Abstract
Glial cell line-derived neurotrophic factor (GDNF) supports the viability of midbrain dopamine (DA) neurons that degenerate in Parkinson’s disease. Middle aged, 12-month-old, Gdnf heterozygous (Gdnf+/−) mice have diminished spontaneous locomotor activity and enhanced synaptosomal DA uptake compared to wildtype mice. In this study, dopamine transporter (DAT) function in middle-aged, 12-month-old Gdnf+/− mice was more thoroughly investigated using in vivo electrochemistry. Gdnf+/− mice injected with the DAT inhibitor, nomifensine, exhibited significantly more locomotor activity than wildtype mice. In vivo electrochemistry with carbon fiber microelectrodes demonstrated enhanced clearance of DA in the striatum of Gdnf+/− mice, suggesting greater surface expression of DAT than in wildtype littermates. Additionally, 12 month old Gdnf+/− mice expressed greater D2 receptor mRNA and protein in the striatum than wildtype mice. Neurochemical analyses of striatal tissue samples indicated significant reductions in DA and a faster DA metabolic rate in Gdnf+/− mice than in wildtype mice. Altogether, these data support an important role for GDNF in the regulation of uptake, synthesis, and metabolism of DA during aging.
Keywords: In vivo electrochemistry, Dopamine, neurodegeneration, Glial cell-line derived neurotrophic factor, Striatum, Movement disorders, Dopamine transporter
1. Introduction
The decline in motor function associated with aging has been widely demonstrated in animal models of aging (Hebert and Gerhardt, 1998, Willig, et al., 1987, Yurek, et al., 1998, Zhang, et al., 2000) and parallels a similar decline in human aging (Bennett, et al., 1996, Kluger, et al., 1997, Richards, et al., 1993). Dopamine (DA) neuron dysfunction has been related to age-associated motor impairment in both humans (Volkow, et al., 1998) and animals (Gerhardt, et al., 2002, Hebert and Gerhardt, 1998, Yurek, et al., 1998). The loss of functional DA neurons in the substantia nigra pars compacta (SNpc) and consequent loss of striatal DA are a hallmark of Parkinson’s disease (PD) (Marsden, 1990), with its motor symptoms of bradykinesia, rigidity, and tremor (Hornykiewicz and Kish, 1987). The presence of some of these motoric deficits observed in many aged individuals has been termed age-related parkinsonism (Bennett, et al., 1996), and likely involves changes in the functional properties of DA neurons rather than neuronal loss as demonstrated in aged rats and monkeys (Grondin, et al., 2003, Hebert and Gerhardt, 1999, Hebert, et al., 1999, Yurek, et al., 1998).
Glial cell line-derived neurotrophic factor (GDNF) is a member of the transforming growth factor-β superfamily (Lin, et al., 1993). It has been hypothesized that age-related decreases in neurotrophic factor levels contribute to DA neuron degeneration and/or alterations in DA neuron function (Yurek and Fletcher-Turner, 2001). In vivo application of exogenous GDNF to the SN is reported to enhance DA neuron function in normal young (Hebert, et al., 1996), aged (Grondin, et al., 2003, Hebert and Gerhardt, 1997), and lesioned (Hoffer, et al., 1994, Tomac, et al., 1995) animals. In addition, GDNF is neuroprotective and neurorestorative in rat DA systems subjected to neurotoxic doses of methamphetamine (Cass, et al., 2000, Cass, et al., 2006) and produces functional restoration in rhesus monkeys exhibiting 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced parkinsonism (Gash, et al., 1996, Grondin, et al., 2002). GDNF has also been shown to enhance DA neuron function as indicated by 1) increased evoked DA release, 2) augmented locomotor behavior, 3) increased DA content in nigral tissue (Hebert and Gerhardt, 1997, Hebert, et al., 1996), and 4) increased high affinity DA uptake (Lin, et al., 1993). However, the exact role (s) of GDNF in normal aging, specifically the maintenance of DA neuron system function, is not known.
To further elucidate the effects of GDNF, a knockout mouse model was created (Pichel, et al., 1996) which exhibits a partial and stable reduction of the GDNF protein in brain tissues (Boger, et al., 2006). At birth, midbrain DA systems in mice homozygous for the GDNF null mutation (Gdnf−/−) appear unaffected (Moore, et al., 1996); however, DA neuron function after the major apoptotic waves, which occur 2 and 14 days after birth (Mahalik, et al., 1994), cannot be assessed because these mice die at birth due to kidney agenesis (Moore, et al., 1996). Therefore, postnatal in vivo studies have used mice with a partial deletion of the Gdnf gene (Gdnf+/−) (Boger, et al., 2006). GDNF expression in the striatum appears to be critical for appropriate innervation, survival, and differentiation of midbrain DA neurons to their striatal targets during early development (Stromberg, et al., 1993). Indeed, postnatal development of these DA neurons is compromised in the absence of GDNF (Granholm, et al., 2000). Behavioral and immunohistochemical characterization of multiple age groups (4, 8, 12, 16, and 20 months of age) of Gdnf+/− mice compared to wildtype (WT) controls, provides evidence of a unique aging phenotype in the Gdnf+/− mice. A partial GDNF depletion leads to an earlier (12 months of age) loss of tyrosine hydroxylase positive (TH-positive) neurons in the SNpc as well as diminished spontaneous locomotor activity (Boger, et al., 2006). The diminished locomotor activity and decline in TH-positive neurons also show an accelerated age-associated decline from 8 to 12 months in the Gdnf+/− mice while both measures in WT mice of the same age groups are unchanged (Boger, et al., 2006). Young Gdnf+/− mice (6–9 months) exhibit no effect of GDNF reduction on spontaneous or methamphetamine-induced locomotor behavior (Boger, et al., 2006, Boger, et al., 2007, Gerlai, et al., 2001). In vitro synaptosomal preparations from Gdnf+/− mice show increased dopamine transporter (DAT) activity, which has been suggested to predispose DA neurons to methamphetamine-induced toxicity (Boger, et al., 2007). Consistent with the age related changes in DA systems noted above, DAT activity in the striatal pathway is altered in the normal aging process in animal models (Friedemann, 1992, Friedemann and Gerhardt, 1992, Hebert and Gerhardt, 1999, Hebert, et al., 1999), and may be correlated with age-related motor deficits. Such dynamic changes in DA uptake using parameters associated with DAT activity have been characterized by in vivo chronoamperometry using carbon fiber microelectrodes (Cass, et al., 1993, Gerhardt, et al., 1999, Gerhardt, et al., 1986). The high spatial (microns) and temporal (seconds) resolution of these techniques allows for the rapid and sensitive quantification of DA uptake in discrete DA terminal regions.
Based on previous in vitro findings that DA uptake in Gdnf+/− mice was greater than in WT mice (Boger, et al., 2007), the present study investigated this age-associated deficit by using in vivo electrochemistry to address the effects of a partial and chronic genetic reduction of GDNF on the function of DA terminals in the striatum of middle-aged 12-month-old Gdnf+/− mice. The following questions were addressed: (1) Do Gdnf+/− mice differ in behavioral response to DAT modulation via DAT inhibition? (2) Do Gdnf+/− mice exhibit altered DAT activity in vivo, and if so, what is the spatial pattern of this effect within striatal subregions? (3) Do Gdnf+/− mice have altered striatal dopamine D2 receptor expression? (4) Is whole tissue neurochemical content altered in Gdnf+/− mice? Thus, these studies focus on the functional properties of DA neurons in 12-month-old Gdnf+/− mice that demonstrate accelerated age-related motor deficits and loss of TH-positive neurons as compared to WT littermate controls (Boger, et al., 2006).
2. Methods
2.1. Animals
A nonfunctional GDNF allele was generated by replacing part of exon 3 which encodes the GDNF protein with a selectable marker neomycin phosphotransferase expressing cassette. Generation and genotyping of Gdnf+/− mice is described in detail in previous work (Pichel, et al., 1996). Mice were obtained from a colony established at the Medical University of South Carolina. Mice were bred on a C57Bl/6J background consistent with NIH approved protocols. After transfer to the University of Kentucky, mice were acclimated for a minimum of 1 week before experimentation. Male Gdnf+/− mice (12 months of age) were compared with age-matched WT mice in all experiments. Mice were housed 3–4 per cage with food and water provided ad libitum. Mice were maintained under 12:12 h light/dark cycle at an ambient temperature of 20–22° C. Protocols for animal care were in agreement with NIH approved guidelines and compliant with local institutional protocols at the University of Kentucky Medical Center and Medical University of South Carolina. Procedures were in strict agreement with the Guide for the Care and Use of Laboratory Animals.
2.2. Locomotor activity
Locomotor activity (total distance traveled) was assessed with a Digiscan Animal Activity Monitor system (Omnitech Electronics Model RXYZCM (8); TAO, Columbus, OH, USA), details of which have been previously described (Halberda, et al., 1997). Animals of each genotype (N = 8) were injected with 0.9% NaCl (0.01ml/g body weight, i.p.) and placed into the activity boxes for a 1 hr period of habituation. After the habituation period, the mice were injected with the DAT inhibitor, nomifensine (7.5 mg/kg, i.p.) and placed into the activity boxes for a 2 hr recording period.
