Abstract
Restrictive dermopathy (RD) results in stillbirth or early neonatal death. RD is characterized by prematurity, intrauterine growth retardation, fixed facial expression, micrognathia, mouth in the “o” position, rigid and tense skin with erosions and denudations and multiple joint contractures. Nearly all 25 previously reported neonates with RD had homozygous or compound heterozygous null mutations in the ZMPSTE24 gene. Here, we report 3 new cases of RD; all died within three weeks of birth. One of them had a previously reported homozygous c.1085dupT (p.Leu362PhefsX19) mutation, a second case had a novel homozygous c.1020G>A (p.Trp340X) null mutation in ZMPSTE24, and finally, a stillborn with features of RD except for the presence of tapering rather than rounded, bulbous digits, harbored no disease-causing mutations in LMNA or ZMPSTE24. In the newborn with a novel ZMPSTE24 mutation, unique features included butterfly-shaped thoracic 5th vertebra and the bulbous appearance of the distal clavicles. Skin biopsies from both the stillborn fetus and the newborn with c.1020G>A ZMPSTE24 mutation showed absence of elastic fibers throughout the dermis. This report provides evidence of genetic heterogeneity among RD and concludes that there may be an additional locus for RD which remains to be identified.
Keywords: Restrictive dermopathy, ZMPSTE24, Lamin A/C, mandibuloacral dysplasia
INTRODUCTION
Restrictive dermopathy (RD; OMIM 275210) results in stillbirth or early neonatal death and is characterized by prematurity, intrauterine growth retardation (IUGR), fixed facial expression, micrognathia, mouth in the “o” position, rigid and tense skin with erosions and denudations, and multiple joint contractures. Nearly all neonates with RD have homozygous or compound heterozygous null mutations in the zinc metalloproteinase (ZMPSTE24) gene (1–7). ZMPSTE24 is critical for post-translational proteolytic cleavage of carboxy terminal residues of prelamin A in two steps to form mature lamin A (8), an integral nuclear lamina protein encoded by LMNA gene.
Thus far, 25 patients with RD have been reported to harbor ZMPSTE24 mutations (1–7, 9–11), but many of them lacked detailed phenotypic information (4, 5). Here, we report clinical features and genotyping of 3 new cases of RD.
Patients and Methods
A written informed consent was obtained from the parents of RD 600.3 and RD 500.3, and the study was approved by the Institutional Review Board at UT Southwestern. A written consent for autopsy was obtained from the parents of RD 200.3 at the Sanjay Gandhi Post Graduate Institute of Medical Sciences in Lucknow, India.
RD 600.3
This female infant was born via vaginal delivery at 34 weeks gestation to nonconsanguineous, healthy parents (Fig. 1) with one previous stillbirth who had features of restrictive dermopathy. A prenatal diagnosis of 70% mosaic trisomy 20 was made. Prenatal ultrasound revealed a short chest and small stomach. Polyhydramnios was noted early on, but in the few weeks prior to delivery, oligohydramnios was present. Mother underwent oxytocin augmentation for nonreassuring fetal heart tracings. The birth weight was 1615 g. Apgar scores were 1 at 1 minute and 5 at 5 minutes. The baby was immediately resuscitated, intubated and transferred to the neonatal intensive care unit.
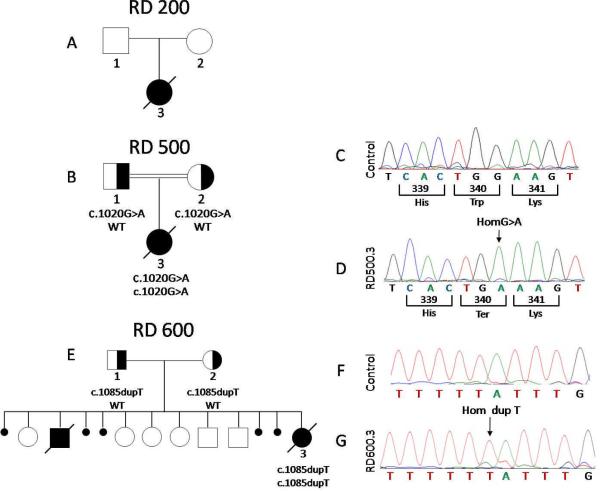
Figure 1. Pedigrees and chromatograms of patients with restrictive dermopathy.
