Abstract
ABCA12 is a member of the large superfamily of ATP-binding cassette (ABC) transporters, which bind and hydrolyze ATP to transport various molecules across limiting membranes or into vesicles. The ABCA subfamily members are thought to be lipid transporters. ABCA12 is a keratinocyte transmembrane lipid transporter protein associated with the transport of lipids in lamellar granules to the apical surface of granular layer keratinocytes. Extracellular lipids, including ceramide, are thought to be essential for skin barrier function. ABCA12 mutations are known to underlie the three main types of autosomal recessive congenital ichthyoses: harlequin ichthyosis, lamellar ichthyosis and congenital ichthyosiform erythroderma. ABCA12 mutations lead to defective lipid transport via lamellar granules in the keratinocytes, resulting in malformation of the epidermal lipid barrier and ichthyosis phenotypes. Studies of ABCA12-deficient model mice indicate that lipid transport by ABCA12 is also indispensable for intact differentiation of keratinocytes.
Key words: ABCA12, congenital ichthyosiform erythroderma, harlequin ichthyosis, lamellar granules, lamellar ichthyosis
Introduction
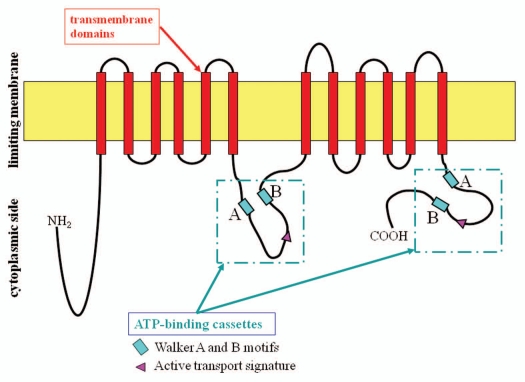
ABCA12 is a member of the large superfamily of ATP-binding cassette (ABC) transporters,1 which bind and hydrolyze ATP to transport various molecules across limiting membranes or into vesicles.2 The ABCA subfamily members are thought to be lipid transporters.3 The ABC transporter A12 (ABCA12) is known to be a key molecule in keratinocyte lipid transport (Fig. 1).4–6 ABCA12 is a keratinocyte transmembrane lipid transporter protein associated with the transport of lipids in lamellar granules to the apical surface of granular layer keratinocytes.4 This article reviews the importance of ABCA12 as a keratinocyte lipid transporter in the context of keratinocyte differentiation and skin lipid barrier formation.
Figure 1.
ABCA12 protein structure and domains. Analysis of the predicted structure of the ABCA12 protein reveals features typical of ABCA transporters.1
ABCA12 and Other ABCA Transporters
Several genetic diseases have been shown to be caused by mutations in ABCA subfamily genes. The ABCA subfamily, of which the ABCA12 gene is a member, comprises 12 full transporters and one pseudogene (ABCA11) that are essential for lipid transport and secretion.7 Three ABCA genes of the same subfamily as ABCA12 have been also implicated in the development of genetic diseases affecting cellular lipid transport. In the phylogenetic tree of ABCA subfamily proteins, ABCA3 is very close ABC12.1 ABCA3 is known to aid lipid secretion from alveolar type II cells via lamellar granules,8 and an ABCA3 deficiency recently was reported to underlie a fatal lung surfactant deficiency in newborns,9 a condition that often leads to death shortly after birth.
