Abstract
Functional imaging research has identified fronto-parietal attention networks involved in visual search, with mixed evidence regarding whether different networks are engaged when the search target differs from distracters by a single (elementary) versus multiple (conjunction) features. Neural correlates of visual search, and their potential dissociation, were examined here using integrity of white matter connecting the fronto-parietal networks. The effect of aging on these brain-behavior relationships was also of interest. Younger and older adults performed a visual search task and underwent diffusion tensor imaging (DTI) to reconstruct two fronto-parietal (superior and inferior longitudinal fasciculus, SLF and ILF) and two midline (genu, splenium) white matter tracts. As expected, results revealed age-related declines in conjunction, but not elementary, search performance; and in ILF and genu tract integrity. Importantly, integrity of the SLF, ILF, and genu tracts predicted search performance (conjunction and elementary), with no significant age group differences in these relationships. Thus, integrity of white matter tracts connecting fronto-parietal attention networks contributes to search performance in younger and older adults.
Visual search refers to the ability to locate an object of interest (i.e., a target) among other elements in the visual environment (i.e., distracters). For example, it enables us to find an ingredient in the pantry or a friend in the crowd. In the laboratory, visual search tasks typically involve searching for a single target (e.g., the letter T) within an array of distracters (e.g., multiple letter Ls). Task difficulty can be manipulated by varying the number of items within the array (i.e., set size) or the similarity between the target and distracters (i.e., search type). For search type, the target may differ from distracters on a single feature (elementary search) or a combination of more than one feature (conjunction search). In general, slower and less accurate performance is observed when there are more distracter items to be searched (i.e., higher versus lower set sizes), especially when the target is less distinct from the distracters (i.e., conjunction versus elementary search types)(Wolfe, 1998).
These behavioral patterns have been attributed, at least in part, to attention processes that guide visual search (Egeth and Yantis, 1997; Theeuwes, 1993; Yantis, 2000). Top-down guidance of attention refers to situations in which a priori knowledge of target features is used to facilitate target identification. Bottom-up attention, on the other hand, is engaged when inherent features of the target draw attention. Based on initial observations that the effect of set size was present for conjunction search but absent for elementary search, researchers originally proposed a dissociation between attention processes involved in these distinct search conditions (e.g., Treisman and Gelade, 1980). That is, conjunction search was thought to rely on top-down processes that guided attention during a serial search of the array, whereas elementary search was proposed to rely on bottom-up processes operating in parallel that draw attention. However, under certain circumstances, set size effects are absent for conjunction search (e.g., Nakayama and Silverman, 1986) and present for elementary search (e.g., Carter, 1982), suggesting more of a continuum in the magnitude of set size effects for both search types (Wolfe et al., 1989). Thus, an alternative theory, gaining currency in recent literature, is that similar attention processes (top-down and bottom-up) may be engaged during both conjunction and elementary search.
Functional imaging research has identified different fronto-parietal networks underlying top-down and bottom-up attention processes (Corbetta and Shulman, 1998, 2002; Corbetta et al., 1995). Top-down, knowledge-driven shifts of attention call upon a dorsal fronto-parietal network that includes superior frontal (e.g., frontal eye field) and dorsal parietal (e.g., intraparietal sulcus) regions, whereas bottom-up, stimulus-driven reorienting engages a ventral fronto-parietal network that includes inferior frontal (e.g., middle and inferior frontal gyrus) and temporoparietal (e.g., temporoparietal junction) regions. Activity within these visual attention regions has been observed during visual search performance, providing moderate support for the theory that similar attention processes contribute to both search types. That is, overlapping frontal and parietal regions are engaged during both conjunction and elementary search conditions, though the magnitude and extent of these activations has been shown to vary across search types (e.g., Donner et al., 2002; Leonards et al., 2000) (c.f., Corbetta et al., 1995).
The fronto-parietal attention networks involved in visual search require coordination of processing across distributed brain regions. Thus, in addition to examining functional activity within task-relevant regions, our understanding of the neural substrates of visual search would be enhanced by examining structural connections between those regions. Using diffusion tensor imaging (DTI), it is possible to examine the integrity of white matter tracts that connect frontal and parietal brain regions. DTI measures the diffusion (i.e., movement) of molecular water (Basser et al., 1994; Pierpaoli and Basser, 1996). By following the trajectory of the primary diffusion directions of adjacent voxels, DTI tractography analyses allow for reconstruction of white matter tracts between regions of interest (see Jones, 2008). Measures of integrity, such as fractional anisotropy (FA), can then be calculated to assess the directional coherence of diffusion within a given tract. Additional measures of diffusion in the primary (axial diffusivity, AD) and perpendicular (radial diffusivity, RD) directions provide information about whether effects seen for FA are due to differences in the degree of myelination and axonal loss or shrinkage, respectively (Song et al., 2003; Song et al., 2002; c.f., Wheeler-Kingshott and Cercignani, 2009).
White matter integrity correlates of visual search are currently unknown. One previous study did use DTI in conjunction with a visual search paradigm (Madden et al., 2007). However, relationships between white matter integrity and search performance were not directly examined. Nonetheless, given the known functional substrates of visual search, two fronto-parietal tracts that may be relevant are the superior (SLF) and inferior (ILF) longitudinal fasciculi (Wakana et al., 2004). The SLF traverses superior frontal and parietal regions that correspond to the dorsal fronto-parietal attention network, whereas the ILF runs between fronto-temporal and occipitoparietal regions that correspond to the ventral fronto-parietal attention network. Other tracts whose integrity may also contribute to search performance are the genu and splenium of the corpus callosum, which may be involved in coordinating interhemispheric processing across frontal (genu) and parietal (splenium) regions within the attention networks.
