzymes.
The enzymes that catalyze these four steps have been recently characterized in mammals.
Condensation (1st Step). ELOs that are responsible for the condensation step first were found in yeasts, and later in plants and mammals. Seven ELO homologues (ELOVL 1–7) have been identified in mammals. ELOVLs exhibit tissue-specific distribution and distinct substrate specificities. Further details of ELOVLs are described in the next section (see below).
Reduction (2nd Step). 3-ketoacyl-CoA reductase (KAR), which has a homologous relationship with the short chain dehydrogenase superfamily, catalyzes 3-ketoacyl-CoA to 3-hydroxyacyl-CoA. The gene encoding this enzyme is a homologue of yeast Ybr159p.9
Dehydration (3rd Step). Four 3-hydroxyacyl-CoA dehydratases (HACD) that catalyze the dehydration of the 3-hydroxyacyl-CoA have been characterized in mammals. Both HACD1 and HACD2 have similarities in their sequences to yeast Phs. Like ELOVLs, four HASDs show tissue specific expression profiles.10 Although substrate specificities of HACD have not been completely elucidated, HACD2 and HACD3 are ubiquitously expressed, and the other two HACD levels, HACD1 and HACD4, are high in heart and leukocyte in humans,10 suggesting that appropriate HACDs are likely utilized for specific FA elongation in combination with ELOVL expressed in the same tissue. Yet, expression profile on HACD in epidermis has not been characterized.
Reduction (4th Step). The trans-2,3-enoyl-CoA reductase (TER) that catalyzes 3-hydroxyacyl-CoA to fatty acyl-CoA, is 32% identical to the yeast trans-2,3-enoyl-CoA reductase and a homologue of Yeast Tsc13p.9 Although all other FA elongation enzymes (step 1 through 3) contain a ER retention motif (KKXX), TER does not have such motif.9 In contrast to ELOVLs and HACDs, only two reductases (KAR and TER) that account for the second and last step of elongation have been identified. It is unclear whether these identified reductases serve to reduce any chain lengths of 3-ketoacyl-CoA or 3-hydroxyacyl-CoA.
VLCFA in Tissues
VLCFA (C26–40) are present as saturated, monounsaturated and polyunsaturated forms. In contrast to FA containing hydrocarbon chain lengths of 16–22, which are widely distributed in most living organisms including mammals, VLCFA (C26–40) are found in limited species and tissues mainly in yeasts, plants (seed oils, plant waxes, cutin, suberin) and mammals (brain, retina, skin, hair, testis and spermatozoa). Brain and retina contain VLCFA-PUFA in phosphatidylcholine,4,11 while VLCFA are contained in Cer, glycosphingolipids and sphingomyelins (SM) in spermatozoa.12,13 VCLFA have been shown predominantly in Cer and glucosylceramide (GlcCer), but trace levels are in SM, in epidermis.14
ELOVL
ELOVL have been the most characterized enzymes in the FA elongation complex, including substrate specificities and expression profile in tissues. Pertinently, physiological and pathological roles of certain ELOVL in mammals, including in skin, have been elucidated. Hence, ELOVLs are further discussed in this review below.
Seven mammalian homologues (ELOVL1–7) of the yeast ELO gene products exhibit substrate specificities as well as tissue specific distribution.15 While the expression profile of ELOVL7 has not been elucidated, distribution of the other six ELOVL have been examined, i.e., ELOVL1, ELOVL5 and ELOVL6 are ubiquitously expressed, while ELOVL2, ELOVL3 and ELOVL4 highly express in liver/testis, skin/liver and retina/brain/skin, respectively (note: whole skin was used in this study).24 Yet, the expression profile/level of other ELOVLs has not been completely defined in comparison to other tissues.
ELOVLs contain five transmembrane regions, a histidine-rich motif (HXXHH) and a ER retention signal (KKXX) (reviewed in ref. 7).