Another important member of the ABCA subfamily is ABCA1. Mutations in the human ABCA1 gene underlie familial alpha-lipoprotein deficiency syndrome (Tangier disease), which suggests that ABCA1 is a major regulator of high-density lipoprotein metabolism.10–12
ABCA2, ABCA3 and ABCA7 mRNA levels were reported to be upregulated after sustained cholesterol influx,13,14 suggesting that ABCA transporters are involved in the transmembrane transport of endogenous lipids.15 From these facts, transporters in the ABCA subfamily are thought to be involved in the transmembrane transport of cholesterol.16–18 Interestingly, ABCA3, a member of the same protein superfamily as ABCA12, functions in pulmonary surfactant lipid secretion through the production of similar lamellar-type granules within lung alveolar type II cells.8,9
The Role of ABCA12 in the Transport of Lipids into Lamellar Granules
Extracellular lipids, including ceramide, are thought to be essential for skin barrier function.19 Mutations in the ABCA12 gene (ABCA12) were reported to underlie the devastating phenotype seen in harlequin ichthyosis (HI) patients,4,20 the most severe keratinization disorder. ABCA12 mutations underlying HI are thought to have major disruptive defects on ABCA12 lipid transporter function, resulting in the HI phenotype.4 We reported that ABCA12 is localized in lamellar granules (LGs) in the granular layer keratinocytes and might work in lipid transport through LGs to form the intercellular lipid layers in the stratum corneum.4 We have analyzed the epidermal localization of ABCA12 in comparison with the localization of Golgi apparatus markers and LG-associated proteins together with transglutaminase 1, because LGs are thought to be a part of the continuous tubular network that originates from the Golgi apparatus and extends to the cell membrane.5 We employed antibodies to well-established marker molecules of each part of the Golgi apparatus-LG-cell membrane network, i.e., the GM130, anti-TGN-46 and anti-transglutaminase 1 antibodies (B.C1), as markers for cis-Golgi, trans-Golgi and cell membrane, respectively. Our results show that ABCA12 localizes throughout the entire Golgi apparatus to LGs at the cell periphery, mainly in the granular layer keratinocytes. These results suggest that ABCA12 works in the transport of lipids from the Golgi apparatus to LGs in the granular layer cells.5 Double-labeling immunofluorescence staining of cultured keratinocytes clearly indicates that ABCA12 is localized from the Golgi apparatus (colocalized with cis-Golgi marker GM130 and trans-Golgi marker TGN-46) to the cell periphery (close to the plasma membrane stained with transglutaminase 1). ABCA12 fails to colocalize with TGase1, a cell membrane-bounding protein, both in vivo and in cultured keratinocytes and ABCA12 is thought to distribute only very sparsely on the cell membrane.5
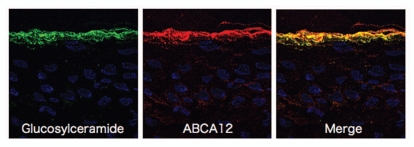
In normal human epidermis, ABCA12 is expressed throughout, but mainly in the upper spinous and granular layers.5 Immunofluorecent double labeling reveals that the majority of ABCA12 colocalizes with glucosylceramide in the cytoplasm within the upper spinous and granular cells (Fig. 2).5 Immunofluorescence labeling on ultrathin cryosections clearly reveals localization of ABCA12 and glucosylceramide. In immunofluorescence labeling under light microscopy, ABCA12 and glucosylceramide staining almost completely overlap within the granular layer keratinocytes.5 Post-embedding immunoelectron microscopy reveals both ABCA12 and glucosylceramide in the LGs of the uppermost granular layer keratinocytes.5 Under immunoelectron microscopy using ultrathin cryosections, glucosylceramide labeling is seen with the lamellar structures in the LGs. ABCA12 immunogold labeling is observed on or close to the membrane surrounding LGs in the uppermost granular layer cells.5
Figure 2.
Immunofluorescence labeling using ultrathin cryosections as substrates reveal that glucosylceramide (green) and ABCA12 (red) overlap in the granular layers (derived from ref. 5).
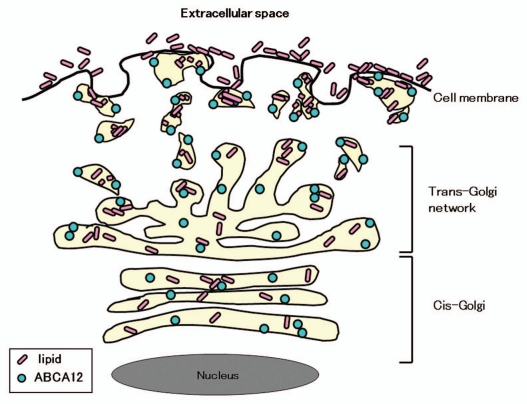
We can hypothesize that ABCA12 is likely to be a membrane lipid transporter that functions in the transport of lipids from the trans-Golgi network to LGs at the keratinocyte periphery (Fig. 3).4,5 Recently, it was confirmed biochemically that ABCA12 deficiency impairs glucosylceramide accumulation in lamellar granules and that ABCA12 transports glucosylceramide to the inner side of lamellar granules.6 In addition, ceramide was reported to upregulate ABCA12 expression via PPAR delta-mediated signaling pathway, providing a substrate-driven, feed-forward mechanism for regulation of this key lipid transporter.21 More recently, studies using Abca12-/- mice suggested that ABCA12 plays an important role in the normal differentiation of epidermal keratinocytes.22
Figure 3.