What is well-known is that white matter integrity is significantly affected in healthy aging. Relative to younger adults, older adults show decreased FA, especially in frontal white matter (e.g., genu of the corpus callosum)(Madden et al., 2009a; Sullivan and Pfefferbaum, 2006). Age-related FA decreases are often accompanied by increased RD, indicative of mild age-related demyelination and possibly axonal damage (Bennett et al., 2010a; Burzynska et al., 2010). Compromised white matter integrity in aging disrupts communication between interconnected brain regions, ultimately leading to age-related declines in cognition (e.g., Andrews-Hanna et al., 2007; O'Sullivan et al., 2001). Thus, it is not surprising that visual search, like many other cognitive functions, is affected in aging. Older adults show larger set size effects for conjunction search, but not elementary search, compared to younger adults (Foster et al., 1995; Humphrey and Kramer, 1997; Plude and Doussard-Roosevelt, 1989).
In response to this neural insult, older adults may rely on different neural substrates in order to perform the same task (see Reuter-Lorenz, 2002). In fact, functional imaging studies have shown that visual search performance in younger and older adults is predicted by activity within different brain regions (Madden et al., 2007; Madden et al., 2002). At least one DTI study similarly reported significant age group differences in relationships between white matter integrity and performance on a different type of visual target detection task (Madden et al., 2004). However, other DTI studies have observed statistically similar relationships between white matter integrity and performance on implicit learning (Bennett et al., 2010b) and task switching (Gold et al., 2010) paradigms in younger and older adults. Thus, the influence of white matter integrity on cognitive performance may be independent of age.
In the present study, our primary aim was to assess white matter integrity correlates of visual search. A secondary aim was to determine whether these relationships between white matter integrity and visual search performance varied in healthy aging. Younger and older adults completed a visual search task in which set size (3, 9, or 15 items) and search type (elementary, conjunction) were manipulated. Participants also underwent DTI scanning, and tractography was used to reconstruct two bilateral white matter tracts corresponding to the dorsal (SLF) and ventral (ILF) fronto-parietal attention networks, and two additional midline tracts of the corpus callosum (genu and splenium).
For our first aim, we hypothesized that integrity of the SLF and ILF (and possibly the genu and splenium) tracts would predict visual search performance in both younger and older adults. However, given evidence that both top-down and bottom-up attention processes contribute to both search types, it was unknown if SLF and ILF tract integrity would differentially relate to conjunction and elementary search performance, respectively. For our second aim, age group differences were expected in visual search performance (for conjunction but not elementary search) and white matter integrity (especially in the frontal genu, SLF, and ILF tracts). However, relationships between visual search performance and white matter integrity were predicted to be equivalent in younger and older adults.
Method
Participants
Fourteen younger (24.0 ± 4.0 years old, range of 18–30 years, 7 female) and 19 older (63.2 ± 6.6 years old, range of 52–75 years, 11 female) adults were recruited from the University of Texas at Dallas and advertisements in local newspapers. Participants obtained High School degrees, with 57% of younger adults and 79% of older adults having earned Bachelor's degrees or higher. They had normal or corrected-to-normal vision. All participants performed within the age-expected range (scores ≥ 26) on two brief measures used to screen for general cognitive functioning: the Mini-Mental State Examination (Folstein et al., 1975) and Telephone Interview for Cognitive Status (Brandt et al., 1988). Informed consent was obtained from each participant, and they received either payment or course credit for their participation. The University of Texas at Dallas Institutional Review Board approved the experimental procedures.
Prior to participation, individuals were screened for conditions that would affect their ability to complete the computer-based task (e.g., uncorrected vision, arthritis, and back problems that would make it difficult to see items presented on the computer screen or comfortably push the response buttons), prevent them from being able to enter the MRI scanner (e.g., being pregnant, having ferrous metal implants, having difficulty lying in the supine position for 30 minutes, and being claustrophobic), or influence their cognitive functioning and/or contribute to white matter pathology (e.g., stroke, dementia, diabetes, and uncontrolled depression or hypertension).
Scanning Protocol
Participants were scanned using a Philips Achieva 3.0 Tesla MRI system at the University of Texas Southwestern Advanced Imaging Research Center. Participants lay in the supine position in the scanner with an 8-element, SENSE, receive-only head coil. A mirror mounted on the head coil allowed them to view the computer screen during scanning. Fitted padding was used to minimize head movements.
A single diffusion weighted echo planar imaging sequence was acquired using gradient values of b = 0 and b = 1000 s/mm2 applied in 30 orthogonal directions and the following parameters: scan time = 4:39 minutes, TR = 5630 ms, TE = 51 ms, FOV = 224 × 224 mm, 65 axial interleaved slices, and 2 mm3 spatial resolution. Functional images were also acquired while participants performed the Visual Search task (see below), from which only the behavioral data is reported here. The entire scanning session took approximately 60 minutes.
