Abstract
Background
We hypothesized that circulating levels of lipid peroxidation products in patients with severe sepsis are associated with development of pulmonary, renal, hepatic, circulatory and coagulation failure.
Methods
Plasma levels of F2-isoprostanes and isofurans were measured by mass spectroscopy on ICU day 2 in 50 critically ill patients with severe sepsis.
Results
F2-isoprostane levels were higher in patients who developed renal failure compared to not [65 pg/ml (IQR 44–112) vs. 44 pg/ml(IQR 29–54), p = 0.009] as were isofuran levels [1223 pg/ml (IQR 348–2531) vs. 329 pg/ml (IQR 156-1127), p = 0.009]. Plasma F2-isoprostane levels were higher in patients who developed hepatic failure compared to not [72 pg/ml (IQR 44–112) vs. 44 pg/ml (IQR 30–65), p = 0.023] and there was also a trend for higher isofuran levels [1411 pg/ml (IQR 298-1965) vs. 525 pg/ml (IQR 160–1223), p = 0.14]. Coagulation failure (thrombocytopenia) was associated with higher isofuran levels. Circulatory failure and acute lung injury were not associated with elevated levels of isoprostanes or isofurans. Patients with isoprostane levels above the 25th percentile had higher mortality (42%) compared to patient with levels below the 25th percentile (8%, p = 0.03).
Conclusions
Plasma levels of F2-isoprostanes and isofurans are associated with renal, hepatic, and coagulation failure but not with circulatory or pulmonary failure in severe sepsis suggesting that lipid peroxidation is a prominent feature of septic multi-system organ failure. Plasma isoprostanes and isofurans may be useful for monitoring oxidative stress in treatment trials of antioxidant therapies in severe sepsis.
Introduction
Sepsis is a common, life threatening syndrome with an estimated incidence of 750,000 cases per year in the United States (1). Mortality is high and is frequently caused by multiple organ failure. Despite decades of research, the mechanisms of organ failure in sepsis are still incompletely understood. Inflammation, and activation of the coagulation and complement cascades are important pathways (2). Oxidant injury has also been shown to play a role in organ failure, but has been studied primarily in animal models (3). Investigation of the role of oxidant injury in sepsis-induced multiple organ failure in humans has been hampered by the lack of stable, specific biomarkers of oxidative stress that can be measured accurately and non-invasively in patients with severe sepsis (4).
First described in humans in 1990 (5), isoprostanes are products of lipid peroxidation that are formed non-enzymatically and can be measured in blood, urine or tissues (6). Because of their stability and high specificity, the F2-isoprostanes are currently considered to be the most reliable biomarkers of in vivo oxidative stress and lipid peroxidation (7,8). In addition to serving as stable, specific markers of in vivo oxidative stress, F2-isoprostanes have potent biological activity including vasoconstriction (9) and activation of thromboxane and prostanoid receptors (6), suggesting that they may be mediators as well as markers of oxidant stress-induced tissue injury. Unlike isoprostanes, whose formation is disfavored at elevated oxygen tensions, the recently described isofurans (10) are lipid peroxidation products whose formation is favored by elevated oxygen tension. Thus, the combined measurement of both F2-isoprostanes and isofurans has been proposed for quantitation of oxidative stress in conditions such as critical illness that may involve hyperoxia (11).
In the current study, we hypothesized that levels of circulating F2-isoprostanes and isofurans in critically ill patients with severe sepsis would be associated with development of organ failure including pulmonary, renal, hepatic, circulatory and coagulation failure. We tested this hypothesis in 50 consecutive patients with severe sepsis enrolled in a large observational study of biomarkers in critical illness.
Methods
Patients
Patients were selected from a large observational cohort study [the Validating Acute Lung Injury biomarkers for Diagnosis (VALID) study] of diagnostic biomarkers for ARDS that has been enrolling patients in the Vanderbilt Medical Center medical, surgical, cardiovascular and trauma intensive care units since 2006 with a planned enrollment of 2550 patients (current enrollment 2000 patients) (12). Inclusion criteria for the VALID study include admission to the intensive care unit (ICU) with continued admission to the ICU on day two and age greater than or equal to 18 years. Exclusion criteria include chronic lung disease requiring supplemental oxygen, uncomplicated overdose, cardiac arrest prior to enrollment, transfer orders from the ICU written or planned within the next 4 hours or ICU admission for greater than 48 hours prior to Vanderbilt ICU admission. Fifty sequential patients who met diagnostic criteria for severe sepsis (13) at the time of enrollment and who had an enrollment plasma sample drawn were selected for inclusion in the current study. The VALID study was approved by the Vanderbilt Institutional Review Board (#051065). Informed consent was obtained from the patient or surrogate whenever possible; however, given the minimally invasive nature of the study, a waiver of consent was granted by the Institutional Review Board.