A. RD 200 Pedigree. No disease-causing mutations were found in either ZMPSTE24 or LMNA
B. RD 500 Pedigree. Affected individual with homozygous c.1020G>A (p.W340X) mutation of ZMPSTE24 is shown as filled black symbol, whereas heterozygous subjects are shown as half filled symbols. The parents of the affected subject were second cousins. WT indicates wild type allele.
C,D: Chromatograms from direct sequencing of exon 8 of ZMPSTE24 gene showing normal sequence from a control individual (C), and from the proband RD 500.3 showing homozygous c.1020G>A mutation (D)
E. RD 600 Pedigree. Affected individual with homozygous c.1085dupT mutation of ZMPSTE24 is shown as filled black symbol, whereas heterozygous subjects are shown as half filled symbols. A previous male fetus stillborn at 33 weeks gestation was described as having features consistent with RD. Five spontaneous abortions occurred between 11–12 wks gestation.
F,G: Chromatograms from direct sequencing of exon 9 of ZMPSTE24 gene showing normal sequence from a control individual (F) and from the proband RD 600.3 showing homozygous c.1085dupT mutation (G).
Upon examination, she had tight, fragile skin with prominent superficial vasculature. An 8–9 cm neck laceration extended circumferentially around the neck from the left ear to past the right ear towards the back of the head. It was estimated to be 8–10 mm deep and had outward curling ragged edges along the entire length. The right external jugular vein appeared exposed. There was minimal range of motion of the large and small joints. Her mouth was open and tightly fixed in an O-shaped position. There was almost no nasal bridge with a minimal pointed tip. Hands were small with relatively long fingers, and nails were unremarkable. She was also noted to have large fontanelles, low-set and posteriorly rotated ears, downslanting eyes, and micrognathia. An echocardiogram showed a small patent ductus arteriosus shunting left to right and radiographs showed short dysplastic clavicles. After a prolonged hospital course requiring mechanical ventilation, antibiotics for severe sepsis, total parental nutrition, and nonoligouric renal insufficiency, the infant died on day-of-life 16.
RD 500.3
This female infant was born via vaginal delivery at 33 weeks gestation to consanguineous parents of Mexican origin (Fig. 1). The mother had preterm premature rupture of membranes. An abdominal sonogram showed IUGR. Labor was complicated by severe preeclampsia requiring magnesium sulfate therapy and oxytocin augmentation for non-reassuring fetal heart tracings. Upon delivery, the baby was cyanotic and hypotonic. The birth weight was 1192 g. Apgar scores were 1 at 1 minute and 4 at 5 minutes. The baby was immediately resuscitated, intubated and transferred to the neonatal intensive care unit.
Upon examination, she had thin, taught, translucent skin with prominent superficial vasculature. Extremities were rigid with flexural joint contractures at the elbows, fingers, knees, and ankles and extensor contractures of the wrists. Her mouth was in a fixed O-shaped position. Fingers showed rounded and bulbous tips consistent with acro-osteolysis. She was also noted to have enlarged anterior fontanelles, low-set and hypoplastic ears, absent eyelashes and eyebrows, eyelids that did not close completely, small pinched nose, micrognathia, and multiple skin erosions on the chest and extensor surfaces.
The patient's karyotype was 46, XX. Her hemoglobin and hematocrit were 12.0 g/dL [normal 14.5–22.5] and 36.1% [normal 45.0–67.0], respectively, requiring transfusion with packed red blood cells. Arterial blood pH was 6.96. The remainder of the blood chemistries and blood counts were normal at birth. Blood urea nitrogen was 18 mg/dL and glucose was 126 mg/dL. Her serum triglycerides were 130 mg/dL [normal 50–150] on day-of-life 4 and 51 mg/dL on day-of-life 5.
