Abstract
Objectives
The objective of this study was to identify the role of dimethylarginine dimethylaminohydrolase-1 (DDAH1) in degrading the endogenous NOS inhibitors ADMA and L-NMMA.
Methods and results
We generated a global-DDAH1 gene deficient (DDAH1−/−) mouse strain to examine the role of DDAH1 in ADMA and L-NMMA degradation, and the physiological consequences of loss of DDAH1. Plasma and tissue ADMA and L-NMMA levels in DDAH1−/− mice were several fold higher than in wild type mice, but growth and development of these DDAH1−/− mice was similar to their wild type littermates. Although the expression of DDAH2 was unaffected, DDAH activity was undetectable in all tissues tested. These findings indicate that DDAH1 is the critical enzyme for ADMA and L-NMMA degradation. Blood pressure was ~20 mmHg higher in the DDAH1−/− mice than in wild type mice, but no other cardiovascular phenotype was found under unstressed conditions. Crossing DDAH1+/− male with DDAH1+/− female mice yielded DDAH1+/+ mice, DDAH1+/− mice and DDAH1−/− mice at anticipated ratios of 1:2:1, indicating that DDAH1 is not required for embryonic development in this strain.
Conclusions
Our findings indicate that DDAH1 is required for metabolizing ADMA and L-NMMA in vivo, while DDAH2 had no detectable role for degrading ADMA and L-NMMA.
Keywords: Asymmetric dimethylarginine, dimethylarginine dimethylaminohydrolase 1, nitric oxide, knockout mice
Introduction
Nitric oxide (NO) exerts important biological functions 1 by stimulating guanylate cyclase to generate cGMP 2, inhibiting mitochondrial respiration by competing with oxygen at cytochrome oxidase 3, 4, or inducing s-nitrosylation 5 to regulate protein stability and function. NO production is restrained by the endogenous NOS inhibitors asymmetric dimethylarginine (ADMA) and Ng-monomethyl-L-arginine (L-NMMA) 6, 7. In intact animals, infusion of ADMA or L-NMMA increases vascular resistance and blood pressure 1, 8. Cardiovascular diseases including hypertension 9, coronary artery disease 10, 11, stroke 10–12, congestive heart failure 13, 14, atherosclerosis 15 and diabetes 16 are associated with increased plasma levels of ADMA with a decreased ratio of L-arginine to ADMA 17. Furthermore, increased plasma ADMA is a strong independent predictor of both mortality and major nonfatal cardiovascular events in patients after myocardial infarction, coronary artery disease and stroke 10, 11, 18.
Dimethylarginine dimethylaminohydrolase 1 (DDAH1) 19 and DDAH2 20 are encoded by two different genes. DDAH1 was initially identified as the enzyme degrading ADMA and L-NMMA 19. Recent studies have demonstrated that loss-of-function DDAH1 mutations are associated with increases in the occurrence of coronary heart disease, thrombosis and stroke 10, 21. DDAH2 was also reported to have enzyme activity for degrading ADMA and L-NMMA in vitro that was similar to DDAH1 20. It consequently has been assumed that in vivo metabolism of NOS inhibitors would reflect the combined abundance of both isoforms. As DDAH2 is more abundant than DDAH1 in lung, heart and vascular endothelial cells 22–24, it has been assumed that DDAH2 is the dominant enzyme regulating ADMA and L-NMMA in the cardiovascular system 25. However, using an endothelial specific DDAH1 gene deficient mouse strain, we found that endothelial DDAH1 is important for degrading ADMA and maintaining NO bioavailability 26. Moreover, a recent study reported that while homozygous global DDAH1 gene deletion was embryonic lethal, heterozygous DDAH1 gene deficient mice had increased tissue ADMA and decreased NO production in isolated aortic rings 27. Thus, while there is evidence that DDAH1 contributes to vascular DDAH activity, the contribution of DDAH1 versus DDAH2 in ADMA and L-NMMA degradation in vivo has not been established.
To determine the importance of DDAH1 for in vivo metabolism of the endogenous NOS inhibitors, we generated a global DDAH1 gene deficient (DDAH1−/−) mouse strain. These mice are viable with normal growth and development; indicating that, at least in this strain, DDAH1 is not required for embryonic development. Using stable isotope labeled ADMA or L-NMMA as substrate, we found that ADMA and L-NMMA degradation was undetectable in all DDAH1 deficient tissues tested, even though DDAH2 expression was not altered in those tissues. These results demonstrated that DDAH1 is essential for metabolizing endogenous NOS inhibitors in vivo. Our findings help to resolve the controversy regarding the relative importance of DDAH1 and DDAH2 in degrading ADMA and L-NMMA. Namely, our data indicate that in vivo clearance of ADMA and L-NMMA is dependent on DDAH1 with no detectable role for DDAH2.
