Abstract
Background
Zoledronic acid, a bisphosphonate, delays progression of bone metastases in adult malignancies. Bone is a common metastatic site of advanced neuroblastoma. We previously reported efficacy of zoledronic acid in a murine model of neuroblastoma bone invasion prompting this Phase I trial of zoledronic acid with cyclophosphamide in children with neuroblastoma and bone metastases. The primary objective was to determine recommended dosing of zoledronic acid for future trials.
Procedure
Escalating doses of intravenous zoledronic acid were given every 28 days with oral metronomic cyclophosphamide (25 mg/m2/day). Toxicity, response, zoledronic acid pharmacokinetics, bone turnover markers, serum IL-6, and sIL-6R were evaluated.
Results
Twenty-one patients, median age 7.5 (range 0.8 - 25.6) years were treated with 2 mg/m2 (n=4), 3 mg/m2 (n=3), or 4 mg/m2 (n=14) zoledronic acid. Fourteen patients were evaluable for dose escalation. A median of one (range 1-18) courses was given. Two dose limiting toxicities (Grade 3 hypophosphatemia) occurred at 4 mg/m2 zoledronic acid. Other Grade 3-4 toxicities included hypocalcemia (n=2), elevated transaminases (n=1), neutropenia (n=2), anemia (n=1), lymphopenia (n=1), and hypokalemia (n=1). Osteosclerosis contributed to fractures in one patient after 18 courses. Responses in evaluable patients included 1 partial response, 9 stable disease (median 4.5 courses, range 3-18), and 10 progressions. Zoledronic acid pharmacokinetics were similar to adults. Markers of osteoclast activity and serum IL-6 levels decreased with therapy.
Conclusions
Zoledronic acid with metronomic cyclophosphamide is well tolerated with clinical and biologic responses in recurrent/refractory neuroblastoma. The recommended dose of zoledronic acid is 4 mg/m2 every 28 days.
Keywords: Phase I, neuroblastoma, bisphosphonate
Introduction
High-risk neuroblastoma (NB) regimens achieve only 40% long term event-free survival [1] despite aggressive therapy including surgical resection, myeloablative chemotherapy, focal radiation therapy, and 13-cis-retinoic acid. Novel treatment modalities are required to improve outcome.
Zoledronic acid, a nitrogen-containing bisphosphonate, inhibits the mevalonate pathways critical for protein prenylation and geranyl-geranylation and is a potent inducer of apoptosis [2]. Because of its unique ability to bind with high affinity to the bone matrix, zoledronic acid is a potent inhibitor of osteoclasts [2]. Bisphosphonates, including zoledronic acid, have shown efficacy as adjuvant agents against bone metastases in patients with breast and prostate cancer, multiple myeloma, and other malignancies by significantly delaying or reducing the incidence of skeletal related events (SRE), including pathologic fractures and the need for palliative radiation [3,4,5]. More recently, zoledronic acid was associated with improved survival in women with premenopausal breast cancer treated with hormonal therapies [6].
Cyclophosphamide has known activity against neuroblastoma. Although children with newly diagnosed high-risk neuroblastoma typically receive high doses of cyclophosphamide as part of standard frontline therapy [7,8], resistant tumors may still respond to low dose protracted (metronomic) regimens of cyclophosphamide [9,10] theoretically through both anti-angiogenic and direct anti-tumor effects. Adult studies of metronomic cyclophosphamide for advanced malignancies have been well tolerated and associated with stable disease [11,12].
We previously demonstrated the efficacy of bisphosphonates including zoledronic acid, in a murine neuroblastoma bone invasion model [13]. We showed that zoledronic acid combined with high dose cyclophosphamide and topotecan significantly inhibited osteolytic lesions in mice even when administered four weeks after tumor cell inoculation [13]. Zoledronic acid’s effects were limited to bone lesions in this model. Cyclophosphamide also enhanced the inhibition of the proliferation of neuroblastoma cell lines observed with zoledronic acid alone [13]. These data showing additive activity of zoledronic acid with cyclophoshamide formed the basis for our Phase I trial design combining oral metronomic cyclophosphamide with escalating doses of zoledronic acid in children with refractory/resistant neuroblastoma and bone metastases. The aims of this trial were to define the maximum tolerated dose of zoledronic acid in this combination and the pediatric toxicity profile of zoledronic acid, and to obtain tumor response data, pharmacokinetics, and correlative biologic data within the confines of a Phase I trial.
Methods
Eligibility
Patients with high-risk NB < 30 years of age at study entry with recurrent/progressive disease, refractory disease (less than partial response to frontline therapy), or biopsy proven disease after at least a partial response to frontline therapy were eligible. Patients were required to have bone metastases at entry defined as at least one MIBG avid bone site or progression at a bone site based on bone scan findings if tumor was MIBG-nonavid. Other eligibility criteria included: Karnofsky or Lansky performance status > 50%; life expectancy of > 2 months; ANC > 750/μl; platelet count > 50,000/μl; normal serum creatinine for age; ionized serum calcium > 1.0 mmol/L; and adequate hepatic function. Prior autologous stem cell transplant was allowed. Exclusion criteria included prior use of bisphosphonates.