Visual Search Task
On each trial, participants viewed arrays of colored T's (green, red) presented on a black background (see Supplementary Figure 1). They were instructed to respond to the presence of a target upright green T among the other distractor Ts, which was present on half of the trials. Search type was manipulated by having trials in which the target could only be discriminated from distracters by a single feature (elementary search) or the conjunction of two or more features (conjunction search). For elementary search trials, distracters were red upright and inversed Ts (only color differed between target and distracter Ts). For conjunction search trials, distracters were red upright and green inversed Ts (color and orientation differed between target and distracter Ts). Array set size was also manipulated by having 3, 9, or 15 items within each array. Arrays were presented for 2 s, followed by a variable inter-trial interval (0.5 – 0.75 s). Participants completed three runs of 60 trials each, with 10 randomly presented trials for each combination of search type and set size.
Imaging Data Analysis
Pre-processing
Diffusion-weighted data were separately processed for each participant using the University of Oxford's Center for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL) release 4.0 (http://www.fmrib.ox.ac.uk/fsl)(Smith et al., 2004). The first volume that did not have gradient applied (i.e., the first b = 0 image) was used to generate a binary brain mask with the Brain Extraction Tool. These data were then corrected for head movement and eddy current distortions using Eddycorrect, which aligns all the volumes. Finally, DTIfit was used to independently fit diffusion tensors to each voxel, with the brain mask limiting the fitting of tensors to brain space. The output of DTIfit yielded a voxelwise map of FA (and each of three primary diffusion directions: λ1, λ2, and λ3) for each participant.
Tractography
Probabilistic fiber tracking was conducted separately in each participant using FMRIB's Diffusion Toolbox (FDT) (Behrens et al., 2003). First, diffusion parameters were estimated at each voxel using BEDPOSTX, in which Markov Chain Monte Carlo sampling generates a probability distribution function of the primary diffusion direction. ProbtrackX was then used to estimate the distribution of connections between seed and waypoint regions (see below). To generate this connectivity distribution, 5,000 streamline samples were initiated from all voxels within the seed regions, traveling along the probability distribution functions of local voxels (steplength = 0.5 mm, curvature threshold = 0.2), until they terminated in voxels within the target regions. The output contains a connectivity value for each voxel that represents the number of streamline samples that passed through that voxel.
Two bilateral association tracts (superior longitudinal fasciculus, SLF; inferior longitudinal fasciculus, ILF) and two midline corpus callosum tracts (genu, splenium) were generated from seed, waypoint, and exclusion masks that were traced in individual space (see Figure 1). For SLF and ILF tracts, seed and waypoint regions were rectangular masks 10 axial slices high, 20 sagittal slices across, and 2 coronal slices deep. These masks were traced separately for the left and right hemispheres, and a midline exclusion mask (3 sagittal slices thick at the midline) was used to restrict tracking to each hemisphere. The SLF seed masks were traced in superior white matter on the two most posterior coronal slices that contained the splenium of the corpus callosum, and two waypoint masks were traced 30 and 60 coronal slices anterior to the seed mask. The ILF seed masks were traced in occipital white matter 30 coronal slices anterior to the most posterior slice of the brain, and a single waypoint mask was traced in fronto-temporal white matter 60 slices anterior to the seed mask. All SLF and ILF masks were centered around anterior-posterior oriented white matter, identified by viewing images of the primary diffusion direction rendered by FA for each individual (these tracts appeared green in the rendered images). For the genu and splenium tracts, seed regions were rectangular masks 10 axial slices high, 5 sagittal slices across, and 20 coronal slices wide, centered around the most anterior and posterior portions of the corpus callosum, respectively. Waypoint masks were traced (3 coronal slices thick) 30 coronal slices posterior to the most anterior slice of the brain for the genu tract, and 30 coronal slices anterior to the most posterior slice of the brain for the splenium tract.
Figure 1.
Population maps illustrate locations of the two bilateral association and two midline callosum tracts of interest in younger (top) and older (middle) adults. Example seed (yellow), waypoint (green), and exclusion (red) masks used to generate these tracts are also shown (bottom), overlaid on the standard MNI152 2 mm3 brain. Individual tracts in diffusion space were transformed into standard space. To remove spurious tracts, images were thresholded to show only voxels common to at least two younger (red-yellow) or older (blue-light blue) adults. Axial slices are presented in radiological orientation (right = left). SLF = superior longitudinal fasciculus. ILF = inferior longitudinal fasciculus.
Resulting tracts were thresholded at 15% of the maximum connectivity value, leaving only those voxels with a high likelihood of being connected to the seed regions. In contrast to previous studies that have used a constant proportion of the total number of permutations for all participants (e.g., 25/25,000 in Johansen-Berg et al., 2007), the proportional threshold used here accommodates for individual differences in connectivity values for each tract, maximizing tract size and quality (see Bennett et al., 2010b). To illustrate these tracts (see Figure 1), population maps were created by transforming thresholded tracts from diffusion space into standard space, and then overlaying tracts from each individual onto the standard MNI152 2 mm3 brain.
Tract-based measures of integrity
Mean FA values were calculated for all six tracts for each participant. Thresholded tracts were binarized and multiplied by the individual's FA map, leaving just the FA values for voxels within that tract, which were then averaged. Measures of AD (λ1) and RD ((λ2 +λ3)/2) were also calculated for each tract by multiplying thresholded tracts by the individual's diffusion maps (λ1, λ2, and λ3).