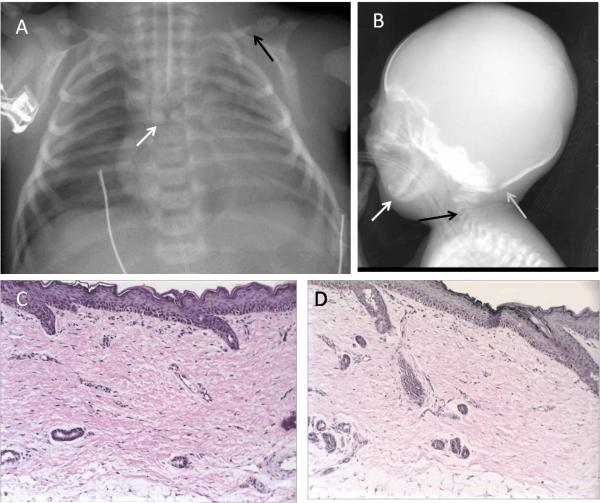
A chest radiograph showed butterfly vertebra at the thoracic 5 level (Fig. 2A). Clavicles were dysmorphic with a pseudoarthrosis at the juncture of the middle and distal one-thirds with the distal portions bulbous in appearance (Fig. 2A). Radiograph of the skull revealed soft tissue swelling in the parietal regions, prominent sutures, micrognathia, and hypoplastic cervical vertebral bodies (Fig. 2B). Echocardiogram showed moderate patent ductus arteriosus and patent foramen ovale. A right thigh skin biopsy showed the epidermis and dermis to be slightly attenuated, and the dermis showed marked fibrosis and parallel collagen bundles (Fig. 2C). An elastic tissue stain, Verhoeff van Gieson, showed complete absence of elastic fibers throughout the dermis (Fig. 2D). The infant was maintained on mechanical ventilation, parenteral nutrition, antibiotics and pain medications and died on day-of-life 6.
Figure 2. Radiographs and histopathology of skin biopsy of RD 500.3.
A. Anterior-posterior chest radiograph of patient RD 500.3 shows a butterfly vertebra at the thoracic 5 level (white arrow). Clavicles were dysmorphic with a pseudoarthrosis at the juncture of the middle and distal one-thirds with the distal portions bulbous in appearance (black arrow).
B. Lateral radiograph of the skull of patient RD 500.3 reveals prominent sutures (gray arrow), micrognathia (white arrow), and hypoplastic cervical vertebral bodies (black arrow).
C. Hematoxylin and eosin stain of skin biopsy shows the epidermis and dermis to be slightly attenuated, and the dermis shows marked fibrosis and parallel collagen bundles.
D. An elastic tissue stain, Verhoeff van Gieson, shows complete absence of elastic fibers throughout the dermis.
RD 200.3
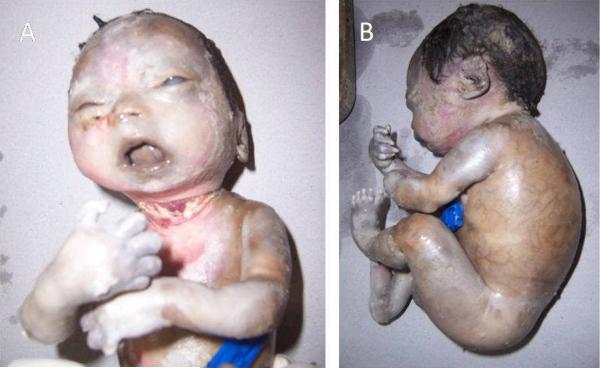
This was a stillborn male fetus born to Asian Indian primigravada mother at 32 weeks gestation. Prenatal period was significant for oligohydramnios with premature rupture of membranes and preterm delivery. Upon birth, the fetus was noted to have tight skin with contractures in the neck, elbows, hip joints, and digits in hands and feet. Post-mortem examination showed the fetus weighed 750 grams and was 48 cm long. Pinched nose, posteriorly rotated ears, tapering of the digits of hands and feet, superficial vasculature, absent eyelashes and eyebrows, and skin erosions were also noted (Fig. 3). The heart, lungs, liver, kidneys, gastrointestinal tract, and genitourinary tract looked normal. The brain tissue was autolysed. The placenta was normal. Skin histology revealed thin epidermis and dermis. Epidermis displayed few variably sized keratohyaline granules. The dermis revealed loose fibrous connective tissue without any elastic fibers. The karyotype was 46, XY.
Figure 3. Clinical features of RD 200.3.