The study was approved by the Institutional Review Board at each participating site of the New Approaches to Neuroblastoma Therapy (NANT consortium) (www.nant.org). Informed consent was obtained from the patient or their parent/guardian, and assent was obtained as appropriate before protocol enrollment.
Drug Administration
Cyclophosphamide was administered at a fixed dose of 25 mg/m2/day orally once daily for 28 days with zoledronic acid administered at least one hour after cyclophosphamide as a 15 minute intravenous (IV) infusion on day 0. Courses were repeated every 28 days without interruption. Patients were required to take daily age-appropriate Vitamin D and calcium replacements. Patients were monitored weekly during the first course and every other week during subsequent courses. Administration of oral cyclophosphamide, Vitamin D and calcium were documented within patient diaries.
Trial Design
Patients were accrued in cohorts of three per dose level. The first dose level utilized 2 mg/m2 zoledronic acid (approximately 80% of adult MTD), with subsequent dose escalations to 3 mg/m2 and 4 mg/m2 using a standard “3 + 3” cohort design [14]. zoledronic acid doses were capped at 4 mg. The maximum tolerated dose (MTD) was defined as the highest dose at which < 1 of 6 patients experienced first-course dose limiting toxicity (DLT) at least possibly attributed to treatment. Due to the observation of hypophosphatemia, the study was amended to continue enrollment at 4 mg/m2 (level 3e) until 3 patients completed a minimum of 2 courses to further evaluate hypophosphatemia with more frequent monitoring during the second course. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria (version 3.0). Hematologic DLTs were defined as Grade 4 neutropenia or platelet count < 20,000 that did not resolve to eligibility criteria within 14 days of withholding cyclophosphamide. Patients with bone marrow metastases documented on required bone marrow exam at study entry were considered not evaluable for hematological toxicity. Non-hematological DLT was defined as any Grade 3-4 non-hematological toxicity with the following exceptions: renal DLTs were defined as previously described for zoledronic acid clinical trials [5,15] as an increase in creatinine by > 0.4 mg/dL from baseline if baseline was < 1.4 mg/dL, or an increase by > 1 mg/dL if baseline creatinine was > 1.4 mg/dL; grade 3-4 hypocalcemia responsive to calcium replacement or resolving after 1-2 dose reductions; grade 3 nausea/vomiting; grade 3 fever; and grade 3-4 infection (unless associated with Grade 3 symptoms in other organs). Grade 3-4 asymptomatic hypophosphatemia was excluded from non-hematological DLT for level 3e.
Courses started every 28 days provided toxicity resolved to eligibility criteria and there was no disease progression. Patients with reversible DLT were offered further modified treatment, depending on the DLT. For example, patients with hematologic DLT had cyclophosphamide reduced by 25%, while zoledronic acid was reduced by 25% for dose limiting hypocalcemia.
Tumor response
Tumor was evaluated at baseline, after courses one and three, and then after every three courses. Overall response was graded according to the NANT Response Criteria, modified from the International Neuroblastoma Response Criteria [16] using the RECIST method [17] of longest dimension for measurable tumor on CT/MRI and Curie score [18] for MIBG response. Only complete responses were described in bone marrow, defined as no morphologic tumor on two serial samples. Two central reviewers evaluated CT/MRI and MIBG scans for all patients with overall response of stable disease or better. A single central reviewer reviewed bone marrow slides.
Pharmacokinetics of Zoledronic Acid
Participation in pharmacokinetic (PK) studies was optional. Peripheral blood was collected before zoledronic acid infusion then at 15 minutes, 1 hour, 3 hours, 8 hours and 24 hours following infusion during course one. Blood was processed, frozen, and PK assays performed by Novartis via radioimmunoassay as previously described [19]. The data were analyzed graphically by construction of plasma zoledronic acid concentration versus time profiles. Non-compartmental PK methods were utilized to determine area under the curve of plasma zoledronic acid for 24 hours post-dose (AUC24h) and maximum concentration (Cmax).
To characterize the urinary excretion of zoledronic acid during course one, complete collection of urine was performed by pooling the urine over 0-4 hours, 4-12 hours, 12-24 hours and 24-48 hours time intervals following the dose administration. A 10-mL aliquot of each pooled collection was assayed for zoledronic acid using a validated radioimmunoassay method as previously described [19]. The zoledronic acid concentration in each urine collection was multiplied by the estimated urine volume in order to estimate the quantity of zoledronic acid excreted during each collection interval and to provide data for assessing the cumulative excretion of zoledronic acid over a period of 24 hours.
Biologic Assays
Bone Resorption Markers
Participation in bone resorption studies was optional. Peripheral blood and urine collected prior to treatment and after courses one and three were analyzed for type I collagen C-telopeptide (serum) and N-telopeptide (urine) levels in clinical laboratories of the participating institutions using standard immunoassays.
IL-6 and sIL-6 Receptor Levels
Participation in cytokine studies was optional. Peripheral blood collected prior to treatment and after courses one and three was analyzed for IL-6 and soluble IL-6 receptor (sIL-6R). Control samples from normal children were obtained from IRB-approved banking protocols. Levels of human IL-6 and sIL-6R in serum were determined in triplicate aliquots by quantitative Enzyme Linked Immunoabsorbance Assay (ELISA) according to the manufacturer’s protocol (Quantikine Immunoassay kit from R&D).
