Abstract
Background
Melanoma patients who develop brain metastases (B-Met) have limited survival and are excluded from most clinical trials. In this study, we sought to identify primary tumor characteristics and clinical features predictive of B-Met development and post-B-Met survival.
Methods
We studied a prospectively accrued cohort of 900 melanoma patients to identify clinicopathologic features of primary melanoma (e.g. thickness, ulceration, mitotic index, lymphovascular invasion) that are predictive of B-Met development and post-B-Met survival. Associations between clinical variables present at the time of B-Met diagnosis (e.g. extracranial metastases, B-Met location, presence of neurological symptoms) and post-B-Met survival were also assessed. Univariate associations were analyzed using Kaplan-Meier survival analysis, and the effect of independent predictors assessed using a multivariate Cox proportional hazards regression.
Results
89 (10%) of the 900 patients developed B-Met. Ulceration and site of primary on the head and neck were independent predictors of B-Met development on multivariate analysis (p=0.001 and p=0.003, respectively). Clinical variables predictive of post-B-Met survival on multivariate analysis included the presence of neurological symptoms (p=0.008) and extracranial metastases (p=0.04). Ulceration was the only primary tumor characteristic that remained a significant predictor of post-B-Met survival on multivariate analysis (p=0.04).
Conclusions
Primary tumor ulceration was the strongest predictor of B-Met development and remained an independent predictor of decreased post-B-Met survival in a multivariate analysis inclusive of primary tumor characteristics and clinical variables. Our results suggest that patients with ulcerated primaries should be prospectively studied to determine if heightened surveillance for B-Met can improve clinical outcome.
Keywords: melanoma, brain, metastasis, outcomes, survival, ulceration
INTRODUCTION
The development of brain metastases (B-Met) heralds a dismal prognosis for melanoma patients. They have a median survival of less than 6 months1, 2, and their poor prognosis precludes entry into most clinical trials of novel agents. Surgery, chemotherapy, and radiosurgery can extend survival in select patients, but a definitive cure conferring long term survival is unattainable for the vast majority of B-Met patients2–4.
Relative to other solid malignancies, it is more common in melanoma that B-Mets represent the only site of metastatic involvement5. This observation has led to speculation that melanoma has a brain-specific tropism, and investigations utilizing animal models of spontaneous melanoma B-Met to elucidate the molecular mechanisms of site-specific metastases are currently underway6. At the present time, however, the mechanism driving this predilection is unknown, and we are unable to utilize molecular markers to predict which primary melanoma patients will ultimately develop B-Met or which patients will have the best chance for extended survival following the development of B-Met. Previous retrospective studies have identified clinical variables present at the time of B-Met diagnosis that are predictive of post-B-Met survival including age, the number of lesions, and the presence of extracranial disease3, 7, 8. These studies may have clinical implications for the appropriate selection of B-Met patients most likely to benefit from additional, possibly more aggressive treatment. The lack of effective treatment for melanoma B-Met in terms of improving overall survival, however, makes it less likely that variables identified at the time of B-Met diagnosis will ultimately impact mortality in a way that is clinically meaningful. There remains a need for the identification of prognostic variables present early in the melanoma course that can identify patients at highest risk for B-Met who may benefit from increased surveillance and/or prophylactic interventions targeting the central nervous system (CNS).
Mitotic index has been incorporated into the AJCC staging system9, and recent reports suggesting that ulceration may be an independent predictor of better clinical response to both adjuvant interferon and to adjuvant vaccination with antigenic peptides emphasize the prognostic relevance of primary tumor characteristics10–12. Although thickness and ulceration of the primary tumor factor into the staging of melanoma patients with primary and nodal disease, the influence of primary tumor characteristics on the clinical course of stage IV melanoma has not been defined. Staging of patients with distant metastases is dictated primarily by the site of metastatic involvement with primary tumor characteristics no longer playing a role. Previously published studies assessing the impact of primary tumor characteristics on post-B-Met survival are retrospective1, 3. In the current study, we identified primary tumor characteristics and clinical features that are predictive of B-Met development and post-B-Met survival in a cohort of 900 prospectively accrued melanoma patients.
METHODS
Study population
Patients receiving treatment for primary or metastatic melanoma at New York University (NYU) Langone Medical Center were prospectively enrolled in the Interdisciplinary Melanoma Cooperative Group (IMCG) database13 from August 2002 to October 2008. The study was approved by the Internal Review Board (IRB) of NYU, and all patients signed informed consent at time of enrollment.
IMCG patients with evidence of B-Met as documented by the attending clinical oncologist were identified and medical charts were reviewed for clinical variables including: location of B-Met (frontal, temporal, occipital lobe, etc.), laterality (uni- vs. bilateral), number of metastatic lesions (at time of initial B-Met diagnosis and cumulative), presence of extracranial metastases at B-Met diagnosis, first metastatic site at stage IV diagnosis, total number of visceral metastases, presence of neurological symptoms, total number of symptoms at B-Met diagnosis, evidence of hydrocephalus, leptomeningeal lesions, and hemorrhage. Information regarding B-Met-directed treatments including craniotomy, radiosurgery, whole-brain radiation, and systemic therapy (chemo-, immuno-, and biotherapy trials) was also obtained. In the case of patients who expired during the follow-up period, the attending medical oncologist was contacted to determine if B-Met was considered to be the cause of death.