2.3 In vivo electrochemistry measurements of dopamine clearance
High-speed in vivo chronoamperometric recordings of DA clearance were carried out using Nafion®-coated carbon fiber microelectrodes (electrode tips were ~30 μm o.d. × 150 μm length; Quanteon, LLC, Lexington, KY) as previously described (Cass and Gerhardt, 1994, Thomas, et al., 2007). The sensitivity and selectivity for DA versus other endogenous electroactive molecules were enhanced by the use of a high temperature Nafion® coating (5% solution, 1 coat at 200°C, Aldrich Chemical Co., Milwaukee, WI (Gerhardt and Hoffman, 2001). High-speed chronoamperometric electrochemical measurements were made using the FAST-16 system (Quanteon, LLC, Nicholasville, KY). A square wave potential (0.0 to +0.55 V, 200ms total) was applied to the carbon fiber microelectrode versus the Ag/AgCl reference electrode at a frequency of 1 Hz. The resulting oxidation of DA and subsequent reduction of dopamine-o-quinone was digitally integrated during the last 80 ms of each 100 ms pulse.
The microelectrodes were calibrated in vitro in 0.05 M phosphate buffered saline solution. Parameters quantified included: linearity of response to DA, selectivity, and limit of detection (LOD) for DA. Data are expressed as mean ± SEM. The microelectrodes showed linear responses to serial additions of DA (2–6 μM, effective beaker concentrations) with correlation coefficients (R2) of 0.9978 to 0.9998. Additions of ascorbate (250 μM) were used to determine the selectivity of the recordings for DA that averaged 908 ± 166:1 (N = 13). The LOD for DA was 11 nM ± 3 with a signal to noise ratio ≥ 3 (N = 13). Changes in DA in vivo were expressed as changes from a stable baseline response using the calibration curve for a particular microelectrode. DA clearance in the mouse striatum was studied in vivo using microelectrodes attached to a single barrel micropipette. A micropipette (1 mm o.d., 0.58 i.d., glass, A-M Systems, Inc., Everett, WA) with an inner tip diameter of 10–15 μm was positioned 180–220 μm from the carbon fiber electrode tip with sticky wax (Kerr Corp., Orange, CA, USA). The micropipette was filled with a DA solution (100 μM DA, 200 μM ascorbate, in 0.9% NaCl). The solution was filtered (0.22 μm) and pH adjusted between 7.2 and 7.4 before in vivo use.
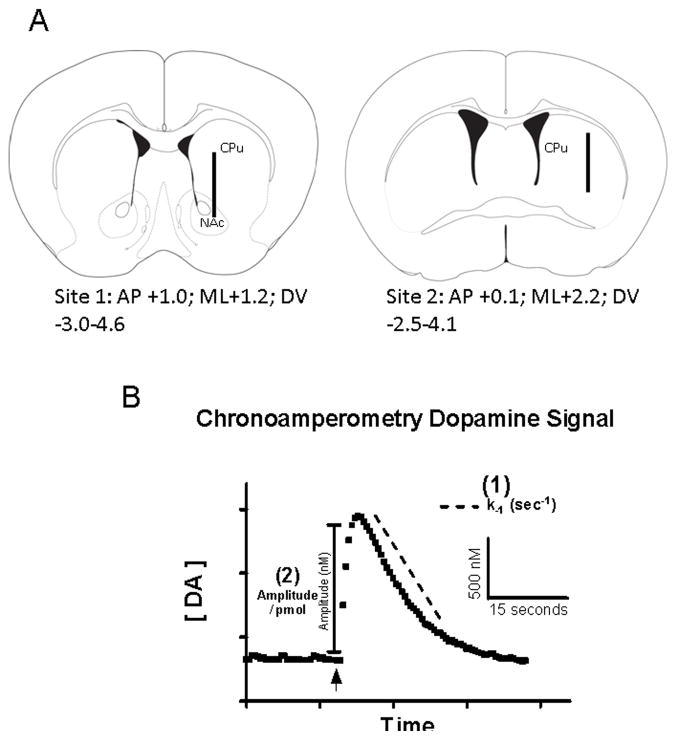
WT (N=7) or Gdnf+/− (N=5) mice were anesthetized, placed into a stereotaxic frame and rested on a re-circulating water bath connected to a heating pad (Gaymar Industries, Inc., Orchard Park, NY), which was maintained at 37° (Thomas, et al., 2007). A craniotomy was performed for access to the striatum and the overlying dura was reflected prior to insertion a carbon fiber microelectrode/micropipette assembly into the brain. A burr hole distant from the recording site(s) was created and used for the placement of a miniature Ag/AgCl reference electrode (0.008 in. Teflon coated wire; A-M Systems Inc., Carlborg, WA, USA) in contact with brain tissue (Hascup, et al., 2007). Two striatal recording tracts per hemisphere were used to characterize DA uptake in the left hemisphere. The electrode assembly was stereotaxically placed (Kopf Instruments, Tujunga, CA, USA) in contact with dura and lowered via a microdrive apparatus at 400 μm increments. Stereotaxic coordinates from bregma were (mm) Site 1 (rostral-medial): anterior-posterior +1.0, medial-lateral +1.2, dorsal-ventral −3.0 to −4.6; Site 2 (caudal-lateral): anterior-posterior +0.1, medial-lateral +2.2, dorsal-ventral −2.5 to −4.1 (incisor bar positioned with lambda level to bregma; see Fig. 1A; (Franklin and Paxinos, 2001). The microelectrode recordings were allowed to reach a stable baseline response in the mouse cortex for a minimum of 1 hour after initial placement into the brain. The microelectrode was then lowered to the targeted recording area in the striatum for studies of DA uptake through the local application of the DA solution. The DA solution was applied by pressure ejection (Palmer, et al., 1980) using a Picosprizter III (Parker-Hannifin, NJ) and solution volume applied was monitored using a stereomicroscope fitted with a reticule (Friedemann and Gerhardt, 1992). The solution volume was adjusted in order to obtain DA signals of equivalent amplitudes, which were subsequently analyzed for clearance kinetics (Cass and Gerhardt, 1995, Cass, et al., 1993). When the resulting peak from DA application had returned to a stable baseline, the recording assembly was then lowered 400 μm and the recordings were allowed to baseline for a minimum of 5 minutes before DA application at the next recording depth. All DA signals used for analysis exhibited mean reduction/oxidation ratios of 0.78 ± 0.01 (mean ± SEM, N = 107 signals), consistent with the normal redox profile for DA (Gerhardt and Hoffman, 2001, Gerhardt, et al., 1986).
Figure 1.
(A) Microelectrode placement through the mouse dorsal striatum and nucleus accumbens. The diagrams show the approximate location of the microelectrode at site 1: AP+1.0 and site 2: AP+0.1 (relative to bregma; modified from Franklin and Paxinos (2001)). The microelectrode was moved in increments of 400 μm throughout the dorsal and ventral parts of the striatum. (B) Analysis parameters of the in vivo electrochemical DA signals. Following local application of DA solution (indicated by arrow), the amplitude (nM) is determined by calculating the change from a stable baseline using the calibration curves generated from each microelectrode prior to their use. The first order exponential decay constant, k−1 (sec −1) (1), is calculated from the decay portion of the DA signal fitted to the slope of the linear regression of the natural log transformation of the data. By dividing the resulting DA signal amplitude (nM) by the amount (picomole) of DA solution applied the amplitude per picomole (nM/pmol) (2) is calculated.
After electrochemical recordings, animals were decapitated while under anesthesia and the brains were dissected and frozen until sectioning (40 μm) on a cryostat (Microm, Zeiss, and Thornwood, NY, USA). Sections were mounted on glass slides, stained with cresyl violet acetate and coverslipped to confirm the microelectrode placements in the targeted striatal regions. Figure 1 illustrates the electrode placements in schematics of the striatum (A) and a typical dopamine chronoamperometric signal (B).
2.4 Semiquantitative in situ hybridization histochemistry
Semi-quantitative In situ hybridization of the dopamine D2 receptor was performed as previously described (Wang and McGinty, 1995). Briefly, sections were cut at 12 μm with a cryostat through the striatum of each mouse (n=4 for Gdnf+/− and n=5 for WT) and thaw-mounted onto Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA, USA). These sections were pretreated to fix and defat the tissue and block non-specific hybridization. A 1:1 mixture of synthetic cDNA oligodeoxynucleotide probes complementary to the long form of D2 receptor mRNA (D2244; corresponding to bases 728–763) and the short form of D2 receptor mRNA (D2415; corresponding to bases 704–739) was labeled using alpha-[35S]-dATP (Amersham Biosciences, Piscataway, NJ, USA) and terminal deoxynucleotidyl transferase (Roche Diagnostics Corporation, Indianapolis, IN, USA). Sections were immersed in 5.0×105 c.p.m./20 ml hybridization buffer/section overnight (15 h) at 37°C in a humid environment and then washed and air dried before being placed into a film cassette with 14C standards (American Radiolabeled Chemicals, St Louis, MO, USA) and Kodak Biomax film (Rochester, NY, USA) for 2 weeks. Quantitation of the mRNA hybridization signals was performed using NIH image 1.62 software as previously described (Wang and McGinty, 1995).
2.5 Immunoblotting
Striatal samples from 12 month old WT and Gdnf+/− mice (20μg) were loaded in duplicate and separated on 4–12% NuPAGE Bis Tris gels (Invitrogen, Carlsbad, CA) at 150V for 45 minutes (N=7 per genotype). After separation onto the gel, the gel was transferred via wet transfer onto a nitrocellulose membrane for one hour at 30V. The membrane was then removed from the cassette and blocked for one hour in 5% non-fat milk in PBS at room temperature and subsequently incubated overnight at room temperature in a dopamine D2 receptor primary antibody (1:200, mouse monoclonal, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, sc-5303). After 24 hours, blots were washed in PBS-T (0.1% Tween-20) then incubated in secondary antibody (1:5000, donkey anti-mouse IgG, Jackson Immunoresearch, West Grove, PA, 715-035-151) in 5% non-fat milk in PBS for one hour at room temperature. Blots were then washed in PBS-T then imaged on a Kodak Image Station 4000 (4 exposures, 15 sec each) using Immobilon chemiluminescent reagent (Millipore, Bellerica, MA). For loading control, blots were incubated with anti-actin antibody for one hour at room temperature, then incubated in secondary antibody, then finally re-imaged under the same settings. The samples were normalized to actin. Integrated density of the bands was analyzed using AlphaEaseFC (FluorChem 9900) and results are reported as percent of wildtype.