Statistical Analysis of Biologic Results
A general linear model was used to test for a course effect, adjusting for the fact that not all patients had assessments at all times. If the overall course effect was significant at the 0.05 level, then pair wise comparisons were performed by Tukey-Kramer method of multiple comparisons. Prior to analysis, data were transformed using either the logarithm (for IL-6 and N-telopeptide) or the square root (for sIL-6R and C-telopeptide). Means and confidence intervals were converted back to original scale.
Results
Patient Characteristics
A total of 21 patients were enrolled at nine NANT institutions between September 2005 and January 2008. Their clinical characteristics are described in Table I. Seventyseven courses were given with a median number of one course per patient (range 1 to 18). Doses were capped at 4 mg for 5 patients, all at dose level 3.
Table I.
Patient Characteristic (n=21)
| N (%) | |
|---|---|
| Male/Female | 15/6 |
| Median Age (Range) | 7.5 (0.8 – 25.6) years |
| Stage at Diagnosis | |
| 1 | 1 (5%) |
| 3 | 1 (5%) |
| 4 | 19 (90%) |
| Tumor Biology at Diagnosis | |
| MYCN amplified | 5 (24%) |
| MYCN non-amplified | 14 (67%) |
| Unknown/not done | 2 (10%) |
| Favorable histology | 0 (0%) |
| Unfavorable histology | 14 (67%) |
| Unknown/not done | 7 (33%) |
| Prior Therapies | |
| Chemotherapy | 21 (100%) |
| XRT | 16 (76%) |
| HDT/SCR | 13 (62%) |
| MIBG Therapy | 7 (33%) |
| History of Tumor Relapse prior to Study Entry |
21 (100%) |
Toxicity
Dose limiting toxicities are listed in Table II and detailed toxicities are listed in Table III. Both DLTs at dose level 3 were asymptomatic grade 3 hypophosphatemia beginning 2 and 4 days after the dose of zoledronic acid and resolving 14 and 18 days later respectively. One of these patients did not complete course one due to tumor progression and was initially considered inevaluable for dose escalation and replaced. On review, the patient was reclassified as evaluable since a DLT had occurred. The replacement patient also experienced grade 3 asymptomatic hypophosphatemia. The original study design followed laboratory values weekly during the first course but not during subsequent courses, and therefore could not adequately determine if hypophosphatemia was isolated to the initial course or also occurred after the second or subsequent courses [20]. Therefore, the protocol was amended to assess 3 additional patients completing a minimum of two courses with weekly laboratory monitoring. Based on prior study data, we expected six to nine additional patients would be required. Seven patients entered dose level 3e, but only one completed two courses at the prescribed doses. Six were inevaluable due to early tumor progression (n=4), refusal to take therapy (n=1), and a non-protocol mandated dose reduction. Only 1/7 patients on dose level 3e had grade 3 hypophosphatemia during course one. Enrollment was discontinued at this point because the objective of the amendment was unlikely to be achieved, and no other significant toxicity had been observed which would alter the zoledronic acid dose recommended for further studies.
Table II.
Evaluable Patients and Dose Limiting Toxicity (DLT)
| Dose Level |
# Entered | # Eligible |
# Evaluable for Dose Escalation Decisions |
# Patients with DLT in Course 1 |
Type of DLT |
|---|---|---|---|---|---|
| 1 | 4 | 4 | 3* | 0 | |
| 2 | 3 | 3 | 3 | 0 | |
| 3 | 7 | 7 | 7 | 2 | Grade 3 Hypophosphatemia, asymptomatic |
| 3e | 7# | 6 | 1 | 0 |
One patient did not take cyclophosphamide as prescribed during course one
One patient enrolled but never received either drug
Table III.
