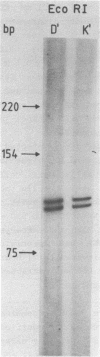
Abstract
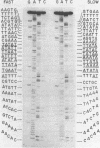
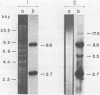
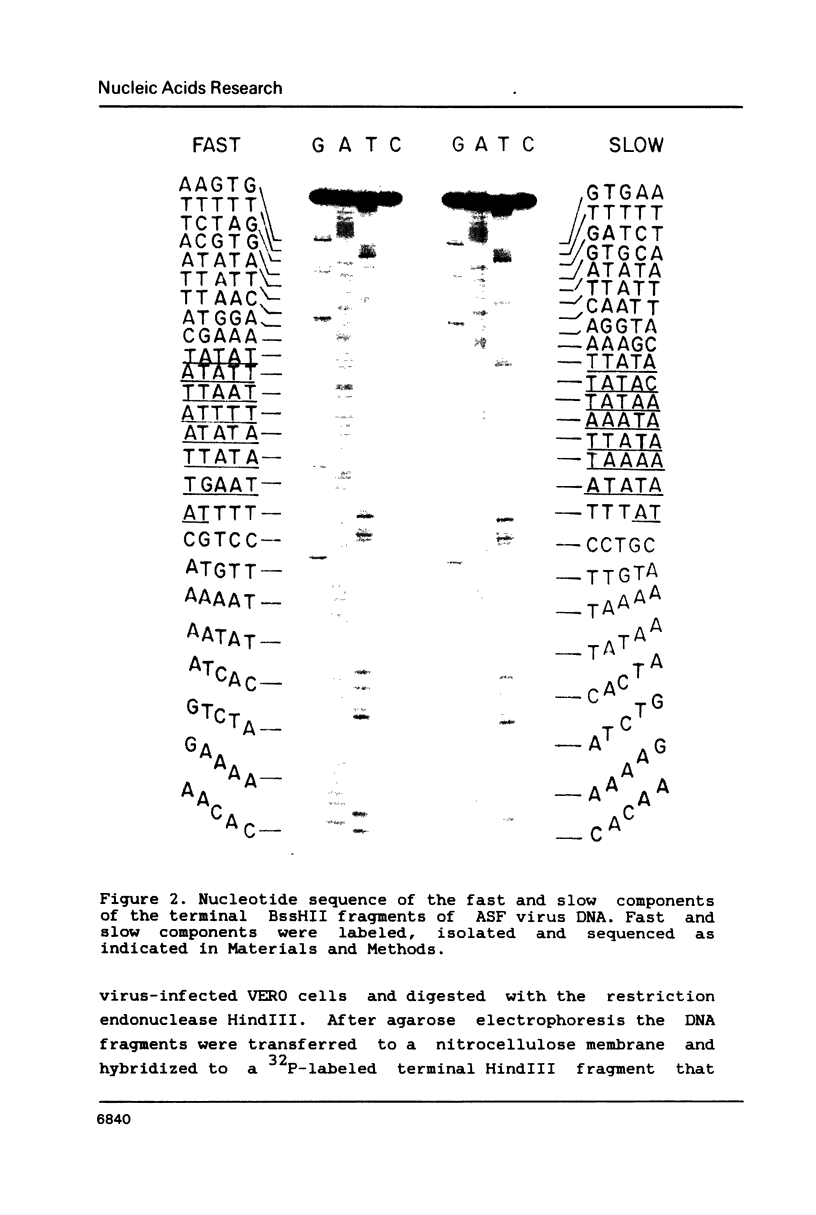
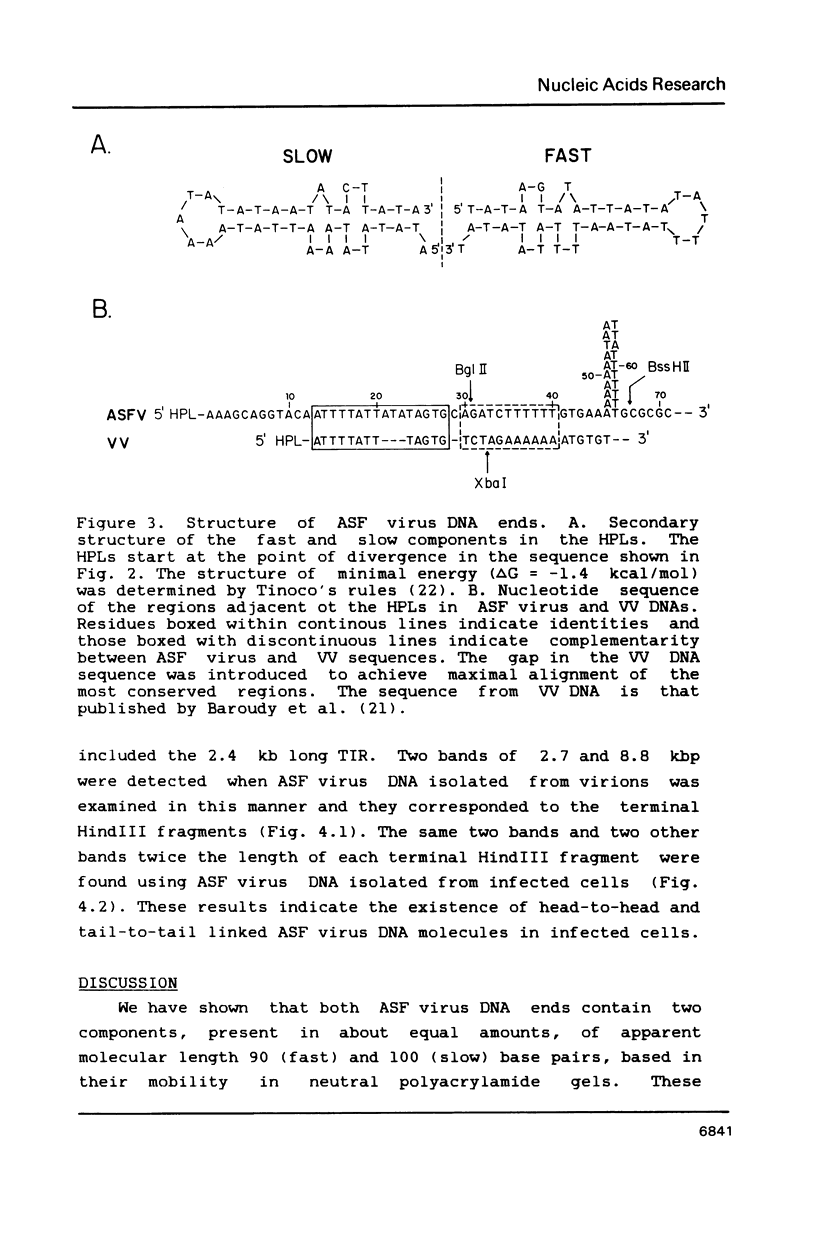
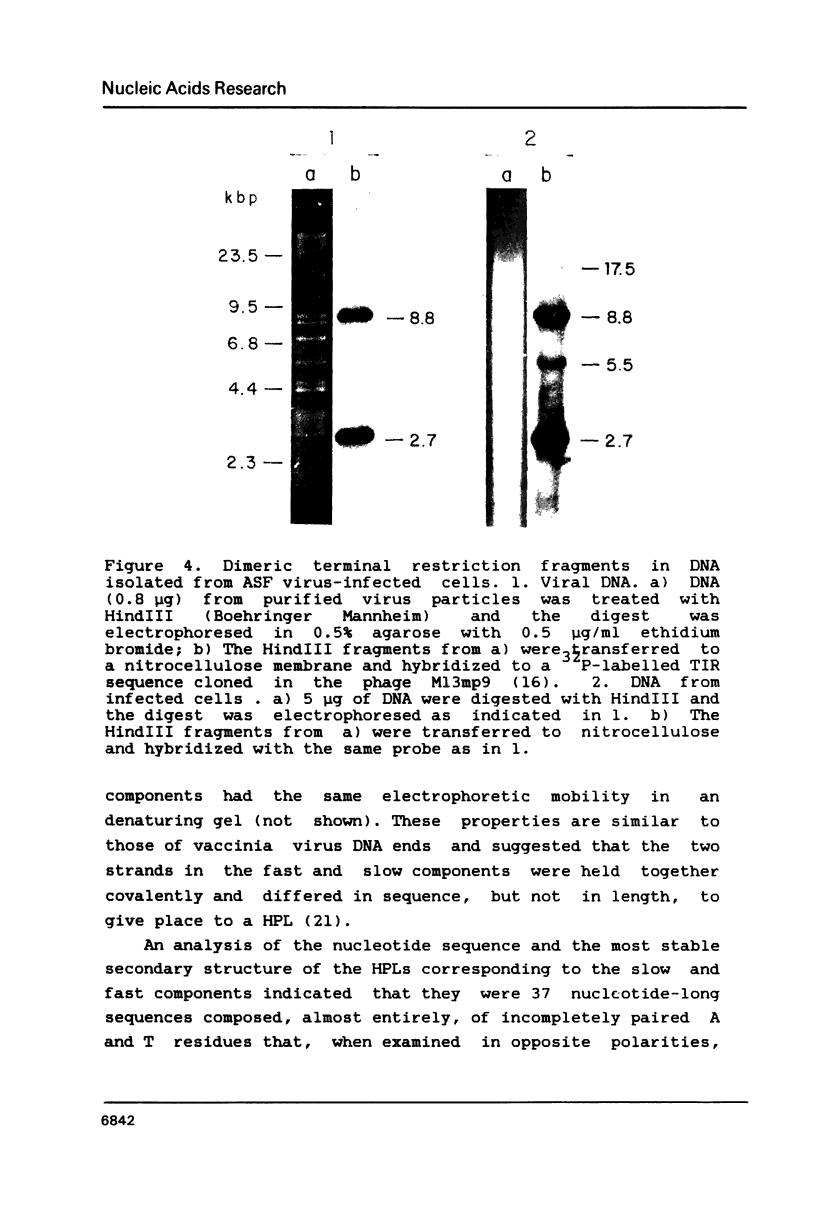
The ends of African swine fever virus genome are formed by a 37 nucleotide-long hairpin loop composed, almost entirely, of incompletely paired A and T residues. The loops at each DNA end were present in two equimolar forms that, when compared in opposite polarities, were inverted and complementary (flip-flop), as in the case of poxvirus DNA. The hairpin loops of African swine fever and vaccinia virus DNAs had no homology, but both DNAs had a 16 nucleotide-long sequence, close to the hairpin loops, with an homology of about 80%. An analysis of African swine fever virus replicating DNA showed head-to-head and tail-to-tail linked molecules that may be replicative intermediates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Blasco R., Ley V., Beloso A., Talavera A., Viñuela E. Restriction site map of African swine fever virus DNA. Virology. 1984 Mar;133(2):258–270. doi: 10.1016/0042-6822(84)90393-3. [DOI] [PubMed] [Google Scholar]
- Baroudy B. M., Venkatesan S., Moss B. Incompletely base-paired flip-flop terminal loops link the two DNA strands of the vaccinia virus genome into one uninterrupted polynucleotide chain. Cell. 1982 Feb;28(2):315–324. doi: 10.1016/0092-8674(82)90349-x. [DOI] [PubMed] [Google Scholar]