All tracts were visually checked for quality. Due to significant distortions (e.g., pixilated tracts, branching to non-target regions) some individuals' tracts were excluded from analyses of the genu (four older adults) and splenium (one younger and two older adults). Note that analyses including these tracts either remained significant, p < .05, or became marginally significant trends, p < .08 (e.g., the relationship between overall reaction time and splenium tract FA).
Results
Age Group Differences in Visual Search Performance
Age Group (young, old) × Search Type (elementary, conjunction) × Set Size (3, 9, 15) ANOVAs were conducted separately for mean reaction time on correct trials and mean accuracy, with Age Group as a between-subject variable, and Search Type and Set Size as within-subject variables. To minimize the effect of outliers, trials in which reaction times were greater than two standard deviations above an individuals' mean response time were removed from the analyses (~9% of all trials). These data are presented in Figure 2.
Figure 2.
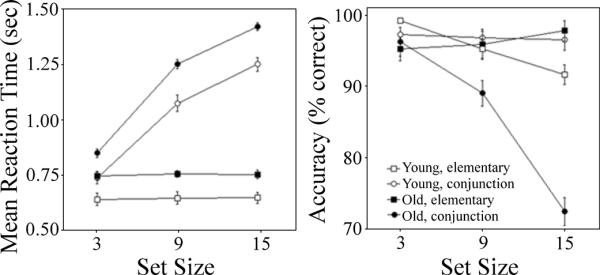
Mean reaction times for correct trials (left) and proportion of correct responses (right) are presented. Responses to elementary (square) and conjunction (circle) search types are plotted separately across the three set sizes for younger (open shapes) and older (closed shapes) adults.
For reaction time, a significant Search Type effect revealed faster responses to the elementary (699.5 ± 101.0 ms) versus conjunction (1054.9 ± 221.7 ms) search condition, F(1, 31) = 1332.91, partial η2 = 0.98, p < .001. A significant Set Size effect showed faster responses to set size 3 (745.0 ± 117.4 ms) relative to set sizes 9 (919.4 ± 247.0 ms) and 15 (967.1 ± 288.3 ms), F(2, 62) = 460.95, partial η2 = 0.94, p < .001. These main effects were modulated by a Search Type × Set Size interaction, in which a measurable set size effect (i.e., the difference in response to set size 15 minus set size 3) was observed in the conjunction (430.6 ms) but not elementary (13.7 ms) search condition, F(2, 62) = 523.45, partial η2 = 0.94, p < .001.
A significant Age Group effect revealed that older adults (931.2 ± 245.6 ms) responded slower than younger adults (803.9 ± 232.0 ms), F(1, 31) = 19.19, partial η2 = 0.38, p < .001. The Age Group × Search Type interaction indicated that this age group difference was magnified for the conjunction (147.1 ms) versus elementary (107.3 ms) search condition, F(1, 31) = 4.26, partial η2 = 0.12, p < .05. A marginally significant Age Group × Search Type × Set Size interaction, F(2, 62) = 2.65, partial η2 = 0.08, p < .08, was followed up with Age Group × Set Size ANOVAs conducted separately for each search type. These analyses revealed a significant Age Group × Set Size interaction for conjunction search, F(2, 62) = 3.20, partial η2 = 0.09, p < .05, but not elementary search, p > .77. Thus, as expected, greater set size effects were observed in reaction time for older versus younger adults, especially in conjunction search (447.2 versus 407.9 ms) relative to elementary search (17.5 versus 8.6 ms).
For accuracy, a significant Search Type effect revealed more accurate responses in the elementary (98.9 ± 1.9%) versus conjunction (95.9 ± 5.5%) search condition, F(1, 31) = 29.60, partial η2 = 0.51, p < .001. A significant Set Size effect showed more accurate responses to set size 3 (98.6 ± 2.5%) relative to set sizes 9 (97.6 ± 3.1%) and 15 (96.0 ± 6.2%), Set Size, F(2, 62) = 6.72, partial η2 = 0.18, p < .01. These main effects were modulated by a Search Type × Set Size interaction, in which a measurable set size effect was seen in the conjunction (5.6%) but not elementary (0.0%) search condition, F(2, 62) = 11.59, partial η2 = 0.28, p < .001.
A significant Age Group effect revealed that older adults (96.9 ± 5.1%) responded less accurately than younger adults (98.1 ± 3.0%), F(1, 31) = 5.81, partial η2 = 0.16, p < .02. Significant Age Group × Search Type, F(1, 31) = 4.95, partial η2 = 0.14, p < .04, and Age Group × Set Size, F(2, 62) = 3.42, partial η2 = 0.10, p < .04, interactions were modulated by a significant Age Group × Search Type × Set Size interaction, F(2, 62) = 3.29, partial η2 = 0.10, p < .05. As expected, this three-way interaction revealed greater set size effects for older versus younger adults, which were limited to the conjunction (8.2 versus 2.1%), but not elementary (−0.6% versus −0.5%), search condition. Taken together, these behavioral results are consistent with studies showing that age group differences in visual search performance are magnified for conjunction search relative to elementary search conditions (e.g., Foster et al., 1995; Humphrey and Kramer, 1997; Plude and Doussard-Roosevelt, 1989).
Age Group Differences in White Matter Integrity
Separate between-group t-tests compared FA in younger and older adults for each tract of interest. Similar analyses were also performed for the diffusivity measures (AD and RD). These data are presented in Table 1.
Table 1.