Primary tumor characteristics assessed included thickness, histological subtype, anatomic site, mitotic index (few=2, moderate=3–5, many=5+/HPF), regression, ulceration, and lymphovascular invasion (LVI). Cases with reported LVI were reviewed by the IMCG pathologist (FD) for histopathologic confirmation and assessed microscopically for LVI, which was defined as the presence of tumor cells within the peritumoral endothelial-lined spaces.
Statistical methods
Descriptive statistics were calculated for baseline demographic and clinicopathologic characteristics. For categorical variables, the frequency distribution between patients with and without B-Met was evaluated using the chi-square test. For analyses pertaining to primary tumor characteristics, patients with melanoma of unknown primary or patients without complete information for a given variable were excluded from the analysis. The Wilcoxon rank-sum test was used to compare median primary tumor thickness between patients with and without B-Met. The independent effect of demographic and clinicopathologic variables on B-Met development were assessed by multivariate logistic regression analysis. Referent categories for categorical (indicator) variables in the multivariate logistic regression model were defined based on the category of each predictor variable that had the lowest risk of B-Met development as observed in the univariate analyses. Post-B-Met survival (defined as the day of B-Met detection to the date of last follow-up or death) was estimated by Kaplan-Meier survival analysis, and univariate associations between clinicopathologic variables and post-B-Met survival were assessed by the log-rank test. The independent effect of predictors of post-B-Met survival identified by univariate analysis was assessed by multivariate Cox proportional hazards regression analysis. Adjusted hazard ratios were computed and 95% confidence intervals for the hazard ratio are presented to assess the precision of the obtained estimates. Median follow-up time (from the date of initial melanoma diagnosis) was computed based on survivors in the non-B-Met cohort. Follow-up time for the B-Met patients was calculated based on B-Met survivors and based on the total cohort. All p-values are two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in SAS Version 9.2 and Stata Version 10.0 (Stata Corporation, College Station, Texas).
RESULTS
There were 89 patients who presented with or developed melanoma B-Met during the study period (2002–2008). The mean age at B-Met diagnosis was 60.3 years (SD 16), and 60% of the patients were male (Table 1). The majority of patients (n=46, 52%) were designated as AJCC stage I or II at the time of initial melanoma diagnosis (Table 1 and Figure 1), and the median time from initial melanoma diagnosis to B-Met was 2.1 years (range: 2 months −13.3 years, Table 1). Median follow-up time, based on all B-Met patients (n=89) was 2.7 years (range: 0.4–17.7 years). At the end of the study period, 70 (79%) of the B-Met patients had died with melanoma, 13 (15%) were alive with melanoma, and 6 (7%) were alive without melanoma. The median post-B-Met survival was 5.8 months (range: 0.2–76.8 months). The incidence density for B-Met in our cohort was 3.19 cases per 100 person-years at risk, which translates to 8 cases per 100 patients at risk (based on a median length of follow-up of 2.4 years among survivors in the cohort. At the time of B-Met diagnosis 63% (n=56) of patients had evidence of extra-cranial disease. Regarding treatment modalities, 70% of patients (n=62) received 2–4 B-Met directed interventions which included craniotomy (36%, n=32), gamma knife radiosurgery (48%, n=43), temozolomide (65%, n=58), or another type of systemic therapy other than temozolomide (79%, n=70). Patients who received craniotomy had the highest median survival of the cohort (7.9 months) which was 2.1 months longer than the overall median survival (5.8 months).
Table 1.
Baseline and primary tumor characteristics of patients who developed brain metastases (B-Met) identified from a cohort of 900 melanoma patients with prospective clinical follow-up.
| Variable | Patients without B-Met (n=811)* |
Patients with B-Met (n=89)* |
p-value† |
|---|---|---|---|
| Age at initial melanoma diagnosis (years) | 0.87 | ||
| Mean, SD | 57.1, 17.2 | 57.3, 16.1 | |
| Age at B-Met diagnosis (years) | NA | ||
| Mean, SD | NA | 60.3, 16.0 | |
| Sex | 0.25 | ||
| Male | 433 (53%) | 53 (60%) | |
| Female | 378 (47%) | 36 (40%) | |
| Time from initial melanoma diagnosis to B-Met (years) | NA | ||
| Median (range) | NA | 2.1 (2 mos - 13.3 years) | |
| Follow-up (years)§ | 0.23 | ||
| Median (range) | 2.4 (0.1 – 17.3) | 2.7 (0.4 – 17.7) | |
| AJCC stage at initial melanoma diagnosis** | <0.0001 | ||
| I | 501 (62%) | 24 (27%) | |
| II | 117 (14%) | 22 (25%) | |
| III | 149 (18%) | 31 (35%) | |
| IV | 44 (5%) | 11 (13%) | |
| Thickness (mm) | |||
| Median (range) | 0.95 (0.12 – 30) | 1.85 (0.21 – 30) | <0.0001 |
| <=2 | 596 (76%) | 40 (53%) | <0.0001 |
| >2 | 186 (24%) | 35 (47%) | |
| Anatomic site | 0.002 | ||
| Axial | 322 (41%) | 33 (43%) | |
| Head/Neck | 119 (15%) | 22 (29%) | |
| Extremity | 343 (44%) | 21 (28%) | |
| Histologic subtype | <0.0001 | ||
| Nodular | 199 (27%) | 40 (54%) | |
| Superficial spreading | 439 (59%) | 28 (38%) | |
| Other | 101 (14%) | 6 (8%) | |
| Ulceration | <0.0001 | ||
| Present | 132 (17%) | 36 (48%) | |
| Absent | 647 (83%) | 39 (52%) | |
| Regression | 0.75 | ||
| Present | 195 (27%) | 18 (25%) | |
| Absent | 535 (73%) | 54 (75%) | |
| Mitotic index (mitoses/mm2) | <0.0001 | ||
| Many | 136 (19%) | 32 (45%) | |
| Moderate | 110 (16%) | 12 (17%) | |
| Few | 260 (37%) | 19 (27%) | |
| None | 200 (28%) | 8 (11%) | |
| Lymphovascular invasion | 0.01 | ||
| Present | 64 (9%) | 13 (18%) | |
| Absent | 660 (91%) | 59 (82%) |
Sum may be less than total cohort due to patients with melanoma of unknown primary (n=27 non-B-Met cohort; n=13 B-Met cohort) or lack of data for given variable.