Detailed Toxicities*
| Toxicity Type | Maximum Grade of Toxicity | |||||
|---|---|---|---|---|---|---|
| Dose Level 1(n=4) | Dose Level 2 (n=3) | Dose Level 3 (n=13) | ||||
| Grade 1- 2 |
Grade 3-4 |
Grade 1- 2 |
Grade 3-4 |
Grade 1- 2 |
Grade 3- 4 |
|
| Non-heme | ||||||
| Hypocalcemia | 3 | 2 | 1 | 1 | 7 | 1 |
| Pain^ | 1 | 3 | 1 | |||
| Hypophosphatemia | 2 | 2 | 3 | |||
| Nausea/Vomiting | 1 | 5 | ||||
| Fever | 1 | 2 | 4 | |||
| SGOT (AST) | 2 | 3 | ||||
| Hypercalcemia | 4 | |||||
| Fatigue | 4 | |||||
| Hypermagnesemia | 1 | 2 | ||||
| Infection | 1 | 1 | 1 | |||
| Anorexia | 1 | 2 | ||||
| Heme$ | ||||||
| Evaluable for Heme Toxicity |
n=3 | n=1 | n=5 | |||
| Leukopenia | 3 | 1 | 4 | |||
| Anemia | 2 | 1 | 1 | |||
| Lymphopenia | 1 | 3 | ||||
| Neutropenia | 1 | 2 | 1 | |||
| Thrombocytopenia | 1 | 1 | ||||
Toxicities possibly, probably, or definitely related to treatment occurring in more than 10% of patients as determined in the first course of protocol therapy (n=20)
Pain described at various sites
In patients evaluable for hematologic toxicity
A 2-year-old male (dose level 3) developed diffuse osteosclerosis detected after nine courses that progressed on serial radiographs. He was removed from study treatment after 18 courses, but continued to receive cyclophosphamide off-study. Three months after his final dose of zoledronic acid he developed a low-trauma femoral fracture felt to be associated with osteosclerosis. One-year off-therapy, a rib fracture was found on routine follow-up CT. These sites were neither sites of tumor nor sites of prior radiation therapy. No other patient experienced a fracture. Reports from plain films done per protocol to monitor bone response were reviewed in the four other patients who received at least 5 doses of zoledronic acid. Osteosclerosis was not found in a 15 year old during 5 courses of zoledronic acid, or in a 26 year old who received 12 courses. A 4 year old had osteosclerosis after 6 courses. A 7 year old male had dense metaphyseal bands in the distal femurs and proximal tibia/fibulae after 4 courses, which persisted after course 11.
A dose of 4 mg/m2 zoledronic acid is recommended for future pediatric trials as the grade 3 hypophosphatemia observed is self-correcting and has no clinical sequelae. Further identification of risk factors for osteosclerosis is required to determine if duration of therapy is related to this toxicity.
Pharmacokinetics
Seven patients had pharmacokinetic studies. A total of 4 patients contributed urinary excretion data, but complete collection was obtained in 2 patients only. Cmax and AUC24h values for each participating patient are presented in Table IV. Too few patients participated in pharmacokinetic studies to draw conclusions about variations with dosing. The systemic plasma exposures are concordant with values for adult cancer patients with normal renal function who participated in pharmacokinetic studies during their first dose of zoledronic acid 4 mg, approximately equivalent to 2.1 mg/m2 given their mean BSA of 1.9 m2. The average Cmax and AUC24h in this adult population were 309+/− 71 ng/mL and 408+/− 90 ng•hour/mL respectively [19].
Table IV.
PK Results for Subjects Consenting to Optional Pharmacokinetic Studies
| Zoledronic acid Dose mg/m2/dose$ |
Age (yr) |
Cmax (ng/mL) |
AUC24h (ng•h/mL) |
Urinary excretion. (% dose) |
|---|---|---|---|---|
| 2 | 15 | 223 | 449 | 40 |
| 9 | 351 | 330 | ||
| 26 | 187 | 334 | ||
| 4 | 120 | 139* | 34 | |
|
| ||||
| 3 | 3 | 496 | 478 | |
|
| ||||
| 4 | 2 | 76.1 | 460 | |
| 8 | 401 | 447 | ||
AUC8h
No patient participating in pharmacokinetic studies had dose capped at 4 mg.
Biologic Assays
Bone Resorption Studies
Normal telopeptide values varied by clinical lab, therefore values are reported here in relationship to the upper limit of normal (ULN) value for that patient. Mean N-telopeptide values were elevated in patients before treatment with a mean of 6.09 × ULN and 95% confidence interval (CI): (4.22, 8.79) × ULN and decreased significantly after course 1 (2.14 CI:(1.29, 3.54) × ULN) and again after course 3 (1.00 CI: (0.54, 1.84) × ULN) (Figure 1A). Mean C-telopeptide values were elevated before treatment with a mean of 2.09 × ULN and CI (1.47, 2.83) × ULN) and decreased after course 1 (1.26 CI: (0.65, 2.06) × ULN) but remained stable after course 3 (1.48 CI: (0.63, 2.68) × ULN) (Figure 1B).
Figure 1.
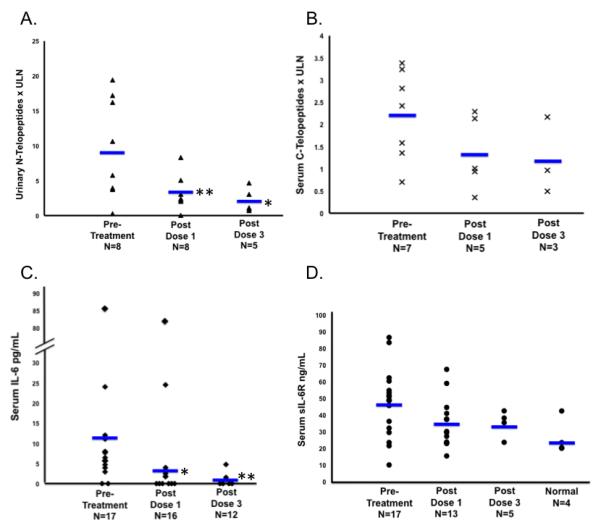
Biologic assays determined prior to treatment and after courses 1 and 3. Each point represents individual subject values. Bars represent mean values; A. Urinary N-tetlopeptide levels; B. Serum C-telopeptide levels; C. Serum IL-6 levels; D. Serum sIL-6R levels; * p<0.05; ** p<0.01
Serum IL-6 and s-IL6R Levels
Serum IL-6 values were elevated before treatment (mean of 5.9 pg/mL and CI:(4.2, 8.2) pg/mL; normal <4 pg/mL) decreased significantly after course 1 (2.9 CI:(1.7, 4.5) pg/mL) and decreased further after course 3 (0.5 CI:(0.0, 1.6) pg/mL) (Figure 1C.) The mean serum s-IL6R level from four normal pediatric controls was 26.5 +/− 10.7 ng/mL. Serum values in our patients before treatment was mean of 44.4 CI:(38.8, 50.4) ng/mL, and did not significantly change after one course (37.2 CI:(30.8, 44.2) ng/mL) or 3 courses (40.0 CI:(29.8, 51.7) ng/mL) (Figure 1D).