The distinctive roles of each ELOVL in generating different VLCFA species have been elucidated, while recent studies using different chain lengths of FA as substrates further clarified the substrate specificities and their products.15 ELOVL1, ELOVL3, ELOVL4 and ELOVL6 account for the elongation of both saturated and monosaturated VLCFA synthesis, i.e., ELOVL1, substrate specificity: C20-26, C20-22:1; ELOVL3, C18–26, C20–22:1; and ELOVL6, C12–16, C18:1, while ELOVL2 and ELOVL5 elongate polyunsaturated FA (PUFA), i.e., C20–22:4, C20–22:5 and ELOVL5, C18–20:4, C20:5.15 ELOVL4 utilizes both saturated and PUFA as substrates (≥C24, C24:4, C24:5, C24:6) to synthesize VLCFA.15
Roles of VLCFA in Epidermis
In addition to free VLCFA form, VLCFA are utilized as constituents of amide-like FA in Cer and GlcCer in epidermis. In particular, acylceramides (acylCer) (Fig. 1) and their glucosylated form [acylglucosylceramide (acylGlcCer)] that contain ω-OH VLCFA are unique to epidermis. Moreover, ω-OH residue is esterified by the predominantly essential FA, linoleate, deficiency of which results in abnormal permeability function.16 Therefore, roles of acylCer and acylGlcCer in skin have been paid attention to since the discovery of these molecules in the early 1980s,17–19 and the importance of acylCer as an epidermal permeability barrier constituent has mounted. In vitro studies using reconstituted membrane models demonstrated the role of acylCer in the establishment of the long periodicity phase (13 nm) that represents normal lamellar membrane structures in the extracellular domains of barrier competent SC.20 Indeed, decreases in acylCer have been shown in cutaneous diseases with abnormal permeability barrier function, e.g., atopic dermatitis,21–23 senile xerosis,24 lamellar ichthyosis,25 Sjögren-Larsson syndrome25 and Dorfman-Chanarin syndrome.26 Moreover, recent findings in cutaneous diseases and their animal models have further suggested the importance of acylCer for terrestrial mammalian survival. A mutant Elovl4-knock-in mouse, a model of a rare form of autosomal dominant Stargardt macular dystrophy (STGD3), which lacks normal VLCFA generation due to a single 5 bp deletion coded ER retention signal,27 results in the failure to form FA elongation complex. The homozygous mutant Elovl4 knock-in mouse lacks both acylCer and normal lamellar membranes, and displays abnormal lamellar body contents and postnatal lethality within 4 hrs due to excess water loss.28,29 Elovl4-null mice also show similar features.30 Whereas VLCFA are contained in acylCer and acylGlcCer in epidermis, part of VLCFA is in other Cer species as well as free VLCFA. However, most recent studies further support the essential role of acylCer in barrier formation and function. Deficiencies of both acylCer and bound ω-OH Cer were found in the SC of a patient with Dorfman-Chanarin syndrome, an autosomal recessive, neutral lipid storage disorder with ichthyosis (NLSDI), displaying an abnormal permeability barrier function due to loss-of-function mutations in CGI-58 (α/β-hydrolase domain-containing protein 5) [ABHD5] (that codes a cofactor required for triacylglyceride lipase).26 CGI-58 deficient mice, a model of Dorfman-Chanarin syndrome, also display both acylCer and bound ω-OH Cer deficiencies in parallel with a neonatal lethal skin barrier defect31 (c.f. see in the review of this series by Dr. Rudolf Zechner). Although triacylglyceride accumulation in the SC32 could also contribute to altered lamellar membrane structures, VLCFA generation is not affected by lacking CGI-58 function, suggesting that acylCer rather than other Cer containing VLCFA and/or free VFCFA are more critical lipid species. The insights of Dorfman-Chanarin syndrome also led to elucidation of the synthetic pathway of acylCer (see next section). Finally, the ω-position of the amide-linked FA moiety of ω-OH Cer becomes covalently bound to the carboxy termini of cornified envelope proteins (primarily involucrin) on the external surface of the corneocyte, forming the corneocyte lipid envelope (i.e., bound form of ω-OH Cer.) (reviewed in ref. 33) Although the function of the corneocyte lipid envelope has not been completely defined, it has been proposed that it serves as a scaffold for the deposition of extracellular lamellar bilayers.34 Hence, taken together with these findings, acylCer is a more physiologically important lipid species containing VLCFA compared with other Cer species and/or free VLCFA in epidermis.
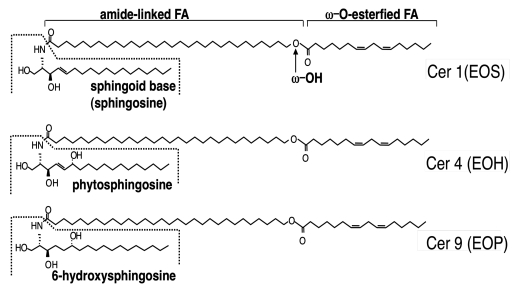
Figure 1.