The DDAH1−/− mice had moderate systemic hypertension with no other obvious phenotype, indicating that deficiency in DDAH activity alone is insufficient to cause structural or functional cardiovascular abnormality under unstressed conditions. The moderate hypertension in the DDAH1−/− mice is consistent with a role for DDAH1 in modulating vascular tone and regulating blood pressure in vivo 26, 28. This novel DDAH1−/− mouse strain will be a valuable tool to test whether abnormal DDAH1 function will exacerbate the development of cardiovascular disease under stress conditions.
Methods
Generation of global DDAH1−/− mice
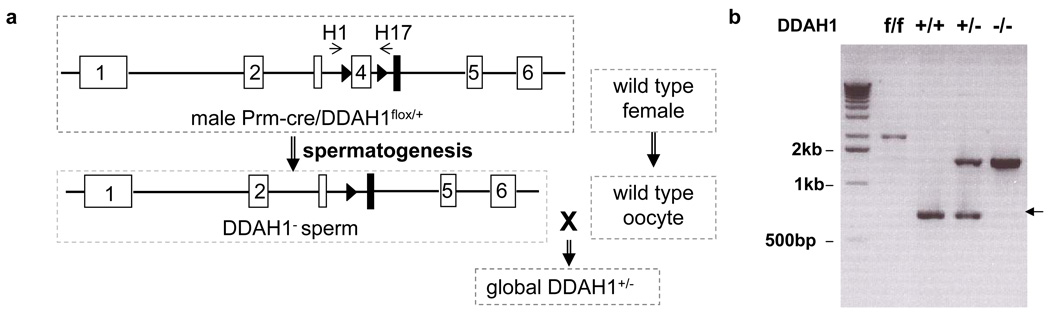
The DDAH1flox/flox mice 26 were crossed with protamine (Prm)-Cre mice (129-Tg(Prm-cre)58Og/J, Jackson Laboratory). The DDAH1 gene was deleted in the sperm of the male double heterozygote Prm-Cre/DDAH1flox/+ mice. When these male mice were crossed with wild type female breeders, DDAH1+/− mice were generated. The homozygote global DDAH1−/− was generated by inbreeding of the heterozygotes. PCR was performed for genotyping of the offspring using primer pairs 5’-AAT CTG CAC AGA AGG CCC TCA A-3’ and 5’- GGA GGA TCC ATT GTT ACA AGC CCT TAA CGC-3’ for the wild type allele and 5’- TGC AGG TCG AGG GAC CTA ATA ACT-3’ and 5’- AAC CAC ACT GCT CGA TGA AGT TCC-3’ for the knockout allele.
Measurement of ADMA, L-NMMA, SDMA, L-arginine content and DDAH activity
Tissue and plasma ADMA, L-NMMA, SDMA and L-arginine were measured using a high-throughput liquid chromatographic-tandem mass spectrometric method 29. A stable-isotope based technique was used for determination of DDAH activity 30.
siRNA transfection
Human umbilical vein endothelial cells were transfected with DDAH1 and/or DDAH2 specific siRNA (Santa Cruz Biotechnology). Three days after transfection, the transfection medium was removed and the cells were incubated in EBM-2 (Lonza) for another 24hrs. Then the media was collected and the amount of ADMA in the medium was determined by a validated ELISA method (DLD Diagnostika GmbH, Hamburg, Germany) 31.
Measurement of total nitrogen oxides (NOx)
Osmotic Minipumps (Alzet®, Charles River, Germany) containing saline or Nω-nitro-L-arginine methyl ester (L-NAME, 50mg/kg/day) 32, 33 were implanted subcutaneously in the back to deliver drug into mice for 72 hours 34. Previous studies have demonstrated that L-NAME ranging from 33.7–67.4mg/kg/day was effective in blocking NOS activity32, 33. Total plasma, urinary and tissue NOx content was determined using the colorimetric assay kit from Cayman Chemical Company according to the protocol provided by the manufacturer.
Echocardiography and measurement of blood pressure
Mice were anesthetized with 1.5% isoflurane. Echocardiographic images were obtained with a Visualsonics Veve 770 system as previously described 35, 36. For aortic pressure measurement, a 1.2 Fr. pressure catheter (Scisense Inc. Ontario Canada) was introduced into the right common carotid artery and advanced into the ascending aorta 26. L-arginine was administrated intravenously at a dose of 400mg/kg, a dose that has been reported to increase plasma L-arginine ~2.8 fold 37. Tail blood pressure was determined in conscious mice with the XBP 1000 system (Kent Scientific) as we previously described 26.
Statistical analysis
More than 5 mice from each strain were used in each assay. Student’s t-test was performed to compare data between groups. P<0.05 was considered statistically significant. Results are presented as mean ± standard error.
Results
DDAH1−/− mice grow and develop normally
By breeding DDAH1flox/flox mice generated in our laboratory with Protamine-Cre transgenic mice, we generated global heterozygous DDAH1 gene deficient (DDAH1+/−) mice (Figure 1a). Crossing DDAH1+/− male with DDAH1+/− female mice yielded DDAH1+/+ mice, DDAH1+/− mice and DDAH1−/− (global DDAH1 deficient) mice at anticipated ratios of 1:2:1. Genomic DNA PCR showed that exon4 of DDAH1 was deleted from the genome of the DDAH1−/− mice (Figure 1b). Both DDAH1+/− and DDAH1−/− mice grew similarly to DDAH1+/+ mice up to 3 months of age. Thus, the global DDAH1−/− mice are viable with normal growth and development. These findings indicate that DDAH1 is not required for embryonic development in this knockout strain.