Tumor Response
Tumor response in the 20 evaluable patients is shown in Table V. One patient refused therapy after enrollment and was inevaluable. One patient at dose level 3 had a partial response after 10 courses consisting of resolution of multiple MIBG avid sites, which was maintained for 18 courses. This patient initially presented with intermediate risk stage 4 neuroblastoma at 4 months of age and had tumor progression on intermediate-risk based chemotherapy and again on high-risk based induction chemotherapy. Nine patients had stable disease for a median of 3 courses (range 2-12). The median time to progression was 0.9 (95% CI: 0.8, 2.7) months, and median overall survival was 12.3 (95% CI: 8.3, 19.5) months.
Table V.
Disease Response
| Dose Level | Patient # | Sites of Tumor at Entry |
Total Number Courses |
Best Overall Response |
Reason Off Therapy |
|---|---|---|---|---|---|
| 1 | 1 | MIBG | 3 | SD | PD |
| 2 | MIBG | 12 | SD * | PD | |
| 3 | BM, MIBG | 1 | PD | PD | |
| 4 | MIBG | 6 | SD * | PD | |
| 2 | 5 | MIBG | 3 | SD | PD |
| 6 | CT, BM MIBG, |
1 | PD | PD | |
| 7 | BM, MIBG | 1 | PD | PD | |
| 3 | 8 | MIBG | 18 | PR * | Osteosclerosis |
| 9 | CT, MIBG | 1 | PD | PD | |
| 10 | CT, BM, MIBG, |
1 | PD | PD | |
| 11 | BM, MIBG | 1 | PD | PD | |
| 12 | CT, BM, MIBG, |
1 | PD | PD | |
| 13 | CT, BM, MIBG, |
5 | SD * | PD | |
| 14 | MIBG | 3 | SD | PD | |
| 15 | BM, MIBG | 1 | PD | PD | |
| 16 | BM, MIBG | 3 | SD | PD | |
| 17 ** | CT, BM, MIBG |
NE | Patient/parent withdrawal from therapy |
||
| 18 | BM, MIBG | 12 | SD * | SD after course 12, stopped for parent/physician choice |
|
| 19 | CT, MIBG | 2 | SD | PD | |
| 20 | CT, MIBG | 1 | PD | PD | |
| 21 | BM, MIBG | 1 | PD | PD |
SD: stable disease; PD: progressive disease; PR: Partial response; CR: Complete Response; NE: Not evaluable
Response confirmed by central review if SD for more than 3 courses
Withdrew prior to receiving treatment therefore inevaluable for response.
Discussion
Zoledronic acid has been incorporated into breast cancer, multiple myeloma, and prostate cancer therapy because of its ability to delay new bone lesions, fractures, and diminish pain [3,4,5]. Bisphosphonate use in children has been limited to primary bone disorders such as osteogenesis imperfecta [21,22] and steroid-induced osteopenia [23]. We report the first study to use zoledronic acid in children with cancer. Both zoledronic acid and orally administered metronomic cyclophosphamide were extremely well tolerated. One partial response to the combination maintained after 18 courses was noted, with nine stable disease responses maintained for 2-12 months.
As in adult studies, hypocalcemia occurred in over 50% of patients, but was mitigated with vitamin D and calcium supplementation. Hypophosphatemia, also a known toxicity of bisphosphonates, was seen only in our highest dose level. In a study of 34 children with primary bone disorders who received 0.025 mg/kg zoledronic acid, hypocalcemia and hypophosphatemia occurred in 74 and 82% of subjects (respectively) within 48 hours of infusion and was most prevalent with the first dose of zoledronic acid [20]. Our study was originally designed to follow labs weekly in the first course and less frequently in subsequent courses, therefore preventing us from describing the frequency of this toxicity in subsequent courses. Cohort 3e was added to the original design of this trial to address this issue, unfortunately we were unable to accrue sufficient numbers of patients within the timeframe of this study to answer this question. This toxicity should continue to be carefully followed in future studies, particularly in the context of understanding the impact of bisphosphonates on bone metabolism in children who have not yet completed bone growth.