- Baroudy B. M., Venkatesan S., Moss B. Structure and replication of vaccinia virus telomeres. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):723–729. doi: 10.1101/sqb.1983.047.01.083. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. N., Brown F. Purification and physicochemical characteristics of African swine fever virus. J Gen Virol. 1976 Sep;32(3):509–518. doi: 10.1099/0022-1317-32-3-509. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Carrascosa A. L., Moreno M. A., Viñuela E. Titration of African swine fever (ASF) virus. J Gen Virol. 1976 Sep;32(3):471–477. doi: 10.1099/0022-1317-32-3-471. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Carrascosa A. L., Viñuela E. Isolation and properties of the DNA of African swine fever (ASF) virus. J Gen Virol. 1976 Sep;32(3):479–492. doi: 10.1099/0022-1317-32-3-479. [DOI] [PubMed] [Google Scholar]
- Kuznar J., Salas M. L., Viñuela E. DNA-dependent RNA polymerase in African swine fever virus. Virology. 1980 Feb;101(1):169–175. doi: 10.1016/0042-6822(80)90493-6. [DOI] [PubMed] [Google Scholar]
- Kuznar J., Salas M. L., Viñuela E. Nucleoside triphosphate phosphohydrolase activities in African swine fever virus. Arch Virol. 1981;69(3-4):307–310. doi: 10.1007/BF01317347. [DOI] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Ley V., Almendral J. M., Carbonero P., Beloso A., Viñuela E., Talavera A. Molecular cloning of African swine fever virus DNA. Virology. 1984 Mar;133(2):249–257. doi: 10.1016/0042-6822(84)90392-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Graves R. L. The mechanism of cytoplasmic orthopoxvirus DNA replication. Cell. 1981 Dec;27(2 Pt 1):391–401. doi: 10.1016/0092-8674(81)90422-0. [DOI] [PubMed] [Google Scholar]