Structures of AcylCer. Abbreviation for Cer structures are according to Robson et al. and Motta et al. N, A and EO indicate amide-linked FA species: N, non-OH FA; and EO, ω-O-esterified FA. A, S, P and H indicate sphingoid base structures: S, sphingosine (or sphingenine); P, 4-hydroxysphinganine (or phytosphingosine); and H, 6-hydroxysphingosine (or 6-hydroxysphingenine). Late stages of differentiated keratinocytes produce heterogeneous Cer molecules (at least ten species) due to different combination of sphingoid base and amide-linked FA species, while acylCer are unique to the epidermis.
AcylCer Formation
AcylCer synthesis occurs in the late stages of keratinocyte differentiation.35,36 In human epidermis, three distinct acylCer species which contain a different sphingoid base; i.e., sphingosine, 6-hydroxy sphingosine and 4-hydroxysphinganine (or phytosphingosine), for the acylCer species Cer 1 (EOS), Cer 4 (EOH), and Cer 9 (EOP), respectively33 (Fig. 1), have been identified. AcylCer formation requires unique synthetic steps, including VLCFA synthesis, ω-hydroxylation, and ω-O-esterification (Fig. 2).
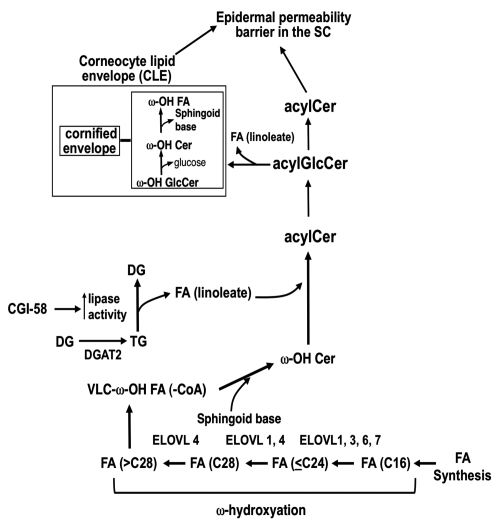
Figure 2.
Updated most likely pathway for acylCer formation (November 2010). AcylCer formation requires unique biochemical steps, which include VLFA synthesis, ω-hydroxylation and ω-O-esterification. ELOVL4 and a cytochrome P-450 enzyme account for VLFA synthesis and ω-hydroxylation, respectively. Linoleic acid, an essential FA, is a predominant lipid species in ω-O-esterified FA. CE, cornified envelope; LA, linoleic acid; Sp, sphingoid base; and DGAT2, acylCoA:diacylglycerol acyltransferase 2.
VLCFA synthesis.
Elovl3-, Elovl4-, Elovl5-,37 Elovl6-null mice,38 and mutant Elov4 knock-in mice have been generated to date (November 2010). Only homozygous Elovl4-null and Elov4 knock-in mice are lethal due to epidermal permeability barrier defects.28–30 VLCFA containing hydrocarbon chain length of 28 levels do not differ in epidermis of normal Wt mice and of homozygous mutant ELOVL4 knock-in mice that lack acyl-Cer production.28 However, since increased VLCFA containing hydrocarbon chain length of 26 and absence of >C28 are evident in epidermis from homozygous mutant Elovl4-knock-in mice,28 ELOVL4 is responsible for VLCFA being required for acylCer amide-linked FA production.28 Skin phenotypes and epidermal Cer profiles of Elovl5-null (-/-),37 and Elovl6-null38 mice are not reported, while those mice are not neonatal lethal. Although Elovl3-null mice show normal epidermal permeability barrier function at basal condition and contain normal levels of acylCer, a barrier recovery was delayed following acute barrier perturbation,39 suggesting that ELOVL3 function is not compensated by other ELOVLs. Therefore, both ELOVL1, 3, 6 and/or 7 can be involved in generate substrate of VLCFA catalyzed by ELOVL4, and ELOVL1, 3, 6 and/or 7, expressed in skin, can compensate each other to synthesize VLCFA, if any of them are defective.
Cer synthesis.