This study did not encounter the rare toxicities reported with potent bisphosphonates, including nephrotoxicity and osteonecrosis of the jaw. Bisphosphonate associated nephrotoxicities, including toxic acute tubular necrosis and collapsing focal segmental glomerulosclerosis [24], occur in approximately 5% of patients [25] usually after repeated dosing. Patients on this trial were required to have normal renal function, yet all had received prior potentially nephrotoxic platinum therapy. Osteonecrosis of the jaw, a rare but severe toxicity with an incidence between <1% and 12% [26] in adults on prolonged bisphosphonate use, has not been reported in children. These toxicities may not have been encountered on this study because of the small number of patients. Continued vigilance for rare toxicities is warranted as children receive zoledronic acid in future studies.
We observed osteosclerosis in two children and dense metaphaseal bands in a third. Osteosclerosis of the bone was routinely observed in our preclinical murine trials [13]. Considering that bisphosphonates specifically inhibit osteoclast activity, it is conceivable that their inhibitory activity on bone resorption leads to osteosclerosis. Because of limited use of bisphophonates in children with actively growing bones, the impact of specific risk factors such as age, rate of bone growth, and gender remain undefined. Reports of fractures after prolonged bisphosphonate use are increasing as these agents are incorporated into various treatment modalities. One adolescent patient developed fractures after 2.5 years of bisphophonate therapy for idiopathic bone pain [27]. Femoral shaft fractures have also been associated with prolonged bisphosphonate, particularly aledronate, use in women with osteoporosis [28,29]. Based on the published cases and our own experience, future studies of zoledronic acid in children should address the identification of risk factors for osteosclerosis.
Serum and urinary type I collagen telopeptides are surrogate markers of osteoclast activity and bone remodeling. Both decreased during the first three courses of Zoledronic acid in our study, consistent with suppression of osteoclast activity. Elevated bone resorption markers are common in multiple myeloma and metastatic breast cancer [30,31] and can correlate with tumor responses [31,5]. In this study, we demonstrate for the first time that such elevations are present in children with neuroblastoma and bone metastases. Normal type I collagen telopeptide values for children are poorly defined [32], limiting conclusions about the degree of elevation, relationship to tumor burden, and therapy response. However, these results support further studies to determine the utility of these and other surrogate bone activity markers with better-defined normal pediatric values (such as bone specific alkaline phosphatase, and dihydroxypyrimide [33]) in neuroblastoma bone metastases.
The elevated levels of IL-6 prior to treatment with zoledronic acid and cyclophosphamide are consistent with a previous report by Egler et al, who found significantly elevated peripheral blood IL-6 levels in neuroblastoma patients with high-risk features [34]. Similarly, elevations of IL-6 at diagnosis are associated with poor outcomes in patients with multiple myeloma [35], melanoma [36], prostate cancer [37] and other tumors. Our previous work indicates that this pleomorphic cytokine promotes growth and survival of neuroblastoma cells [38] and plays an important role in the tumor interactions with the bone microenvironment. However, tumor cells are not the source of IL-6; in the bone microenvironment, neuroblastoma cells stimulate IL-6 production from mesenchymal cells and monocytes [38]. The decrease in IL-6 levels after treatment with zoledronic acid and cyclophosphamide may be due to zoledronic acid’s interference in the bony micro-environment. Alternatively, the decrease may have resulted from systemic immune-modulation by low-dose cyclophosphamide. Our data strongly support further investigation of this cytokine as both a biomarker and potential therapeutic target.
Elevated sIL-6R serum levels have been reported in multiple myeloma, breast cancer, colon cancer, non-Hodgkin lymphoma and hepatocellular carcinoma [39,40] and typically correlated with advanced disease stage and poor outcome [41]. sIL-6R may orginate from either tumor cells or stromal and inflammatory cells. In colon and breast cancer, sIL-6R is released by polymorphonuclear cells and peripheral blood mononuclear cells [42,43]. Multiple myeloma and prostate cancer cells also express high levels of IL-6R surface membrane bound receptors that could be shed by proteases produced in the tumor microenvironment [44]. We have recently reported that sIL-6R is not produced by neuroblastoma cells [38] and preliminary studies in the laboratories of YDC and RCS (personal communication, R.C. Seeger) indicate that sIL-6R is primarily expressed by peripheral blood monocytes. sIL-6R levels from newly diagnosed neuroblastoma patients with high-risk features tended to be lower than normal controls in the report by Egler et al. [34]. In our study, patient sIL-6R levels were higher than normal children and did not significantly change with treatment. Although the reason for this difference is presently unclear, the patient populations differed since Egler et al, performed studies in newly diagnosed and untreated patients, and our study was in patients with refractory or relapsed tumor after prior intensive chemotherapy. The tumor microenvironments may differ significantly between these groups. This aspect is currently under further investigation.
In summary, the combination of zoledronic acid every 28 days with oral metronomic cyclophosphamide (25 mg/m2/day) was well tolerated and associated with prolonged disease stability. The maximum tolerated dose of zoledronic acid based on this study was 4 mg/m2/dose, max 4 mg. Although 2 of 7 children had grade 3 hyposphosphatemia on this dose, the toxicity was self resolving and asymptomatic, and is therefore considered tolerable. This combination is well-suited to study with additional agents that may enhance its anti-tumor efficacy because of its favorable toxicity profile. These data have prompted a successor study in the NANT consortium adding the antiangiogenic agent, bevacizumab, to further target the microenvironment in order to inhibit neuroblastoma progression.
