Abstract
Background
RTOG 0515 is a Phase II prospective trial designed to quantify the impact of PET/CT compared to CT alone on radiation treatment plans (RTPs) and to determine the rate of elective nodal failure for PET/CT derived volumes.
Methods
Each enrolled patient underwent definitive radiation therapy for NSCLC (≥60 Gy) and had two RTP datasets generated: gross tumor volume (GTV) derived with CT alone and with PET/CT. Patients received treatment using the PET/CT-derived plan. The primary endpoint, the impact of PET/CT fusion on treatment plans was measured by differences of the following variables for each patient: GTV, number of involved nodes, nodal station, mean lung dose (MLD), volume of lung exceeding 20 Gy (V20), and mean esophageal dose (MED). Regional failure rate was a secondary endpoint. The nonparametric Wilcoxon matched-pairs signed-ranks test was used with Bonferroni adjustment for an overall significance level of 0.05.
Results
RTOG 0515 accrued 52 patients, 47 of whom are evaluable. The follow-up time for all patients is 12.9 months (2.7–22.2). Tumor staging was as follows: II = 6%; IIIA = 40%; and IIIB = 54%. The GTV was statistically significantly smaller for PET/CT-derived volumes (98.7 vs. 86.2 cc; p<0.0001). MLDs for PET/CT plans were slightly lower (19 vs. 17.8 Gy; p=0.06). There was no significant difference in the number of involved nodes (2.1 vs. 2.4), V20 (32% vs. 30.8%), or MED (28.7 vs. 27.1 Gy). Nodal contours were altered by PET/CT for 51% of patients. One patient (2%) has developed an elective nodal failure.
Conclusions
PET/CT-derived tumor volumes were smaller than those derived by CT alone. PET/CT changed nodal GTV contours in 51% of patients. The elective nodal failure rate for GTVs derived by PET/CT is quite low, supporting the RTOG standard of limiting the target volume to the primary tumor and involved nodes.
Keywords: Lung Cancer, FDG-PET, Mediastinal nodal staging, mediastinum
Introduction
The use of positron emission tomography (PET) with 2-[18F]fluoro-2-deoxy-D-glucose (FDG) for staging newly-diagnosed non-small cell lung cancer (NSCLC) has expanded rapidly over the past 15 years.(1) With the expansion of diagnostic PET and PET/CT, the ability for radiation oncologists to use these images for radiation treatment planning has become widespread.(2) The overall goal of this comparative trial is to determine the impact of FDG-PET on specific radiation treatment planning parameters including tumor volumes, the number and location of involved nodes, dosimetric measures of normal tissue radiation dose, and failure in elective lymph nodes (i.e. non-targeted lymph nodes).
Materials and Methods
Patient Selection
Because this trial was primarily a comparison study of CT versus PET/CT for radiation treatment planning, the patient eligibility criteria were intentionally inclusive. Patients had pathologically proven Stage II or III(3) non-small cell lung cancer (NSCLC) and received either radiation alone or chemoradiation therapy as primary management of their lung cancer. The minimal radiation dose for inclusion was ≥60 Gy. Tissue heterogeneity calculation algorithms were required. Intensity-modulated radiation therapy was not allowed. Patients must have been ≥18 years old and have a Zubrod performance status of 0–2. Patient workup included a history and physical examination, contrast-enhanced chest and abdomen CT, contrast-enhanced magnetic resonance imaging or CT of the brain, and a diagnostic PET or PET/CT each within 8 weeks of registration. The diagnostic PET or PET/CT could serve as the radiation treatment planning CT as long as the imaging was acquired under conditions simulating radiation therapy. These restrictions included use of a flat tabletop and the same immobilization as was employed for radiation treatments.
PET Acquisition
Participating facilities could use either a dedicated PET scanner or a dedicated PET/CT scanner. PET scanners that were credentialed for ongoing PET/CT trials within the American College of Radiology Imaging Network (ACRIN 6668 and 6665) were automatically approved for this trial. PET imaging guidelines established within ACRIN scientific protocols were used, including both technical and patient preparation parameters (http://www.acrin.org/CORELABS/PETCORELABORATORY/PETSOPS/tabid/484/Default.aspx). Software fusion of separately obtained planning CT and planning PET was allowed as long as the PET was obtained under the conditions used in radiation therapy, as noted above. In this circumstance, the patient was immobilized supine with the arms overhead. Image co-registration was achieved with 6–8 fiducial markers placed externally, either on the patient or on the immobilization device itself. For co-registration, metallic markers were used as external fiducials for the CT portion. Markers containing FDG were used for the PET portion. If PET/CT was used for radiation treatment planning, the patient positioning requirement was the same, but no fiducials were required.