Scheme of ABCA12 distribution from the cis-Golgi, trans-Golgi network to lamellar granules in the upper spinous and granular layer keratinocytes (derived from ref. 5).
ABCA12 Mutations and Ichthyoses
ABCA12 mutations are known to underlie the three main types of autosomal recessive congenital ichthyoses: harlequin ichthyosis (HI), lamellar ichthyosis (LI) and congenital ichthyosiform erythroderma (CIE). Harlequin ichthyosis is the most severe ichthyosis subtype. Affected patients show plate-like scales over the whole body, severe eclabium and ectropion.
In 2010, a review of the literature was performed to identify all known ABCA12 mutations in patients with ARCI and 56 ABCA12 mutations were described (online database: www.derm-hokudai.jp/ABCA12/) in 66 unrelated families, including 48 HI, 10 LI and 8 CIE families.23 Mutations have been reported among autosomal recessive congenital ichthyosis patients with African, European, Pakistani/Indian and Japanese backgrounds in most parts of the world. Of the 56 mutations, 36% (20) are nonsense, 25% (14) are missense, 20% (11) comprise small deletions, 11% (6) are splice site, 5% (3) are large deletions and 4% (2) are insertion mutations. At least 62.5% (35) of all the reported mutations are predicted to result in truncated proteins. There is no apparent mutation hot spot in ABCA12, although mutations underlying the LI phenotype are clustered in the region of the first ATP-binding cassette.24
In HI-affected epidermis, several morphologic abnormalities have been reported, including abnormal lamellar granules in the keratinocyte granular layer and a lack of extracellular lipid lamellae within the stratum corneum.25–28 Lack of ABCA12 function subsequently leads to disruption of lamellar granule lipid transport in the upper keratinizing epidermal cells, resulting in malformation of the intercellular lipid layers of the stratum corneum in HI.4 Cultured epidermal keratinocytes from an HI patient carrying ABCA12 mutations demonstrate defective glucosylceramide transport, and this phenotype is recoverable by in vitro ABCA12 corrective gene transfer.4 Intracytoplasmic glucosylceramide transport has been studied using cultured keratinocytes from a total of three patients harboring ABCA12 mutations. One patient was homozygous for the splice site mutation c.3295-2A>G4 and another was compound heterozygous for p.Ser387Asn and p.Thr1387del.29 Only one heterozygous mutation, p.Ile1494Thr, was identified in the other patient.30 Cultured keratinocytes from all three patients showed apparently disturbed glucosylceramide transport, although this assay is not quantitative.
In addition, defective lamellar granule formation was observed in the skin of two CIE patients with ABCA12 mutations.30 Electron microscopy revealed that, in the cytoplasm of granular layer keratinocytes, abnormal, defective lamellar granules are assembled with some normal-appearing lamellar granules.30
Formation of the intercellular lipid layers is essential for epidermal barrier function. In ichthyotic skin with ABCA12 deficiency, defective formation of the lipid layers is thought to result in a serious loss of barrier function and a likely extensive compensatory hyperkeratosis.31
One hypothetical pathomechanism for ABCA12 deficiency in autosomal recessive congenital ichthyosis is explained by the “differentiation defect theory,” which is derived from the clinical features of HI patients. Fetuses affected with HI start developing the ichthyotic phenotype while they are in the amniotic fluid, where stratum corneum barrier function is not required. According to this theory, barrier defects cannot be involved directly in the pathogenesis of the HI phenotype, at least during the in utero fetal period. In light of this, disturbed keratinocyte differentiation is speculated to play an important role in the pathogenesis of the HI phenotype. In fact, three-dimensional culture studies reveal that HI keratinocytes differentiate poorly according to morphologic criteria and show reduced expression of keratin 1 and defective conversion from profilaggrin to filaggrin.32
In an ABCA12-ablated organotypic co-culture system, which is an in vitro model of HI skin, the expression of keratinocyte late differentiation-specific molecules is dysregulated.33 The expression of specific proteases associated with desquamation (kallikrein 5 and cathepsin D) is dramatically reduced in the ABCA12-ablated organotypic co-culture system.33 In this model system, ABCA12 ablation results in a premature terminal differentiation phenotype.33 Furthermore, in mutant mice carrying a homozygous spontaneous missense mutation, loss of Abca12 function leads to the premature differentiation of basal keratinocytes.34 In contrast, in our Abca12-/- HI model mice, immunofluorescence and immunoblotting of Abca12-/- neonatal epidermis revealed defective profilaggrin/filaggrin conversion and reduced expression of the differentiation-specific molecules (loricrin, kallikrein 5 and transglutaminase 1), although their mRNA expression is upregulated.22 These data suggest that ABCA12 deficiency may lead to disturbances in keratinocyte differentiation during fetal development, resulting in an ichthyotic phenotype at birth. These observations suggest that ABCA12 deficiency might have global effects on keratinocyte differentiation, resulting in both impaired terminal differentiation and premature differentiation of the epidermis.