A. The infant had tight skin with skin breakdown in the neck, contractures in the elbows and digits in hands, and mouth in fixed “o” position. A large circumferential neck laceration can be appreciated.
B. Prominent superficial vessels are seen in the posterior and lateral truncal region. There is normal number of digits on hands and feet, but fingers appear tapered. Scalp hair in the temporal and occipital regions appears normal.
METHODS
Mutational Analysis
The exons and splice-site junctions of lamin A/C (LMNA) and zinc metalloproteinase (ZMPSTE24) genes were sequenced using genomic DNA as previously described (12, 13). In addition, approximately 2 kb of the promoter regions of LMNA and ZMPSTE24 were sequenced in RD200.3 (primers available on request)
RESULTS
A homozygous mutation in ZMPSTE24, c.1085dupT (p.Leu362PhefsX19), was found in RD 600.3. Both the parents harbored heterozygous c.1085dupT mutation in ZMPSTE24. No disease causing mutations were observed in sequencing of LMNA.
A homozygous missense mutation in ZMPSTE24, c.1020G>A (p.Trp340X), was found in RD 500.3. This patient also harbored a homozygous SNP, c.IVS5+18T>G (rs16827109), in ZMPSTE24. Both the parents had heterozygous c.1020G>A mutation in ZMPSTE24. No disease causing mutations were observed in sequencing of LMNA.
No disease causing mutations were observed in RD 200.3 upon sequencing of either LMNA or ZMPSTE24 genes. The patient harbored a heterozygous c.1698C>T, p.His566His [rs4641 with minor allele frequency (MAF) in Asian subjects of 17–27%] SNP in LMNA and the following SNPs in ZMPSTE24: homozygous c.IVS5-50T>G (rs6677717, allele frequency not known), heterozygous c.651T>C, p.Asp217Asp (rs2076697, MAF 17–26%) and heterozygous c.IVS5+18T>G (rs16827109, MAF 9–27%). Sequencing of the 2.30 kb LMNA promoter region revealed the following variants: homozygous c.−1214T>C (rs2485661; MAF 46%) and homozygous c.−1242T>C (rs2485662; MAF 9–12%). The sequencing of the 2.15 kb ZMPSTE24 promoter region revealed the following variants: heterozygous c.−326G>A (rs3775483; MAF 11%), heterozygous c.−1269C>G (rs7548758, MAF 7–50%), heterozygous c.−1355G>C (not listed) and homozygous c.−2036T>C (rs9326050, MAF 30–47%). All nucleotides are numbered from the first nucleotide of translation start codon ATG.
DISCUSSION
We describe detailed phenotype and genetic analysis of three cases of RD. Two newborns were found to have homozygous mutations in ZMPSTE24, while one stillborn male did not have any disease causing mutation in either ZMPSTE24 or LMNA. Most of the features of our cases, such as IUGR, joint contractures, skin breakdown, micrognathia, and acro-osteolysis, are typical of RD. Histologically, skin findings, including parallel collagen bundles and an absence of elastic fibers, are also consistent with previously reported cases of RD confirmed to be due to ZMPSTE24 mutations (9). However, butterfly thoracic 5 vertebra has not been previously noted. Also, although clavicles are often reported as hypoplastic or dysmorphic (2, 3, 9), the bulbous appearance of the distal portion of the clavicles in RD500.3 is a novel observation.
Homozygous or compound heterozygous ZMPSTE24 mutations were first reported in 10 newborns with RD by Navarro et al. in 2005 (4). Since then, near all other cases of RD have also harbored null mutations in ZMPSTE24 (7). Interestingly, more than 50% of the patients with RD and ZMPSTE24 mutations harbored a homozygous c.1085dupT mutation (1, 3–5, 10) while an additional 17% were compound heterozygotes involving a c.1085dupT mutation on one allele (4, 5). One of our patients had homozygous 1085dupT mutation while another had a novel homozygous c.1020G>A (p.Trp340X) mutation.