Target Volumes
PET images were interpreted by a nuclear physician or radiologist from the institution entering the patient on trial. Standardized uptake values were not used to delineate the tumor and involved nodes. Primary and nodal gross tumor volume (GTV) contours were based on the volume of the lung mass or enlarged lymph node on CT. Traditionally within RTOG trials, nodes measuring > 10 mm in short axis and/or pathologically positive have been contoured as nodal GTV. Therefore, lymph nodes having short axis diameter > 10 mm and/or demonstrating increased FDG uptake relative to mediastinal background on PET were contoured and irradiated as nodal GTV. The distinction between benign-appearing lymph nodes versus malignant-appearing lymph nodes based on FDG-PET, was left to the interpreting nuclear radiologist at the treatment facility. Likewise, the shape of the primary tumor on CT was used for GTV contouring, without using specific window and level settings on the PET component. In the case of lung atelectasis, the delineation of the mass based on the PET/CT was determined by the treating radiation oncologist and nuclear physician/radiologist. GTV contours were expanded volumetrically 15 mm in both CT-only and PET/CT datasets to create the planning treatment volume (PTV). No separate clinical target volume (CTV) was generated. Clinically uninvolved nodal regions (elective lymph nodes) were not intentionally irradiated. The radiation dose (≥60 Gy) was prescribed so that 95% of the dose covered 100% of the PTV for both planned datasets. Regardless of the total prescribed dose, the same dose was required to be used for planning both the CT-derived and PET/CT-derived radiation therapy plans.
Two plans were generated for each patient; one using PET/CT and one using CT alone. The treating radiation oncologist was responsible for defining radiation volumes and delivering radiation therapy based on PET/CT. The responsibility for defining the radiation treatment volumes for the CT-derived plan (comparison study) was assigned to one of two alternating radiation oncologists per facility. This responsibility was alternated and tracked for each of the ten enrolling facilities. The physicians contouring the CT-only plans were not given access to the PET or PET/CT images.
Central Review
Prior to enrollment of its first patient, each facility was required to complete a protocol-specific dry-run case for the PET/CT fusion dataset by successfully completing electronic transfer of images, contours, and radiation doses to the Image-guided Therapy Center (ITC, St. Louis, Missouri). This was typically a non-protocol patient where PET/CT fusion was used for radiation treatment planning. The test case was reviewed for legibility only. Once approved for enrollment, facilities submitted three types of data; PET/CT credentialing data, PET/CT images, and two radiation therapy treatment plans for central review by the principal investigator. The PET/CT fusion and CT-only datasets were reviewed for accuracy of image fusion, GTV contours, normal lung tissue contours, esophagus contours, and resultant dose-volume histograms for GTV, total lung, and esophagus.
Statistical Methods
Study Endpoints
The primary endpoint of the study was to compare the CT- and PET/CT-based treatment plans of the absolute value of the differences for each patient with respect to the following six measures: the GTV, the number of involved nodes, the location of the involved nodal stations, and for accepted measures in normal tissue toxicity including total mean lung dose (MLD), the volume of normal lung exceeding 20 Gy (V20) (both lungs minus PTV), and the mean esophageal dose (MED). The secondary endpoint was to determine the rate of elective nodal failures (nodal failures in regions not intentionally irradiated to definitive doses).
Patient Follow Up
Following treatment completion, patients were seen in follow up at 3 months, then every six months for two years, and yearly thereafter. History and physical examination, Zubrod performance status, and computed tomography of the chest and abdomen was required for each follow up visit. Brain (CT or MRI) and/or PET imaging was left to the discretion of the treating physician.
Study Design
The sample size was designed using data from patients treated on RTOG 9311 to calculate standard deviations (SD) for each of the five measures. A one-sided, one-sample paired t-test was used with 85% statistical power and overall significance level of 0.05 (0.01 for each measure). In order to detect a clinically meaningful difference of 15 cc in absolute value GTV and the estimated SD of 26.2, a sample size of 38 patients was required. A clinically meaningful difference in number of involved nodes was 1. The required sample size to detect this magnitude of difference with an estimated STD of 0.82 was 11 patients. Mean lung dose, V20, and MED of NSCLC patients using CT scans were estimated to be 15 Gy with SD of 4.61 Gy, 24% with SD of 8.9%, and 22 Gy with SD of 12.8 Gy), respectively, from RTOG 9311 data. The required sample sizes to detect a clinically meaningful difference of 20% or 3 Gy in MLD, 4.8% in V20, and 30% or 6.6 Gy in MED, were 30, 42, and 45 patients, respectively. A sample size of 45 patients assures at least 85% statistical power to detect the clinically meaningful difference for each of the five measurements.