Acknowledgments
Support: National Cancer Institute CA81403, Children’s Neuroblastoma Cancer Foundation, Dougherty Family Foundation, Alex’s Lemonade Stand Foundation, Evan Dunbar Memorial Foundation, and Neuroblastoma Children’s Cancer Society, National Institutes of Health, M01-RR00188, General Clinical Research Center
Footnotes
The results of this study have been previously presented at ASCO 2009 meeting (Proc ASCO 27: No15S (May 20 Supplement), 2009: 10022
References
- 1.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J.Clin.Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green JR, Clezardin P. Mechanisms of bisphosphonate effects on osteoclasts, tumor cell growth, and metastasis. Am J Clin Oncol. 2002;25:S3–S9. doi: 10.1097/00000421-200212001-00002. [DOI] [PubMed] [Google Scholar]
- 3.Hatoum HT, Lin SJ, Smith MR, Barghout V, Lipton A. Zoledronic acid and skeletal complications in patients with solid tumors and bone metastases: analysis of a national medical claims database. Cancer. 2008;113:1438–1445. doi: 10.1002/cncr.23775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91:1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J.Natl.Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 6.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N.Engl.J.Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 7.McWilliams NB, Hayes FA, Green AA, et al. Cyclophosphamide/doxorubicin vs. cisplatin/teniposide in the treatment of children older than 12 months of age with disseminated neuroblastoma: a Pediatric Oncology Group Randomized Phase II study. Med.Pediatr.Oncol. 1995;24:176–180. doi: 10.1002/mpo.2950240307. [DOI] [PubMed] [Google Scholar]
- 8.Green AA, Hayes FA, Hustu HO. Sequential cyclophosphamide and doxorubicin for induction of complete remission in children with disseminated neuroblastoma. Cancer. 1981;48:2310–2317. doi: 10.1002/1097-0142(19811115)48:10<2310::aid-cncr2820481029>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.Browder T, Butterfield CE, Kraling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 10.Kerbel RS, Viloria-Petit A, Klement G, Rak J. ‘Accidental’ anti-angiogenic drugs. anti-oncogene directed signal transduction inhibitors and conventional chemotherapeutic agents as examples. Eur.J.Cancer. 2000;36:1248–1257. doi: 10.1016/s0959-8049(00)00092-7. [DOI] [PubMed] [Google Scholar]
- 11.Glode LM, Barqawi A, Crighton F, Crawford ED, Kerbel R. Metronomic therapy with cyclophosphamide and dexamethasone for prostate carcinoma. Cancer. 2003;98:1643–1648. doi: 10.1002/cncr.11713. [DOI] [PubMed] [Google Scholar]
- 12.Colleoni M, Rocca A, Sandri MT, et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann.Oncol. 2002;13:73–80. doi: 10.1093/annonc/mdf013. [DOI] [PubMed] [Google Scholar]
- 13.Peng H, Sohara Y, Moats RA, et al. The activity of zoledronic Acid on neuroblastoma bone metastasis involves inhibition of osteoclasts and tumor cell survival and proliferation. Cancer Res. 2007;67:9346–9355. doi: 10.1158/0008-5472.CAN-06-4508. [DOI] [PubMed] [Google Scholar]
- 14.Le TC, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J.Natl.Cancer Inst. 2009;101:708–720. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen LS, Gordon DH, Dugan W, Jr., et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer. 2004;100:36–43. doi: 10.1002/cncr.11892. [DOI] [PubMed] [Google Scholar]
- 16.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J.Natl.Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Matthay KK, Edeline V, Lumbroso J, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J.Clin.Oncol. 2003;21:2486–2491. doi: 10.1200/JCO.2003.09.122. [DOI] [PubMed] [Google Scholar]
- 19.Skerjanec A, Berenson J, Hsu C, et al. The pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with varying degrees of renal function. J.Clin.Pharmacol. 2003;43:154–162. doi: 10.1177/0091270002239824. [DOI] [PubMed] [Google Scholar]
- 20.Hogler W, Yap F, Little D, et al. Short-term safety assessment in the use of intravenous zoledronic acid in children. J.Pediatr. 2004;145:701–704. doi: 10.1016/j.jpeds.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 21.Zeitlin L, Rauch F, Plotkin H, Glorieux FH. Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta types I, III, and IV. Pediatrics. 2003;111:1030–1036. doi: 10.1542/peds.111.5.1030. [DOI] [PubMed] [Google Scholar]
- 22.Falk MJ, Heeger S, Lynch KA, et al. Intravenous bisphosphonate therapy in children with osteogenesis imperfecta. Pediatrics. 2003;111:573–578. doi: 10.1542/peds.111.3.573. [DOI] [PubMed] [Google Scholar]