Measurements
All measurements were made in plasma obtained on the morning of ICU day 2 at the time of enrollment into the VALID study. Plasma was collected either from central venous access catheters or venipuncture in EDTA tubes, centrifuged immediately at 3000 × g and the plasma fraction stored at -80C to prevent ex vivo formation of lipid peroxidation products. Because immunoassays for F2-isoprostanes are much less reliable and accurate than mass spectrometric assays (14), plasma F2-isoprostanes and isofurans were simultaneously measured by stable isotope dilution negative ion chemical ionization gas chromatography mass spectrometry as described (10,15).
Clinical Data
Clinical data were collected daily through the morning of ICU day 5 as part of the VALID study and included baseline demographics, clinical diagnoses, hemodynamics, ventilator parameters and laboratory values. Severity of illness was assessed by the Acute Physiology And Chronic Health Evaluation II (APACHE II) (16) and Simplified Acute Physiology Score II (SAPS II) (17) scoring systems at the time of enrollment. Patients were assessed daily for the presence or absence of renal, hepatic, circulatory, and coagulation dysfunction using the Brussels Organ Failure Scoring System (18): patients were classified as having renal failure if serum creatinine was ≥ 2.0 mg/dL, hepatic failure if serum total bilirubin was ≥ 2.0 mg/dL, circulatory failure if systolic blood pressure was ≤ 90 mmHg or there was a need for any vasopressor, and coagulation failure if platelet count was ≤ 80,000/mm3 at any time during the study period. Patients were also assessed daily for diagnoses of sepsis, severe sepsis, acute lung injury, and acute respiratory distress syndrome using published definitions (13,19,20). The lowest PaO2/FiO2 and SpO2/FiO2 ratio (21) for each study day was calculated. Clinical outcomes including duration of mechanical ventilation, days of unassisted ventilation, length of ICU stay and hospital mortality were collected.
Statistical Analysis
F2-isoprostane and isofuran levels in the plasma were not normally distributed and are expressed as median [interquartile range (IQR)]. Differences in isoprostane and isofuran levels between groups were compared using the Mann-Whitney U Test. For bivariate correlations, the Spearman correlation coefficient was calculated. For comparison of categorical variables across quartiles of isoprostane or isofuran levels, the linear-by-linear association test was utilized. For comparison of categorical variables between two groups, Fisher's exact test was used. For all analyses, a P value less than or equal to 0.05 was considered to be statistically significant.
Power Calculation
Because levels of F2-isoprostanes and isofurans have not previously been measured in critically ill patients, the power calculation was based on estimates from previously published data. Normal levels of plasma F2-isoprostanes are 35 +/- 6 pg/ml (mean ± SD) (15). In a study of 29 hospitalized geriatric patients, mean plasma levels were approximately 40 ± 29 pg/ml (22). Based on the prediction that mean plasma levels would be somewhat higher, on average, in patients with severe sepsis (60 ± 30 pg/ml), we had an 84% power to detect an increase of 25 pg/ml in patients with a specific organ failure with 25 patients in each group.
Results
Patient characteristics
Patient characteristics are shown in Table 1. The median plasma F2-isoprostane level measured on the morning of ICU day 2 was 47 pg/ml (IQR 36 – 76). The median plasma isofuran level measured on the morning of ICU day 2 was 549 pg/ml (IQR 211 – 1743). Plasma F2-isoprostane levels and isofuran levels were significantly correlated (r = 0.45, p = 0.001). Plasma isofuran levels were modestly correlated with severity of illness as measured by the APACHE II score (r = 0.31, p = 0.028). Neither plasma F2-isoprostane nor isofuran levels were associated with the SAPS II score. Current smokers had higher isofuran levels [1120 pg/ml (IQR 367 – 2520) vs. 329 pg/ml (IQR 176 – 1411), p = 0.056) but not isoprostane levels.
Table 1. Clinical characteristics of 50 patients with severe sepsis.
| Patient Characteristic | n (%) or mean ± SD |
|---|---|
| Male | 28 (56%) |
| Age | 56 ± 15 |
| Current smoker | 19 (38%) |
| Alcohol abuse | 13 (26%) |
| Medical ICU | 45 (90%) |
| APACHE II* | 25 ± 7 |
| SAPS II** | 51 ± 16 |
| PaO2/FiO2 ratio (lowest in prior 24 h) | 186 ± 132 |
| SpO2/FiO2 ratio (lowest in prior 24 h) | 224 ± 108 |
| Vasopressors at enrollment | 21 (42%) |
| Source of sepsis | |
| Pneumonia | 25 (50%) |
| Urinary tract infection | 8 (16%) |
| Endovascular infection | 5 (10%) |
| Abdominal sepsis | 3 (6%) |
| Acute cholecystitis | 3 (6%) |
| Cellulitis | 3 (6%) |
| Meningitis | 2 (4%) |
| Osteomyelitis | 1 (2%) |
| Comorbidities | |
| Diabetes mellitus | 14 (28%) |
| Chronic liver disease | 5 (10%) |
| Chronic kidney disease | 7 (14%) |
| Hematologic malignancy | 6 (12%) |
| Non-hematologic malignancy | 8 (16%) |
| Organ failures (through ICU day 5) | |
| Renal failure | 19 (38%) |
| Hepatic failure | 15 (30%) |
| Coagulation failure | 18 (36%) |
| Circulatory failure | 40 (80%) |
| Acute lung injury | 25 (50%) |
| Acute respiratory distress syndrome | 23 (46%) |
| Hospital mortality | 17 (34%) |
Renal failure
Plasma F2-isoprostane levels were significantly higher in patients who had renal failure as defined by a serum creatinine greater than 2.0 mg/dL compared to those that did not [65 pg/ml (IQR 44 – 112) vs. 44 pg/ml (IQR 29 – 54, p = 0.009] (Figure 1a). This difference remained significant when the two patients with end stage renal disease on dialysis were excluded (p = 0.007) and when the seven patients with any chronic kidney disease were excluded (p = 0.006). The percentage of patients with renal failure increased by quartile of plasma F2-isoprostane level; in the lowest quartile, only 17% of patients had renal failure compared to 64% in the highest quartile (Figure 1b, p = 0.013 for trend across quartiles).