HI patients often die in the first week or two of life. However, those that survive beyond the neonatal period phenotypically improve within several weeks after birth. To clarify the mechanisms of phenotypic recovery, we studied grafted skin and keratinocytes from Abca12-disrupted (Abca12-/-) mice.22 Abca12-/- skin grafts kept in a dry environment exhibited dramatic improvements in all the abnormalities seen in the model mice. Increased transepidermal water loss, a parameter of barrier defect, is remarkably decreased in grafted Abca12-/- skin. Ten-passage sub-cultured Abca12-/- keratinocytes show restoration of intact ceramide distribution, differentiation-specific protein expression and profilaggrin/filaggrin conversion, which are defective in the primary-culture.22 These observations suggest that, during maturation, Abca12-/- epidermal keratinocytes regain normal differentiation processes, although the exact mechanisms of this restoration remain unknown.22
ABCA12-deficient Animal Models
Recently, bioengineered disease models were established to investigate the ichthyotic pathomechanisms that result from defective ABCA12 function and to aid the development of innovative treatments for ichthyosis with ABCA12 deficiency.
We transplanted cultured keratinocytes from patients with HI and succeeded in reproducing HI skin lesions in immunodeficient mice.35 These reconstituted HI lesions show similar changes to those observed in HI patients' skin. In addition, we generated Abca12-disrupted (Abca12-/-) mice that closely reproduced the human HI phenotype, showing marked hyperkeratosis with eclabium and skin fissures.36 Lamellar granule abnormalities and defective ceramide distribution were remarkable in the epidermis. Skin permeability assays of Abca12-/- mouse fetuses revealed severe skin barrier dysfunction after the initiation of keratinization. Surprisingly, the Abca12-/- mice also demonstrated lung alveolar collapse immediately after birth. Lamellar bodies in alveolar type II cells from Abca12-/- mice lack normal lamellar structures.36 The level of surfactant protein B, an essential component of alveolar surfactant, is reduced in the Abca12-/- mice.36 Another group independently developed Abca12-/- mice and these also had the clinical features of HI.37
A study in one Abca12-disrupted HI model mouse indicates that a lack of desquamation of skin cells, rather than enhanced proliferation of basal-layer keratinocytes accounts for the five-fold thickening of the Abca12-/- stratum corneum determined by in vivo skin proliferation measurements.37 It was suggested that this lack of desquamation is associated with a profound reduction in skin linoleic esters of long-chain omega-hydroxyceramides and a corresponding increase in their glucosylceramide precursors. Omega-hydroxyceramides are required for correct skin barrier function, and these results from HI model mice establish that ABCA12 activity is required for the generation of the long-chain ceramide esters that are essential for the development of normal skin structure and function.37
In addition, a mouse strain carrying a homozygous spontaneous missense mutation was reported to show skin manifestations similar to ichthyosis.34 Lipid analysis of Abca12 mutant epidermis revealed defects in lipid homeostasis, suggesting that Abca12 plays a crucial role in maintaining lipid balance in the skin.34 The cells from the Abca12 mutant mouse have severely impaired lipid efflux and intracellular accumulation of neutral lipids.34 Abca12 was also demonstrated as a mediator of Abca1-regulated cellular cholesterol efflux.34 Injection of a morpholino designed to target a splice site at the exon 4/intron 4 junction to block Abca12 pre-mRNA processing induced altered skin surface contours, disorganization of the melanophore distribution, pericardial edema and enlargement of the yolk sac at 3 days post-fertilization in the larvae of zebrafish. It was also associated with premature death at around 6 days post-fertilization. These results suggest that Abca12 is an essential gene for normal zebrafish skin development and provide novel insight into the function of ABCA12 (reported at the Annual Meeting of the Society for Investigative Dermatology 2010; Abstract, Frank et al. J Invest Dermatol 2010; 130:86).