Figure 1.
Generation of the DDAH1−/− mouse strain was achieved by crossing DDAH1flox/flox with Protamine-Cre mice. Exon4 of DDAH1 was deleted in the sperm of Protamine-Cre/DDAH1flox/+ during spermatogenesis. Heterozygous global DDAH1 mice were generated by crossing male Prm-cre/DDAH1flox/+ to wild type female mice (a). Genomic DNA PCR shows that exon4 of DDAH1 was deleted in the DDAH1−/− mice (b).
DDAH1−/− does not affect DDAH2 expression
To determine whether DDAH1 deficiency might cause compensatory upregulation of DDAH2 expression, the protein content of DDAH2 was determined in several organs of DDAH1−/− mice. Although DDAH1 protein was not detectable in kidney, brain, liver, lung (Figure 2a) or other tissues from DDAH1−/− mice, the protein levels of DDAH2 were not altered in these organs (Figure 2a,b). DDAH2 mRNA content was also unchanged in tissues from the DDAH1−/− mice (Supplementary Figure I). DDAH1−/− had no significant effect on the expression of eNOS, protein arginine methyltransferase-1, protein arginine methyltransferase-3, or cationic amino acid transporter in brain, kidney, lung (Supplementary Figure II) and other tissues tested.
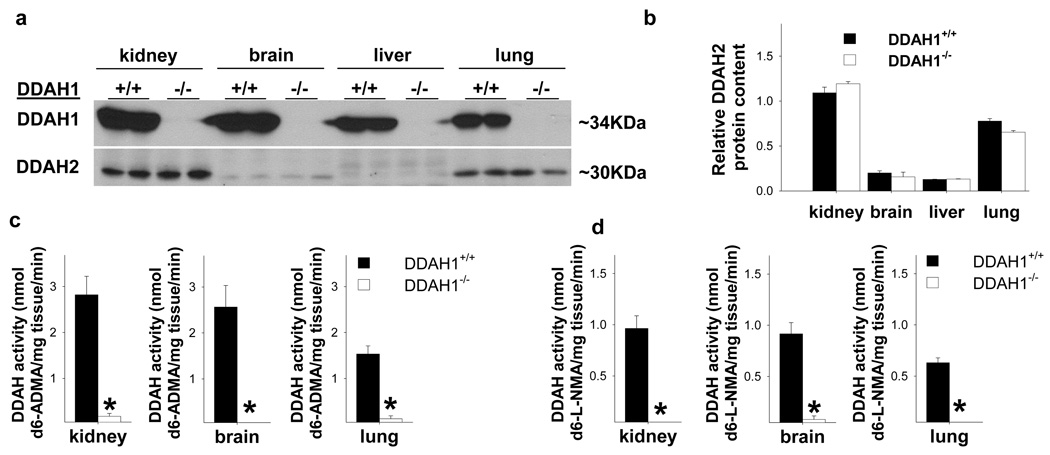
Figure 2.
Global-DDAH1−/− mice reveal that DDAH1 is essential for degradation of ADMA and L-NMMA. DDAH1−/− abolished DDAH1 protein expression in all tissues tested (a), but had no effect on DDAH2 protein expression (b). DDAH1−/− abolished DDAH activity in kidney, brain and lung as tested using either stable isotope labeled d6-ADMA (c) or d6-L-NMMA as substrate (d). * p<0.05 compared with samples from wild type littermates.
ADMA degradation was not detectable in tissues from DDAH1−/− mice
Normal tissues continuously generate ADMA. To avoid interference from endogenous ADMA, we performed the DDAH activity assay using stable isotope labeled ADMA as substrate. Enzyme activity for degrading ADMA was undetectable in all tested tissues from the DDAH1−/− mice (Figure 2c), indicating that DDAH1 is responsible for majority, if not all, of enzyme activity for metabolizing ADMA in these tissues.
L-NMMA degradation was not detectable in tissues from DDAH1−/− mice
Because DDAH1 and DDAH2 might have different substrate preference, we went on to determine the effect of DDAH1 deficiency on L-NMMA degradation. Using stable isotope labeled d6-L-NMMA as substrate, we found that the activity for metabolizing L-NMMA was also abolished in all tissues tested from the DDAH1−/− mice (Figure 2d). Since DDAH2 expression was not changed in the DDAH1−/− mice, these results indicate that DDAH2 did not have a detectable contribution in the degradation of L-NMMA in these tissues.