By Wilcoxon rank-sum test or chi-square test as appropriate.
Calculated based on all patients in the B-Met cohort (n=89) from the time of initial melanoma diagnosis and based on survivors (n=726) in the non-B-Met cohort (n=811) from the time of initial melanoma diagnosis. Median follow-up time based on survivors in the B-Met cohort (n=19 of 89) was 3.3 years (range: 0.4–17.7 years). P=0.22 for comparison of median follow-up time among survivors in both cohorts (median=2.4 years for non-B-Met vs. 3.3 years for B-Met, respectively).
Excludes 1 patient with mucosal melanoma in B-Met cohort.
Abbreviations: SD, standard deviation; NA, not applicable; AJCC, American Joint Committee on Cancer
Figure 1.
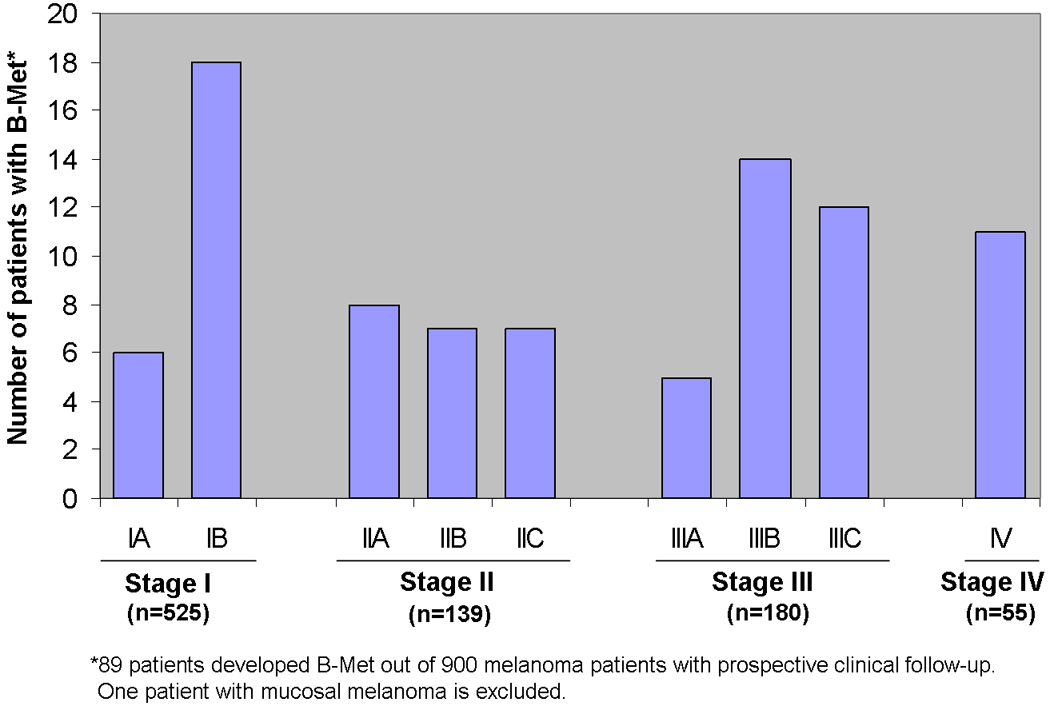
Melanoma patients who developed brain metastases (B-Met) stratified by AJCC clinical stage at time of initial melanoma diagnosis.
To assess the clinical and primary tumor characteristics associated with B-Met development, the 89 B-Met patients identified from the cohort of 900 were compared to the 811 patients who did not develop B-Met during the study period. The mean age at initial melanoma diagnosis was the same for both B-Met and non-B-Met patients (57 years)(Table 1). A higher percentage of B-Met patients were male (60% vs. 53%), although the difference was not statistically significant (p=0.25). Median follow-up time, based on survivors, for the non-B-Met cohort (n=726 survivors out of 811 patients without B-Met) was 2.4 years (range: 0.1–17.3 years). There were 85 deaths (10.5%) in the non-B-Met cohort: 63 died with melanoma, 16 died without melanoma, and 6 died with melanoma status unknown. There was no significant difference in the median follow-up time between the B-Met and non B-Met cohort (2.7 versus 2.4 years, respectively; p=0.23).