Fig. 1. Plasma levels of both F2-isoprostanes and isofurans are elevated in severe sepsis patients with renal failure.
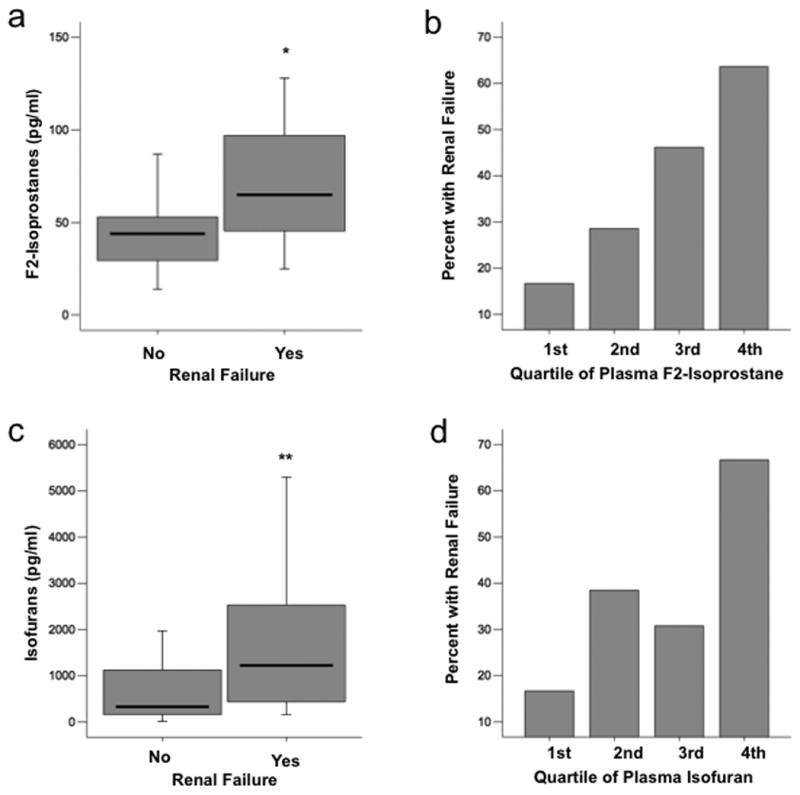
A, Boxplot summary of plasma levels of F2-isoprostanes in patients who had or developed renal failure (n = 19) compared with those who did not (n = 31) *P = 0.009. B, Comparison of percent of patients who had or developed renal failure between quartiles of plasma F2-isoprostane level. First quartile is the lowest quartile (0 – 25th percentile). P = 0.013 for trend across quartiles. C, Boxplot summary of plasma levels of isofurans in patients who had or developed renal failure (n = 19) compared with those who did not (n = 31) **P = 0.009. D, Comparison of percent of patients who had or developed renal failure between quartiles of plasma isofuran level, P = 0.026 for trend across quartiles. For boxplots, horizontal line represents median, box encompasses 24th to 75th percentile, and error bars encompass 10th to 90th percentile.
Plasma isofuran levels were also significantly higher in patients who had renal failure as defined by a serum creatinine greater than 2.0 mg/dL [1223 pg/ml (IQR 348 – 2531 vs. 329 pg/ml (IQR 156 - 1127), p = 0.009] (Figure 1c). This difference remained significant when the two patients with end stage renal disease on dialysis were excluded (p = 0.018) and when the seven patients with any chronic kidney disease were excluded (p = 0.022). The percentage of patients with renal failure increased by quartile of plasma isofuran level; in the lowest quartile, only 17% of patients had renal failure compared to 67% in the highest quartile (Figure 1d, p = 0.026 for trend across quartiles).
Hepatic failure
Plasma F2-isoprostane levels were significantly higher in patients who had hepatic failure as defined by a serum total bilirubin greater than 2.0 mg/dL compared to those that did not [72 pg/ml (IQR 44 – 112) vs. 44 pg/ml (IQR 30 – 65, p = 0.023] (Figure 2a). This difference remained significant when the five patients with chronic liver disease were excluded (p = 0.030). The percentage of patients with hepatic failure increased by quartile of plasma F2-isoprostane level; in the lowest quartile, only 8% of patients had hepatic failure compared to 55% in the highest quartile (Figure 2b, p = 0.045 for trend across quartiles). Of note, alcohol abuse, which was present in 8 of 15 patients who developed hepatic failure, was also associated with quartile of plasma F2-isoprostane level. Only 8% of patients in the lowest quartile of plasma isoprostane levels had a history of alcohol abuse compared to 45% in the highest quartile (p = 0.045 for trend across quartiles).