ZMPSTE24 mutations also cause autosomal recessive mandibuloacral dysplasia (MAD; OMIM 248370 and 608612) (13–16). In contrast to RD, MAD patients with ZMPSTE24 mutations harbor a null mutation on one allele and a missense mutation on the other allele (13–16). We have previously documented, using a yeast halo assay, that null mutations have no ZMPSTE24 activity whereas the missense mutants have partial loss or nearly normal activity (13, 16, 17). Thus, the variable manifestations of the two disorders could be explained by varying amounts of prelamin A accumulation.
Only eight cases of MAD due to ZMPSTE24 mutations have been reported thus far (13–16, 18). Both MAD and RD patients with ZMPSTE24 mutations manifest prematurity, micrognathia, small pinched nose, sparse or absent hair, enlarged fontanelles, dysplastic clavicles and acro-osteolysis (Table 1). However, MAD due to ZMPSTE24 deficiency is not lethal within the newborn period with death reported at ages 3, 28, and 37 years (13–15). MAD patients do not present with IUGR, fixed facial expression, mouth in the “o” position, or skin erosions and denudations (Table 1). Development of contractures and joint stiffness is also delayed in MAD patients.
Table 1.
Comparison of clinical features of previously published and our patients with restrictive dermopathy and mandibuloacral dysplasia due to ZMPSTE24 mutations.
| Finding | Previously reported RD cases (n=25) (1–7, 9–11) | Our cases | ZMPSTE24 MAD cases (n=8) (13–18) | ||
|---|---|---|---|---|---|
| RD 600.3 | RD 500.3 | RD 200.3 | |||
| IUGR | 12/14 (86%) | + | + | NA | 0 |
| Birth weiqht: Normal: Low (<2500 g) : Very low(<1500 g): Extremely low (<1000 g) | 0:0:7:0 (7) | 1615 g | 1192 g | 750 g | 2:2:0:0 (4) |
| Birth at ≤ 33 weeks gestation | 16/17 (94%) | − | + | + | 1/5 (20%) |
| Tight, thin, translucent, or shiny skin | 20/20 (100%) | + | + | + | 6/6 (100%) |
| Skin erosions | 19/19 (100%) | + | + | + | 0 |
| Micrognathia | 17/17 (100%) | + | + | NA | 6/6 (100%) |
| Small pinched nose | 7/7 (100%) | + | + | NA | 4/4 (100%) |
| Mouth in the o position | 19/19 (100%) | + | + | NA | 0 |
| Sparse or absent hair, eyelashes or eyebrows | 4/4 (100%) | NA | + | + | 3/3 (100%) |
| Low-set dysplastic ears | 6/6 (100%) | + | − | NA | 0 |
| Enlarged fontanelles | 7/7 (100) | + | + | NA | 4/4 (100%) |
| Contractures and rigid posture | 20/20 (100%) | + | + | + | 5/5 (100%) |
| Prominent superficial vasculature | 6/6 (100%) | + | + | + | 2/2 (100%) |
| Dysplastic / hypoplastic clavicles | 4/4 (100) | + | + | NA | 5/5 (100%) |
| Bulbous appearance of distal clavicle | 0 | NA | + | NA | 0 |
| Butterfly vertebrae | 0/1 (0) | NA | + | NA | 0 |
| Death in neonatal period | 14/16 (88%) | + | + | + | 0 |
| Partial lipodystrophy | NA | NA | NA | NA | 5/5 (100%) |
NA: information not available, IUGR, intrauterine growth retardation
Navarro et al. (19) have reported heterozygous abnormal splice inducing mutations (c.IVS11+1G>A and c.1824C>T) in the LMNA gene in two patients age 5 months and 6 months with presumed diagnosis of RD. However, both these mutations cause Hutchinson-Gilford progeria syndrome (HGPS) (20, 21) and therefore, these may have been misdiagnosed. More recently, a newborn with RD was reported to carry a heterozygous c.1821G>A, (p.Val607Val) LMNA mutation (22); however, whether this mutation induces an alternative splicing was not demonstrated.