DDAH1−/− caused accumulation of tissue ADMA and L-NMMA
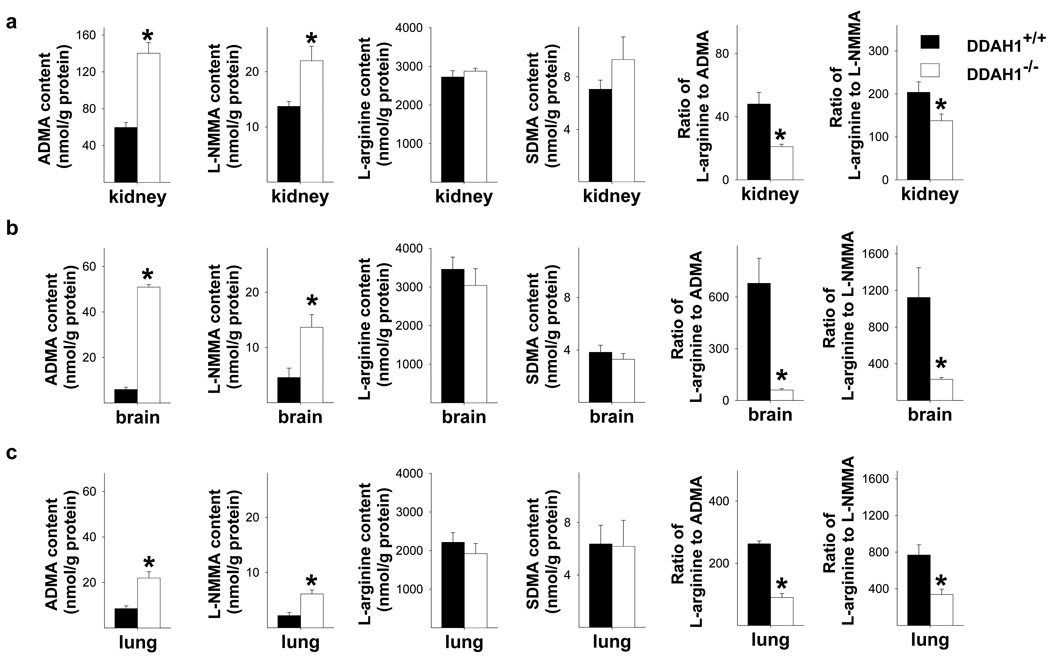
ADMA and L-NMMA tissue content in kidney, brain and lung were significantly increased in the global DDAH1−/− mice as compared with DDAH1+/+ mice (Figure 3a–c), indicating that DDAH1 is pivotal in regulating tissue ADMA and L-NMMA levels. Since tissue L-arginine and SDMA were not different between DDAH1−/− and DDAH1+/+ mice, the ratios of L-arginine to ADMA or L-NMMA, indicators of systemic nitric oxide bioavailability 17, were significantly decreased in these organs (Figure 3a–c). In addition, the ADMA content of mesenteric microvessels was significantly increased in DDAH1−/− mice (64.0 nmol/g protein in DDAH1−/− vs. 31.7 nmol/g protein in wild type mice, p<0.05). Total NOx in mesenteric vessels was significantly decreased in DDAH1−/− mice (Supplemental Figure III).
Figure 3.
DDAH1−/− caused significant increases of ADMA and L-NMMA in kidney (a), brain (b) and lung (c), but had no effect on SDMA or L-arginine content. DDAH1−/− decreased the ratios of L-arginine to ADMA or L-NMMA in these samples (a–c). * p<0.05 compared with controls.
DDAH1−/− caused accumulation of plasma ADMA and L-NMMA and their ratios to L-arginine
DDAH1−/− caused significant increases of plasma ADMA and L-NMMA (Figure 4a, b), but had no effect on plasma L-arginine or SDMA (Figure 4c, d). As a result, DDAH1 gene deletion caused significant decreases of the ratios of L-arginine to ADMA or L-NMMA in plasma (Figure 4e, f). Thus, DDAH1 metabolizes ADMA and L-NMMA in tissue and also clears these NOS inhibitors from the circulation, indicating that DDAH1 acts to maintain systemic homeostasis of the endogenous NOS inhibitors.