- 23.Brumsen C, Hamdy NA, Papapoulos SE. Long-term effects of bisphosphonates on the growing skeleton. Studies of young patients with severe osteoporosis. Medicine (Baltimore) 1997;76:266–283. doi: 10.1097/00005792-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int. 2008;74:1385–1393. doi: 10.1038/ki.2008.356. [DOI] [PubMed] [Google Scholar]
- 25.Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 26.Dodson TB. Intravenous bisphosphonate therapy and bisphosphonate-related osteonecrosis of the jaws. J.Oral Maxillofac.Surg. 2009;67:44–52. doi: 10.1016/j.joms.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Whyte MP, McAlister WH, Novack DV, et al. Bisphosphonate-induced osteopetrosis: novel bone modeling defects, metaphyseal osteopenia, and osteosclerosis fractures after drug exposure ceases. J.Bone Miner.Res. 2008;23:1698–1707. doi: 10.1359/jbmr.080511. [DOI] [PubMed] [Google Scholar]
- 28.Lenart BA, Neviaser AS, Lyman S, et al. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos.Int. 2009;20:1353–1362. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J.Orthop.Trauma. 2008;22:346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 30.Costa L, Demers LM, Gouveia-Oliveira A, et al. Prospective evaluation of the peptide-bound collagen type I cross-links N-telopeptide and C-telopeptide in predicting bone metastases status. J.Clin.Oncol. 2002;20:850–856. doi: 10.1200/JCO.2002.20.3.850. [DOI] [PubMed] [Google Scholar]
- 31.Lipton A, Costa L, Ali S, Demers L. Use of markers of bone turnover for monitoring bone metastases and the response to therapy. Semin.Oncol. 2001;28:54–59. doi: 10.1016/s0093-7754(01)90233-7. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson SA. Vitamin D status and bone biomarkers in childhood cancer. Pediatr.Blood Cancer. 2008;50:479–482. doi: 10.1002/pbc.21406. [DOI] [PubMed] [Google Scholar]
- 33.Tuchman S, Thayu M, Shults J, et al. Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease. J.Pediatr. 2008;153:484–490. doi: 10.1016/j.jpeds.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egler RA, Burlingame SM, Nuchtern JG, Russell HV. Interleukin-6 and soluble interleukin-6 receptor levels as markers of disease extent and prognosis in neuroblastoma. Clin.Cancer Res. 2008;14:7028–7034. doi: 10.1158/1078-0432.CCR-07-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lauta VM. A review of the cytokine network in multiple myeloma: diagnostic, prognostic, and therapeutic implications. Cancer. 2003;97:2440–2452. doi: 10.1002/cncr.11072. [DOI] [PubMed] [Google Scholar]
- 36.Soubrane C, Rixe O, Meric JB, Khayat D, Mouawad R. Pretreatment serum interleukin-6 concentration as a prognostic factor of overall survival in metastatic malignant melanoma patients treated with biochemotherapy: a retrospective study. Melanoma Res. 2005;15:199–204. doi: 10.1097/00008390-200506000-00009. [DOI] [PubMed] [Google Scholar]
- 37.George DJ, Halabi S, Shepard TF, et al. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: results from cancer and leukemia group B 9480. Clin.Cancer Res. 2005;11:1815–1820. doi: 10.1158/1078-0432.CCR-04-1560. [DOI] [PubMed] [Google Scholar]
- 38.Ara T, Song L, Shimada H, et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 2009;69:329–337. doi: 10.1158/0008-5472.CAN-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannitrapani L, Cervello M, Soresi M, et al. Circulating IL-6 and sIL-6R in patients with hepatocellular carcinoma. Ann.N.Y.Acad.Sci. 2002;963:46–52. doi: 10.1111/j.1749-6632.2002.tb04093.x. [DOI] [PubMed] [Google Scholar]
- 40.Kovacs E. Investigation of interleukin-6 (IL-6), soluble IL-6 receptor (sIL-6R) and soluble gp130 (sgp130) in sera of cancer patients. Biomed.Pharmacother. 2001;55:391–396. doi: 10.1016/s0753-3322(01)00079-8. [DOI] [PubMed] [Google Scholar]
- 41.Knupfer H, Preiss R. sIL-6R: more than an agonist? Immunol.Cell Biol. 2008;86:87–91. doi: 10.1038/sj.icb.7100113. [DOI] [PubMed] [Google Scholar]
- 42.Dowdall JF, Winter DC, Andrews E, et al. Soluble interleukin 6 receptor (sIL-6R) mediates colonic tumor cell adherence to the vascular endothelium: a mechanism for metastatic initiation? J.Surg.Res. 2002;107:1–6. doi: 10.1006/jsre.2001.6222. [DOI] [PubMed] [Google Scholar]
- 43.Jablonska E. Release of soluble IL-6 receptor (IL-6sR) in comparison with release of soluble TNF receptors (sTNF-Rs) by PMNs and WBC derived from breast cancer patients. Cancer Lett. 1997;119:79–85. doi: 10.1016/s0304-3835(97)00256-5. [DOI] [PubMed] [Google Scholar]
- 44.Saily M, Koistinen P, Savolainen ER. The soluble form of interleukin-6 receptor modulates cell proliferation by acute myeloblastic leukemia blast cells. Ann.Hematol. 1999;78:173–179. doi: 10.1007/s002770050496. [DOI] [PubMed] [Google Scholar]