The phenotype of the stillborn fetus was consistent with a diagnosis of RD except for the presence of tapering rather than rounded, bulbous digits. The lack of mutations in LMNA and ZMPSTE24 in this fetus suggests additional loci for RD. The presence of two heterozygous SNPs in ZMPSTE24 reduces the likelihood of a large deletion of one allele. Regardless, a heterozygous deletion or c.−1355G>C promoter region variant in ZMPSTE24, without a concomitant null mutation, are not sufficient to cause RD by themselves. Furthermore, we did not find any disease-causing nucleotide alterations in the proximal (approximately 2 kb) promoter regions of LMNA and ZMPSTE24. However, lack of RNA precludes us to determine if this patient harbored any homozygous cryptic intronic mutation in ZMPSTE24. We were also unable to exclude the possibility for small deletions in LMNA.
There is a significant risk of recurrence of RD in subsequent pregnancies. As illustrated in our cases, early diagnosis of RD is difficult given that most affected fetuses have an unremarkable prenatal course. Ultrasound findings of polyhydramnios, decreased fetal movements, and growth retardation are suggestive of RD, but are nonspecific and late findings. A small fixed open mouth on ultrasound is also a late finding (23) and as such is not useful in prenatal genetic counseling. Skin biopsy at 20 weeks gestation has been attempted (24), but failed to predict the presence of RD likely because fetal skin development is not complete by then. Thus, the best option for early prenatal diagnosis is genetic testing of DNA obtained via chorionic villus sampling or amniocentesis. This would be mostly done in families who have previously had newborns with RD. In one such case (2), prenatal diagnosis revealed the presence of a homozygous ZMPSTE24 mutation; the pregnancy was subsequently terminated.
In conclusion, we report a novel and a previously reported homozygous null mutation in ZMPSTE24 in two newborns with RD. We also report a stillborn fetus with features of RD but no mutations in LMNA or ZMPSTE24, suggesting an additional locus may exist for RD.
Acknowledgments
We would like to acknowledge the following physicians who participated in the care of RD 500.3: Lewis Waber, Ponciano Cruz Jr, Timothy Brannon, and Antoanella Calame. We thank Sarah Masood and Crystal Kittisopikul for help with illustrations and mutational screening. This work was supported by the National Institutes of Health grants R01-DK54387, Southwest Medical Foundation and the Indian Council of Medical Research.
Footnotes
Conflicts of interest: Zahid Ahmad: none Shubha Phadke: none Ellen Arch: none Jonathan Glass: none Anil K. Agarwal: none Abhimanyu Garg: none
REFERENCES
- 1.Kariminejad A, Goodarzi P, Thanh Huong le T, et al. Restrictive dermopathy. Molecular diagnosis of restrictive dermopathy in a stillborn fetus from a consanguineous Iranian family. Saudi Med J. 2009;30:150–153. [PubMed] [Google Scholar]
- 2.Jagadeesh S, Bhat L, Suresh I, et al. Prenatal diagnosis of restrictive dermopathy. Indian Pediatr. 2009;46:349–351. [PubMed] [Google Scholar]
- 3.Morais P, Magina S, Ribeiro Mdo C, et al. Restrictive dermopathy--a lethal congenital laminopathy. Case report and review of the literature. Eur J Pediatr. 2009;168:1007–1012. doi: 10.1007/s00431-008-0868-x. [DOI] [PubMed] [Google Scholar]
- 4.Navarro CL, Cadinanos J, De Sandre-Giovannoli A, et al. Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of Lamin A precursors. Hum Mol Genet. 2005;14:1503–1513. doi: 10.1093/hmg/ddi159. [DOI] [PubMed] [Google Scholar]
- 5.Moulson CL, Go G, Gardner JM, et al. Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J Invest Dermatol. 2005;125:913–919. doi: 10.1111/j.0022-202X.2005.23846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sander CS, Salman N, van Geel M, et al. A newly identified splice site mutation in ZMPSTE24 causes restrictive dermopathy in the Middle East. Br J Dermatol. 2008;159:961–967. doi: 10.1111/j.1365-2133.2008.08772.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Kuo HH, Huang YC, et al. A case of restrictive dermopathy with complete chorioamniotic membrane separation caused by a novel homozygous nonsense mutation in the ZMPSTE24 gene. Am J Med Genet A. 2009;149A:1550–1554. doi: 10.1002/ajmg.a.32768. [DOI] [PubMed] [Google Scholar]