2.6 Tissue studies of dopamine and metabolites and serotonin and metabolites using high performance liquid chromatography coupled with electrochemical detection (HPLC-EC)
Striatal tissue was collected from a separate cohort of 12 month old Gdnf+/− and WT mice (N=8 per genotype) to mimic the general recording tracks from the chronoamperometry measures by obtaining rostral and caudal tissue samples. Mice used for neurochemistry were anesthetized with isoflurane and euthanized by decapitation. Brains were quickly removed and placed in an ice cold mouse brain mold (ASI instruments, Warren, MI). Rostral and caudal striatal tissue punches from the dorsal striata were obtained bilaterally and placed into pre-weighed microcentrifuge tubes and stored on dry ice. The tubes were weighed again before being stored at −70° C until HPLC-EC analysis.
In preparation for HPLC-EC analysis, samples were sonicated in cold mobile phase (pH = 4.1) with dihydroxybenzylamine (DHBA) as an internal standard. Samples were centrifuged at 16,000 × g for 10 minutes. The supernatants (50 μL) were injected into the HPLC system using dual-coulometric electrochemical detectors (Hall, et al., 1989). The HPLC system detection limits were < 1 ng/g tissue wet weight for the analytes of interest. DA, 3–4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) were identified by peak retention times and analyzed by peak areas using standard concentrations of analytes. Striatal tissue neurochemical content was expressed as ng/gram wet weight of tissue.
2.7 Statistics
2.7.1. Locomotor activity
Basal locomotor activity data collected following saline injections were analyzed with a 2(Genotype) × 4(15-Min Interval) mixed factor ANOVA with repeated measures on the Interval factor. Because of the genotype difference in basal activity data collected following the nomifensine injections were analyzed with a 2(Genotype) × 8 (Time Interval) ANOVA with repeated measures on the interval factor.
2.7.2. In vivo electrochemistry
High–speed chronoamperometric data were analyzed using custom MATLAB®-based software (The MathWorks™, Inc.). Several signal parameters were obtained from each DA signal (shown in Fig. 1B). Signal amplitude (nM) was calculated as the change from baseline after local application of DA. The first order exponential decay constant, k−1 (sec−1), was calculated from the decay portion of the DA signal fitted to the slope of the linear regression of the natural log transformation of the data (Sabeti, et al., 2002). This is indicated (Fig. 1B) by the dashed line on the decay portion of the signal after local application of DA. The first order decay constant, k−1 (sec−1), is a quantitative measure that can be multiplied by the signal amplitude (nM) to yield the uptake rate (nM/sec) (Stephens, et al., 2009). For our purposes we analyzed the raw k−1 as signal amplitude was controlled. A single value for k−1 was obtained from a single peak from each recording depth using peak amplitudes that were matched (Cass, et al., 1992, Cass, et al., 1993). The volume of solution applied to obtain signals with amplitudes ranging from 500–1000 nM was recorded and used to calculate the total amount (pmol) of DA applied. Adjusting the volume of DA applied enables modulation of signal amplitude such that physiologically appropriate concentrations are analyzed and the inherent Michaelis-Menten properties of the DAT are not altered by varied extracellular concentrations of substrate (Hebert and Gerhardt, 1999). In addition, the amplitude of the DA signal (nM) per amount of DA applied (pmol) (Fig. 1B) is a measure of the surface expression of the DAT (Cass and Gerhardt, 1995, Hebert and Gerhardt, 1999). Because prior work demonstrated heterogeneous properties of DA uptake and DAT density, which varies most notably along the dorsal-ventral orientation of the striatum (Hebert and Gerhardt, 1999, Hebert, et al., 1999), uptake data (k−1 and amplitude per picomole DA applied) from the striatum were analyzed from dorsal and ventral subregions at each recording site. Five recording depths separated by 400 μm were used in each recording site. For each animal, data from the two most dorsal recording depths were used to characterize the dorsal striatum (Str) (sites 1 and 2) and the two most ventral sites were used similarly to characterize the ventral striatum/nucleus accumbens (NAc) of the rostral-medial recording site (site 1) or ventral Str of the caudal-lateral recording site (site 2). Data from the dorsal and ventral subregions of all animals were combined, averaged and analyzed by a two-tailed unpaired t-test to compare differences at the same recording subregion between genotypes. Data from the respective dorsal and ventral subregions remained separated by recording site.
2.7.3. Neurochemistry HPLC-EC, In situ hybridization immunohistochemistry, & Immunoblotting
Neurochemical contents of brain tissues from striatal regions were compared between genotypes and analyzed by a two-tailed unpaired t-test.
For all measures, statistical significance was defined by P < 0.05.
3. Results
3.1 Nomifensine-Induced locomotor activity
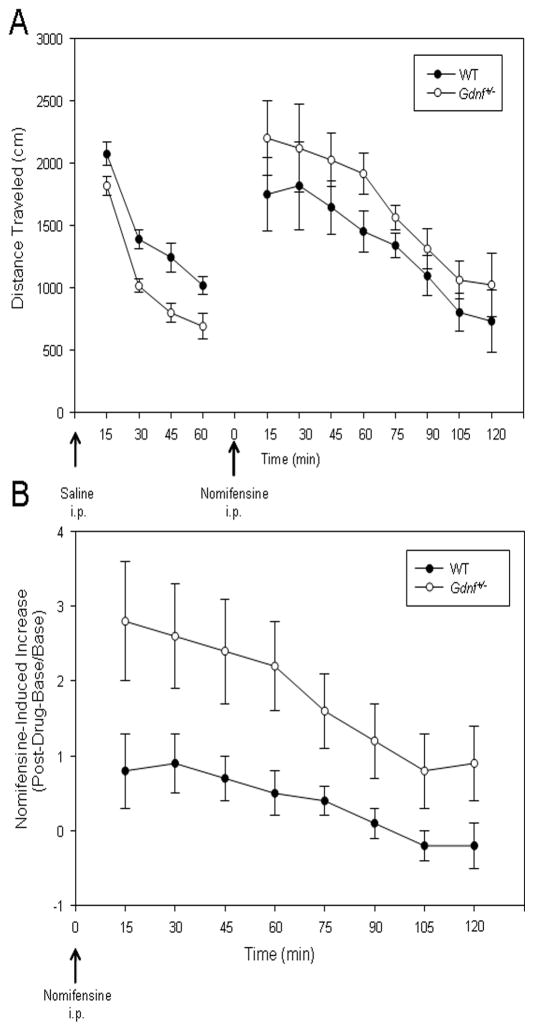
Locomotor activity of the 12-month-old Gdnf+/− and WT mice was investigated to study potential age-related changes in the movement capabilities of the Gdnf+/− mice and the effects of a catecholamine uptake inhibitor on locomotion in these mice. As seen in Fig. 2A, the spontaneous locomotor activity following saline (vehicle) injection was diminished in the 12-month-old Gdnf+/− mice compared to that of WT mice. A 2(Genotype) × 4(15-Min Interval) mixed factor ANOVA with repeated measures on the interval factor provided statistical support for reduced motor activity observed for the Gdnf+/− mice [Genotype: F1,12= 15.179, P < 0.01]. This result confirms results of a previous study which also established a positive correlation for reductions in motor activity and TH-positive neurons in the SNpc (Boger, et al., 2006). Following the habituation period, mice were injected with nomifensine; a known DAT inhibitor that increases locomotor activity (Altar and Marshall, 1988, Hebert and Gerhardt, 1998). Because Gdnf+/− mice were less active than WT controls during the habituation period, data generated following the nomifensine injections are expressed as changes in motor activity relative to the final 15-min base interval after the saline injections. The activity increase relative to basal levels is noted in Fig. 2B and reflects a greater nomifensine-induced increase in locomotor activity for the 12-month-old Gdnf+/− than age-matched WT control mice. A Genotype × Interval ANOVA on these data indicated a significant Genotype × Interval interaction [F7,84=3.119, P<0.01] with greater nomifensine-induced locomotor activity for the Gdnf+/− mice during the earlier intervals of activity.
Figure 2.
Spontaneous and nomifensine-induced locomotor activity in 12 month old Gdnf+/− and WT mice. (A) Saline-injected (indicated by arrow, 0.01ml/g body weight) Gdnf+/− mice showed significantly decreased total distance traveled as compared to saline-treated WT mice [P < 0.01]. (B) Gdnf+/− mice showed greater nomifensine-induced (indicated by arrow, 7.5mg/kg, i.p.) locomotor activity, compared to WT mice [F7,84 = 3.119, P < 0.01].