Fig. 2. Plasma levels of both F2-isoprostanes and isofurans are elevated in severe sepsis patients with hepatic failure.
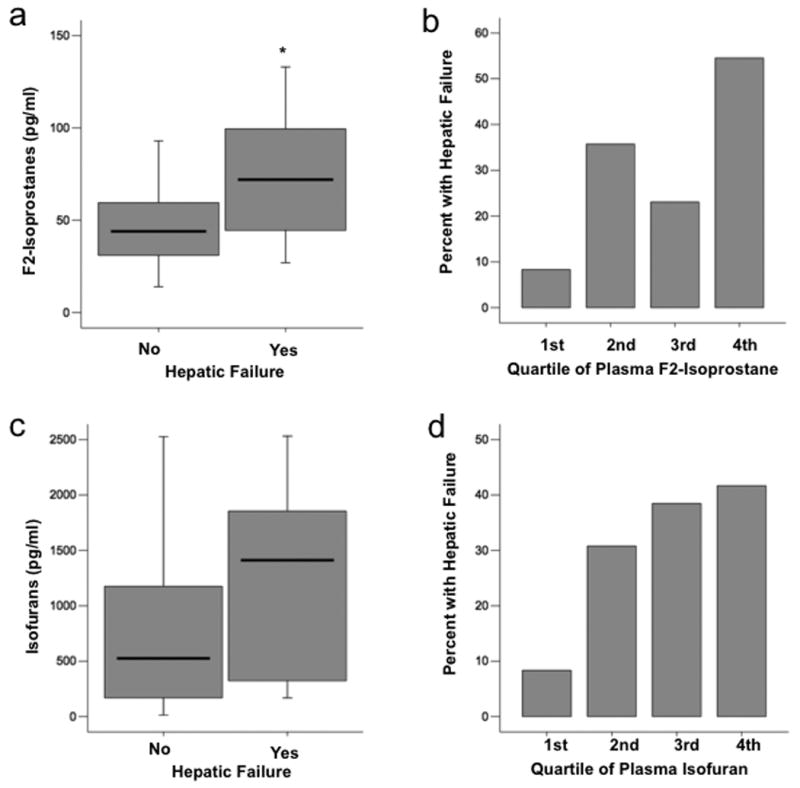
A, Boxplot summary of plasma levels of F2-isoprostanes in patients who had or developed hepatic failure (n = 15) compared with those who did not (n = 35, *P = 0.023). B, Comparison of percent of patients who had or developed hepatic failure between quartiles of plasma F2-isoprostane level. First quartile is the lowest quartile (0 – 25th percentile), P = 0.045 for trend across quartiles. C, Boxplot summary of plasma levels of isofurans in patients who had or developed hepatic failure (n = 15) compared with those who did not (n = 35, P = 0.136). D, Comparison of percent of patients who had or developed hepatic failure between quartiles of plasma isofuran level. P value for trend across quartiles is 0.071. For boxplots, horizontal line represents median, box encompasses 24th to 75th percentile, and error bars encompass 10th to 90th percentile.
Plasma isofuran levels also tended to be higher in patients who had hepatic failure as defined by a serum total bilirubin greater than 2.0 mg/dL [1411 pg/ml (IQR 298 - 1965) vs. 525 pg/ml (IQR 160 - 1223] (Figure 2c) but this trend did not reach significance (p = 0.136). The percentage of patients with hepatic failure increased by quartile of plasma isofuran level; in the lowest quartile, only 8% of patients had hepatic failure compared to 42% in the highest quartile (Figure 2d), however the p value for trend across quartiles did not quite reach significance (p = 0.071).
Because hyperbilirubinemia is not a direct measure of liver synthetic function or liver injury, we assessed both the liver synthetic function and the levels of liver enzymes in patients defined as having hepatic failure. Patients with hepatic failure as defined by bilirubin > 2 mg/dl also had evidence of liver synthetic dysfunction and transaminase elevations. In patients with bilirubin > 2 mg/dl compared to those with bilirubin < 2 mg/dl, INR was 2.3 ± 0.7 vs. 1.5 ± 0.4, p < 0.001 and AST was 525 ± 1243 U/l vs. 56 ± 59 U/l, p = 0.036.
Coagulation failure (thrombocytopenia)
Plasma F2-isoprostane levels were not associated with coagulation failure as defined by a platelet count less that 80,000 cells/μl (p = 0.50). F2-isoprostane levels were also not associated with platelet counts when assessed as a graded measure. In a direct comparison of isoprostane levels to platelet counts measured within the prior 24 hours, the Spearman's correlation coefficient was -0.96, p = 0.51.