Using our Abca12-/- HI model mice, we tried fetal therapy with systemic administration to the pregnant mother mice of retinoid or dexamethasone, which are effective treatments for neonatal HI and neonatal respiratory distress, respectively. However, neither of these improved the skin phenotype nor extended the survival period.36 Retinoids were also ineffective in in vivo studies using cultured keratinocytes from the model mice.22
Conclusion
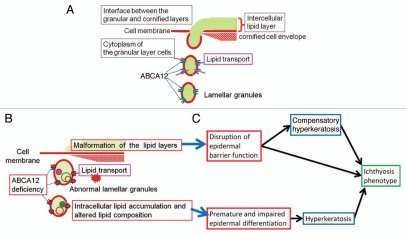
ABCA12 is apparently localized in the membrane of the trans-Golgi network and lamellar granules in the upper epidermis, mainly in the uppermost spinous and granular layer cells. Our own studies and a review of the literature suggest that ABCA12 works in the transport of lipids into the trans-Golgi network and lamellar granules, to accumulate lipids that are essential to skin barrier formation. Consequently, the lipids packed in lamellar granules are secreted to the extracellular space to form intercellular lipid layers in the stratum cornuem, which is important for skin barrier function (Fig. 4). In addition, model mouse studies indicate that lipid transport by ABCA12 is indispensable for intact differentiation of keratinocytes. To elucidate the mechanisms of ABCA12 in keratinocyte differentiation/proliferation, further accumulation of data is needed.
Figure 4.
Physiological role(s) of ABCA12 in lipid trafficking of epidermal keratinocytes and the model of pathogenetic mechanisms in ichthyosis phenotypes caused by ABCA12 deficiency. (A) Model of how ABCA12 transports lipids in epidermal keratinocytes. (B) Model of how loss of ABCA12 function leads to lipid abnormality and lipid barrier malformation in the upper epidermis. (C) It is hypothesized that the combination of lipid barrier defects and disturbed keratinocyte differentiation cause hyperkeratosis and the ichthyosis phenotype (derived from ref. 20).
Acknowledgements
This work was supported in part by a grant-in-aid from the Ministry of Education, Science, Sports and Culture of Japan to M. Akiyama (Kiban B 20390304) and by a grant from the Ministry of Health, Labor and Welfare of Japan (Health and Labor Sciences Research Grants; Research on Intractable Disease; H22-Nanchi-Ippan-177) to M. Akiyama. The ABCA12 mutation database is available at our site: www.derm-hokudai.jp/ABCA12/.
Abbreviations
- ABC
ATP-binding cassette
- ABCA12
ATP-binding cassette transporter sub-family A member 12
- CIE
congenital ichthyosiform erythroderma
- HDL
high-density lipoprotein
- HI
harlequin ichthyosis
- LG
lamellar granule
- LI
lamellar ichthyosis
- PPAR
peroxisome proliferator-activated receptor
References
- 1.Annilo T, Shulenin S, Chen ZQ, Arnould I, Prades C, Lemoine C, et al. Identification and characterization of a novel ABCA subfamily member, ABCA12, located in the lamellar ichthyosis region on 2q34. Cytogenet Genome Res. 2002;98:169–176. doi: 10.1159/000069811. [DOI] [PubMed] [Google Scholar]
- 2.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 3.Peelman F, Labeur C, Vanloo B, Roosbeek S, Devaud C, Duverger N, et al. Characterization of the ABCA transporter subfamily: identification of prokaryotic and eukaryotic members, phylogeny and topology. J Mol Biol. 2003;325:259–274. doi: 10.1016/s0022-2836(02)01105-1. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama M, Sugiyama-Nakagiri Y, Sakai K, McMillan JR, Goto M, Arita K, et al. Mutations in ABCA12 in harlequin ichthyosis and functional rescue by corrective gene transfer. J Clin Invest. 2005;115:1777–1784. doi: 10.1172/JCI24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakai K, Akiyama M, Sugiyama-Nakagiri Y, McMillan JR, Sawamura D, Shimizu H. Localization of ABCA12 from Golgi apparatus to lamellar granules in human upper epidermal keratinocytes. Exp Dermatol. 2007;16:920–926. doi: 10.1111/j.1600-0625.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 6.Mitsutake S, Suzuki C, Akiyama M, Tsuji K, Yanagi T, Shimizu H, et al. ABCA12 dysfunction causes a disorder in glucosylceramide accumulation during keratinocyte differentiation. J Dermatol Sci. 2010;60:128–129. doi: 10.1016/j.jdermsci.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Akiyama M. Pathomechanisms of harlequin ichthyosis and ABCA transporters in human diseases. Arch Dermatol. 2006;142:914–918. doi: 10.1001/archderm.142.7.914. [DOI] [PubMed] [Google Scholar]