Primary tumor characteristics associated with development of B-Met
On univariate analysis, stage, thickness, anatomic site, histologic subtype, ulceration, mitotic index, and LVI were significantly associated with development of B-Met (Table 1). Patients with B-Met had thicker lesions (1.85 mm vs. 0.95 mm, p<0.0001) with a higher percentage of tumors located on the head and neck (29% vs. 15%, p=0.002). The B-Met patients had a higher frequency of nodular melanoma (54% vs. 27%, p<0.0001), ulcerated lesions (48% vs. 17%, p<0.0001), and a higher mitotic index (45% with “many” vs. 19%, p<0.0001). LVI was twice as prevalent in the B-Met group (18% vs. 9%, p=0.01). A multivariate analysis showed that primary anatomic site on the head and neck and ulceration remained independent predictors of B-Met development after adjusting for other variables (adjusted OR=2.52, p=0.003 and adjusted OR=3.08, p=0.001, respectively) (Table 2).
Table 2.
Multivariate analysis of primary melanoma clinicopathologic variables associated with development of brain metastases (B–Met).
| Variable* | OR | 95% CI | p-value |
|---|---|---|---|
| Thickness (mm) | |||
| >2 vs. ≤ 2 | 1.12 | 0.58 – 2.13 | 0.74 |
| Anatomic site | |||
| Head/Neck vs. Extremity/Axial | 2.52 | 1.37 – 4.65 | 0.003 |
| Histologic subtype | |||
| NM vs. SSM/other | 1.82 | 0.97 – 3.39 | 0.06 |
| Ulceration | 3.08 | 1.63 – 5.82 | 0.001 |
| Regression | 1.45 | 0.79 – 2.66 | 0.23 |
| Mitotic index (mitoses/mm2) | |||
| Many vs. None/Few/Moderate | 1.43 | 0.76 – 2.68 | 0.27 |
| Lymphovascular invasion | 1.15 | 0.55 – 2.43 | 0.71 |
Only patients with complete data for a given variable are included in the analysis.
Abbreviations: OR, odds ratio; CI, confidence interval; NM, nodular melanoma; SSM, superficial spreading melanoma
Clinical variables predictive of post-B-Met survival
Eight clinical variables present at the time of B-Met diagnosis were found to be significantly associated with post-B-Met survival on univariate analysis including age >65, the presence of extracranial disease, the number of B-Met lesions (at the time of initial B-Met diagnosis and cumulative), the specific location (frontal lobe vs. other), the localization of disease (unilateral vs. bilateral), the presence of neurological symptoms, the number of neurological symptoms, and the presence of weakness and fatigue (Table 3). Patients with solitary lesions (19%) had the longest median post-B-Met survival (11.9 months) compared to patients with ≥4 cumulative lesions (58%) who survived only 5.1 months (p=0.01). Patients without involvement of the frontal lobe survived twice as long as patients with frontal lobe involvement (10 vs. 4.9 months, p=0.01), and patients presenting with weakness or fatigue at the time of B-Met diagnosis had the lowest median survival of the cohort (2.2 months, p<0.0001). Variables indicative of systemic disease burden such as the presence of extracranial disease at B-Met diagnosis, the number of B-Met lesions, the presence of neurological symptoms at time of B-Met diagnosis, and the presence of weakness and/or fatigue were significant predictors of post-B-Met survival (Table 3).
Table 3.
Univariate analysis of clinical variables present at B-Met diagnosis associated with post-B-Met survival (n=89).
| Variable* | n (%) | Median Survival (months) |
p-value† |
|---|---|---|---|
| Age at B-Met diagnosis (years) | |||
| ≤ 65 | 49 (55) | 7.1 | 0.024 |
| > 65 | 40 (45) | 3.6 | |
| Extracranial disease at B-Met diagnosis | |||
| No | 32 (36) | 7.1 | 0.02 |
| Yes | 56 (63) | 3.7 | |
| Unknown | 1 (1) | ||
| Number of brain lesions | |||
| At B-Met diagnosis | |||
| 1 | 29 (33) | 10.0 | 0.001 |
| 2 | 15 (17) | 5.8 | |
| 3 | 11 (12) | 3.5 | |
| ≥4 | 34 (38) | 4.4 | |
| Cumulative | |||
| 1 | 17 (19) | 11.9 | 0.01 |
| 2 | 11 (12) | 7.2 | |
| 3 | 9 (10) | 6.3 | |
| ≥4 | 52 (58) | 5.1 | |
| B-Met specific location | |||
| Frontal lobe involvement | |||
| Yes | 61 (69) | 4.9 | 0.01 |
| No | 28 (31) | 10.0 | |
| B-Met localization | |||
| Left | 19 (21) | 6.7 | 0.04 |
| Right | 15 (17) | 9.9 | |
| Bilateral | 55 (62) | 4.6 | |
| Neurological symptoms | |||
| No | 26 (29) | 7.2 | 0.05 |
| Yes | 49 (55) | 4.6 | |
| Unknown | 14 (16) | ||
| Number of neurological symptoms | |||
| 0 | 26 (29) | 7.2 | 0.03 |
| 1 | 17 (19) | 6.3 | |
| 2 | 15 (17) | 4.3 | |
| ≥3 | 17 (19) | 3.4 | |
| Unknown | 14 (16) | ||
| Specific neurological symptom§ | |||
| Weakness/Fatigue | |||
| No | 35 (71) | 5.2 | <0.0001 |
| Yes | 14 (29) | 2.2 |
NOTE: percentages may not sum to 100% due to rounding.