Cer are synthesized primarily at the endoplasmic reticulum (ER). Sphingoid base is generated by both De novo pathway and Salvage (or recycle) pathway. De novo pathway L-serine and palmitoyl-CoA are condensed by serine palmitoyl-transferase to form 3-ketosphinganine, followed by its reduction to sphinganine by 3-ketosphinganine reductase, and subsequent N-acylation of sphinganine by Cer synthase(s).40 Six isoforms of Cer synthase (CerS 1–6) preferentially utilize selected carbon chain lengths of acyl-CoA as substrate.41 CerS 3, which is expressed in skin and testis and has broad substrate specificity, including VLCFA, appears to be involved in acylCer formation.42 Following N-acylation, the sphinganine moiety is either desaturated to a sphingenine (or sphingosine) backbone by desaturase-1 (DES-1) or hydroxylated to 4-OH sphinganine (phytosphingosine),43 by desaturase-2 (or DES-2) or to 6-OH sphingosine by an enzyme which has not yet been identified.44 In addition to de novo sphingoid base synthesis (through serine palimitoyltransferase), salvage pathway (or sphingosine recycling) also occurs as GlcCer and SM are hydrolyzed to Cer, followed by hydrolysis to sphingoid base and FA by ceramidases.45 Generated sphingoid base can then be reutilized as substrates for Cer formation.36
FA ω-hydroxylation.
Prior studies indicate that blockade of FA ω-hydroxylation using a specific inhibitor of P-450-type 4 isoforms, aminobenzoltriazol, causes a decrease in ω-OH Cer production and CLE formation accompanied by a barrier abnormality,46 suggesting that P-450-type 4 isoform appears to account for FA ω-hydroxylation.
ω-O-FA esterification.
While it is suggested that FA derived from triacylglyceride is utilized in the ω-O-esterification step to form acylCer,47 both triacylglyceride, linoleate and acylCer content decline in acyl-CoA:diacylglycerol acyltransferase-2 (DGAT2)-deficient mice in parallel with abnormal permeability barrier.48 Moreover, as described above, recent studies further suggest that linoleate produced by the lypolysis of triacylglyceride, which is facilitated by CGI-58, provides FA that is utilized for ω-O-esterification leading to acylCer formation.26,31
Regulation of ELOVL Expression
Increased in both acylCer/acyl-GlcCer generation36 and ELOVL4 mRNA expression occur late in keratinocyte differentiation (Y. Uchida et al. unpublished data). Gene silencing of vitamin D receptor and its coactivator SRC (steroid receptor coactivator) 2 and SRC3 decreased both ELOVL3 and ELOVL4 mRNA expression in cultured primary KC.49 Since vitamin D receptor stimulation increased KC differentiation, it remains to be resolved whether the vitamin D receptor directly binds to promoter regions of ELOVL3 and/or ELOVL4. The regulatory mechanisms of other ELOVLs in epidermis have not been elucidated.
Similar to epidermis, a few studies about the regulation of ELOVL expression have been demonstrated in selected extracutaneous tissues as below. ELOVL1, ELOVL3 and ELOVL6 expression increase after cold stress followed by heat generation to maintain body temperature in murine brown adipose tissues.50 Nutritional status also affects FA metabolism, including changes in ELOVL expression.51 These alterations of ELOVL expression are due to transcriptional levels. ELOVL1 and ELOVL6 mRNA expressions are upregulated by the nuclear liver X receptor (LXR)-sterol regulatory element-binding protein (SREBP-1)-mediated mechanism cultured brown adipocytes.50 In contrast, ELOVL3 mRNA expression is downregulated by LXR-SREBP-1 mechanism and upregulated by adrenergic stimulation.50 Recent studies also characterized the adrenergic mechanism for ELOVL3 as synergistically enhanced by the peroxisome proliferator-activated receptor (PPAR)α activation.52 ELOVL5 mRNA expression is increased by LXRα-SREBP-1c pathway and downregulated by PUFA.53
Acknowledgements
The author thanks Dr. Yukiko Mizutani (Hokkaido University, Sapporo, Japan) for helpful input to the manuscript and also thanks Ms. Joan Wakefield for editorial assistance. Drs. Peter M. Elias and Walter M. Holleran (Department of Dermatology, Veterans Affairs Medical Center and University of California, San Francisco, CA USA) are gratefully acknowledged for critical discussions about the metabolism and roles of acylceramide in skin. These studies were supported by a National Institutes of Health Grant AR 051077.