3.2 Studies of dopamine clearance and uptake
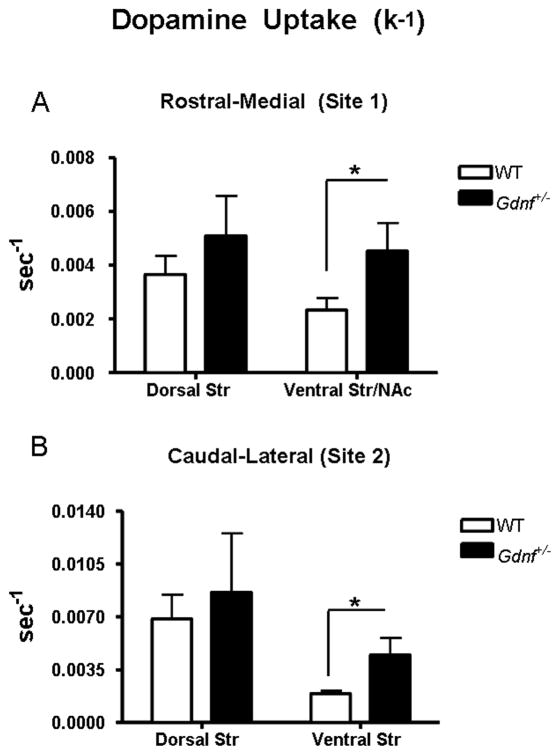
Studies of DA clearance using in vivo electrochemical methods coupled with local application of DA from micropipettes were carried out in the striata of Gdnf+/− and WT mice to further investigate the functional properties of DAT in vivo. These methods have previously been shown to reliably determine the relative density of DAT and its kinetic properties in mice and rats (Thomas, et al., 2007). Local application of DA solution was used to obtain DA signals of equivalent amplitude (771 ± 16, mean ± SEM (nM), N = 107 signals) for DA uptake analysis. Genotypes were compared using k−1 (sec−1) (Fig. 1B), which is the first-order rate constant of the decay of the DA clearance signals. Gdnf+/− mice and WT mice did not show significant differences in the k−1 in the dorsal striatum of either recording site: rostral-medial (site 1) [P = 0.36]; caudal-lateral (site 2) [P = 0.67] (Figs. 3A, 3B). However, increased DA uptake kinetics measured by k−1 were significantly increased in the ventral subregions of both recording sites of the 12 month old Gdnf+/− mice as compared to WT mice [rostral-medial [(site 1): t15 = 2.280, P = 0.04; caudal-lateral (site 2): t22 = 2.437, P = 0.02] (Figs. 3A, 3B). The ventral Str/NAc (recording site 1) of Gdnf+/− mice showed a 96% increase in mean k−1 compared to WT counterparts [WT: 0.0023 ± 0.00045, N = 12; Gdnf+/−: 0.0045 ± 0.0011, N = 5; mean ± SEM (sec−1)]. Similarly, the analogous ventral striatal subregion of recording site 2 showed a 100% increase in DA uptake [WT: 0.0019 ± 0.00016, N = 12 Gdnf+/−: 0.0038 ± 0.00076, N = 12; mean ± SEM (sec−1)].
Figure 3.
Average k−1 (sec−1) from dorsal and ventral striatal recording subregions of Gdnf+/− and WT mice (expressed as mean ± SEM). Local application of DA solution was used to obtain peaks rangeing from 500–1000 nM in amplitude for uptake analysis. The k−1 was analyzed to compare Gdnf+/− mice and WT mice. The k−1 was not significantly changed between genotypes in the dorsal striatum of the rostral-medial recording site 1 (Coordinates: AP +1.0; ML+1.2; DV −3.0–4.6) or caudal-lateral recording site 2 (Coordinates: AP +0.1; ML+2.2; DV −2.5–4.1). The k−1 did show genotype differences exclusive to the ventral Str/NAc of the rostral-medial recording site 1 [*P = 0.0377] (A) and ventral striatum of the caudal-lateral recording site 2 [*P = 0.0233] (B) with significant increases in DA uptake in the Gdnf+/− mouse.
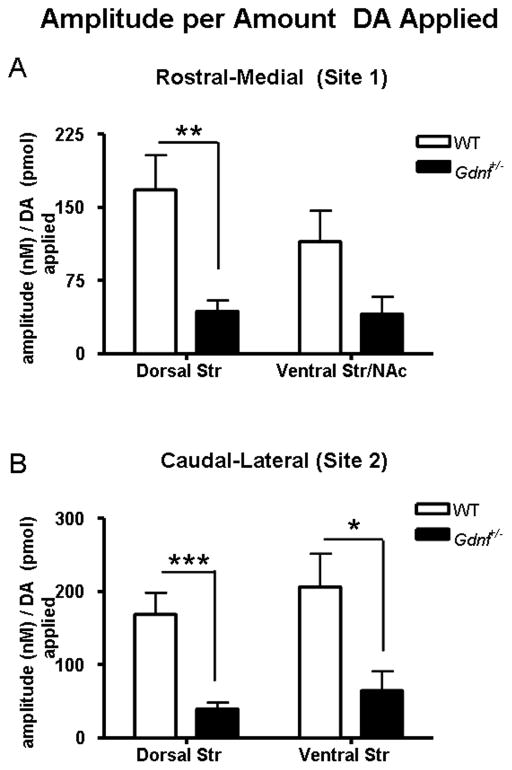
A second series of studies compared the amplitudes of the DA signals achieved per amount of DA solution locally applied, to investigate the surface expression of DAT in the striatal subregions. We have previously shown that the amplitude of the DA signals per amount applied increases as the surface expression of DAT decreases (Cass and Gerhardt, 1995, Hebert and Gerhardt, 1999). The resulting amplitude per picomole varied according to striatal subregion and genotype (Fig. 4). Increased dopamine uptake in the Gdnf+/− mice was evident as the resulting amplitude per picomole of applied DA solution was smaller in the Gdnf+/− mice. This is evident in both dorsal recording subregions in Gdnf+/− mice (Figs. 4A, 4B). There was a 74% decrease in amplitude per unit volume of ejected DA in dorsal striatum of Gdnf+/− mice compared to WT mice (site 1): WT: 167.7 ± 35.6, N = 14; Gdnf+/−: 43.35 ± 11.3, N = 10; mean ± SEM (nM/pmol) (t22 = 2.86, P <0.001) (Fig. 4A). Gdnf+/− mice and WT mice did not show significant differences in the amplitude per picomole in the ventral Str/NAc (site 1) [P = 0.14] (Fig. 4A). Recording site 2 had a 82% decrease in amplitude per picomole in the dorsal striatum (WT: 168.6 ± 29.7, N = 12; Gdnf+/−: 30.23 ± 7.312, N = 10; mean ± SEM (nM/pmol) (t20 = 4.153 P <0.001) and a 69% decrease in the ventral striatum (WT: 206.2 ± 45.2, N = 12; Gdnf+/−: 64.39 ± 26.25, N = 10; mean ± SEM (nM/pmol) (Fig. 4B; t20 = 2.575, P = 0.01). Thus, a significant decrease in the amplitude per picomole of DA that was locally applied, combined with an increase in k−1 indicate increased clearance of exogenously applied DA in Gdnf+/− mice versus WT counterparts and/or a change in the surface expression of DAT on the plasma membrane of DA nerve terminals and varicosities in the striatum of the Gdnf+/− mice.
Figure 4.
Average amplitude per amount of locally applied DA solution (nM/pmol) from dorsal and ventral striatal recording subregions of Gdnf+/− and WT mice (expressed as mean ± SEM). The volume of solution was controlled in order to obtain peaks of amplitudes ranging from 500–1000 nM for uptake analysis. The resulting amplitude per picomole was analyzed to compare Gdnf+/− versus WT mice. The amplitude per picomole was not significantly different between genotypes in the ventral Str/NAc of the rostral-medial recording site 1 (A) (Coordinates: AP +1.0; ML+1.2; DV −3.0–4.6). The dorsal striatum of recording site 1 (A) however showed a significant difference between genotype [**P = 0.0091]. Both recording subregions of recording site 2 (B) (Coordinates: AP +0.1; ML+2.2; DV −2.5–4.1) showed a significant change in amplitude per volume [***P = 0.0005] (dorsal striatum) and [P = 0.0181] (ventral striatum). All striatal subregions with significant genotype differences showed a decrease in the average amplitude per picomole applied in the Gdnf+/− mice.
3.3 Striatal dopamine D2 receptor mRNA and protein expression
In a separate cohort of mice, striatal dopamine D2 receptor mRNA and protein expression was assessed in 12 month old WT and Gdnf+/− mice to determine a potential connection between altered D2 receptor expression and DAT activity (Figure 5). Using in situ hybridization histochemistry, striatal dopamine D2 receptor mRNA expression was determined. At 12 months of age, Gdnf+/− mice had significantly higher dopamine D2 receptor mRNA in the dorsal striatum than WT mice (Figure 5A; t7 = 2.463, P = 0.04). In addition to an increase in striatal dopamine D2 receptor mRNA, immunoblotting for the D2 receptor protein in the striatum was also increased in 12 month old Gdnf+/− mice compared to WT mice (Figure 5B, t12 = 2.645, P = 0.02).
Figure 5.