Only statistically significant variables are displayed.
By log-rank test.
Of the 49 patients presenting with neurologic symptoms.
Abbreviations: B-Met, brain metastases
Primary tumor characteristics predictive of post-B-Met survival
On univariate analysis, ulceration, mitotic index, and LVI were significant predictors of post-B-Met survival (Table 4). Thickness of the primary melanoma, anatomic site, histologic subtype, and regression were not associated with post-B-Met survival. Patients with ulcerated primaries had a median post-B-Met survival of only 4.0 months compared to 9.1 months for patients without ulcerated lesions (p=0.004). The presence of LVI was strongly correlated with survival on univariate analysis. The median post-B-Met survival for patients with primary melanoma positive for LVI was only 1.3 months compared to 6.7 months for patients without LVI (p<0.0001, Table 4). Kaplan-Meier survival curves stratified by ulceration, the presence of LVI, and mitotic index are shown in Figure 2.
Table 4.
Univariate analysis of primary tumor clinicopathologic variables present at initial melanoma diagnosis associated with post-B-Met survival (n=76).*
| Variable† | n (%) | Median Survival (months) |
p-value§ |
|---|---|---|---|
| Ulceration | |||
| Present | 36 (47) | 4.0 | 0.004 |
| Absent | 39 (51) | 9.1 | |
| Unknown | 1 (1) | ||
| Mitotic Index (mitoses/mm2) | |||
| <3 (none/few) | 27 (36) | 9.2 | 0.009 |
| ≥3 (moderate/many) | 44 (58) | 4.9 | |
| Unknown | 5 (7) | ||
| Lymphovascular Invasion | |||
| Present | 13 (17) | 1.3 | <0.0001 |
| Absent | 59 (78) | 6.7 | |
| Unknown | 4 (5) |
NOTE: percentages may not sum to 100% due to rounding.
Excludes 13 patients with melanoma of unknown primary.
Only statistically significant variables are displayed.
By log-rank test.
Figure 2.
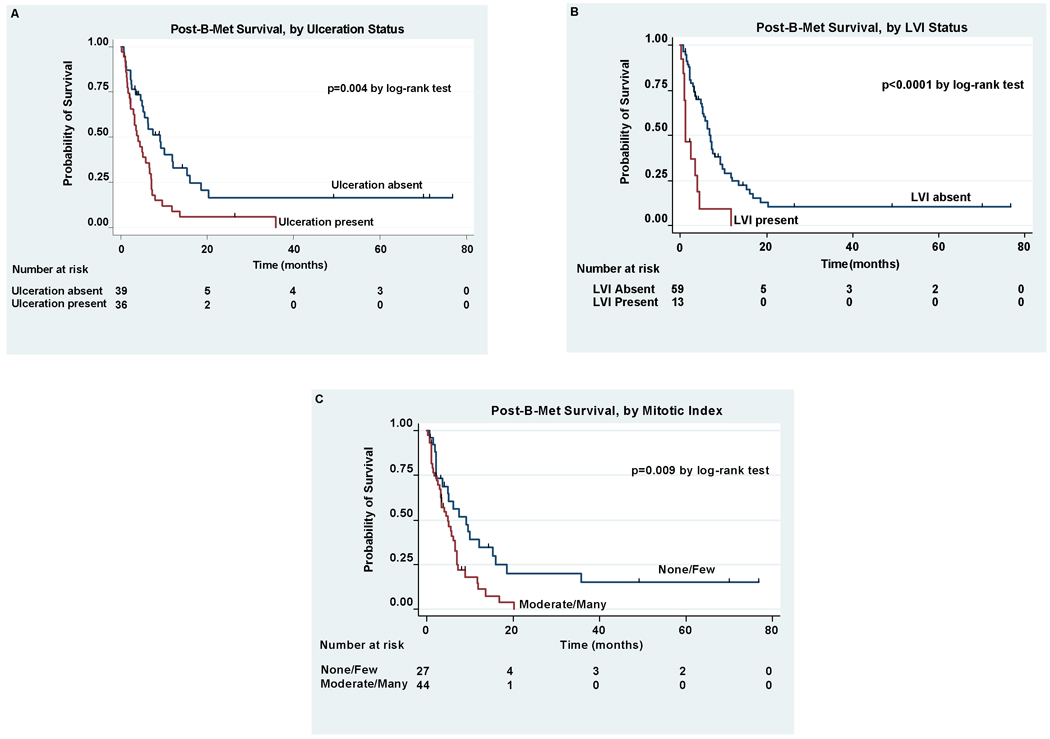
Kaplan-Meier estimates of post-B-Met survival for patients with or without primary tumor ulceration (A), with or without primary tumor LVI (B), and with none/few vs. moderate/many mitotic figures (C).