- 8.Yamano G, Funahashi H, Kawanami O, Zhao LX, Ban N, Uchida Y, et al. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett. 2001;508:221–225. doi: 10.1016/s0014-5793(01)03056-3. [DOI] [PubMed] [Google Scholar]
- 9.Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med. 2004;350:1296–1303. doi: 10.1056/NEJMoa032178. [DOI] [PubMed] [Google Scholar]
- 10.Bodzioch M, Orsó E, Klucken J, Langmann T, Böttcher A, Diederich W, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:316–318. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 11.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 12.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 13.Klucken J, Buchler C, Orso E, et al. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc Natl Acad Sci USA. 2000;97:817–822. doi: 10.1073/pnas.97.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaminski WE, Piehler A, Püllmann K, Porsch-Ozcürümez M, Duong C, Bared GM, et al. Complete coding sequence, promoter region and genomic structure of the human ABCA2 gene and evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun. 2001;281:249–258. doi: 10.1006/bbrc.2001.4305. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz G, Langmann T. Structure, function and regulation of the ABC1 gene product. Curr Opin Lipidol. 2001;12:129–140. doi: 10.1097/00041433-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 17.Hayden MR, Clee SM, Brooks-Wilson A, Genest J, Jr, Attie A, Kastelein JJ. Cholesterol efflux regulatory protein, Tangier disease and familial high-density lipoprotein deficiency. Curr Opin Lipidol. 2000;11:117–122. doi: 10.1097/00041433-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Orsó E, Broccardo C, Kaminski WE, Böttcher A, Liebisch G, Drobnik W, et al. Transport of lipids from Golgi to plasma membrane is defective in Tangier disease patients and Abc1-deficient mice. Nat Genet. 2000;24:192–196. doi: 10.1038/72869. [DOI] [PubMed] [Google Scholar]
- 19.Lee HK, Nam GW, Kim SH, Lee SH. Phytocomponents of triterpenoids, oleanolic acid and ursolic acid, regulated differently the processing of epidermal keratinocytes via PPAR-alpha pathway. Exp Dermatol. 2006;15:66–73. doi: 10.1111/j.0906-6705.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 20.Kelsell DP, Norgett EE, Unsworth H, Teh MT, Cullup T, Mein CA, et al. Mutations in ABCA12 underlie the severe congenital skin disease harlequin ichthyosis. Am J Hum Genet. 2005;76:794–803. doi: 10.1086/429844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang YJ, Uchida Y, Lu B, Kim P, Mao C, Akiyama M, et al. Ceramide stimulates ABCA12 expression via peroxisome proliferator-associated receptor delta in human keratinocytes. J Biol Chem. 2009;284:18942–18952. doi: 10.1074/jbc.M109.006973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanagi T, Akiyama M, Nishihara H, Ishikawa J, Sakai K, Miyamura Y, et al. Self-improvement of keratinocyte differentiation defects during skin maturation in ABCA12 deficient harlequin ichthyosis model mice. Am J Pathol. 2010;177:106–118. doi: 10.2353/ajpath.2010.091120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akiyama M. ABCA12 mutations and autosomal recessive congenital ichthyosis: A review of genotype/phenotype correlations and of pathogenic concepts. Hum Mutat. 2010;31:1090–1096. doi: 10.1002/humu.21326. [DOI] [PubMed] [Google Scholar]