References
- 1.Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 2.Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 3.Aveldano MI, Sprecher H. Very long chain (C24 to C36) polyenoic fatty acids of the n-3 and n-6 series in dipolyunsaturated phosphatidylcholines from bovine retina. J Biol Chem. 1987;262:1180–1186. [PubMed] [Google Scholar]
- 4.Sharp P, Johnson D, Poulos A. Molecular species of phosphatidylcholine containing very long chain fatty acids in human brain: enrichment in X-linked adrenoleukodystrophy brain and diseases of peroxisome biogenesis brain. J Neurochem. 1991;56:30–37. doi: 10.1111/j.1471-4159.1991.tb02558.x. [DOI] [PubMed] [Google Scholar]
- 5.Grogan WM. Metabolism of arachidonate in rat testis: characterization of 26–30 carbon polyenoic acids. Lipids. 1984;19:341–346. doi: 10.1007/BF02534785. [DOI] [PubMed] [Google Scholar]
- 6.Poulos A, Sharp P, Johnson D, Easton C. The occurrence of polyenoic fatty acids with greater than 22 carbon atoms in mammalian spermatozoa. Biochem J. 1986;240:891–895. doi: 10.1042/bj2400891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonard AE, Pereira SL, Sprecher H, Huang YS. Elongation of long-chain fatty acids. Prog Lipid Res. 2004;43:36–54. doi: 10.1016/s0163-7827(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 8.Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res. 2006;45:237–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Moon YA, Horton JD. Identification of two mammalian reductases involved in the two-carbon fatty acyl elongation cascade. J Biol Chem. 2003;278:7335–7343. doi: 10.1074/jbc.M211684200. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda M, Kanao Y, Yamanaka M, Sakuraba H, Mizutani Y, Igarashi Y, et al. Characterization of four mammalian 3-hydroxyacyl-CoA dehydratases involved in very longchain fatty acid synthesis. FEBS Lett. 2008;582:2435–2440. doi: 10.1016/j.febslet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Aveldano MI. A novel group of very long chain polyenoic fatty acids in dipolyunsaturated phosphatidylcholines from vertebrate retina. J Biol Chem. 1987;262:1172–1179. [PubMed] [Google Scholar]
- 12.Furland NE, Oresti GM, Antollini SS, Venturino A, Maldonado EN, Aveldaño MI. Very long-chain polyunsaturated fatty acids are the major acyl groups of sphingomyelins and ceramides in the head of mammalian spermatozoa. J Biol Chem. 2007;282:18151–18161. doi: 10.1074/jbc.M700709200. [DOI] [PubMed] [Google Scholar]
- 13.Rabionet M, van der Spoel AC, Chuang CC, von Tümpling-Radosta B, Litjens M, Bouwmeester D, et al. Male germ cells require polyenoic sphingolipids with complex glycosylation for completion of meiosis: a link to ceramide synthase-3. J Biol Chem. 2008;283:13357–13369. doi: 10.1074/jbc.M800870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida Y, Hara M, Nishio H, Sidransky E, Inoue S, Otsuka F, et al. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J Lipid Res. 2000;41:2071–2082. [PubMed] [Google Scholar]
- 15.Ohno Y, Suto S, Yamanaka M, Mizutani Y, Mitsutake S, Igarashi Y, et al. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci USA. 2010;107:18439–18444. doi: 10.1073/pnas.1005572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burr GO, Burr MM. On the Nature and Role of Fatty Acids Essential in Nutrition. J Biol Chem. 1930;86:587–621. [Google Scholar]
- 17.Gray GM, White RJ, Majer JR. 1-(3′-O-acyl)-beta-glucosyl-N-dihydroxypentatriacontadienoylsphingosine, a major component of the glucosylceramides of pig and human epidermis. Biochim Biophys Acta. 1978;528:127–137. doi: 10.1016/0005-2760(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 18.Wertz PW, Downing DT. Ceramides of pig epidermis: structure determination. J Lipid Res. 1983;24:759–765. [PubMed] [Google Scholar]
- 19.Bowser PA, Nugteren DH, White RJ, Houtsmuller UM, Prottey C. Identification, isolation and characterization of epidermal lipids containing linoleic acid. Biochim Biophys Acta. 1985;834:419–428. doi: 10.1016/0005-2760(85)90016-5. [DOI] [PubMed] [Google Scholar]