A multivariate Cox proportional hazards regression model inclusive of 2 clinical variables (presence of neurological symptoms and presence of extracranial metastases) and 2 primary tumor characteristics (ulceration and LVI) revealed 3 factors independently predictive of post-B-Met survival. Clinical variables that remained statistically significant on multivariate analysis included the presence of neurological symptoms (HR=2.5, p=0.008) and the presence of extracranial metastases at time of diagnosis (HR=2.0, p=0.04) (Table 5). The presence of ulceration was the only primary tumor characteristic that remained a significant predictor of post-B-Met survival on multivariate analysis (HR=2.5, p=0.04).
Table 5.
Multivariate analysis of clinical variables and primary tumor characteristics associated with post-B-Met survival.
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| Presence of neurological symptoms | 2.5 | 1.27 – 4.93 | 0.008 |
| Extracranial metastases | 2.0 | 1.05 – 3.87 | 0.04 |
| Ulceration | 2.5 | 1.07 – 6.03 | 0.04 |
| Lymphovascular invasion | 2.0 | 0.80 – 4.93 | 0.14 |
Abbreviations: HR, hazard ratio; CI, confidence interval
DISCUSSION
Our study of 900 prospectively enrolled melanoma patients shows that primary tumor characteristics maintain their prognostic relevance in patients with advanced melanoma. Patients with primary ulcerated melanoma on the head and neck may be at higher risk for development of B-Met and shorter post-B-Met survival. Consistent with prior studies, the presence of neurological symptoms and the presence of extracranial disease at B-Met diagnosis were the most significant clinical features predictive of post-B-Met survival.
Ulceration was the primary tumor characteristic most strongly associated with the development of B-Met and was the only feature of the primary melanoma significantly associated with post-B-Met survival on multivariate analysis. Increasing evidence demonstrates that the presence of ulceration portends a particularly poor prognosis independent of tumor thickness, prompting investigations into the biology of ulceration and the mechanism by which it adversely influences the clinical course. One such study revealed that elevated serum levels of pro-invasive matrix metalloproteinase-8 (MMP-8) were significantly associated with the presence of LVI, ulceration, and bleeding14. Thus, it is possible that ulceration is a marker of increased propensity for hematogenous dissemination, extracellular matrix invasion, and the subsequent development of B-Met independent of tumor thickness. Previous studies also suggest that only a small degree of ulceration is necessary to adversely affect outcome which is consistent with our data showing that ulceration remained a significant adverse prognostic factor independent of thickness15.
Conversely, some investigations of recurrent melanoma suggest that, by the time B-Met develops, primary tumor characteristics such as ulceration are no longer relevant and the subsequent trophic interactions with the neural microenvironment ultimately dictate the kinetics of tumorigenesis and the ultimate clinical course16. Our data suggest that ulceration of the primary does maintain its prognostic capacity even for advanced patients, thus it is possible that the presence of ulceration is indicative of a unique underlying biologic signature that promotes invasion and metastasis. Further investigation is required to determine the biologic characteristics of the primary melanoma that confer the propensity to metastasize to the brain. Given our findings, however, it is possible that the biologic mediators of ulceration may also play a role in the subsequent penetration of the blood-brain barrier. A recent study of brain metastases in breast cancer patients reported that expression levels of novel gene ST6GALNAC5 in primary breast tumors specifically promotes metastasis to the brain by enhancing adhesion and passage through the blood-brain barrier17. The association between ulceration and brain metastasis noted in the current study suggests that similar investigations are warranted in melanoma. To this end, our group is currently utilizing gene expression array, SNP array, microRNA array, and other genomic tools to identify a specific genetic signature present in the primary melanoma of patients with B-Met compared to patients with other sites of distant metastases.
Our prospectively collected survival data showing a median post-B-Met survival of 5.8 months is consistent with contemporary studies reporting median post-B-Met survival ranging from 3.8 to 5.2 months1, 3, 7, 18. A previous study of a cohort of 175 melanoma B-Met patients accrued between 1972–1978 showed an overall median post-B-Met survival of only 2.3 months19. Although our results show an increase in post-B-Met survival of approximately 3.5 months over the past 30 years, it may be a reflection of lead time bias conferred by the introduction of imaging techniques such as MRI. It is also possible, however, that advances in the treatment of CNS metastases including stereotactic radiosurgery (SRS) account for the modest increase observed in our data. Previous studies have demonstrated that aggressive treatment consisting of SRS followed by a biochemotherapy regimen consisting of dacarbazine or temozolomide plus interferon-α-2B or interleukin-2 can achieve a median post-B-Met survival of over 2 years in patients with limited CNS involvement and relatively well-controlled extra-cranial disease20–22. These studies also suggest that patients with B-Mets amenable to SRS or neurosurgery prior to the administration of combined biochemotherapy have response rates equivalent to patients without B-Met and thus should not be excluded from clinical trials20, 22. The success of SRS and neurosurgery depends largely upon patient selection, and survival rates are better for patients with controlled extra-cranial disease and a single B-Met21. Proponents of multidisciplinary treatment approaches for B-Met patients emphasize the need for early detection and subsequent aggressive treatment20.