Age group differences in white matter integrity
| FA |
AD(× 10−3 mm2/sec) |
RD(× 10−4 mm2/sec) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Tracts | Younger | Older | t | Younger | Older | t | Younger | Older | t |
| ILF Left | .59 ± .03 | .54 ± .04 | −4.5* | 1.34 ± 0.04 | 1.29 ± 0.06 | −2.9* | 4.78 ± 0.29 | 5.12 ± 0.37 | 2.9* |
| ILF Right | .57 ± .04 | .53 ± .03 | −3.0* | 1.30 ± 0.05 | 1.27 ± 0.06 | −1.4 | 4.82 ± 0.37 | 5.18 ± 0.34 | 2.9* |
| SLF Left | .49 ± .05 | .47 ± .03 | −1.8 | 1.08 ± 0.04 | 1.09 ± 0.05 | 0.2 | 4.94 ± 0.39 | 5.21 ± 0.29 | 2.3† |
| SLF Right | .47 ± .05 | .45 ± .03 | −1.2 | 1.07 ± 0.04 | 1.08 ± 0.05 | 0.7 | 5.13 ± 0.39 | 5.37 ± 0.28 | 2.1* |
| Genu | .53 ± .03 | .48 ± .03 | −4.2* | 1.30 ± 0.05 | 1.31 ± 0.06 | 0.1 | 5.11 ± 0.35 | 5.77 ± 0.55 | 3.8* |
| Splenium | .61 ± .03 | .60 ± .03 | −0.6 | 1.50 ± 0.09 | 1.47 ± 0.06 | −0.9 | 5.05 ± 0.52 | 5.03 ± 0.31 | −0.1 |
Notes. Fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD) values are presented as mean ± standard deviation for each white matter tract separately for younger and older adults.
significant effects at p < .008 (Bonferroni-corrected for 6 comparisons per measure of white matter integrity).
significant effects at p < .05.
ILF = inferior longitudinal fasciculus. SLF = superior longitudinal fasciculus.
As expected, significantly higher FA in younger adults compared to older adults was observed in the genu and bilateral ILF tracts, η2s > 0.24, ps < .01. For the diffusivity measures, only left ILF showed a significant group difference in AD, η2 = 0.24, p < .01, with higher values for younger versus older adults. However, RD was significantly lower in younger adults compared to older adults in the genu, bilateral ILF, and bilateral SLF tracts, η2s > 0.12, ps < .05. This pattern of white matter aging is consistent with other work suggesting mild age-related changes in white matter microstructure that affect axonal packing density (e.g., demyelination and possibly axonal loss or shrinkage)(Bennett et al., 2010a; Song et al., 2003; Song et al., 2002).
White Matter Integrity Predicts Visual Search Performance
Correlations between FA and overall search performance
To address the primary aim of examining white matter integrity correlates of visual search performance, correlations were conducted between FA from the tracts of interest and measures of overall search speed (average response time across all trials) and overall search accuracy (average proportion correct across all trials). For each analysis related to this aim, all participants were included regardless of age group. These data are presented in Table 2 and Figure 3.
Table 2.
Correlations between white matter integrity and overall search performance
| Overall Search Speed |
Overall Search Accuracy |
|||||
|---|---|---|---|---|---|---|
| Tracts | FA | AD | RD | FA | AD | RD |
| ILF Left | −.42† | −.20 | +.38† | +.25 | +.15 | −.19 |
| ILF Right | −.40† | −.21 | +.40† | +.18 | +.16 | −.11 |
| SLF Left | −.45† | −.12 | +.46* | +.07 | −.12 | −.19 |
| SLF Right | −.34† | −.04 | +.39† | −.11 | −.18 | −.04 |
| Genu | −.68* | −.13 | +.55* | +.45† | +.00 | −.41† |
| Splenium | −.38† | −.08 | +.29 | +.05 | +.05 | +.00 |
Notes. Pearson correlation r values are presented for each comparison between visual search performance and white matter tract integrity, when data was collapsed across age group.
significant effects at p < .008 (Bonferroni-corrected for 6 comparisons per performance measure).
significant effects at p < .06.
FA = fractional anisotropy. AD = axial diffusivity. RD = radial diffusivity. ILF = inferior longitudinal fasciculus. SLF = superior longitudinal fasciculus.
Figure 3.
Scatterplots show relationships between FA from the tracts of interest and measures of overall search speed (average response time across all trials) and overall search accuracy (average proportion correct across all trials). Pearson R values and regression lines corresponds to analyses that included both younger (closed circles) and older (open circles) adults. SLF = superior longitudinal fasciculus. ILF = inferior longitudinal fasciculus. * = significant effect at p < .008 (Bonferroni-corrected for 6 comparisons)
Overall search speed was significantly correlated with FA from all tracts, rs > −0.34, ps < .06, with faster responses associated with better integrity (i.e., higher FA). Only the relationship between overall search speed and genu FA survived Bonferroni-correction for multiple comparisons (i.e., six measures of tract FA), r = −0.68, p < .001.
Overall search accuracy was significantly related to genu tract FA, r = 0.45, p < .02, with more accurate responses being associated with higher integrity (i.e., higher FA). This relationship did not survive when corrected for multiple comparisons.
Correlations between FA and performance for each search type
Follow-up analyses tested for a dissociation between white matter integrity correlates of conjunction and elementary search performance. Correlations were conducted between FA from the tracts of interest and measures of search speed and search accuracy calculated separately for each search type.