In contrast to F-2 isoprostanes, plasma isofuran levels were significantly higher in patients who had coagulation failure compared to those that did not [1452 pg/ml (IQR 485 – 2040 vs. 314 pg/ml (IQR 157 - 1119), p = 0.01] (Figure 3a). The percentage of patients with thrombocytopenia increased by quartile of plasma isofuran level; in the lowest quartile, no patients had thrombocytopenia compared to 50% in the highest quartile (Figure 3b, p = 0.008 for trend across quartiles). In a direct comparison of plasma isofuran levels to platelet counts measured within the prior 24 hours, the Spearman's correlation coefficient was - 0.42, p = 0.002.
Fig. 3. Plasma levels of isofurans are elevated in severe sepsis patients with coagulation failure.
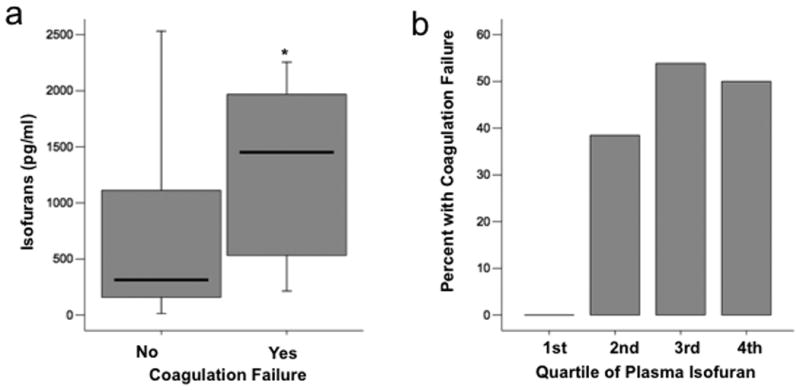
A, Boxplot summary of plasma levels of isofurans in patients who had or developed coagulation failure (n = 18) compared with those who did not (n = 32, *P = 0.01). B, Comparison of percent of patients who had or developed coagulation failure between quartiles of plasma isofuran level, P = 0.008 for trend across quartiles. For boxplot, horizontal line represents median, box encompasses 24th to 75th percentile, and error bars encompass 10th to 90th percentile.
Circulatory failure
Neither plasma F2-isoprostane levels nor plasma isofuran levels were significantly associated with the presence of circulatory failure as defined by a systolic blood pressure less than or equal to 90 mmHg or need for a vasopressor agent. Median plasma isoprostane levels were 43 pg/ml (IQR 34 – 57) in patients without shock and 51 (IQR 35 -76) in patients with shock, p = 0.32. Median plasma isofuran levels were 1266 pg/ml (IQR 671 -2528) in patients without shock and 358 (IQR 183 - 1702) in patients with shock, p = 0.092.
Acute lung injury/acute respiratory distress syndrome
Neither plasma F2-isoprostane levels nor plasma isofuran levels were significantly associated with the presence of acute lung injury or acute respiratory distress syndrome or the need for mechanical ventilation. Median plasma isoprostane levels were 47 pg/ml (IQR 30 – 85) in patients without acute lung injury and 47 (IQR 41 - 71) in patients with acute lung injury, p = 0.99. Median plasma isofuran levels were 329 pg/ml (IQR 185 - 1662) in patients without acute lung injury and 611 (IQR 284 - 1855) in patients with acute lung injury, p = 0.41. Likewise, F2-isoprostane and isofuran levels were not associated with the degree of hypoxemia as measured by the paO2/FiO2 ratio or the SpO2/FiO2 ratio. The ratio of plasma isofurans to F2-isoprostanes tended to be higher in patients with acute respiratory distress syndrome but this difference was not statistically significant [14.8 (IQR 4.7 – 39.6) vs. 7.4 (3.7 – 21.1), p = 0.14).
Clinical outcomes
Overall, plasma F2-isoprostane and isofuran levels did not differ significantly between patients who did or did not survive the hospitalization nor were they associated with the duration of unassisted ventilation. However, there appeared to be a threshold effect for isoprostane levels (Figure 4) such that patients with levels above the 25th percentile had substantially higher mortality (42%) compared to patient with levels below the 25th percentile (8%, p = 0.03).
Fig. 4. Hospital mortality was lower in patients with the lowest quartile of plasma isoprostane levels compared with the second, third, and fourth quartiles.
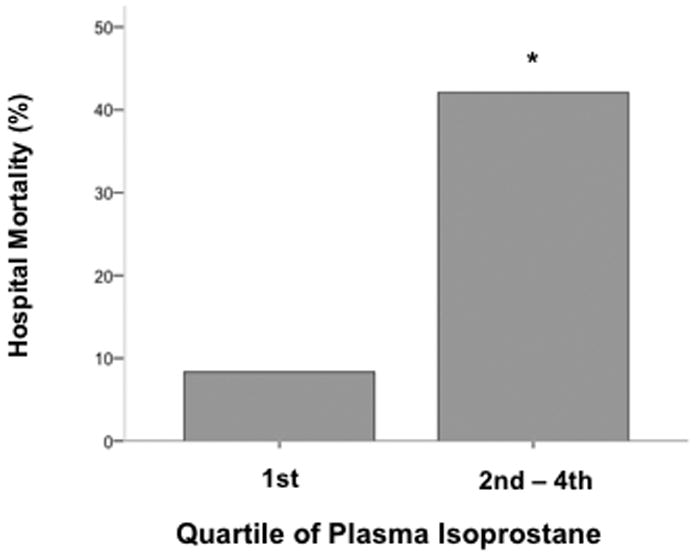
*P = 0.03 compared with first quartile.