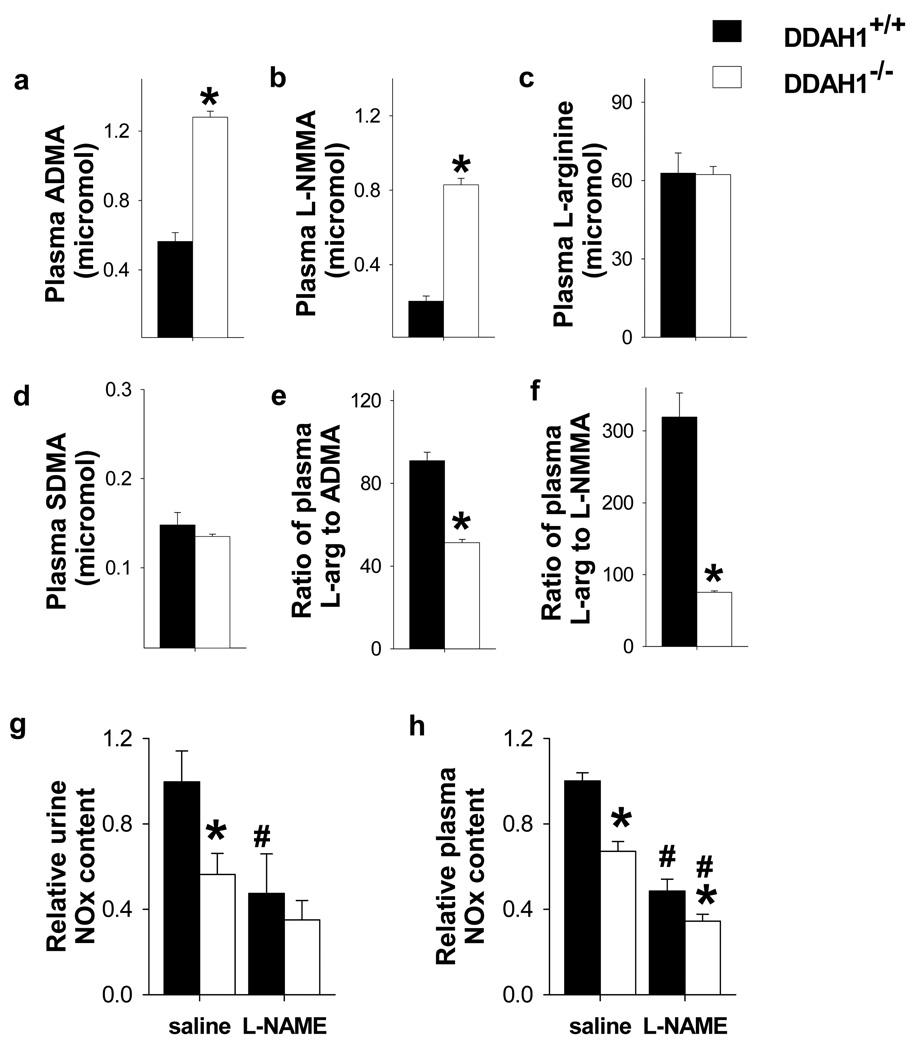
Figure 4.
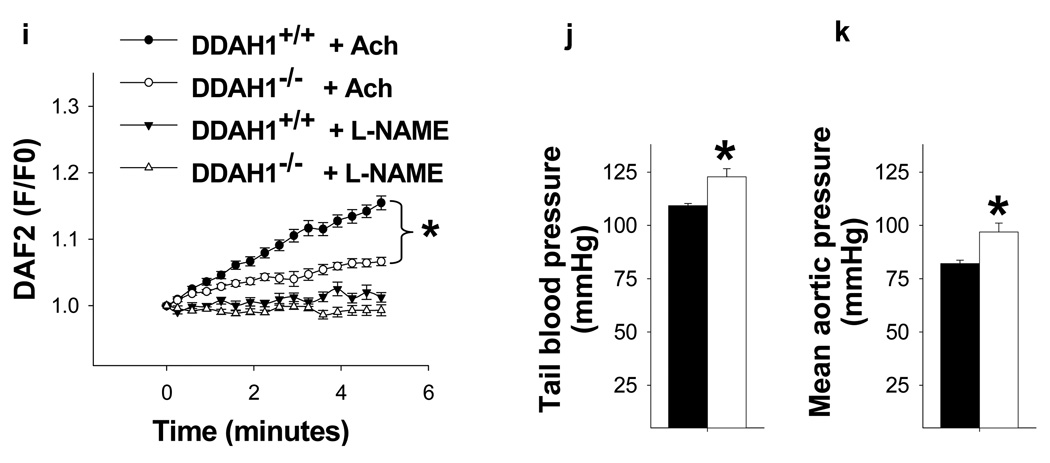
NO signaling was impaired in the DDAH1−/− mice. DDAH1−/− increased ADMA (a) and L-NMMA (b) content in plasma, but had no effect on plasma L-arginine (c) and SDMA (d) levels. The ratios of L-arginine to ADMA (e) and L-NMMA (f) in the DDAH1−/− were significantly decreased. Total NOx in the urine and plasma of DDAH1−/− mice were also significantly decreased; L-NAME caused further decreases of NOx, but ~40% of both urinary and plasma NOx were resistant to NOS inhibition with L-NAME (g, h). DDAH1−/− decreased acetylcholine induced NO generation in aortic rings (i) and increased blood pressure (j, k). *p<0.05 compared with corresponding wild type controls. #p<0.05 compared with saline treated controls.