For search speed, both conjunction and elementary search speed were significantly correlated with FA from left SLF, bilateral ILF, and genu tracts, rs > −.36, ps < .04. Elementary search speed was also significantly related to splenium tract FA, r = −.38, p < .04. Only the relationships with genu FA survived Bonferroni-correction for multiple comparisons (conjunction search speed r = −0.66, p < .001; elementary search speed r = −0.72, p < .001). These findings are similar to those observed for overall search speed, which may reflect the fact that overall search speed was highly correlated with both conjunction search speed, r = +.93, p < .001, and elementary search speed, r = +.97, p < .001.
For search accuracy, conjunction search accuracy was significantly related to FA in genu, r = +.66, p < .001, and the left and right ILF, rs > +.42, ps < .02. Relationship between conjunction search accuracy and FA in the genu and left ILF survived Bonferroni-correction for multiple comparisons. Elementary search accuracy was not significantly related to FA in any tract, rs < +.16, ps > .39. Taken together, results revealed that both conjunction (speed and accuracy) and elementary (speed only) search performance were related to integrity of the genu, with conjunction search performance (accuracy) also relating to integrity of the left ILF.
Controlling for motor speed
Unplanned multiple regression analyses were performed to determine whether relationships between FA and search performance were independent of individual differences in motor speed. Separate models revealed that, after controlling for motor speed (Number Copy task; Salthouse, 1993), FA in the genu remained a significant predictor of overall search speed, overall search accuracy, and conjunction search accuracy, ps < .04; FA in the left SLF remained a significant predictor of overall search speed, p < .02; and FA in the left ILF remained a significant predictor of conjunction search accuracy, p < .04. Thus, in general, relationships with integrity of the genu and left SLF tracts are likely due to higher FA influencing visual search-specific performance, whereas relationships between ILF and splenium tract integrity and visual search performance mostly reflect the influence of higher FA on more general motor processing.
Correlations between diffusivity and search performance
Additional analyses assessed whether white matter integrity-visual search performance relationships were also seen for diffusivity measures, which may provide insight into the neural mechanisms underlying the effects seen with FA. Correlations were conducted between AD and RD from the tracts of interest and measures of overall search performance. These data are presented in Table 2.
There were no significant relationships between AD in any tract and either measure of overall search performance. However, RD in bilateral SLF, bilateral ILF, and genu tracts was significantly related to overall search speed, rs > +.38, ps < .04, with faster responses associated with better integrity (i.e., lower RD). Relationship between overall search speed and FA in the genu and left SLF survived Bonferroni-correction for multiple comparisons. RD in the genu was also significantly related to overall search accuracy, r = −.41, p < .03, with more accurate responses associated with better integrity, though this relationship did not survive the correction for multiple comparisons. Thus, all previously reported relationships between FA and overall search speed (except for the splenium tract) and overall search accuracy were also seen for RD, but not AD. This pattern of results, which has previously been reported (Davis et al., 2009; Madden et al., 2009b), is consistent with the view that individual differences cognition reflect differences in underlying white matter, such as mild changes in microstructures that affect axonal packing density (e.g., demyelination and possibly axonal loss or shrinkage)(Bennett et al., 2010a; Song et al., 2003; Song et al., 2002).
Age Constancy of White Matter Integrity Correlates of Visual Search Performance
To address the secondary aim of determining whether relationships between white matter integrity and visual search performance vary in aging, correlations between FA from the tracts of interest and measures of overall search speed and overall search accuracy were conducted separately for each age group. When the FA-search performance relationships appeared to be different for the younger and older groups, regression analyses were used to test whether age group was a significant moderator of the effect (i.e., whether relationships between FA and search performance were significantly different in younger versus older adults).
The previously reported relationship between overall search accuracy and genu tract FA did not survive when the groups were analyzed separately. However, overall search speed remained significantly related to FA in the left SLF, r = −0.55, p < .05, and genu, r = −0.61, p < .02, tracts for younger but not older adults. Follow-up regression analyses were conducted separately for each of these tracts using search speed as the outcome variable, and age group, tract FA, and the interactions between age group and FA as predictor variables. Results revealed that the interaction term was not a significant predictor of overall search speed in either model, ps > .22, indicating that age group did not moderate these FA-search speed relationships. It is unlikely that the current sample size (n=33 across age groups) was insufficient for detecting this effect because a power analysis, using values from the only previous DTI aging study to report a significant moderation (Madden et al., 2004), revealed that a minimum sample size of 17 is required to detect comparable regression effects (R2=.472, α=.05, 3 predictors, desired power level=0.8).
Discussion
The present study aimed to examine white matter integrity correlates of visual search performance, and whether or not these relationships varied in younger and older adults. Results revealed that visual search performance was significantly related to the integrity of white matter tracts that connect fronto-parietal attention networks (SLF, ILF), as well as tracts that may be involved in interhemispheric processing within frontal and parietal regions (genu, splenium). However, integrity of the SLF and ILF tracts did not differentially predict conjunction and elementary search performance, respectively. Consistent with previous research, behavioral results revealed significant age-related declines in performance for conjunction but not elementary search (e.g., Plude and Doussard-Roosevelt, 1989), and DTI results revealed age-related decreases in frontal white matter tract integrity (genu, bilateral ILF), with declines in FA accompanied by age-related increases in RD but not AD (e.g., Davis et al., 2009; Madden et al., 2009b; Sullivan et al., 2010). However, relationships between visual search performance and white matter integrity did not differ in younger and older adults.