Our data do not include details on the timing or sequence of treatment modalities, thus a direct comparison of survival rates based on the type of intervention was not undertaken. We do report, however, that only 36% of our patients accrued between 2002–2008 underwent craniotomy which is the same percentage reported in a previous retrospective study of B-Met patients accrued 10 years earlier (1991–2001).1 Although it is likely that the highly significant association between craniotomy and improved outcome observed in previous studies is related to selection bias, our results suggest that there has been no improvement in increasing the number of patients eligible to undergo surgical resection. It is possible that heightened surveillance protocols for patients with ulcerated lesions on the head and neck may be warranted, but we recognize that our data cannot be extrapolated to make specific recommendations regarding screening or secondary prevention. Although there are currently no prospective data to support specific follow-up regimens for melanoma, there are many who believe that more structured recommendations might result in earlier detection of solitary B-Mets that can be cured with SRS or neurosurgery. Surveillance guidelines for melanoma remain controversial and are typically left to the discretion of the treating physician as dictated by clinical signs and symptoms23. Radiographic surveillance for asymptomatic patients with localized melanoma is not recommended due to low sensitivity for the detection of occult metastases and the high rate of non-specific findings which have the potential to result in a series of further unnecessary and potentially risky diagnostic procedures24, 25. Similarly, the cumulative effect of long term radiation exposure in a patient with completely excised thin melanoma who is most likely cured of disease must also be considered26.
Given the limited progress of treatment development for melanoma B-Met patients over the past 30 years, it is possible that adjuvant, preventive strategies targeting the CNS may be warranted for patients at very high risk for the development of B-Met. Our results suggest that patients with ulcerated lesions may fall into this category. The current standard of care for melanoma does not include treatments designed to prevent CNS involvement. By contrast, recent data demonstrating that prophylactic cranial irradiation (PCI) reduces the number of symptomatic brain metastases and improves overall and cancer specific survival for patients with small cell lung cancer has lead to the recommendation that all patients who demonstrate a complete response to primary treatment be considered for PCI.20,21 Considering that the mean age at melanoma diagnosis is relatively young, the benefit in terms of life years lost may justify the consideration of prophylactic treatment in patients at high risk for B-Met who would not otherwise be considered candidates for adjuvant therapy. A recent study utilizing real-time in vivo imaging to capture the single events of the metastatic cascade in a mouse model of melanoma B-Met revealed discrete points when cells are the most inefficient at proliferating and thus likely the most vulnerable to inhibition27. In melanoma cells, the most inefficient step occurred early in the cascade, in the transition from single perivascular cell to micrometastasis. This was in contrast to lung cancer cells that were most inefficient at a later point in the cascade, in the transition from micrometastasis to macrometastasis, suggesting that melanoma cells en route to the brain might be targeted most effectively at an early point in the metastatic cascade27. Similarly, a subset of melanoma cells remained dormant over the course of the experiment yet showed a high degree of motility when they ultimately reached the brain via pre-existing vessels, whereas lung cancer cells demonstrated no such motility27. These results offer insight into the possible molecular mechanism underlying the delayed presentation of melanoma B-Met in some patients and may provide a rationale for treating patients at high risk for B-Met with prophylactic therapy early in the clinical course. Similarly, another animal study in breast cancer found that mice whose orthotopic tumors responded to treatment with a toll like receptor agonist prior to direct intra-cranial injection of tumor cells showed reduced tumor viability and eventual clearing of brain tumors compared to mice that had not been pre-treated28. Thus, rather than subjecting melanoma patients to PCI as is done in lung cancer, an alternative strategy could be to design a clinical trial in which patients with localized melanoma designated as high risk for B-Met based on clinicopathologic variables and the presence of a specific molecular signature are offered adjuvant treatment to evaluate the possibility of preventing future B-Mets.
In summary, our study revealed clinical and primary tumor characteristics associated with the development of B-Met and post-B-Met survival. Ulceration of the primary tumor was the strongest predictor of B-Met development and was an independent predictor of reduced post-B-Met survival. Our results emphasize the importance of considering primary melanoma tumor characteristics, especially ulceration, in the development of risk stratification and surveillance protocols early in the clinical course. Further investigations into the biology of ulceration and the molecular mechanism underlying its adverse impact on clinical outcome may be warranted.
ACKNOWLEDGEMENTS
We acknowledge the data management assistance provided by Ting Tu, BS and Daniel Krich, BA. The preliminary findings were presented at ASCO 2009, Orlando, FL.
Grant Support: This work was supported by the Chemotherapy Foundation, the National Cancer Institute Cancer Center Support Grant (5 P30 CA 016087-27), and the Marc Jacobs Campaign to support melanoma research.
Footnotes
Financial Disclosures: None to disclose.