Discussion
Although the role of oxidative stress in sepsis has been studied in animal models, both observational studies and clinical trials of antioxidants in humans have been hampered by the lack of stable markers of oxidative stress and lipid peroxidation that can be measured accurately and non-invasively (7). The discovery of in vivo production of lipid peroxidation products including isoprostanes and more recently isofurans has greatly facilitated the study of oxidative stress and lipid peroxidation in the clinical setting. In the current study we demonstrate that plasma levels of both isoprostanes and isofurans are highly associated with renal, hepatic and coagulation failure in critically ill patients with severe sepsis, indicating that lipid peroxidation is a prominent feature of multi-system organ failure in sepsis. The strong association of these products of lipid peroxidation with organ failure in sepsis suggests that isoprostanes and isofurans are good candidates for monitoring oxidative stress in treatment trials of antioxidant therapies in severe sepsis.
Although oxidative stress has previously been estimated by less accurate and less specific methods, there have been very few prior studies of F2-isoprostanes in critical illness and no prior studies of isofurans. Mishra and colleagues (23) reported plasma F2-isoprostane levels (measured by enzyme immunoassay) in 60 consecutive patients admitted to a single intensive care unit. Almost half of the patients were post-operative and only 10 patients had severe sepsis. Plasma F2-isoprostane levels were higher in non-survivors compared to survivors, (9.2 ± 2.6 pg/ml in survivors vs. 12.0 ± 4.9 pg/ml in non-survivors, p = 0.01). The absolute concentrations of F2-isoprostanes measured by immunoassay in this study cannot be compared to levels measured by mass spectrometry. Immunoassays for F2-isoprostanes are much less reliable and accurate than mass spectrometric assays (14). Carpenter et al.(24) measured F2-isoprostanes in the exhaled breath condensate of mechanically ventilated patients with acute lung injury compared to patients with other lung diseases intubated for elective procedures. Levels of F2-isoprostanes were high in patients with acute lung injury compared to controls (87 ± 28 pg/ml vs. 7 ± 4 pg/ml, p = 0.007) suggesting that lipid peroxidation in the lung compartment is a prominent feature of acute lung injury. Plasma levels were not measured in that study. Finally, in an abbreviated report, Powers et al. (22) measured plasma F2-isoprostanes in 29 hospitalized geriatric patients some of whom may have had severe sepsis. Based on the figure in that study, the range of plasma levels was approximately 10 – 130 pg/ml and the median was approximately 30 pg/ml. In our study, the range of plasma F2-isoprostane levels is broader (14 to 470 pg/ml) and the median is higher, 47 pg/ml. The severity of illness also is higher in the current study (median APACHE II of 25 compared to ∼21 in the geriatric study) likely due to the inclusion of only critically ill patients. Thus, the current study represents the first comprehensive assessment of both plasma isoprostane and isofuran levels in patients with severe sepsis.
Both isoprostanes and isofurans were highly associated with renal failure as measured by a serum creatinine level greater than 2.0 mg/dl. Both chronic and acute kidney disease have been previously associated with increased levels of oxidative stress. In chronic hemodialysis patients, both free and phospholipid-esterified plasma F2-isoprostane levels were substantially higher than in healthy control patients (25), a factor that might contribute to the high levels of cardiovascular morbidity and mortality in hemodialysis patients. Although other less specific markers of oxidative stress have been measured in acute kidney injury (26) and hepatorenal syndrome (27), to our knowledge, neither plasma isoprostanes nor isofurans have been reported previously in patients with acute kidney injury due to severe sepsis. In the current study, the association of plasma isoprostane and isofuran levels with acute renal failure persisted even when patients with chronic kidney disease were excluded, suggesting that chronic kidney disease was not driving the association between elevated levels and renal failure. In experimental models, isoprostanes have been implicated in the pathogenesis of acute kidney injury. Urinary excretion of F2-isoprostanes increased by 3-fold following ischemia-reperfusion of the rat kidney (28). Infusion of the isoprostane 8-Iso-PGF2α into the rat renal artery induced a reduction in glomerular filtration rate that was associated with increased glomerular afferent arteriolar resistance due to potent preglomerular vasoconstriction mediated through thromboxane A2 receptor activation (28). Whether plasma isoprostanes and isofurans are directly injurious to the human kidney cannot be determined from the current study although the prior success of antioxidants (29) in some forms of clinical acute kidney injury supports an injurious role for these mediators in humans.