Previous functional imaging research revealed that visual search engages regions within fronto-parietal attention networks (Corbetta and Shulman, 1998, 2002; Corbetta et al., 1995). Interestingly, however, the role of white matter tracts that connect these distributed neural networks had not been examined. Of particular interest in the present study were the SLF and ILF, which correspond to the dorsal and ventral fronto-parietal networks, respectively. As expected, integrity of both tracts (FA and RD) was a significant predictor of overall search performance (search speed).
Given that interhemispheric processing is important for efficient cognitive performance, especially in aging (Daselaar and Cabeza, 2005), two additional tracts of interest were the genu and splenium of the corpus callosum. Integrity of these tracts was also a significant predictor of visual search performance, with genu tract integrity showed the strongest relationships (i.e., highest Pearson r values) to measures of both search speed and search accuracy. Thus, these corpus callosum tracts may be involved in coordinating processing between bilateral frontal (genu) and parietal (splenium) regions that comprise the fronto-parietal attention networks. Together with the results for the SLF and ILF tracts, the current data show that integrity of white matter tracts connecting fronto-parietal attention networks (both intra-and inter-hemispheric connections) contributes to visual search performance in younger and older adults.
Whereas initial theories proposed a dissociation between attention processes engaged during distinct search conditions (e.g., Treisman and Gelade, 1980), the more recently accepted view is that top-down and bottom-up attention processes are both involved in conjunction and elementary search (e.g., Humphrey and Kramer, 1997; Madden, 2007; Yantis, 1996). In the present study, follow-up analyses were designed to test whether or not conjunction and elementary search performance were both related to the integrity of similar white matter tracts. Unfortunately, interrelationships among the reaction time measures made it difficult to assess these relationships using search speed. For search accuracy, however, integrity of the left SLF tract (RD) and bilateral ILF tracts (FA and RD) significantly predicted conjunction search performance, but not elementary search performance. In combination with functional imaging research showing overlapping fronto-parietal activity for both search types (e.g., Donner et al., 2002; Leonards et al., 2000), this finding supports the theory that similar attention processes contribute to both conjunction (and elementary) search conditions.
The previously described relationships between white matter tract integrity and visual search performance were observed when both younger and older adults were included in the analyses. When age groups were examined separately, FA in the left SLF and genu tracts remained significant predictors of overall search speed (and conjunction and elementary search speed) in younger adults, but not older adults. However, follow-up moderation analyses revealed that the magnitude of the relationships between integrity in these tracts and overall search speed did not significantly differ across age groups. Thus, for the tracts examined here, white matter integrity correlates of visual search appear to be similar for younger and older adults. This pattern of age-equivalence is unlikely to result from methodological issues such as limited power to detect age group differences (see power analysis results). There is also little evidence to support the notion that the present results represent task-specific effects because statistically similar white matter integrity-performance relationships in younger and older adults have been previously observed using other cognitive paradigms (e.g., Bennett et al., 2010b; Gold et al., 2010; Kennedy and Raz, 2009).
Age constancy in relationships between white matter integrity and cognitive performance may indicate that individual- and age-related cognitive differences are attributed to monotonic decreases in structures that support neural connectivity (i.e., white matter)(Rypma et al., 2006). Such declines in brain structure can in turn affect the efficiency of brain functioning (see Motes et al., 2010; Rypma et al., 2006; Rypma and D'Esposito, 1999; Rypma and Prabhakaran, 2009). Previous research has shown that relationships between neural activity and cognitive performance (i.e., neural efficiency) are moderated by age group such that lower neural activity is observed in better versus worse performing younger adults, whereas greater neural activity is observed in better versus worse performing older adults (e.g., Persson et al., 2004; Reuter-Lorenz et al., 2000; Rypma et al., 2007). In contrast, relationships between white matter integrity and cognitive performance (i.e., structural efficiency) might not be moderated by age group, despite mean age group differences for each measure independently. Future research combining DTI and functional imaging techniques will be necessary to test whether or not the previously reported age group differences in neural efficiency (i.e., brain function-performance relationships) arise from the relatively simple linear age-related differences in structural efficiency that we have observed here.
Generalized age-related slowing has been shown to account for age-related differences in visual search performance (Madden and Whiting, 2004), highlighting the importance of separating the contribution of white matter tract integrity to task-specific (i.e., visual search-related performance) versus task-general (i.e., general motor speed) effects. After controlling for motor speed, results revealed that FA in the genu and left SLF tracts remained significant predictors of overall search performance. This finding suggests that better integrity (i.e., higher FA) in the genu and SLF tracts influences task-specific performance independent of task-general processes. In contrast, relationships between ILF and splenium tract integrity and search performance may be due to higher FA in these tract influencing motor speed in a way that is not necessarily specific to the visual search task performed here.
Consistent with neural correlates of visual search identified in functional imaging research, the present study showed that visual search performance was related to integrity of tracts connecting fronto-parietal networks involved in top-down (SLF) and bottom-up (ILF) guidance of attention, and tracts involved in interhemispheric communication between these frontal (genu) and parietal (splenium) regions. Integrity of the SLF and ILF tracts were both related to conjunction search performance, as would be predicted by the theory that both top-down and bottom-up attention processes contribute to conjunction and elementary search. Results also revealed that these white matter integrity correlates of visual search were not statistically different in younger versus older adults. These findings will be followed-up in future studies examining interactions between structural and functional correlates of visual search, and the effect of aging on these relationships.