Analysis Methods
The differences of CT and PET/CT fusion in the five measurements for the primary endpoint were obtained by subtracting each patient’s CT measurements from the PET/CT fusion measurements. A nonparametric Wilcoxon matched-pairs signed-ranks test was applied. The test for each measurement was performed at a significance level of 0.01 using the Bonferroni multiple-comparisons adjustment. The percent of nodal locations in agreement described the strength of agreement of the two scans regarding the location of involved and uninvolved nodes. For each patient, the percent of nodal locations in agreement as determined by CT only vs. PET/CT was calculated by lymph node stations defined by the AJCC Cancer Staging Manual, 6th ed.(3) The mean percent of this measure was combined into a summary statistic for all patients. A 95% confidence interval using the normal approximation for a proportion was constructed. Nodal failure was defined by the Response Evaluation Criteria in Solid Tumors (RECIST) on follow up CT imaging.(4)
Results
Between February 2006 and February 2008, fifty-two patients were enrolled from eight participating centers. Forty-seven of the 52 were evaluable and are the subjects of this analysis. The other five patients were not evaluable for the following reasons; metastatic disease detected on planning PET/CT, malignant pleural effusion detected on PET/CT, chest CT performed >8 weeks prior to registration (n=2); and PET/CT not available for planning because of technical problems. The primary and secondary endpoint data were scored separately and submitted to RTOG for statistical review. Imaging datasets for 47 patients were also sent to the ITC. 13 datasets were not reviewable because of incomplete data submission (either PET/CT images, PET/CT contours, or CT-only contours). There were no differences in patient characteristics between the reviewable and non-reviewable plans. The non-reviewable datasets were randomly distributed. The remaining 34 datasets were reviewed. 32 patients had plans that were protocol compliant. 2 plans were not per protocol; one patient had an FDG-avid node that was not included within the planning target volume (PTV) and another patient had bilateral mediastinal non-FDG avid nodal stations included within the treated PTV.
The median follow up time was 12.9 months (range 2.7 – 22.2 months) for all patients and 13.6 months (range 5.9 – 22.2 months) for surviving patients. Pretreatment characteristics are shown in Table 1. The median age was 64 years (range 46–84 years), and 36% of patients were women. Most had good or excellent performance status and had Stage III disease.
Table 1.
Pretreatment Characteristics (n=47)
| Age | ||
| Median | 64 | |
| Range | 46–84 | |
| n | % | |
| Gender | ||
| Male | 30 | 64 |
| Female | 17 | 36 |
| Zubrod Performance Status | ||
| 0 | 28 | 60 |
| 1 | 15 | 32 |
| 2 | 4 | 9 |
| Stage | ||
| IIA | 1 | 2 |
| IIB | 2 | 4 |
| IIIA | 19 | 40 |
| IIIB | 25 | 53 |
| Histology | ||
| Squamous | 18 | 38 |
| Adenocarcinoma | 10 | 21 |
| Large cell undifferentiated | 1 | 2 |
| Non-small cell carcinoma, NOS | 18 | 38 |
| Neoadjuvant Chemotherapy | ||
| N/A - Registered prior to revision | 11 | 23 |
| No | 18 | 38 |
| Yes | 18 | 38 |
Table 2 shows results for the primary endpoints. The mean GTV for PET/CT was 86.2 cm3 vs. 98.7 cm3 for CT alone (p <0.0001). Figure 1 shows an example of reduced GTV using PET-defined tumor volumes in a case of left upper lobe atelectasis. The resultant MLD for the PET/CT-derived target volume was slightly smaller at 17.8 Gray (Gy) vs. 19 Gy, although this did not reach statistical significance (p =0.06). There was no significant difference between the PET/CT- or CT-derived number of involved nodes (p=0.41), V20 (p=0.21), or MED (p=0.3).
Table 2.
Differences Between CT Only and PET/CT
| Variable | CT only | PET/CT | Difference (PET/CT – CT only) | p-value† |
|---|---|---|---|---|
| GTV Primary Volume (cm3) | <0.0001‡ | |||
| Mean (standard deviation) | 98.7 (102.5) | 86.2 (88.1) | −12.5 (61.5) | |
| Median (Range) | 66.1 (2.3–441.7) | 59.9 (0.7–471.2) | −4.4 (−215.3–112.9) | |
| Number of Included Nodes | 0.41 | |||
| Mean (standard deviation) | 2.1 (2.1) | 2.4 (2.3) | 0.2 (2.0) | |
| Median (Range) | 2 (0–10) | 2 (0–12) | 0 (−6–10) | |
| Mean Lung Dose | 0.06 | |||
| Mean (standard deviation) | 19.0 (8.0) | 17.8 (7.2) | −1.2 (4.0) | |
| Median (Range) | 19.0 (7.2–49.0) | 16.3 (6.3–43.6) | −0.4 (−13.1–6.4) | |
| V20 | 0.21 | |||
| Mean (standard deviation) | 32.0 (13.8) | 30.8 (14.4) | −1.1 (6.2) | |
| Median (Range) | 31 (10–80) | 27 (9–74) | −1 (−13–13) | |
| Mean Esophageal Dose | 0.30 | |||
| Mean (standard deviation) | 28.7 (12.6) | 27.1 (10.8) | −1.4 (9.4) | |
| Median (Range) | 26.8 (0.9–57.5) | 25.1 (8.9–57.1) | −1.8 (−25.3–21.3) |
Wilcoxon Matched-Pairs Signed-Ranks test
Figure 1.