- 24.Lefèvre C, Audebert S, Jobard F, Bouadjar B, Lakhdar H, Boughdene-Stambouli O, et al. Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum Mol Genet. 2003;12:2369–2378. doi: 10.1093/hmg/ddg235. [DOI] [PubMed] [Google Scholar]
- 25.Dale BA, Holbrook KA, Fleckman P, Kimball JR, Brumbaugh S, Sybert VP. Heterogeneity in harlequin ichthyosis, an inborn error of epidermal keratinization: variable morphology and structural protein expression and a defect in lamellar granules. J Invest Dermatol. 1990;94:6–18. doi: 10.1111/1523-1747.ep12873301. [DOI] [PubMed] [Google Scholar]
- 26.Milner ME, O'Guin WM, Holbrook KA, Dale BA. Abnormal lamellar granules in harlequin ichthyosis. J Invest Dermatol. 1992;99:824–829. doi: 10.1111/1523-1747.ep12614791. [DOI] [PubMed] [Google Scholar]
- 27.Akiyama M, Kim DK, Main DM, Otto CE, Holbrook KA. Characteristic morphologic abnormality of harlequin ichthyosis detected in amniotic fluid cells. J Invest Dermatol. 1994;102:210–213. doi: 10.1111/1523-1747.ep12371764. [DOI] [PubMed] [Google Scholar]
- 28.Akiyama M, Dale BA, Smith LT, Shimizu H, Holbrook KA. Regional difference in expression of characteristic abnormality of harlequin ichthyosis in affected fetuses. Prenat Diagn. 1998;18:425–436. [PubMed] [Google Scholar]
- 29.Akiyama M, Sakai K, Sugiyama-Nakagiri Y, Yamanaka Y, McMillan JR, Sawamura D, et al. Compound heterozygous mutations including a de novo missense mutation in ABCA12 led to a case of harlequin ichthyosis with moderate clinical severity. J Invest Dermatol. 2006;126:1518–1523. doi: 10.1038/sj.jid.5700295. [DOI] [PubMed] [Google Scholar]
- 30.Natsuga K, Akiyama M, Kato N, Sakai K, Sugiyama-Nakagiri Y, Nishimura M, et al. Novel ABCA12 mutations identified in two cases of non-bullous congenital ichthyosiform erythroderma associated with multiple skin malignant neoplasia. J Invest Dermatol. 2007;127:2669–2673. doi: 10.1038/sj.jid.5700885. [DOI] [PubMed] [Google Scholar]
- 31.Akiyama M. Harlequin ichthyosis and other autosomal recessive congenital ichthyoses: the underlying genetic defects and pathomechanisms. J Dermatol Sci. 2006;42:83–89. doi: 10.1016/j.jdermsci.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Fleckman P, Hager B, Dale BA. Harlequin ichthyosis keratinocytes in lifted culture differentiate poorly by morphologic and biochemical criteria. J Invest Dermatol. 1997;109:36–38. doi: 10.1111/1523-1747.ep12276450. [DOI] [PubMed] [Google Scholar]
- 33.Thomas AC, Tattersall D, Norgett EE, O'Toole EA, Kelsell DP. Premature terminal differentiation and a reduction in specific proteases associated with loss of ABCA12 in harlequin ichthyosis. Am J Pathol. 2009;174:970–978. doi: 10.2353/ajpath.2009.080860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyth I, Hacking DF, Hilton AA, Mukhamedova N, Meikle PJ, Ellis S, et al. A mouse model of harlequin ichthyosis delineates a key role for Abca12 in lipid homeostasis. PLoS Genet. 2008;4:1000192. doi: 10.1371/journal.pgen.1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamanaka Y, Akiyama M, Sugiyama-Nakagiri Y, Sakai K, Goto M, McMillan JR, et al. Expression of the keratinocyte lipid transporter ABCA12 in developing and reconstituted human epidermis. Am J Pathol. 2007;171:43–52. doi: 10.2353/ajpath.2007.061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanagi T, Akiyama M, Nishihara H, Sakai K, Nishie W, Tanaka S, et al. Harlequin ichthyosis model mouse reveals alveolar collapse and severe fetal skin barrier defects. Hum Mol Genet. 2008;17:3075–3083. doi: 10.1093/hmg/ddn204. [DOI] [PubMed] [Google Scholar]
- 37.Zuo Y, Zhuang DZ, Han R, Isaac G, Tobin JJ, McKee M, et al. ABCA12 maintains the epidermal lipid permeability barrier by facilitating formation of ceramide linoleic esters. J Biol Chem. 2008;283:36624–36635. doi: 10.1074/jbc.M807377200. [DOI] [PMC free article] [PubMed] [Google Scholar]