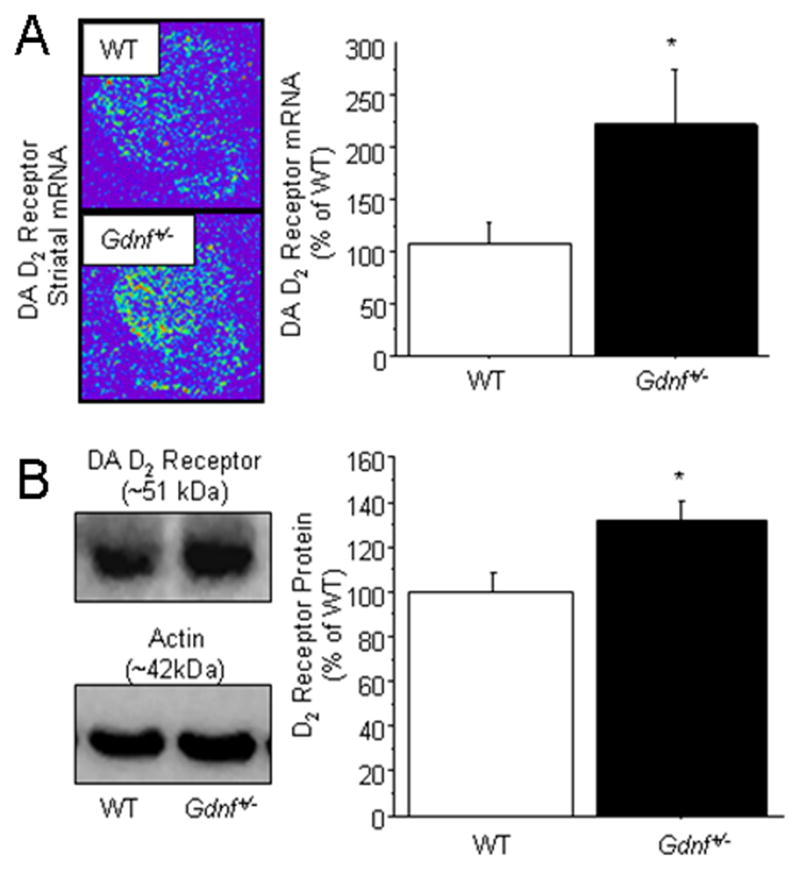
Striatal dopamine D2 receptor mRNA and protein expression in Gdnf+/− vs. WT mice. (A) In situ hybridization immunohistochemical detection revealed a significantly greater expression of the dopamine D2 receptor mRNA in the dorsal striatum of Gdnf+/− mice compared to WT controls [*P = 0.0433]. (B) Protein levels of the dopamine D2 receptor were significantly greater in the dorsal striatum of Gdnf+/− mice compared to WT mice [*P = 0.0214].
3.4 Striatal tissue measures of dopamine and metabolites and serotonin and metabolites by HPLC-EC
To determine if there were concurrent alterations in DA synthesis or metabolism in addition to altered DA uptake, analysis of DA and its metabolites (DOPAC, HVA) was performed on tissue samples harvested from rostral (site 1: AP+1.0) and caudal (site 2: AP+0.1) sections of the mouse striatum in a separate cohort of 12 month old male Gdnf+/− and WT mice (N = 8 per genotype; Table 1). The 12 month old Gdnf+/− mice had lower striatal tissue levels of DA than WT mice in both sets of brain coordinates [site 1: AP+1.0, t13 = 3.541, P <0.01; site 2: AP+0.1, t13 = 2.281, P = 0.04]. The genotypes did not differ in tissue content of DOPAC or HVA at either set of coordinates (Table 1). DA turnover was significantly elevated at both recording sites in Gdnf+/− mice as indicated by the increase in ratios of HVA:DA [site 1: AP+1.0, t13 = 3.672, P = 0.0028; site 2: AP +0.1, t13 = 3.835, P <0.01] and (DOPAC + HVA):DA [site 1: AP+1.0, t13=2.504, P = 0.03; site 2: AP+0.1, t13 = 4.099, P <0.01]. In addition, 12-month-old Gdnf+/− mice differed in the DOPAC:DA ratio compared to WT mice in the caudal striatum [site 2, AP+0.1, t13 = 2.264, P = 0.04]. Thus, Gdnf+/− mice demonstrated decreased DA tissue storage, which likely contributed to the decreases see in the DA turnover ratios.
Table 1.
Dorsal Striatum Tissue Levels of Dopamine (DA) & metabolites (DOPAC, HVA). Serotonin (5-HT) & metabolite 5-Hydroxyindoleacetic acid (5HIAA) and turnover ratios in 12 month old WT and Gdnf+/− male mice.
| Striatal Region | DA (ng/g) | DOPAC (ng/g) | HVA (ng/g) | DOPAC: DA | HVA: DA | (DOPAC +HVA): DA | 5-HT (ng/g) | 5-HIAA (ng/g) | 5-HIAA:5-HT | |
|---|---|---|---|---|---|---|---|---|---|---|
| WT | AP: +1.0 | 19509±141 | 3198±73 | 2087±29 | 0.164±0.011 | 0.107±0.004 | 0.272±0.014 | 874±10 | 590±6 | 0.680±0.030 |
| Gdnf+/− | AP: +1.0 | 15749±1044* | 2907±250 | 2050±96 | 0.184±0.007 | 0.132±0.005* | 0.316±0.010* | 854±51 | 635±19 | 0.757±0.043 |
| WT | AP: +0.1 | 8656±253 | 1796±47 | 1359±25 | 0.210±0.010 | 0.162±0.011 | 0.372±0.019 | 903±23 | 653±17 | 0.726±0.025 |
| Gdnf+/− | AP: +0.1 | 6026±924* | 1487±244 | 1297±61 | 0.244±0.011* | 0.222±0.010* | 0.466±0.010* | 867±53 | 755±56 | 0.879±0.053* |
p < 0.05, WT versus Gdnf+/−, same striatal region
Values shown are mean ± SEM
Whole tissue levels of 5-HT and its metabolite 5-HIAA were also investigated by HPLC-EC from striatal tissues as outlined above (Table 1). The genotypes did not differ in tissue levels of 5-HT or the metabolite, 5HIAA, at either recording site (p>0.05). However, the ratio of 5-HIAA:5-HT in the caudal region of the striatum (site 2) was significantly greater in Gdnf+/− mice compared to WT mice [t13=2.388, P = 0.03].
4. Discussion
The present studies reveal dynamic alterations in DA neurotransmission in the striatum of middle-aged Gdnf+/− mice. Locomotor experiments indicated lower spontaneous locomotor activity of the 12-month-old Gdnf+/− mice than in WT mice that confirms our previous report (Boger, et al., 2006). By contrast, nomifensine produced greater relative increases in locomotor activity of the Gdnf+/− mice, suggesting alterations in DAT function and/or postsynaptic DA signaling and furthermore demonstrating that a partial Gdnf deletion did not cause inability to move in these behavioral tasks. In vivo electrochemical recordings of DA uptake indicated a higher rate, k−1, of DA uptake in the ventral striatal subregions of Gdnf+/− mice compared to WT mice. In addition, Gdnf+/− mice showed significant decreases in the amplitude per picomole DA applied in multiple striatal subregions compared to WT mice. Thus, the chronoamperometric studies confirm that Gdnf+/− mice have increased high-affinity DA uptake in vivo consistent with an increase in the relative Bmax. Assessment of striatal tissue levels of DA and DA metabolites indicated lower DA levels for the Gdnf+/− mice, which is consistent with the lower spontaneous locomotor activity and lower number of TH-positive neurons in the SNpc of these mice (Boger, et al., 2006). Taken together, these data indicate robust functional changes in DA neurons of middle-aged Gdnf+/− mice, supporting a role for GDNF in age-related changes in DA neuron function (Yurek and Fletcher-Turner, 2001, Yurek, et al., 1998).
4.1 Middle-aged Gdnf+/− mice show enhanced behavioral sensitivity to DAT inhibition by nomifensine
The diminished spontaneous locomotor activity baseline activity observed for the 12-month-old Gdnf+/− mice confirms results of a previous study on Gdnf+/− mice (Boger, et al., 2006). Consistent with previous reports, nomifensine injections increased spontaneous motor activity in both genotypes, which is consistent with the DAT inhibition produced by nomifensine (Altar and Marshall, 1988, Marshall and Altar, 1986). Although the DAT inhibitor elevated the activity of both genotypes, the response was greater for the Gdnf+/− mice. Interestingly, the enhanced response to nomifensine seen for middle-aged Gdnf+/− mice in the current study was not observed in previous experiment on younger (3 months of age) Gdnf+/− mice compared to their age-matched WT mice (Boger, et al., 2007). The combined results of the two experiments show that GDNF and the aging process interact to influence progressive alteration in DA systems, an interpretation consistent with the increasing DAT activity observed in aging (from 3 to 12 months of age) Gdnf+/− mice and not WT counterparts (Boger, et al., 2007). Diminished DA uptake is evident in aged rats (Friedemann, 1992, Hebert and Gerhardt, 1999) and is accompanied by an attenuated effect of nomifensine on locomotor behavior (Stanford, et al., 2002) and reductions in DAT density (Hebert, et al., 1999). Considering these previous studies in our model, the increased sensitivity to nomifensine suggests an increase in high affinity DA uptake by the DAT in the Gdnf+/− mice, which was confirmed by the in vivo chronoamperometric studies discussed below.
Previous work showing both comparatively weak inhibition of the serotonin transporter by nomifensine (Tuomisto, 1977) and a lack of effect of serotonin uptake inhibition on locomotor activity (Hebert and Gerhardt, 1998) support a minor if any contribution from serotonergic systems in these studies. Further evidence is provided by the striatal tissue analysis for 5-HT and 5-HIAA discussed below.