DDAH1−/− decreased NO production and increased blood pressure
To determine the impact of DDAH1−/− on systemic NO production, total nitrogen oxides (NOx) were measured in urine and plasma from fasting mice drinking deionized water. Both urinary and plasma NOx content were significantly decreased in the DDAH1−/− mice, implying that accumulation of NOS inhibitors in the DDAH1 KO mice inhibited NOx generation. (Figure 4g, h). The NOS inhibitor L-NAME decreased urinary and plasma NOx in both DDAH1−/− and wild type mice. After L-NAME, the difference of urinary NOx content between DDAH1−/− and wild type mice was no longer statistically significant, and the difference of plasma NOx content between DDAH1−/− and wild type mice was reduced. Of note, approximately 40% of both urine and plasma NOx content remained after L-NAME, consistent with previous reports that NOx was also generated by non NOS sources. DDAH1−/− also significantly decreased acetylcholine induced NO generation by aortic rings (Figure 4i). Previous studies have demonstrated that infusion of ADMA or L-NMMA caused vasoconstriction in vivo 8. Consistent with this, we found that the increased levels of ADMA and L-NMMA in the DDAH1−/− mice were associated with a moderate significant increase of tail blood pressure measured in the awake state (Figure 4j) as well as direct catheter measurement of aortic pressure (Figure 4k). The moderate increase of blood pressure in DDAH1−/− mice was similar to the increase of blood pressure in our endothelial specific DDAH1−/− mice 26 and in global eNOS−/− mice 38. We also determined ADMA clearance by mesenteric microvessels from wild type mice and DDAH1−/− mice. ADMA degradation was undetectable in mesenteric vessels from DDAH1−/− mice (data not shown). The elevated blood pressure in the DDAH1−/− mice was normalized by infusion of L-Arginine at a dose of 400mg/kg (Supplementary Figure IV) 37.
DDAH1−/− has no effect on structure of the kidney, lung or heart
DDAH1−/− had no evident effect on the gross or histologic appearance of the kidneys, lungs or heart. In addition, left ventricular dimensions and function were unchanged in DDAH1−/− mice (Supplementary Table I).
Selective gene silencing of DDAH1 but not DDAH2 caused ADMA accumulation in cultured HUVEC
We also determined the effect of selective gene silencing of DDAH1 and DDAH2 on ADMA accumulation in HUVEC using specific siRNA. DDAH1 gene silencing decreased DDAH1 expression ~80% and significantly increased the ADMA level in the culture medium. DDAH2 gene silencing abolished DDAH2 expression but had no detectable effect on ADMA content in the culture medium (Supplementary Figure V). This result is consistent with previous reports that selective DDAH2 gene silencing had no effect on ADMA content in cultured bovine aortic endothelial cells 39 or in rats 40.
Discussion
The new findings of this study are: (i) a strain of viable DDAH1−/− mice was developed that grow and develop normally; (ii) DDAH1−/− caused accumulation of ADMA and L-NMMA in plasma and tissue; (iii) DDAH1−/− had no effect on DDAH2 expression in any tissue tested; (iv) tissue DDAH activity for ADMA and L-NMMA was not detectable in all tissues tested from the DDAH1−/− mice; and (v) DDAH1−/− resulted in moderate hypertension and impaired endothelial sprouting. These findings indicate that DDAH1 is essential for degrading ADMA and L-NMMA in vivo, but is not essential for embryonic development in this knockout strain. Moreover, our data fail to detect a role for DDAH2 in degrading ADMA or L-NMMA, a finding that helps to resolve the controversy regarding DDAH1 and DDAH2 in degrading the NOS inhibitors.
The accumulation of ADMA and L-NMMA in plasma and tissues of DDAH1−/− mice is similar to our findings in endo-DDAH1−/− mice 26. The decreased ratios of L-arginine to ADMA or L-NMMA, the decreases of urinary and plasma NOx in the DDAH1−/− mice, and the decreased NO generation by aortic rings in response to acetylcholine establish an important role for DDAH1 in maintaining systemic and tissue NO bioavailability. The moderate increase of blood pressure in the DDAH1−/− mice implies that chronic accumulation of endogenous NOS inhibitors results in constriction of the resistance vessels where blood pressure is controlled, and is in agreement with the loss of DDAH activity in mesenteric resistance vessels.
The stable isotope labeled ADMA or L-NMMA technique represents the most reliable method for DDAH activity analysis 30. Using stable isotope labeled ADMA or L-NMMA as substrate, DDAH activity was essentially undetectable in lung and kidney from the DDAH1−/− mice, despite the fact that DDAH2 was highly expressed (and unchanged as compared with wild type mice). Since DDAH2 is highly abundant in many of these tissues, the findings imply that DDAH2 does not contribute to the degradation of ADMA or L-NMMA. Future studies using global DDAH2 KO mice will be useful to further explore the physiologic role of DDAH2 in ADMA degradation.