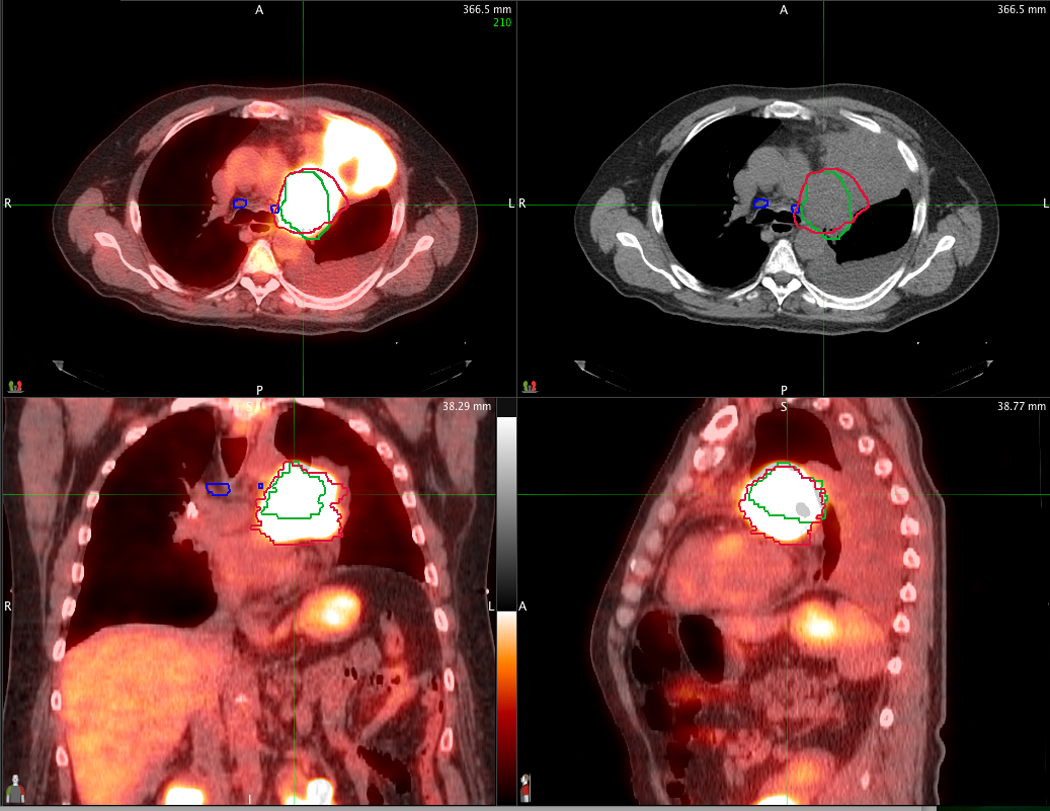
An example of the difference between the defined GTV between PET/CT (red contour) and CT alone (green contour) in a patient with left upper lobe atelectasis. PET/CT images are displayed in axial, sagittal and coronal planes through the tumor. The corresponding CT alone axial image is displayed on the upper right.
The agreement between the location of nodes treated for each plan is shown in Tables 3 and 4. Complete agreement for all 13 mediastinal and hilar nodal stations occurred in 23 of 47 patients (49%). Disagreements between PET/CT and CT-only nodal stations occurred in 24 patients (51%), and was mainly confined to one or two stations (20/24 patients). The mean agreement is 92% (95% CI=88, 96). Figures 2 and 3 show examples of disagreement for the supraclavicular and subcarinal nodal stations, respectively. Nodal failure in non-targeted lymph nodes has occurred in only one patient (2%) (Table 5).
Table 3.