4.2 Middle-aged Gdnf+/− mice show increased DA uptake in vivo
Aged rats are reported to have changes in DA uptake and release kinetics in the dorsal striatum, which are postulated to contribute to age-related motor deficits in these animals (Hebert and Gerhardt, 1998). In the current study, the increase in the decay constant, k−1, in the ventral striatum (caudal-lateral, site 2) and ventral Str/NAc (rostral-medial, site 1) indicates a more selective effect on mesoaccumbens (A10) DA projections versus nigrostriatal (A9) DA terminals, which are more widespread in the dorsal striatum (Bentivoglio and Morelli, 2005). We would expect limbic-related behaviors, particularly those influenced by DAT modulation, to be differentially affected in Gdnf+/− mice. Our findings of mesoaccumbens modulation in 12-month-old Gdnf+/− mice are interesting in light of prior findings that GDNF supplementation attenuates behavioral adaptations following administration of addictive drugs(Ghitza, et al., 2009, Messer, et al., 2000)(Messer, et al., 2000). Although reward and reinforcing behaviors are traditionally attributed to mesolimbic DA projections (Di Chiara and Imperato, 1988), DA neurotransmission in the NAc, and surrounding ventral striatum may influence motor activity as well (Koob, et al., 1981, Pijnenburg, et al., 1976), a function historically associated with nigrostriatal DA projections (Kirik, et al., 1998). With consideration of placement variation between animals, striatal heterogeneity (Glynn and Yamamoto, 1989), and the diffuse anatomical boundaries of mesolimbic and nigrostriatal projections, it is unlikely either of the ‘ventral’ subregions studied reflect mesoaccumbens projections exclusively (Beckstead, et al., 1979, Fallon and Moore, 1978). We believe DA innervation from the SNpc likely contributes to the uptake changes seen in the ventral subregions. Indeed, analysis of the amplitude per picomole of DA solution applied indicates uptake changes in both dorsal subregions (sites 1 and 2) and the ventral striatum (site 2). Previous work has shown that the positive relationship between DA signal amplitude from increasing amounts of locally applied DA solution is linear (Cass and Gerhardt, 1995, Hebert and Gerhardt, 1999). The relationship between the amount of locally applied DA and the resulting amplitude is associated with normal DAT function, and was previously shown to reflect an in vivo Bmax, which allows for comparison of the relative number of functional DA uptake sites (Cass and Gerhardt, 1995, Cass, et al., 1993, Hoffman and Gerhardt, 1998). The increased striatal DA uptake for Gdnf+/− mice indicated by the chronoamperometric clearance experiments provides in vivo confirmation of our earlier in vitro work indicating increased DAT activity in striatal synaptosomes from Gdnf+/− mice (~30% increase in DA uptake compared to age-matched WT mice) (Boger, et al., 2007). Since the in vitro experiments indicated increased DAT activity in tissue from Gdnf+/− mice with no overall change in DAT protein expression, upregulation of DAT expression doesn’t likely account for the current findings. An increase in Bmax due to increased membrane expression of the DAT supports the enhanced sensitivity to DAT inhibition by nomifensine seen in the locomotor data and chronoamperometric studies. Indeed, increased surface expression of the DAT in Gdnf+/− mice would explain how DAT protein levels are not different between genotypes despite increased DAT activity in vitro (Boger, et al., 2007). Future studies will be conducted to determine the surface expression of the DAT in Gdnf+/− vs. WT mice. Underlying mechanisms of interest regarding DAT surface expression include decreased internalization of the transporter via attenuated activity of protein kinase C (PKC), which has been implicated in molecular control of endocytosis of the DAT (Sorkina, et al., 2005).
Altered DA uptake is common to aging studies of rats (Hebert and Gerhardt, 1998, Yurek, et al., 1998) and the Gdnf+/− mice. The former involves diminished uptake while our model examined here appears to show increased DA uptake with an enhanced response to nomifensine rather than the attenuated response observed in prior studies in aged rats (Hebert and Gerhardt, 1998). Changes in DAT activity have been postulated to help maintain DA signaling in lesioned (Hoffman and Gerhardt, 1998, van Horne, et al., 1992) and aging (Hebert and Gerhardt, 1999, Hebert, et al., 1999) animal models of DA dysfunction; perhaps modulating signal duration by increasing the amount of DA and/or time synaptic DA is available. We postulate that increased DAT activity observed for Gdnf+/− mice is accompanied by relative decreases in tonic levels of synaptic and extracellular DA resulting in decreased post-synaptic DA receptor activation.
4.3 Middle-aged Gdnf+/− mice show increased dopamine D2 receptor mRNA and protein
Decreased DA receptor activation would support diminished spontaneous motor activity in Gdnf+/− mice reported in this study and previous work (Boger, et al., 2006). However, here we show an increase in DA D2 receptor mRNA as well as protein in the striatum of 12 month old Gdnf+/− mice compared to WT mice(Boger, et al., 2004). Therefore, if the reduced availability of tonic levels of synaptic DA in Gdnf+/− mice is accompanied by an increase in DA D2 receptor activity, the exaggerated motor activity response to nomifensine might by accounted for by the drug-induced increases in synaptic DA. Thus, the effective stimulus response is exacerbated in the Gdnf+/− mice due to 1) enhanced sensitivity to nomifensine and 2) increased D2 receptor activation. Definitive conclusions about the involvement of the D2 autoreceptor in uptake changes cannot be made from protein and mRNA assays, which detect primarily post-synaptic D2 receptors (Levey, et al., 1993). The contribution of D2 autoreceptors is possible and needs to be directly investigated in the future. However, local inhibition of DA D2 receptors has been shown to produce a more modest ~40% decrease in DA clearance in amp/pmol (Cass and Gerhardt, 1994) as compared to the ~80% decrease in amp/pmol for DA clearance in the middle-aged Gdnf+/− mice. Additionally, PKC mediated regulation of the DAT membrane trafficking discussed previously can function independently of DA D2 receptors (Furman, et al., 2009). Although protein and mRNA levels are significantly increased, profound receptor changes are unlikely considering the modest decline (30%) in tissue DA levels which do not fulfill required deficits (>90%) for receptor supersensitivity phenomena (Hefti, et al., 1980). Future studies will be conducted to further investigate striatal D2 receptor surface expression and activation as well as tonic extracellular DA levels in the Gdnf+/− mice, which will prove useful to evaluate receptor activation.
Boger et al., (2007) showed increased susceptibility to methamphetamine-induced toxicity in Gdnf+/− mice and attributed the susceptibility to increased DAT activity. Although the mechanisms of increased DAT activity have not been clearly defined, we would predict increased DAT activity and/or surface expression of DAT to render the Gdnf+/− mouse more susceptible to other DA neurotoxins like MPTP and 6-OHDA, which confer toxicity largely through the DAT (Gainetdinov, et al., 1997, Storch, et al., 2004). In 6-hydroxydopamine (6-OHDA) lesioned rats, high affinity DA uptake by DAT is diminished due to a profound loss of striatal DA terminals following 6-OHDA lesioned rats (Hoffman and Gerhardt, 1998). However, our previous work showed decreases in TH-positive fiber density and TH-positive neurons in the SN of Gdnf+/− mice (Boger, et al., 2006), with increased DAT activity (current data). An increase in fiber density is also not supported by the overall decrease in striatal DA content discussed below. Regarding potential involvement of serotonergic systems in the electrochemistry clearance studies, prior work shows the clearance properties of locally applied DA in the striatum to be unchanged following selective inhibition of 5-HT transporters (Cass and Gerhardt, 1995). Tissue neurochemistry, however, includes analysis of 5-HT and 5-HIAA discussed below.
Altogether, these data support an increased DA clearance capacity in middle-aged Gdnf+/− mice, which may be explained by changes in the DAT activity, affinity and/or density of DAT membrane expression as seen also in the aged rat (Hebert, et al., 1999) and human brain (Volkow, et al., 1994). It is unclear if the GDNF depletion in this model varies within the striatum as previous characterization studies have focused on the entire striatum, excluding the NAc (Boger, et al., 2006). A differential effect of GDNF on DA terminals in the striatal subregions and/or differential spatial expression in this model could contribute to the current findings. These results require further investigation to determine the definitive mechanism(s), which underlie the apparent uptake changes.
4.4 Middle-aged Gdnf+/− mice demonstrate decreased DA synthesis and increased DA turnover without regional alterations in 5-HT
HPLC-EC analyses of DA and its metabolites were conducted to determine if DA synthesis and metabolism are altered in the striatum of Gdnf+/− mice. Tissues from rostral-medial and caudal-lateral striatal regions (recording sites 1 and 2) showed decreases in DA tissue levels (average ~25%), indicative of decreased DA synthesis. Decreased DA synthesis is not surprising given the loss of TH-positive cell bodies in the SN of Gdnf+/− mice (Boger, et al., 2006). Although there is evidence for decreased striatal TH staining density (Boger, et al., 2007), it is also possible that the relative activity of TH is reduced due to dephosphorylation, an aspect of TH activity shown to be enhanced by GDNF supplementation in vivo (Salvatore, et al., 2009, Salvatore, et al., 2004). Given the nature of the global partial depletion of GDNF protein in the brain in this model, both nigral and striatal TH phosphorylation may be diminished. The increased ratio of metabolites to DA indicate increased DA metabolism, but this is likely due to the decreased levels of DA, as the DA metabolites DOPAC and HVA were not significantly different in the Gdnf+/− mice.
HPLC-EC quantification of 5-HT and its metabolites were carried out to examine serotonergic changes in Gdnf+/− mice. There were no changes in whole tissue 5-HT and metabolite (5-HIAA) levels (p>0.05) at the two recording sites which support little contribution of the serotonergic systems in the outlined studies.
4.5 Conclusions
Motor and pathological manifestations associated with this Gdnf+/− mouse model become apparent later in life and progress gradually (Boger, et al., 2006). This slower time course contrasts with other DA lesion models in which the onset of neurodegeneration is comparatively rapid and severe (Ichitani, et al., 1994, Kirik, et al., 1998, Lee, et al., 1996, Sauer and Oertel, 1994), and deficits may recover with time (Yuan, et al., 2005). The Gdnf+/− mouse provides a novel model of DA dysfunction, which uniquely models subtle aspects of the human DA dysfunction seen in aging. The regulation of GDNF in aging and DA dysfunction in general has yet to be clearly defined. There are contradictory results regarding the regulation of GDNF in DA dysfunction and in age-associated movement disorders like PD. It has been suggested that GDNF isoforms may be differentially expressed in PD with an increase in isoform I (Backman, et al., 2006). Conversely, decreases in GDNF levels have been reported in the SN of PD patients (Jenner and Olanow, 1998). In animal models of DA dysfunction, both increased (Nakajima, et al., 2001) and unchanged levels of striatal GDNF (Smith, et al., 2003) have been reported following 6-OHDA administration. Our studies reveal changes in DA neurochemistry, uptake, and consequential behavioral implications in a mouse model with a chronic and partial genetic reduction of GDNF, which appears to manifest age-related changes in DA function at 12 months of age. Taken together, these studies support a unique role for GDNF in the regulation of DA and DA neuron development and maintenance. Further studies are necessary to determine the specific mechanisms which underlie the apparent neurochemical and kinetic changes in DA regulation as well as the implications of these changes.