Supplementary Material
Acknowledgments
This research was funded by grant R01 AG029523 from the National Institute on Aging/National Institutes of Health. There are no actual or potential conflicts of interest related to this work. Preliminary findings from this project were presented at the Dallas Aging and Cognition Conference in Dallas, TX in January, 2010.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Hunes S, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010a;31:378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard JH, Jr., Howard DV. White matter integrity correlates of implicit sequence learning in healthy aging. Neurobiol Aging. 2010b doi: 10.1016/j.neurobiolaging.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsuchiat Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- Burzynska AZ, Preuschhof C, Backman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR. Age-related differences in white-matter microstructure: Region-specific patterns of diffusivity. Neuroimage. 2010;49:2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Carter RC. Visual search with color. J Exp Psychol Hum Percept Perform. 1982;8:127–136. doi: 10.1037//0096-1523.8.1.127. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Human cortical mechanisms of visual attention during orienting and search. Philos Trans R Soc Lond B Biol Sci. 1998;353:1353–1362. doi: 10.1098/rstb.1998.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270:802–805. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Daselaar S, Cabeza R. Age-related changes in hemispheric organization. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. Oxford University Press; New York: 2005. pp. 325–353. [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of aging on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner TH, Kettermann A, Diesch E, Ostendorf F, Villringer A, Brandt SA. Visual feature and conjunction searches of equal difficulty engage only partially overlapping frontoparietal networks. Neuroimage. 2002;15:16–25. doi: 10.1006/nimg.2001.0951. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annu Rev Psychol. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foster JK, Behrmann M, Stuss DT. Aging and visual search: Generalized cognitive slowing of selective deficit in attention? Aging and Cognition. 1995;2:279–299. [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2010;31:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey DG, Kramer AF. Age differences in visual search for feature, conjunction, and triple-conjunction targets. Psychol Aging. 1997;12:704–717. doi: 10.1037//0882-7974.12.4.704. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007;36(Suppl 2):T16–21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44:936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonards U, Sunaert S, Van Hecke P, Orban GA. Attention mechanisms in visual search -- an fMRI study. J Cogn Neurosci. 2000;12(Suppl 2):61–75. doi: 10.1162/089892900564073. [DOI] [PubMed] [Google Scholar]
- Madden DJ. Aging and Visual Attention. Curr Dir Psychol Sci. 2007;16:70–74. doi: 10.1111/j.1467-8721.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009a;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. Journal of Cognitive Neuroscience. 2009b;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study. Neurobiology of Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Langley LK, Hawk TC, Coleman RE. Aging and attentional guidance during visual search: functional neuroanatomy by positron emission tomography. Psychology and Aging. 2002;17:24–43. doi: 10.1037//0882-7974.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL. Age-related changes in visual attention. Advances in Cell Aging and Gerontology. 2004;15:41–88. [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Motes MA, Biswal BB, Rypma B. Age-dependent relationships between prefrontal cortex aactivation and processing speed. Cognitive Neuroscience. 2010 doi: 10.1080/17588928.2010.512974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Silverman GH. Serial and parallel processing of visual feature conjunctions. Nature. 1986;320:264–265. doi: 10.1038/320264a0. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Persson J, Sylvester CY, Nelson JK, Welsh KM, Jonides J, Reuter-Lorenz PA. Selection requirements during verb generation: differential recruitment in older and younger adults. Neuroimage. 2004;23:1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Plude DJ, Doussard-Roosevelt JA. Aging, selective attention, and feature integration. Psychol Aging. 1989;4:98–105. doi: 10.1037/0882-7974.4.1.98. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA. New visions of the aging mind and brain. TRENDS in Cognitive Sciences. 2002;6:394–400. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, D'Esposito M. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proceedings of the National Academy of Sciences U S A. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation-performance relations in delayed-response tasks: a multiple component analysis. Cortex. 2007;43:65–76. doi: 10.1016/s0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: The mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37:207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Speed mediation of adult age differences in cognition. Developmental Psychology. 1993;29:722–738. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saudners J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiology of Aging. 2010;31:464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J. Visual selective attention: a theoretical analysis. Acta Psychol (Amst) 1993;83:93–154. doi: 10.1016/0001-6918(93)90042-p. [DOI] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cogn Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott CAM, Cercignani M. About “axial” and “radial” diffusivities. Magnetic Resonance in Medicine. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Visual Search. In: Pashler H, editor. Attention. Psychology Press; East Sussex, UK: 1998. pp. 13–73. [Google Scholar]
- Wolfe JM, Cave KR, Franzel SL. Guided search: an alternative to the feature integration model for visual search. J Exp Psychol Hum Percept Perform. 1989;15:419–433. doi: 10.1037//0096-1523.15.3.419. [DOI] [PubMed] [Google Scholar]
- Yantis S. Attentional capture in vision. In: Kramer AF, Coles MGH, Logan GD, editors. Converging operations in the study of visual selective attention. American Psychological Association; Washington, DC: 1996. pp. 45–76. [Google Scholar]
- Yantis S. Goal-directed and stimulus-driven determinants of attentional control. In: Monsell S, Driver J, editors. Attention and Performance XVIII. MIT Press; Cambridge: 2000. pp. 71–107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