- Ortín J., Enjuanes L., Viñuela E. Cross-links in African swine fever virus DNA. J Virol. 1979 Sep;31(3):579–583. doi: 10.1128/jvi.31.3.579-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Salas M. L., Kuznar J., Viñuela E. Effect of rifamycin derivatives and coumermycin A1 on in vitro RNA synthesis by African swine fever virus. Brief report. Arch Virol. 1983;77(1):77–80. doi: 10.1007/BF01314866. [DOI] [PubMed] [Google Scholar]
- Salas M. L., Kuznar J., Viñuela E. Polyadenylation, methylation, and capping of the RNA synthesized in vitro by African swine fever virus. Virology. 1981 Sep;113(2):484–491. doi: 10.1016/0042-6822(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Almendral J. M., Talavera A., Viñuela E. Terminal and internal inverted repetitions in African swine fever virus DNA. Virology. 1984 Mar;133(2):271–275. doi: 10.1016/0042-6822(84)90394-5. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Viñuela E. African swine fever virus. Curr Top Microbiol Immunol. 1985;116:151–170. doi: 10.1007/978-3-642-70280-8_8. [DOI] [PubMed] [Google Scholar]
- Wardley R. C., Hamilton F., Wilkinson P. J. The replication of virulent and attenuated strains of African swine fever virus in porcine macrophages. Arch Virol. 1979;61(3):217–225. doi: 10.1007/BF01318056. [DOI] [PubMed] [Google Scholar]