Acknowledgments
This work was supported by USPHS grants AG023630, IT32 DA022738, NS39787, DA017186, AG13494 and NSF grant EEC-0310723.
Footnotes
Disclosures
Dr. Greg Gerhardt is the owner of the manufacturer of the FAST – 16 mk II systems, Quanteon, LLC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altar CA, Marshall JF. Neostriatal dopamine uptake and reversal of age-related movement disorders with dopamine-uptake inhibitors. Ann N Y Acad Sci. 1988;515:343–54. doi: 10.1111/j.1749-6632.1988.tb33006.x. [DOI] [PubMed] [Google Scholar]
- Backman CM, Shan L, Zhang YJ, Hoffer BJ, Leonard S, Troncoso JC, Vonsatel P, Tomac AC. Gene expression patterns for GDNF and its receptors in the human putamen affected by Parkinson’s disease: a real-time PCR study. Mol Cell Endocrinol. 2006;252(1–2):160–6. doi: 10.1016/j.mce.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175(2):191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgrim DM, Evans DA. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334(2):71–6. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Morelli M. Dopamine Circuits and Receptors. In: Dunnett SB, Bentivoglio M, Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Elsevier B.V.; 2005. pp. 44–56. [Google Scholar]
- Boger HA, Granholm AC, Jin L, Nelson ME, Page G, McGinty JF. Striatal gene expression profile of 12 month old GDNF heterozygous mice. Soc Neurosci Abstr. 2004;30:725.17. [Google Scholar]
- Boger HA, Middaugh LD, Huang P, Zaman V, Smith AC, Hoffer BJ, Tomac AC, Granholm AC. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp Neurol. 2006;202(2):336–47. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Patrick KS, Ramamoorthy S, Denehy ED, Zhu H, Pacchioni AM, Granholm AC, McGinty JF. Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice. J Neurosci. 2007;27(33):8816–25. doi: 10.1523/JNEUROSCI.1067-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA. Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci Lett. 1994;176(2):259–63. doi: 10.1016/0304-3940(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA. In vivo assessment of dopamine uptake in rat medial prefrontal cortex: comparison with dorsal striatum and nucleus accumbens. J Neurochem. 1995;65(1):201–7. doi: 10.1046/j.1471-4159.1995.65010201.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Mayfield RD, Curella P, Zahniser NR. Differences in dopamine clearance and diffusion in rat striatum and nucleus accumbens following systemic cocaine administration. J Neurochem. 1992;59(1):259–66. doi: 10.1111/j.1471-4159.1992.tb08899.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Manning MW, Bailey SL. Restorative effects of GDNF on striatal dopamine release in rats treated with neurotoxic doses of methamphetamine. Ann NY Acad Sci. 2000;914:127–36. doi: 10.1111/j.1749-6632.2000.tb05190.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Peters LE, Harned ME, Seroogy KB. Protection by GDNF and other trophic factors against the dopamine-depleting effects of neurotoxic doses of methamphetamine. Ann NY Acad Sci. 2006;1074:272–81. doi: 10.1196/annals.1369.024. [DOI] [PubMed] [Google Scholar]
- Cass WA, Zahniser NR, Flach KA, Gerhardt GA. Clearance of exogenous dopamine in rat dorsal striatum and nucleus accumbens: role of metabolism and effects of locally applied uptake inhibitors. J Neurochem. 1993;61(6):2269–78. doi: 10.1111/j.1471-4159.1993.tb07469.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180(3):545–80. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 2001. [Google Scholar]
- Friedemann MN. In vivo Electrochemical Studies of Dopamine Diffusion and Clearance in the Striatum of Young and Aged Fischer-344 Rats. Age. 1992;15(1):23–8. [Google Scholar]
- Friedemann MN, Gerhardt GA. Regional effects of aging on dopaminergic function in the Fischer-344 rat. Neurobiol Aging. 1992;13(2):325–32. doi: 10.1016/0197-4580(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Furman CA, Chen R, Guptaroy B, Zhang M, Holz RW, Gnegy M. Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: live-cell imaging using total internal reflection fluorescence microscopy. J Neurosci. 2009;29(10):3328–36. doi: 10.1523/JNEUROSCI.5386-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69(3):1322–5. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380(6571):252–5. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Cass WA, Yi A, Zhang Z, Gash DM. Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J Neurochem. 2002;80(1):168–77. doi: 10.1046/j.0022-3042.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Hoffman AF. Effects of recording media composition on the responses of Nafion-coated carbon fiber microelectrodes measured using high-speed chronoamperometry. J Neurosci Methods. 2001;109(1):13–21. doi: 10.1016/s0165-0270(01)00396-x. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Ksir C, Pivik C, Dickinson SD, Sabeti J, Zahniser NR. Methodology for coupling local application of dopamine and other chemicals with rapid in vivo electrochemical recordings in freely-moving rats. J Neurosci Methods. 1999;87(1):67–76. doi: 10.1016/s0165-0270(98)00158-7. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Rose GM, Hoffer BJ. Release of monoamines from striatum of rat and mouse evoked by local application of potassium: evaluation of a new in vivo electrochemical technique. J Neurochem. 1986;46(3):842–50. doi: 10.1111/j.1471-4159.1986.tb13048.x. [DOI] [PubMed] [Google Scholar]
- Gerlai R, McNamara A, Choi-Lundberg DL, Armanini M, Ross J, Powell-Braxton L, Phillips HS. Impaired water maze learning performance without altered dopaminergic function in mice heterozygous for the GDNF mutation. Eur J Neurosci. 2001;14(7):1153–63. doi: 10.1046/j.0953-816x.2001.01724.x. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: A review. Neurosci Biobehav Rev. 2009 doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn GE, Yamamoto BK. In vivo neurochemical and anatomical heterogeneity of the dopamine uptake system in the rat caudate putamen. Brain Res. 1989;481(2):235–41. doi: 10.1016/0006-8993(89)90799-3. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Reyland M, Albeck D, Sanders L, Gerhardt G, Hoernig G, Shen L, Westphal H, Hoffer B. Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons. J Neurosci. 2000;20(9):3182–90. doi: 10.1523/JNEUROSCI.20-09-03182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin R, Cass WA, Zhang Z, Stanford JA, Gash DM, Gerhardt GA. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J Neurosci. 2003;23(5):1974–80. doi: 10.1523/JNEUROSCI.23-05-01974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Yi A, Cass WA, Maswood N, Andersen AH, Elsberry DD, Klein MC, Gerhardt GA, Gash DM. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125(Pt 10):2191–201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- Halberda JP, Middaugh LD, Gard BE, Jackson BP. DAD1- and DAD2-like agonist effects on motor activity of C57 mice: differences compared to rats. Synapse. 1997;26(1):81–92. doi: 10.1002/(SICI)1098-2396(199705)26:1<81::AID-SYN9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hall ME, Hoffer BJ, Gerhardt GA. Rapid and Sensitive Determination of Catecholamines in Small Tissue Samples by High-Performance Liquid-Chromatography Coupled with Dual-Electrode Coulometric Electrochemical Detection. Lc Gc-Magazine of Separation Science. 1989;7(3):258–65. [Google Scholar]
- Hascup KN, Rutherford EC, Quintero JE, Day BK, Nickell JR, Pomerleau F, Huettl P, Burmeister J, Gerhardt GA. Second-by-Second Measures of L-Glutamate and Other Neurotransmitters Using Enzyme-Based Microelectrode Arrays. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience, Frontiers in Neuroengineering. CRC Press; Boca Raton, FL: 2007. pp. 407–50. [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J Pharmacol Exp Ther. 1997;282(2):760–8. [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in Fischer 344 rats. Brain Research. 1998;797(1):42–54. doi: 10.1016/s0006-8993(98)00370-9. [DOI] [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Age-related changes in the capacity, rate, and modulation of dopamine uptake within the striatum and nucleus accumbens of Fischer 344 rats: An in vivo electrochemical study. J Pharmacol Exp Ther. 1999;288(2):879–87. [PubMed] [Google Scholar]
- Hebert MA, Larson GA, Zahniser NR, Gerhardt GA. Age-related reductions in [3H]WIN 35,428 binding to the dopamine transporter in nigrostriatal and mesolimbic brain regions of the fischer 344 rat. J Pharmacol Exp Ther. 1999;288(3):1334–9. [PubMed] [Google Scholar]
- Hebert MA, Vanhorne CG, Hoffer BJ, Gerhardt GA. Functional effects of GDNF in normal rat striatum: Presynaptic studies using in vivo electrochemistry and microdialysis. J Pharmacol Exp Ther. 1996;279(3):1181–90. [PubMed] [Google Scholar]
- Hefti F, Melamed E, Wurtman RJ. Partial lesions of the dopaminergic nigrostriatal system in rat brain: biochemical characterization. Brain Res. 1980;195(1):123–37. doi: 10.1016/0006-8993(80)90871-9. [DOI] [PubMed] [Google Scholar]