Involved Nodes
| Agree – Included |
Disagree – Included in CT Plan Only |
Disagree – Included in PET/CT Plan Only |
Agree – Not Included |
|||||
|---|---|---|---|---|---|---|---|---|
| Nodal Station | n | % | n | % | n | % | n | % |
| Highest Mediastinal | 2 | 4 | 2 | 4 | 2 | 4 | 41 | 87 |
| Upper Paratracheal – Right | 5 | 11 | 2 | 4 | 6 | 13 | 34 | 72 |
| Upper Paratracheal – Left | 2 | 4 | 1 | 2 | 1 | 2 | 43 | 91 |
| Pre-vascular and Retrotracheal | 5 | 11 | 1 | 2 | 1 | 2 | 40 | 85 |
| Lower Paratracheal – Right (including Azygos nodes) | 16 | 34 | 1 | 2 | 0 | 0 | 30 | 64 |
| Lower Paratracheal – Left (including Azygos nodes) | 1 | 2 | 2 | 4 | 3 | 6 | 41 | 87 |
| Subaortic (A-P window) | 4 | 9 | 3 | 6 | 4 | 9 | 36 | 77 |
| Para-aortic (ascending aortic or phrenic) | 4 | 9 | 0 | 0 | 2 | 4 | 41 | 87 |
| Subcarinal | 12 | 26 | 2 | 4 | 2 | 4 | 31 | 66 |
| Paraesophageal (below carina) | 2 | 4 | 1 | 2 | 1 | 2 | 43 | 91 |
| Pulmonary Ligament | 0 | 0 | 0 | 0 | 0 | 0 | 47 | 100 |
| Hilar – Right | 12 | 26 | 1 | 2 | 5 | 11 | 29 | 62 |
| Hilar – Left | 4 | 9 | 1 | 2 | 5 | 11 | 37 | 79 |
Table 4.
Agreement of CT- and PET/CT Defined Nodal Stations
| % Agreement | n | % |
|---|---|---|
| 23% (3 of 13 agree) | 1 | 2 |
| 54% (7 of 13 agree) | 1 | 2 |
| 69% (9 of 13 agree) | 1 | 2 |
| 77% (10 of 13 agree) | 1 | 2 |
| 85% (11 of 13 agree) | 6 | 13 |
| 92% (12 of 13 agree) | 14 | 30 |
| 100% (13 of 13 agree) | 23 | 49 |
| Mean | 92% | |
| (95% CI) | (88%–96%) | |
Figure 2.
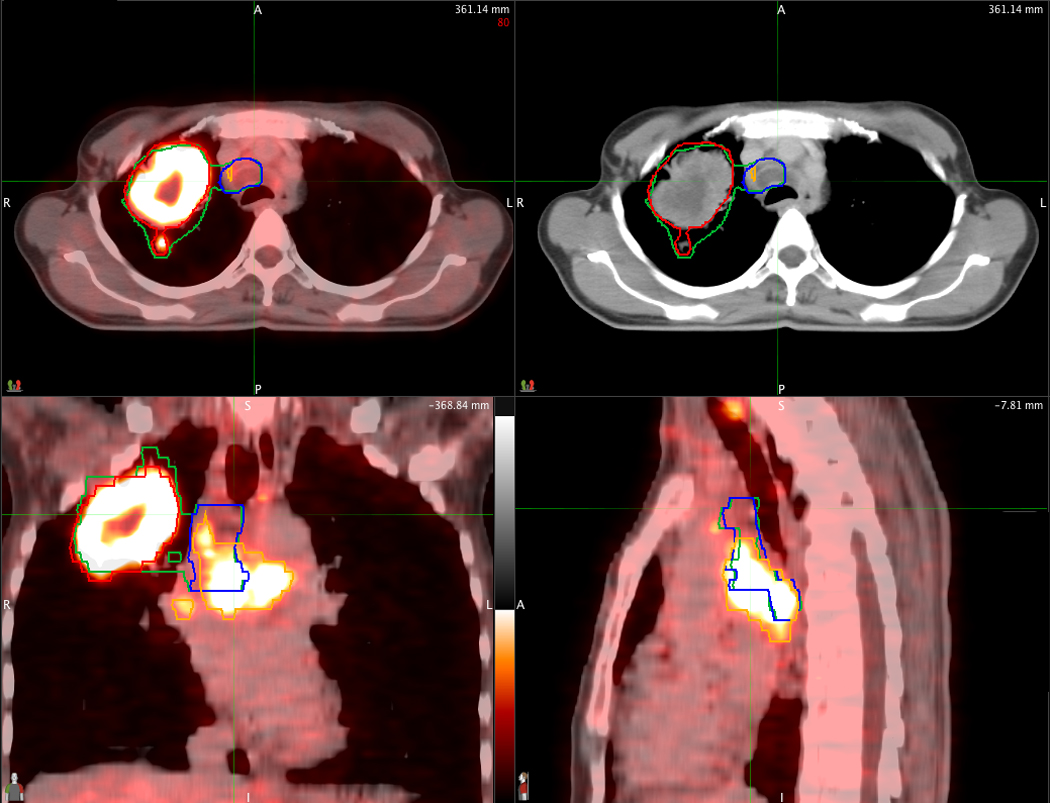
An example of PET/CT-derived nodal GTV (orange contour) which differs from the nodal GTV defined on CT alone (blue contour). PET/CT images are displayed in axial, sagittal and coronal planes through the tumor. The corresponding axial image from CT alone is displayed on the upper right.