The fold increase in tissue ADMA and L-NMMA was less in the kidney than in other tissues, suggesting the possibility that another pathway might contribute to their metabolism. Previous studies have reported that ADMA can be metabolized through an alternate pathway by alanine-glyoxylate aminotransferase 2 (AGXT2), a mitochondrial aminotransferase expressed primarily in the kidney 41. Rodionov et al 42 demonstrated that overexpression of AGXT2 using an adenoviral expression vector caused decreased ADMA levels in the plasma and liver of C57BL/6 mice. However, when rats were injected with radiolabeled ADMA, transamination products of ADMA were detected in the urine but most of the radioactivity appeared as citrulline, implying that ADMA was metabolized principally by DDAH 43. In agreement with that report, the disappearance of radioisotope labeled ADMA and L-NMMA was nearly undetectable in kidney tissue from our DDAH1−/− mice, implying that DDAH1 was principally responsible for ADMA and L-NMMA degradation in the kidney. Our data suggests that AGXT2 is likely to make a minimal direct contribution to ADMA degradation. A likely cause for the relatively smaller increase of ADMA and L-NMMA in kidney tissue of DDAH1−/− mice may relate to its capacity to directly excrete ADMA and L-NMMA into the urine.
Our finding that DDAH1−/− mice developed and grew comparably to DDAH1+/+ mice indicates that DDAH1 is not essential for embryonic development in this knockout strain. In addition, Lexicon Pharmaceuticals, Inc has recently generated a viable global DDAH1 KO strain. These findings are in contrast to a previous study in which DDAH1−/− was reported to be embryonic lethal 27. The DNA construct used in the previous study was designed to delete exon1 of DDAH1, while exon4 was targeted in our study. Study of embryonic development in the previous global DDAH1−/− strain showed that only ~5% of blastocysts were DDAH1−/− at embryo day 2 44, suggesting that the developmental defects in those homozygotes occurred before implantation. Since triple eNOS/iNOS/nNOS null mice are reported to be viable 45, the lethality of the previous DDAH1−/− strain is not likely due to NO dependent implantation or placentation defects. It is possible that some important genomic sequence that is critical for embryonic development was disrupted in the previous study so that that the ES cells used to generate their DDAH1−/− strain had defects which contributed to the lethality 27.
In summary, the present data demonstrate that DDAH1 is essential for degrading ADMA and L-NMMA in vivo, but is not required for embryonic development in this DDAH1−/− strain. Our data fail to support an important physiological role for DDAH2 in metabolizing ADMA and L-NMMA. Impaired DDAH1 function caused a moderate increase of blood pressure similar to that in eNOS gene deficient mice 38 and in our endothelial specific DDAH1 deficient mice 26. This novel DDAH1−/− mouse strain will be a valuable tool to test whether abnormal DDAH1 function will exacerbate the development of cardiovascular pathology under stress conditions.
Supplementary Material
Acknowledgements
Sources of Funding
This study was supported by U.S. Public Health Service Grants HL20598, HL021872, R21HL098669, R21HL098719 and R21HL102597 from the National Heart, Lung and Blood Institute and Research Grants 0330136N, 09SDG2170072 and 0160275Z from the American Heart Association. Drs Hu and Zhang are recipients of Scientist Development Awards from the American Heart Association National Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Moncada S. Adventures in vascular biology: a tale of two mediators. Philos Trans R Soc Lond B Biol Sci. 2006;361:735–759. doi: 10.1098/rstb.2005.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Letters. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 4.Cleeter MWJ, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AHV. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide: Implications for neurodegenerative diseases. FEBS Letters. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 5.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends in Molecular Medicine. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer RMJ, Rees DD, Ashton DS, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochemical and Biophysical Research Communications. 1988;153:1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- 7.Vallance P, Leone A, Calver A, Collier JSM. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol. 1992;20:S60–S62. doi: 10.1097/00005344-199204002-00018. [DOI] [PubMed] [Google Scholar]
- 8.Gardiner S, Kemp P, Bennett T, Palmer R, Moncada S. Regional and cardiac haemodynamic effects of NG, NG, dimethyl-L-arginine and their reversibility by vasodilators in conscious rats. Br J Pharmacol. 1993;110:1457–1464. doi: 10.1111/j.1476-5381.1993.tb13985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surdacki A, Nowicki M, Sandmann J, Tsikas D, Boeger RH, Bode-Boeger SM, Kruszelnicka-Kwiatkowska O, Kokot F, Dubiel JS, Froelich JC. Reduced Urinary Excretion of Nitric Oxide Metabolites and Increased Plasma Levels of Asymmetric Dimethylarginine in Men with Essential Hypertension. Journal of Cardiovascular Pharmacology. 1999;33:652–658. doi: 10.1097/00005344-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Ding H, Wu B, Wang H, Lu Z, Yan J, Wang X, Shaffer JR, Hui R, Wang DW. A Novel Loss-of-Function DDAH1 Promoter Polymorphism Is Associated With Increased Susceptibility to Thrombosis Stroke and Coronary Heart Disease. Circ Res. 2010;106:1145–1152. doi: 10.1161/CIRCRESAHA.109.215616. [DOI] [PubMed] [Google Scholar]