- 20.Bouwstra JA, Ponec M. The skin barrier in healthy and diseased state. Biochim Biophys Acta. 2006;1758:2080–2095. doi: 10.1016/j.bbamem.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96:523–526. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Archives for Dermatological Research. Archiv fur Dermatologische Forschung. 1991;283:219–223. doi: 10.1007/BF01106105. [DOI] [PubMed] [Google Scholar]
- 23.Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Dermato-Venereologica. 1998;78:27–30. doi: 10.1080/00015559850135788. [DOI] [PubMed] [Google Scholar]
- 24.Rogers J, Harding C, Mayo A, Banks J, Rawlings A. Stratum corneum lipids: the effect of ageing and the seasons. Arch Dermatol Res. 1996;288:765–770. doi: 10.1007/BF02505294. [DOI] [PubMed] [Google Scholar]
- 25.Paige DG, Morse-Fisher N, Harper JI. Quantification of stratum corneum ceramides and lipid envelope ceramides in the hereditary ichthyoses. Br J Dermatol. 1994;131:23–27. doi: 10.1111/j.1365-2133.1994.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 26.Uchida Y, Cho Y, Moradian S, Kim J, Nakajima K, Crumrine D, et al. Neutral lipid storage leads to acylceramide deficiency, likely contributing to the pathogenesis of Dorfman-Chanarin syndrome. J Invest Dermatol. 2010;130:2497–2499. doi: 10.1038/jid.2010.145. [DOI] [PubMed] [Google Scholar]
- 27.Zhang K, Kniazeva M, Han M, Li W, Yu Z, Yang Z, et al. A 5 bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat Genet. 2001;27:89–93. doi: 10.1038/83817. [DOI] [PubMed] [Google Scholar]
- 28.Vasireddy V, Uchida Y, Salem N, Jr, Kim SY, Mandal MN, Reddy GB, et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (≥C28) and the unique {omega}-O-acylceramides in skin leading to neonatal death. Hum Mol Genet. 2007;16:471–482. doi: 10.1093/hmg/ddl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMahon A, Butovich IA, Mata NL, Klein M, Ritter R, 3rd, Richardson J, et al. Retinal pathology and skin barrier defect in mice carrying a Stargardt disease-3 mutation in elongase of very long chain fatty acids-4. Mol Vis. 2007;13:258–272. [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Sandhoff R, Kono M, Zerfas P, Hoffmann V, Ding BC, et al. Depletion of ceramides with very long chain fatty acids causes defective skin permeability barrier function, and neonatal lethality in ELOVL4 deficient mice. Int J Biol Sci. 2007;3:120–128. doi: 10.7150/ijbs.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radner FP, Streith IE, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, et al. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58) J Biol Chem. 2010;285:7300–7311. doi: 10.1074/jbc.M109.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ujihara M, Nakajima K, Yamamoto M, Teraishi M, Uchida Y, Akiyama M, et al. Epidermal triglyceride levels are correlated with severity of ichthyosis in Dorfman-Chanarin syndrome. J Dermatol Sci. 2010;57:102–107. doi: 10.1016/j.jdermsci.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Uchida Y, Holleran WM. Omega-O-acylceramide, a lipid essential for mammalian survival. J Dermatol Sci. 2008;51:77–87. doi: 10.1016/j.jdermsci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Wertz PW, Madison KC, Downing DT. Covalently bound lipids of human stratum corneum. J Invest Dermatol. 1989;92:109–111. doi: 10.1111/1523-1747.ep13071317. [DOI] [PubMed] [Google Scholar]
- 35.Uchida Y, Behne M, Quiec D, Elias PM, Holleran WM. Vitamin C stimulates sphingolipid production and markers of barrier formation in submerged human keratinocyte cultures. J Invest Dermatol. 2001;117:1307–1313. doi: 10.1046/j.0022-202x.2001.01555.x. [DOI] [PubMed] [Google Scholar]
- 36.Hamanaka S, Nakazawa S, Yamanaka M, Uchida Y, Otsuka F. Glucosylceramide Accumulates Preferentially in Lamellar Bodies in Differentiated Keratinocytes. Br J Dermatol. 2005;152:426–434. doi: 10.1111/j.1365-2133.2004.06333.x. [DOI] [PubMed] [Google Scholar]