Circulating markers of lipid peroxidation were also associated with hepatic and coagulation failure. High levels of both plasma and urinary isoprostanes have been reported in acute alcoholic hepatitis, chronic alcoholic liver disease and alcoholic cirrhosis (30,31). However, lipid peroxidation has not previously been quantified in patients with acute hepatic dysfunction due to severe sepsis. In the current study, the association of elevated levels of isoprostanes and isofurans with acute hepatic failure in sepsis persisted even when patients with chronic liver disease were excluded suggesting that this association is not driven primarily by chronic liver disease. Thrombocytopenia in patients with severe sepsis may be multifactorial, but most often represents disseminated intravascular coagulation due to widespread endothelial activation and injury. The association of circulating markers of lipid peroxidation with coagulation failure as defined by thrombocytopenia has not been previously reported but is not unexpected since oxidative stress has been implicated in endothelial activation, injury and apoptosis in sepsis (32).
That neither circulatory failure nor acute lung injury was associated with elevated markers of oxidant stress is surprising. In the case of acute lung injury, it is possible that oxidant production is localized to the lung. Indeed, several investigators have previously shown increased levels of oxidants (33,34) and decreased levels of antioxidants (35-37) in the alveolar compartment in patients with acute lung injury. In the case of circulatory failure, the degree of end organ ischemia may be a more important determinant of oxidant stress than the degree of vasodilation and hypotension; this is an important area for future investigation.
This study has several limitations. First, the sample size is relatively small. Although the parent cohort study has enrolled larger numbers of patients, accurate measurement of plasma isoprostanes and isofurans by mass spectroscopy has limited throughput. Because of this, the study was primarily powered to demonstrate major differences in plasma levels of isoprostanes and isofurans in association with organ failures in severe sepsis and was underpowered for associations of smaller magnitude. Future studies of larger patient groups may be better able to delineate the relationship between circulating markers of lipid peroxidation and acute lung injury and clinical outcomes. Second, the study measured only products of lipid peroxidation. We recognize that oxidative stress can have other important effects on proteins, carbohydrates, and other mediators, but at present isoprostanes and isofurans represent the most stable and accurate markers of oxidative stress that can be measured non-invasively in humans. Third, measurements were made at only a single timepoint. Sequential analysis over time might provide additional information. Finally, due to the observational nature of the study, the causative role of lipid peroxidation in multi-organ system failure cannot be determined. However, the stepwise association of higher levels of these mediators with increasing organ failures does suggest a possible cause and effect relationship as has been observed in experimental models.
Conclusions
In summary, plasma levels of both isoprostanes and isofurans are associated with renal, hepatic and coagulation failure in critically ill patients with severe sepsis. These findings suggest that lipid peroxidation may be a prominent feature of multi-system organ failure in sepsis. Plasma levels of isoprostanes and isofurans may be useful for monitoring oxidative stress in treatment trials of antioxidant therapies in severe sepsis.
Acknowledgments
This work was funded by National Institutes of Health [HL081332, HL103836]
Abbreviation List
- APACHE II
Acute Physiology And Chronic Health Evaluation II
- ICU
intensive care unit
- IQR
interquartile range
- SAPS II
Simplified Acute Physiology Score II
- VALID Study
Validating Acute Lung Injury biomarkers for Diagnosis Study
Footnotes
Conflict of Interest Disclosure: Dr. Ware has no conflicts of interest to disclose. Dr. Fessel has no conflicts of interest to disclose. Dr. May has no conflicts of interest to disclose. Dr. Roberts has no conflicts of interest to disclose.
Author Contributions. Dr. Ware: contributed to study concept and design, enrolling patients, acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript. Dr. Fessel: contributed to study concept and design, analysis and interpretation of data and critical revision of the manuscript. Dr. May contributed to study concept and design, enrolling patients and critical revision of the manuscript. Dr. Roberts contributed to study concept and design, acquisition, analysis and interpretation of data and critical revision of the manuscript.