Figure 3.
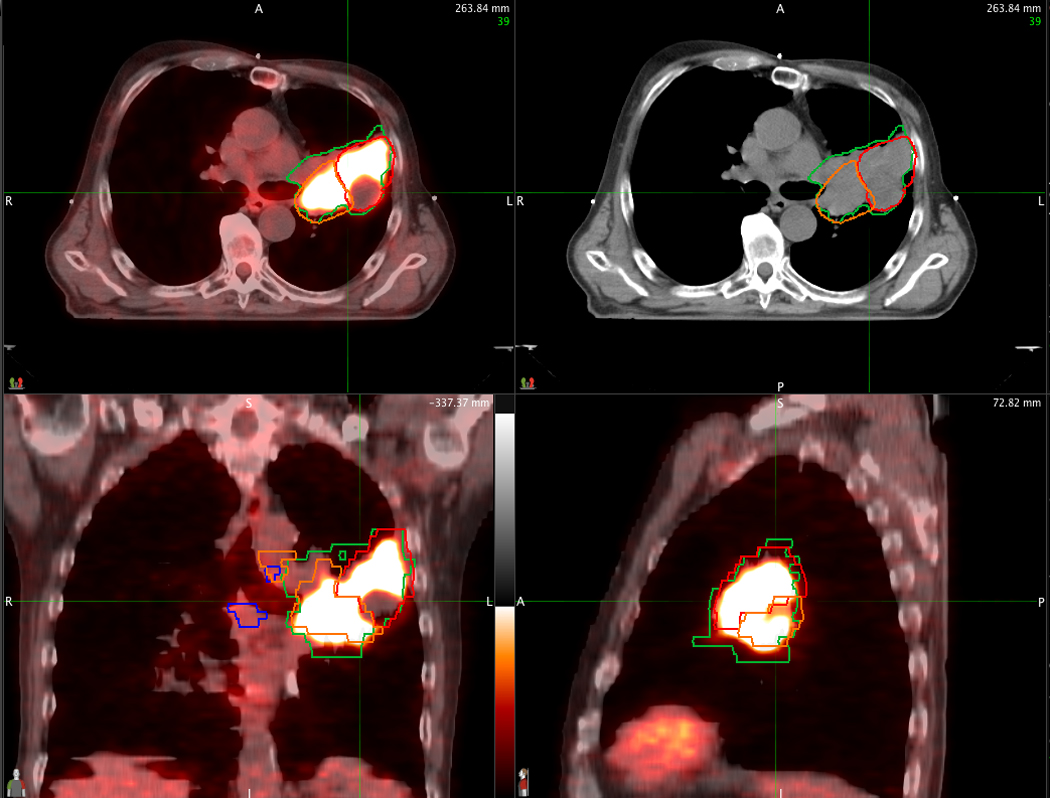
An example of a PET/CT-derived GTV within the left upper lobe (red contour) and left hilum (orange contour), and excluding the subcarinal node, compared to the CT alone-derived GTV that includes the subcarinal node (blue contours). The corresponding axial image from CT alone is displayed on the upper right.
Table 5.
Nodal Failure Rate (n=46*)
| Failure in Non-Targeted Nodes | Failure in Targeted Nodes | |||
|---|---|---|---|---|
| n | % | n | % | |
| Alive, no failure | 29 | 63 | 30 | 65 |
| Failure | 1 | 2 | 1 | 2 |
| Dead, no failure | 16 | 35 | 15 | 33 |
1 patient did not have follow-up information
Discussion
Mediastinal nodal staging of NSCLC with FDG-PET represents a substantial improvement over CT alone. Toloza et al. reported a pooled analysis of sensitivities and specificities for CT and PET compared to pathological staging of the mediastinum.(5) For CT, the pooled sensitivity and specificity were 0.57 and 0.82, respectively. For PET, the pooled sensitivity and specificity were 0.84 and 0.89, respectively. Thus, it is natural to incorporate FDG-PET information for defining radiation therapy volumes for NSCLC.