- Hoffer BJ, Hoffman A, Bowenkamp K, Huettl P, Hudson J, Martin D, Lin LFH, Gerhardt GA. Glial-Cell Line-Derived Neurotrophic Factor Reverses Toxin-Induced Injury to Midbrain Dopaminergic-Neurons In-Vivo. Neuroscience Letters. 1994;182(1):107–11. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Gerhardt GA. In vivo electrochemical studies of dopamine clearance in the rat substantia nigra: effects of locally applied uptake inhibitors and unilateral 6-hydroxydopamine lesions. J Neurochem. 1998;70(1):179–89. doi: 10.1046/j.1471-4159.1998.70010179.x. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson’s disease. Adv Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- Ichitani Y, Okamura H, Nakahara D, Nagatsu I, Ibata Y. Biochemical and immunocytochemical changes induced by intrastriatal 6-hydroxydopamine injection in the rat nigrostriatal dopamine neuron system: evidence for cell death in the substantia nigra. Exp Neurol. 1994;130(2):269–78. doi: 10.1006/exnr.1994.1205. [DOI] [PubMed] [Google Scholar]
- Jenner P, Olanow CW. Understanding cell death in Parkinson’s disease. Ann Neurol. 1998;44(3 Suppl 1):S72–84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152(2):259–77. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Kluger A, Gianutsos JG, Golomb J, Ferris SH, Reisberg B. Motor/psychomotor dysfunction in normal aging, mild cognitive decline, and early Alzheimer’s disease: diagnostic and differential diagnostic features. Int Psychogeriatr. 1997;9(Suppl 1):307–16. doi: 10.1017/s1041610297005048. discussion 17–21. [DOI] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M. Hyperactivity and hypoactivity produced by lesions to the mesolimbic dopamine system. Behav Brain Res. 1981;3(3):341–59. doi: 10.1016/0166-4328(81)90004-8. [DOI] [PubMed] [Google Scholar]
- Lee CS, Sauer H, Bjorklund A. Dopaminergic neuronal degeneration and motor impairments following axon terminal lesion by instrastriatal 6-hydroxydopamine in the rat. Neuroscience. 1996;72(3):641–53. doi: 10.1016/0306-4522(95)00571-4. [DOI] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci U S A. 1993;90(19):8861–5. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LFH, Doherty DH, Lile JD, Bektesh S, Collins F. Gdnf - A Glial-Cell Line Derived Neurotrophic Factor for Midbrain Dopaminergic-Neurons. Science. 1993;260(5111):1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Mahalik TJ, Hahn WE, Clayton GH, Owens GP. Programmed cell death in developing grafts of fetal substantia nigra. Exp Neurol. 1994;129(1):27–36. doi: 10.1006/exnr.1994.1144. [DOI] [PubMed] [Google Scholar]
- Marsden CD. Parkinson’s disease. Lancet. 1990;335(8695):948–52. doi: 10.1016/0140-6736(90)91006-v. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Altar CA. Striatal dopamine uptake and swim performance of the aged rat. Brain Res. 1986;379(1):112–7. doi: 10.1016/0006-8993(86)90262-3. [DOI] [PubMed] [Google Scholar]
- Messer CJ, Eisch AJ, Carlezon WA, Jr, Whisler K, Shen L, Wolf DH, Westphal H, Collins F, Russell DS, Nestler EJ. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26(1):247–57. doi: 10.1016/s0896-6273(00)81154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382(6586):76–9. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Hida H, Shimano Y, Fujimoto I, Hashitani T, Kumazaki M, Sakurai T, Nishino H. GDNF is a major component of trophic activity in DA-depleted striatum for survival and neurite extension of DAergic neurons. Brain Res. 2001;916(1–2):76–84. doi: 10.1016/s0006-8993(01)02866-9. [DOI] [PubMed] [Google Scholar]
- Palmer MR, Wuerthele SM, Hoffer BJ. Physical and physiological characteristics of micropressure ejection of drugs from multibarreled pipettes. Neuropharmacology. 1980;19(10):931–8. doi: 10.1016/0028-3908(80)90001-5. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382(6586):73–6. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Pijnenburg AJ, Honig WM, Van der Heyden JA, Van Rossum JM. Effects of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. Eur J Pharmacol. 1976;35(1):45–58. doi: 10.1016/0014-2999(76)90299-5. [DOI] [PubMed] [Google Scholar]
- Richards M, Stern Y, Marder K, Cote L, Mayeux R. Relationships between extrapyramidal signs and cognitive function in a community-dwelling cohort of patients with Parkinson’s disease and normal elderly individuals. Ann Neurol. 1993;33(3):267–74. doi: 10.1002/ana.410330307. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Adams CE, Burmeister J, Gerhardt GA, Zahniser NR. Kinetic analysis of striatal clearance of exogenous dopamine recorded by chronoamperometry in freely-moving rats. J Neurosci Methods. 2002;121(1):41–52. doi: 10.1016/s0165-0270(02)00229-7. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Gerhardt GA, Dayton RD, Klein RL, Stanford JA. Bilateral effects of unilateral GDNF administration on dopamine- and GABA-regulating proteins in the rat nigrostriatal system. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Zhang JL, Large DM, Wilson PE, Gash CR, Thomas TC, Haycock JW, Bing G, Stanford JA, Gash DM, Gerhardt GA. Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in the rat striatum and substantia nigra. J Neurochem. 2004;90(1):245–54. doi: 10.1111/j.1471-4159.2004.02496.x. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59(2):401–15. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Smith AD, Antion M, Zigmond MJ, Austin MC. Effect of 6-hydroxydopamine on striatal GDNF and nigral GFRalpha1 and RET mRNAs in the adult rat. Brain Res Mol Brain Res. 2003;117(2):129–38. doi: 10.1016/s0169-328x(03)00289-4. [DOI] [PubMed] [Google Scholar]
- Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic. 2005;6(2):157–70. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Stanford JA, Currier TD, Gerhardt GA. Acute locomotor effects of fluoxetine, sertraline, and nomifensine in young versus aged Fischer 344 rats. Pharmacol Biochem Behav. 2002;71(1–2):325–32. doi: 10.1016/s0091-3057(01)00655-4. [DOI] [PubMed] [Google Scholar]
- Stephens ML, Quintero JE, Pomerleau F, Huettl P, Gerhardt GA. Age-related changes in glutamate release in the CA3 and dentate gyrus of the rat hippocampus. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch A, Ludolph AC, Schwarz J. Dopamine transporter: involvement in selective dopaminergic neurotoxicity and degeneration. J Neural Transm. 2004;111(10–11):1267–86. doi: 10.1007/s00702-004-0203-2. [DOI] [PubMed] [Google Scholar]
- Stromberg I, Bjorklund L, Johansson M, Tomac A, Collins F, Olson L, Hoffer B, Humpel C. Glial-Cell Line-Derived Neurotrophic Factor Is Expressed in the Developing But Not Adult Striatum and Stimulates Developing Dopamine Neurons In-Vivo. Exp Neurol. 1993;124(2):401–12. doi: 10.1006/exnr.1993.1214. [DOI] [PubMed] [Google Scholar]
- Thomas TC, Kruzich PJ, Joyce BM, Gash CR, Suchland K, Surgener SP, Rutherford EC, Grandy DK, Gerhardt GA, Glaser PE. Dopamine D4 receptor knockout mice exhibit neurochemical changes consistent with decreased dopamine release. J Neurosci Methods. 2007 doi: 10.1016/j.jneumeth.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373(6512):335–9. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Tuomisto J. Nomifensine and its derivatives as possible tools for studying amine uptake. Eur J Pharmacol. 1977;42(2):101–6. doi: 10.1016/0014-2999(77)90348-x. [DOI] [PubMed] [Google Scholar]
- van Horne CG, Hoffer BJ, Stromberg I, Gerhardt GA. Clearance and diffusion of locally applied dopamine in normal and 6-hydroxydopamine-lesioned rat striatum. J Pharmacol Exp Ther. 1992;263(3):1285–92. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Logan J, Schlyer D, MacGregor R, Hitzemann R, Wolf AP. Decreased dopamine transporters with age in health human subjects. Ann Neurol. 1994;36(2):237–9. doi: 10.1002/ana.410360218. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Ding YS, Gur RC, Gatley J, Logan J, Moberg PJ, Hitzemann R, Smith G, Pappas N. Parallel loss of presynaptic and postsynaptic dopamine markers in normal aging. Ann Neurol. 1998;44(1):143–7. doi: 10.1002/ana.410440125. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Dose-dependent alteration in zif/268 and preprodynorphin mRNA expression induced by amphetamine or methamphetamine in rat forebrain. J Pharmacol Exp Ther. 1995;273(2):909–17. [PubMed] [Google Scholar]
- Willig F, Palacios A, Monmaur P, M’Harzi M, Laurent J, Delacour J. Short-term memory, exploration and locomotor activity in aged rats. Neurobiol Aging. 1987;8(5):393–402. doi: 10.1016/0197-4580(87)90033-9. [DOI] [PubMed] [Google Scholar]
- Yuan H, Sarre S, Ebinger G, Michotte Y. Histological, behavioural and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. J Neurosci Methods. 2005;144(1):35–45. doi: 10.1016/j.jneumeth.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001;891(1–2):228–35. doi: 10.1016/s0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Hipkens SB, Hebert MA, Gash DM, Gerhardt GA. Age-related decline in striatal dopamine release and motoric function in brown Norway/Fischer 344 hybrid rats. Brain Res. 1998;791(1–2):246–56. doi: 10.1016/s0006-8993(98)00110-3. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Andersen A, Smith C, Grondin R, Gerhardt G, Gash D. Motor slowing and parkinsonian signs in aging rhesus monkeys mirror human aging. J Gerontol A Biol Sci Med Sci. 2000;55(10):B473–B80. doi: 10.1093/gerona/55.10.b473. [DOI] [PubMed] [Google Scholar]