REFERENCES
- 1.Sampson JH, Carter JH, Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88(1):11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 2.Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293–1300. doi: 10.1200/JCO.2004.08.140. [DOI] [PubMed] [Google Scholar]
- 3.Meier S, Baumert BG, Maier T, et al. Survival and prognostic factors in patients with brain metastases from malignant melanoma. Onkologie. 2004;27(2):145–149. doi: 10.1159/000076903. [DOI] [PubMed] [Google Scholar]
- 4.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 5.Thompson JF, Morton D, Kroon B. Textbook of Melanoma. vol. 1. London: Martin Dunitz; 2004. [Google Scholar]
- 6.Cruz-Munoz W, Man S, Xu P, Kerbel RS. Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res. 2008;68(12):4500–4505. doi: 10.1158/0008-5472.CAN-08-0041. [DOI] [PubMed] [Google Scholar]
- 7.Raizer JJ, Hwu WJ, Panageas KS, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199–207. doi: 10.1215/15228517-2007-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann MA, Coll SH, Kuchler I, et al. Prognostic factors and impact of treatment in melanoma brain metastases: better prognosis for women? Dermatology. 2007;215(1):10–16. doi: 10.1159/000102028. [DOI] [PubMed] [Google Scholar]
- 9.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimotty PA, Elder DE, Fraker DL, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol. 2007;25(9):1129–1134. doi: 10.1200/JCO.2006.08.1463. [DOI] [PubMed] [Google Scholar]
- 11.Eggermont Ulceration of primary melanoma and responsiveness to adjuvant interferon therapy : Analysis of the adjuvant trials EORTC18952 and EORTC18991 in 2644 patients. J Clin Oncol. 2009;15s(27) suppl abstr 9007. [Google Scholar]
- 12.Baurain Association of primary melanoa ulceration and clinical benefit of adjuvant vaccination with tumor-specific antigen peptides. J Clin Oncol. 2009;27(15s) suppl abstr 3022. [Google Scholar]
- 13.Wich LG, Hamilton HK, Shapiro RL, et al. Developing a multidisciplinary prospective melanoma biospecimen repository to advance translational research. Am J Transl Res. 2009;1(1):35–43. [PMC free article] [PubMed] [Google Scholar]
- 14.Vihinen P, Koskivuo I, Syrjanen K, Tervahartiala T, Sorsa T, Pyrhonen S. Serum matrix metalloproteinase-8 is associated with ulceration and vascular invasion of malignant melanoma. Melanoma Res. 2008;18(4):268–273. doi: 10.1097/CMR.0b013e3283090031. [DOI] [PubMed] [Google Scholar]
- 15.Grande Sarpa H, Reinke K, Shaikh L, et al. Prognostic significance of extent of ulceration in primary cutaneous melanoma. Am J Surg Pathol. 2006;30(11):1396–1400. doi: 10.1097/01.pas.0000213262.61855.7d. [DOI] [PubMed] [Google Scholar]
- 16.Soong SJ, Shaw HM, Balch CM, McCarthy WH, Urist MM, Lee JY. Predicting survival and recurrence in localized melanoma: a multivariate approach. World J Surg. 1992;16(2):191–195. doi: 10.1007/BF02071520. [DOI] [PubMed] [Google Scholar]
- 17.Bos PD, Zhang XH, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panagiotou IE, Brountzos EN, Kelekis DA, Papathanasiou MA, Bafaloukos DI. Cerebral metastases of malignant melanoma: contemporary treatment modalities and survival outcome. Neoplasma. 2005;52(2):150–158. [PubMed] [Google Scholar]
- 19.Madajewicz S, Karakousis C, West CR, Caracandas J, Avellanosa AM. Malignant melanoma brain metastases. Review of Roswell Park Memorial Institute experience. Cancer. 1984;53(11):2550–2552. doi: 10.1002/1097-0142(19840601)53:11<2550::aid-cncr2820531129>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Majer M, Jensen RL, Shrieve DC, et al. Biochemotherapy of metastatic melanoma in patients with or without recently diagnosed brain metastases. Cancer. 2007;110(6):1329–1337. doi: 10.1002/cncr.22905. [DOI] [PubMed] [Google Scholar]
- 21.Mathieu D, Kondziolka D, Cooper PB, et al. Gamma knife radiosurgery in the management of malignant melanoma brain metastases. Neurosurgery. 2007;60(3):471–481. doi: 10.1227/01.NEU.0000255342.10780.52. discussion 81–2. [DOI] [PubMed] [Google Scholar]
- 22.Boasberg PD, O'Day SJ, Kristedja TS, et al. Biochemotherapy for metastatic melanoma with limited central nervous system involvement. Oncology. 2003;64(4):328–335. doi: 10.1159/000070289. [DOI] [PubMed] [Google Scholar]
- 23.Coit D, Andtbacka R, Bichakjian C, et al. Melanoma. Journal of the National Comprehensive Cancer Network. 2009;7(3):250–275. doi: 10.6004/jnccn.2009.0020. [DOI] [PubMed] [Google Scholar]
- 24.Yancovitz M, Finelt N, Warycha MA, et al. Role of radiologic imaging at the time of initial diagnosis of stage T1b–T3b melanoma. Cancer. 2007;110(5):1107–1114. doi: 10.1002/cncr.22868. [DOI] [PubMed] [Google Scholar]
- 25.Buzaid AC, Sandler AB, Mani S, et al. Role of computed tomography in the staging of primary melanoma. J Clin Oncol. 1993;11(4):638–643. doi: 10.1200/JCO.1993.11.4.638. [DOI] [PubMed] [Google Scholar]
- 26.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 27.Kienast Y, von Baumgarten L, Fuhrmann M, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 16(1):116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 28.Xiong Z, Gharagozlou S, Vengco I, Chen W, Ohlfest JR. Effective CpG immunotherapy of breast carcinoma prevents but fails to eradicate established brain metastasis. Clin Cancer Res. 2008;14(17):5484–5493. doi: 10.1158/1078-0432.CCR-07-4139. [DOI] [PubMed] [Google Scholar]