- 37.Moon YA, Hammer RE, Horton JD. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J Lipid Res. 2009;50:412–423. doi: 10.1194/jlr.M800383-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuzaka T, Shimano H, Yahagi N, Kato T, Atsumi A, Yamamoto T, et al. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med. 2007;13:1193–1202. doi: 10.1038/nm1662. [DOI] [PubMed] [Google Scholar]
- 39.Westerberg R, Tvrdik P, Undén AB, Månsson JE, Norlén L, Jakobsson A, et al. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J Biol Chem. 2004;279:5621–5629. doi: 10.1074/jbc.M310529200. [DOI] [PubMed] [Google Scholar]
- 40.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 41.Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: Metabolism, function and role(s) in skin disorders. FEBS Letters. 2006;23:5456–5466. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 42.Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie. 2009;91:784–790. doi: 10.1016/j.biochi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Ternes P, Franke S, Zähringer U, Sperling P, Heinz E. Identification and characterization of a sphingolipid delta 4-desaturase family. J Biol Chem. 2002;277:25512–25518. doi: 10.1074/jbc.M202947200. [DOI] [PubMed] [Google Scholar]
- 44.Stewart ME, Downing DT. A new 6-hydroxy-4-sphingenine-containing ceramide in human skin. J Lipid Res. 1999;40:1434–1439. [PubMed] [Google Scholar]
- 45.Hamanaka S, Nakazawa S, Yamanaka M, Uchida Y, Otsuka F. Glucosylceramide accumulates preferentially in lamellar bodies in differentiated keratinocytes. Br J Dermatol. 2005;152:426–434. doi: 10.1111/j.1365-2133.2004.06333.x. [DOI] [PubMed] [Google Scholar]
- 46.Behne M, Uchida Y, Seki T, de Montellano PO, Elias PM, Holleran WM. Omega-hydroxyceramides are required for corneocyte lipid envelope (CLE) formation and normal epidermal permeability barrier function. J Invest Dermatol. 2000;114:185–192. doi: 10.1046/j.1523-1747.2000.00846.x. [DOI] [PubMed] [Google Scholar]
- 47.Wertz PW, Downing DT. Metabolism of linoleic acid in porcine epidermis. J Lipid Res. 1990;31:1839–1844. [PubMed] [Google Scholar]
- 48.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 49.Oda Y, Uchida Y, Moradian S, Crumrine D, Elias PM, Bikle DD. Vitamin D Receptor and Coactivators SRC2 and 3 Regulate Epidermis-Specific Sphingolipid Production and Permeability Barrier Formation. J Invest Dermatol. 2008;129:1367–1378. doi: 10.1038/jid.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jakobsson A, Jorgensen JA, Jacobsson A. Differential regulation of fatty acid elongation enzymes in brown adipocytes implies a unique role for Elovl3 during increased fatty acid oxidation. Am J Physiol Endocrinol Metab. 2005;289:517–526. doi: 10.1152/ajpendo.00045.2005. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Botolin D, Christian B, Busik J, Xu J, Jump DB. Tissue-specific, nutritional and developmental regulation of rat fatty acid elongases. J Lipid Res. 2005;46:706–715. doi: 10.1194/jlr.M400335-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jorgensen JA, Zadravec D, Jacobsson A. Norepinephrine and rosiglitazone synergistically induce Elovl3 expression in brown adipocytes. Am J Physiol Endocrinol Metab. 2007;293:1159–1168. doi: 10.1152/ajpendo.00213.2007. [DOI] [PubMed] [Google Scholar]
- 53.Qin Y, Dalen KT, Gustafsson JA, Nebb HI. Regulation of hepatic fatty acid elongase 5 by LXRalpha-SREBP-1c. Biochim Biophys Acta. 2009;1791:140–147. doi: 10.1016/j.bbalip.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Robson KJ, Stewart ME, Michelsen S, Lazo ND, Downing DT. 6-Hydroxy-4-sphingenine in human epidermal ceramides. J Lipid Res. 1994;35:2060–2068. [PubMed] [Google Scholar]
- 55.Motta S, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R. Ceramide composition of the psoriatic scale. Biochim Biophys Acta. 1993;1182:147–151. doi: 10.1016/0925-4439(93)90135-n. [DOI] [PubMed] [Google Scholar]