Many centers have adopted PET or PET/CT fusion into radiation treatment planning for NSCLC. There are several reported studies that measure the impact of PET or PET/CT on radiation treatment planning (Table 6). Among these, the overall impact in terms of change in radiation treatment planning based on PET for NSCLC ranged from 34–100%. Changes included the decision of whether or not to treat the patient for cure or palliation, what volumes to treat, or what radiation dose to prescribe. The methods of determining the impact of PET varied from series to series, but included visual side-by-side comparisons, hardware fusions, and software fusions. Since the adoption of FDG-PET into radiation treatment planning is occurring at a rapid pace, the RTOG wanted to conduct a comparative study to measure the impact that will occur in future trials. Although the studies listed in Table 6 make a statement that FDG-PET has an impact, there are no multi-institutional prospective studies with pre-defined endpoints that clearly elucidate the changes that occur.
Table 6.
Impact of FDG-PET on radiation planning in patients with NSCLC
| Author | Number of patients | Fusion method | Impact on radiation planning |
|---|---|---|---|
| Nestle(1) | 34 | Visual | 35% |
| Kiffer(2) | 15 | Visual | 47% |
| Vanuystel(3) | 73 | Software | 67% |
| Munley(4) | 35 | Visual | 34% |
| Brianzoni(5) | 24 | Hardware | 50% |
| Kalff(6) | 105 | Visual | 50% |
| MacManus(7) | 102 | Visual | 67% |
| Mah(8) | 30 | Software | 40% |
| Giraud(9) | 11 | Software | 45% |
| Erdi(10) | 11 | Software | 100% |
| Bradley(11) | 26 | Software | 58% |
| Deniaud-Alexandre(12) | 92 | Visual | 49% |
| Faria(13) | 32 | Hardware | 56% |
Nestle U, Walter K, Schmidt S, et al. 18F-deoxyglucose positron emission tomography (FDG-PET) for the planning of radiotherapy in lung cancer: high impact in patients with atelectasis. Int J Radiat Oncol Biol Phys 1999;44:593–597.
Kiffer JD, Berlangieri SU, Scott AM, et al. The contribution of 18F-fluoro-2-deoxy-glucose positron emission tomographic imaging to radiotherapy planning in lung cancer. Lung Cancer 1998;19:167–177.
Vanuytsel LJ, Vansteenkiste JF, Stroobants SG, et al. The impact of (18)F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) lymph node staging on the radiation treatment volumes in patients with non-small cell lung cancer. Radiother Oncol 2000;55:317–324.
Munley MT, Marks LB, Scarfone C, et al. Multimodality nuclear medicine imaging in three-dimensional radiation treatment planning for lung cancer: challenges and prospects. Lung Cancer 1999;23:105–114.
Brianzoni E, Rossi G, Ancidei S, et al. Radiotherapy planning: PET/CT scanner performances in the definition of gross tumour volume and clinical target volume. Eur J Nucl Med Mol Imaging 2005;32:1392–1399.
Kalff V, Hicks RJ, MacManus MP, et al. Clinical impact of (18)F fluorodeoxyglucose positron emission tomography in patients with non-small-cell lung cancer: a prospective study. J Clin Oncol 2001;19:111–118.
MacManus MP, Hicks RJ, Matthews JP, et al. High rate of detection of unsuspected distant metastases by pet in apparent stage III non-small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys 2001;50:287–293.
Mah K, Caldwell CB, Ung YC, et al. The impact of (18)FDG-PET on target and critical organs in CT-based treatment planning of patients with poorly defined non-small-cell lung carcinoma: a prospective study. Int J Radiat Oncol Biol Phys 2002;52:339–350.
Giraud P, Grahek D, Montravers F, et al. CT and (18)F-deoxyglucose (FDG) image fusion for optimization of conformal radiotherapy of lung cancers. Int J Radiat Oncol Biol Phys 2001;49:1249–1257.
Erdi YE, Rosenzweig K, Erdi AK, et al. Radiotherapy treatment planning for patients with non-small cell lung cancer using positron emission tomography (PET). Radiother Oncol 2002;62:51–60.
Bradley J, Thorstad WL, Mutic S, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;59:78–86.
Deniaud-Alexandre E, Touboul E, Lerouge D, et al. Impact of computed tomography and 18F-deoxyglucose coincidence detection emission tomography image fusion for optimization of conformal radiotherapy in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2005;63:1432–1441.
Faria SL, Menard S, Devic S, et al. Impact of FDG-PET/CT on radiotherapy volume delineation in non-small-cell lung cancer and correlation of imaging stage with pathologic findings. Int J Radiat Oncol Biol Phys 2008;70:1035–1038.
Given that PET/CT has demonstrated superiority over CT alone in staging the mediastinum, patients on this trial were treated using radiotherapy volumes that were defined by PET/CT. Radiation treatment volumes only included FDG-avid disease. Elective nodal irradiation was omitted. Our secondary endpoint was to determine the rate of elective nodal failures using PET/CT-based treatment planning.