- 8.Sinensky M, Fantle K, Trujillo M, et al. The processing pathway of prelamin A. J Cell Sci. 1994;107(Pt 1):61–67. doi: 10.1242/jcs.107.1.61. [DOI] [PubMed] [Google Scholar]
- 9.Thill M, Nguyen TD, Wehnert M, et al. Restrictive dermopathy: a rare laminopathy. Arch Gynecol Obstet. 2008;278:201–208. doi: 10.1007/s00404-008-0676-6. [DOI] [PubMed] [Google Scholar]
- 10.Li C. Homozygosity for the common mutation c.1085dupT in the ZMPSTE24 gene in a Mennonite baby with restrictive dermopathy and placenta abruption. Am J Med Genet A. 152A:262–263. doi: 10.1002/ajmg.a.33163. [DOI] [PubMed] [Google Scholar]
- 11.Smigiel R, Jakubiak A, Esteves-Vieira V, et al. Novel frameshifting mutations of the ZMPSTE24 gene in two siblings affected with restrictive dermopathy and review of the mutations described in the literature. Am J Med Genet A. 152A:447–452. doi: 10.1002/ajmg.a.33221. [DOI] [PubMed] [Google Scholar]
- 12.Simha V, Agarwal AK, Oral EA, et al. Genetic and phenotypic heterogeneity in patients with mandibuloacral dysplasia-associated lipodystrophy. J Clin Endocrinol Metab. 2003;88:2821–2824. doi: 10.1210/jc.2002-021575. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal AK, Fryns JP, Auchus RJ, et al. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12:1995–2001. doi: 10.1093/hmg/ddg213. [DOI] [PubMed] [Google Scholar]
- 14.Shackleton S, Smallwood DT, Clayton P, et al. Compound heterozygous ZMPSTE24 mutations reduce prelamin A processing and result in a severe progeroid phenotype. J Med Genet. 2005;42:e36. doi: 10.1136/jmg.2004.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal AK, Zhou XJ, Hall RK, et al. Focal segmental glomerulosclerosis in patients with mandibuloacral dysplasia owing to ZMPSTE24 deficiency. J Investig Med. 2006;54:208–213. doi: 10.2310/6650.2006.05068. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi Y, Akagi M, Agarwal AK, et al. Severe mandibuloacral dysplasia caused by novel compound heterozygous ZMPSTE24 mutations in two Japanese siblings. Clin Genet. 2008;73:535–544. doi: 10.1111/j.1399-0004.2008.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denecke J, Brune T, Feldhaus T, et al. A homozygous ZMPSTE24 null mutation in combination with a heterozygous mutation in the LMNA gene causes Hutchinson-Gilford progeria syndrome (HGPS): insights into the pathophysiology of HGPS. Hum Mutat. 2006;27:524–531. doi: 10.1002/humu.20315. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad Z, Zakai E, Medne L, et al. Mandibuloacral Dysplasia due to Compound Heterozygous Mutations in ZMPSTE24. Endocrine Review. 2010;31:S795. doi: 10.1002/ajmg.a.33664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navarro CL, De Sandre-Giovannoli A, Bernard R, et al. Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum Mol Genet. 2004;13:2493–2503. doi: 10.1093/hmg/ddh265. [DOI] [PubMed] [Google Scholar]
- 20.Moulson CL, Fong LG, Gardner JM, et al. Increased progerin expression associated with unusual LMNA mutations causes severe progeroid syndromes. Hum Mutat. 2007;28:882–889. doi: 10.1002/humu.20536. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosque E. Complex case study: nursing care of an infant with restrictive dermopathy. J Perinat Neonatal Nurs. 2009;23:171–177. doi: 10.1097/JPN.0b013e3181a39211. [DOI] [PubMed] [Google Scholar]
- 23.van der Stege JG, van Straaten HL, van der Wal AC, et al. Restrictive dermopathy and associated prenatal ultrasound findings: case report. Ultrasound Obstet Gynecol. 1997;10:140–141. doi: 10.1046/j.1469-0705.1997.10020140.x. [DOI] [PubMed] [Google Scholar]
- 24.Hamel BC, Happle R, Steylen PM, et al. False-negative prenatal diagnosis of restrictive dermopathy. Am J Med Genet. 1992;44:824–826. doi: 10.1002/ajmg.1320440622. [DOI] [PubMed] [Google Scholar]