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–87. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007;35:2408–16. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 4.Montuschi P, Barnes P, Roberts LJ., 2nd Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14:703–17. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- 5.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–7. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 7.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–13. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 8.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, 2nd, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 10.Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ., 2nd Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc Natl Acad Sci U S A. 2002;99:16713–8. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fessel JP, Roberts LJ. Isofurans: Novel products of lipid peroxidation that define the occurrence of oxidant injury in settings of elevated oxygen tension. Antioxid Redox Signal. 2005;7:202–9. doi: 10.1089/ars.2005.7.202. [DOI] [PubMed] [Google Scholar]
- 12.Siew E, Ware LB, Gebretsadik T, Shintani AK, Wickersham N, Bossert FR, Ikizler TA. Urine neutrophil gelatinase-asociated lipocalin (uNGAL) for the early detection of acute kidney injury in a prospective mixed adult ICU population. J Am Soc Nephrology. 2009 doi: 10.1681/ASN.2008070673. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bone R, Balk R, Cerra F, Dellinger R, Fein A, Knaus W, Schein R, Sibbald W. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 14.Il'yasova D, Morrow JD, Ivanova A, Wagenknecht LE. Epidemiological marker for oxidant status: comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3-dinor-5,6-dihydro-15-F2t-isoprostane. Ann Epidemiol. 2004;14:793–7. doi: 10.1016/j.annepidem.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Morrow JD, Roberts LJI. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymology. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 17.LeGall J, Lemshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 18.Bernard G. The Brussels Score. Sepsis. 1997;1:43–44. [Google Scholar]
- 19.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R the Consensus Committee. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 20.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the SpO2/FiO2 Ratio and the PaO2/FiO2 Ratio in Patients with Acute Lung Injury or Acute Respiratory Distress Syndrome. Chest. 2007 doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 22.Powers JS, Roberts LJ, 2nd, Tarvin E, Hongu N, Choi L, Buchowski M. Oxidative stress and multi-organ failure in hospitalized elderly people. J Am Geriatr Soc. 2008;56:1150–2. doi: 10.1111/j.1532-5415.2008.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra V, Baines M, Wenstone R, Shenkin A. Markers of oxidative damage, antioxidant status and clinical outcome in critically ill patients. Ann Clin Biochem. 2005;42:269–76. doi: 10.1258/0004563054255461. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter CT, Price PV, Christman BW. Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ARDS. Chest. 1998;114:1653–1659. doi: 10.1378/chest.114.6.1653. [DOI] [PubMed] [Google Scholar]
- 25.Ikizler TA, Morrow JD, Roberts LJ, Evanson JA, Becker B, Hakim RM, Shyr Y, Himmelfarb J. Plasma F2-isoprostane levels are elevated in chronic hemodialysis patients. Clin Nephrol. 2002;58:190–7. doi: 10.5414/cnp58190. [DOI] [PubMed] [Google Scholar]
- 26.Himmelfarb J, McMonagle E, Freedman S, Klenzak J, McMenamin E, Le P, Pupim LB, Ikizler TA The PG. Oxidative stress is increased in critically ill patients with acute renal failure. J Am Soc Nephrol. 2004;15:2449–56. doi: 10.1097/01.ASN.0000138232.68452.3B. [DOI] [PubMed] [Google Scholar]
- 27.Morrow JD, Moore KP, Awad JA, Ravenscraft MD, Marini G, Badr KF, Williams R, Roberts LJ., 2nd Marked overproduction of non-cyclooxygenase derived prostanoids (F2-isoprostanes) in the hepatorenal syndrome. J Lipid Mediat. 1993;6:417–20. [PubMed] [Google Scholar]
- 28.Takahashi K, Nammour TM, Fukunaga M, Ebert J, Morrow JD, Roberts LJ, 2nd, Hoover RL, Badr KF. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 alpha, in the rat. Evidence for interaction with thromboxane A2 receptors. J Clin Invest. 1992;90:136–41. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyner JL, Sher Ali R, Murray PT. Antioxidants. Do they have a place in the prevention or therapy of acute kidney injury? Nephron Exp Nephrol. 2008;109:e109–17. doi: 10.1159/000142935. [DOI] [PubMed] [Google Scholar]
- 30.Aleynik SI, Leo MA, Aleynik MK, Lieber CS. Increased circulating products of lipid peroxidation in patients with alcoholic liver disease. Alcohol Clin Exp Res. 1998;22:192–6. [PubMed] [Google Scholar]
- 31.Meagher EA, Barry OP, Burke A, Lucey MR, Lawson JA, Rokach J, FitzGerald GA. Alcohol-induced generation of lipid peroxidation products in humans. J Clin Invest. 1999;104:805–13. doi: 10.1172/JCI5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvemini D, Cuzzocrea S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic Biol Med. 2002;33:1173–85. doi: 10.1016/s0891-5849(02)00961-9. [DOI] [PubMed] [Google Scholar]
- 33.Baldwin SR, Grum CM, Boxer LA, Simon RH, Ketai LH, Devall LJ. Oxidant activity in expired breath of patients with adult respiratory distress syndrome. Lancet. 1986;1(8471):11–14. doi: 10.1016/s0140-6736(86)91895-7. [DOI] [PubMed] [Google Scholar]
- 34.Chabot F, Mitchell JA, Gutteridge JMC, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J. 1998;11:745–757. [PubMed] [Google Scholar]
- 35.Metnitz PG, Bartens C, Fischer M, Fridrich P, Steltzer H, Druml W. Antioxidant status in patients with acute respiratory distress syndrome. Intensive Care Med. 1999;25:180–185. doi: 10.1007/s001340050813. [DOI] [PubMed] [Google Scholar]
- 36.Bowler RW, Velsor LW, Duda B, Chan ED, Abraham E, Ware LB, Matthay MA, Day BJ. Pulmonary edema fluid anti-oxidants are depressed in acute lung injury. Crit Care Med. 2003;31:2309–2315. doi: 10.1097/01.CCM.0000085090.06078.8C. [DOI] [PubMed] [Google Scholar]
- 37.Lykens MG, Davis WB, Pacht ER. Antioxidant activity of bronchoalveolar lavage fluid in the adult respiratory distress syndrome. Am J Physiol. 1992;262:L169–75. doi: 10.1152/ajplung.1992.262.2.L169. [DOI] [PubMed] [Google Scholar]