It is important to note that the PET-based GTVs were smaller than those derived from CT alone (86.2 vs 98.7 cm3; p<0.0001). There was no difference in the mean number of involved nodal stations (2.4 vs 2.1; p=0.41). Therefore the reduction in GTV is likely the result of improved clarification by PET of tumor margins in cases where there is associated atelectasis (Figure 1). As would be expected, smaller tumor volumes led to reduced mean lung doses (17.8 vs 19.0 Gy; p=0.06).
There were no differences in the V20 values or mean esophagus doses between datasets. Whereas mean lung doses reflect both high-dose and low-dose regions, they were more affected by the differences in the GTV receiving the prescribed dose. V20 values reflect the volume of the 20 Gy isodose line. This low-dose value is not likely to be affected much in cases where the mean number of lymph nodes is similar or, alternatively, where both datasets have atelectasis that is excluded from the total lung volume measurement. Likewise, since the mean number of involved lymph nodal stations was similar on both datasets, the mean esophagus doses would be expected to be similar.
Optimally, clarification of the impact of FDG-PET would include descriptions of agreement or disagreement by lymph node station. Thus, Table 4 shows the level of agreement and disagreement between PET/CT and CT-only plans for each patient. For the purposes of this study, agreement was defined as whether or not a nodal structure was contoured for each of the thirteen different nodal stations. The PET/CT and CT-only contours agreed in 49% of cases. Thus, disagreement occurred 51% of the time and was mainly limited to one or two nodal stations (43%). This represents a substantial change in what is being contoured as GTV in half of the cases. Since the physician contouring the CT-only dataset was blinded to the PET results, and that contouring tasks were alternated between CT-only and PET/CT datasets on a case-to-case basis, we believe that a difference of 51% in contoured nodal stations is likely representative of how PET has impacted GTV contours across clinical practice. These patients were followed for regional failure in order to determine whether nodal volumes defined by PET/CT were adequate. Only one patient experienced local failure in a lymph node outside of the defined GTV, resulting in a 2% elective nodal failure rate. Though our median follow-up period is 12.9 months, a 2% elective nodal failure rate provides verification that nodal GTV contours can be limited to PET-defined tumor volumes on future RTOG studies.
The methods of tumor delineation according to FDG-PET in this study were largely qualitative in nature. The main determinant for the defined tumor volume was based on CT appearance, with PET used as a guide. Methods of quantitative or semi-quantitative contouring, perhaps based on SUV, are needed. Nestle et al. have provided a detailed overview of the multiple factors and pitfalls involved using quantitative FDG-PET for contouring tumor volumes for radiation therapy.(6)
Conclusions
PET/CT-derived tumor volumes for radiotherapy planning of patients with Stages II and III NSCLC were smaller than those derived from CT alone. There were no significant differences in normal tissue dose, represented by V20, mean lung dose and mean esophageal dose between PET/CT and CT derived plans. When comparing plans according to nodal stations, there was 51% disagreement between PET/CT and CT-only nodal gross tumor volumes. This disagreement usually involved one or two nodal stations. All patients in this trial were treated using PET/CT-based radiation therapy plans. With 12.9 months median follow-up time, only 2% have failed in lymph nodes outside of the target volume. We suggest that nodal GTV contours should be limited to PET/CT-defined tumor volumes in future studies.
Acknowledgments
This project was supported by RTOG grant U10 CA21661, CCOP grant U10 CA37422, and ATC grant U24 CA81647 from the National Cancer Institute (NCI). This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts to disclose
References
- 1.Morgensztern D, Goodgame B, Baggstrom MQ, et al. The effect of FDG-PET on the stage distribution of non-small cell lung cancer. J Thorac Oncol. 2008;3:135–139. doi: 10.1097/JTO.0b013e3181622c2c. [DOI] [PubMed] [Google Scholar]
- 2.MacManus M, Nestle U, Rosenzweig KE, et al. Use of PET and PET/CT for radiation therapy planning: IAEA expert report 2006–2007. Radiother Oncol. 2009;91:85–94. doi: 10.1016/j.radonc.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th Edition. New York: Springer-Verlag; 2002. [Google Scholar]
- 4.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003;123:137S–146S. doi: 10.1378/chest.123.1_suppl.137s. [DOI] [PubMed] [Google Scholar]
- 6.Nestle U, Kremp S, Grosu AL. Practical integration of [18F]-FDG-PET and PET-CT in the planning of radiotherapy for non-small cell lung cancer (NSCLC): the technical basis, ICRU-target volumes, problems, perspectives. Radiother Oncol. 2006;81:209–225. doi: 10.1016/j.radonc.2006.09.011. [DOI] [PubMed] [Google Scholar]