- 11.Leong T, Zylberstein D, Graham I, Lissner L, Ward D, Fogarty J, Bengtsson C, Bjorkelund C, Thelle D The Swedish-Irish-Norwegian C. Asymmetric Dimethylarginine Independently Predicts Fatal and Nonfatal Myocardial Infarction and Stroke in Women: 24-Year Follow-Up of the Population Study of Women in Gothenburg. Arterioscler Thromb Vasc Biol. 2008;28:961–967. doi: 10.1161/ATVBAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 12.Wanby P, Teerlink T, Brudin L, Brattström L, Nilsson I, Palmqvist P, Carlsson M. Asymmetric dimethylarginine (ADMA) as a risk marker for stroke and TIA in a Swedish population. Atherosclerosis. 2006;185:271–277. doi: 10.1016/j.atherosclerosis.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Usui M, Matsuoka H, Miyazaki H, Ueda S, Okuda S, Imaizumi T. Increased endogenous nitric oxide synthase inhibitor in patients with congestive heart failure. Life Sciences. 1998;62:2425–2430. doi: 10.1016/s0024-3205(98)00225-2. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Li Y, Zhang P, Traverse JH, Hou M, Xu X, Kimoto M, Bache RJ. Dimethylarginine dimethylaminohydrolase and endothelial dysfunction in failing hearts. Am J Physiol Heart Circ Physiol. 2005;289:H2212–H2219. doi: 10.1152/ajpheart.00224.2005. [DOI] [PubMed] [Google Scholar]
- 15.Kumagai H, Sakurai M, TTakita a, Maruyama Y, Uno S, Ikegaya N, Kato A, Hishida A. Association of Homocysteine and Asymmetric Dimethylarginine With Atherosclerosis and Cardiovascular Events in Maintenance Hemodialysis Patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006;48:797–805. doi: 10.1053/j.ajkd.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Anderssohn M, Schwedhelm E, Lüneburg N, Vasan RS, Böger RH. Review article: Asymmetric dimethylarginine as a mediator of vascular dysfunction and a marker of cardiovascular disease and mortality: an intriguing interaction with diabetes mellitus. Diabetes and Vascular Disease Research. 2010;7:105–118. doi: 10.1177/1479164110366053. [DOI] [PubMed] [Google Scholar]
- 17.Bode-Böger SM, Scalera F, Ignarro LJ. The l-arginine paradox: Importance of the l-arginine/asymmetrical dimethylarginine ratio. Pharmacology & Therapeutics. 2007;114:295–306. doi: 10.1016/j.pharmthera.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Böger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS. Plasma Asymmetric Dimethylarginine and Incidence of Cardiovascular Disease and Death in the Community. Circulation. 2009;119:1592–1600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa T, Kimoto M, Sasaoka K. Purification and properties of a new enzyme, NG,NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J. Biol. Chem. 1989;264:10205–10209. [PubMed] [Google Scholar]
- 20.Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, Whitley GS, Vallance P. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem. J. 1999;343:209–214. [PMC free article] [PubMed] [Google Scholar]
- 21.Valkonen V-P, Tuomainen T-P, Laaksonen R. DDAH gene and cardiovascular risk. Vascular Medicine. 2005;10:S45–S48. doi: 10.1191/1358863x05vm600oa. [DOI] [PubMed] [Google Scholar]
- 22.Arrigoni FI, Vallance P, Haworth SG, Leiper JM. Metabolism of Asymmetric Dimethylarginines Is Regulated in the Lung Developmentally and With Pulmonary Hypertension Induced by Hypobaric Hypoxia. Circulation. 2003;107:1195–1201. doi: 10.1161/01.cir.0000051466.00227.13. [DOI] [PubMed] [Google Scholar]
- 23.Gray GA, Patrizio M, Sherry L, Miller AA, Malaki M, Wallace AF, Leiper JM, Vallance P. Immunolocalisation and activity of DDAH I and II in the heart and modification post-myocardial infarction. Acta Histochemica. 2010;112:413–423. doi: 10.1016/j.acthis.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Tran CTL, Fox MF, Vallance P, Leiper JM. Chromosomal Localization, Gene Structure, and Expression Pattern of DDAH1: Comparison with DDAH2 and Implications for Evolutionary Origins. Genomics. 2000;68:101–105. doi: 10.1006/geno.2000.6262. [DOI] [PubMed] [Google Scholar]
- 25.Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol. 2007;293:H3227–H3245. doi: 10.1152/ajpheart.00998.2007. [DOI] [PubMed] [Google Scholar]
- 26.Hu X, Xu X, Zhu G, Atzler D, Kimoto M, Chen J, Schwedhelm E, Luneburg N, Boger RH, Zhang P, Chen Y. Vascular Endothelial-Specific Dimethylarginine Dimethylaminohydrolase-1-Deficient Mice Reveal That Vascular Endothelium Plays an Important Role in Removing Asymmetric Dimethylarginine. Circulation. 2009;120:2222–2229. doi: 10.1161/CIRCULATIONAHA.108.819912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O'Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- 28.Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger MC, Wang B-y, Tsao PS, Kimoto M, Vallance P, Patterson AJ, Cooke JP. Dimethylarginine Dimethylaminohydrolase Regulates Nitric Oxide Synthesis: Genetic and Physiological Evidence. Circulation. 2003;108:3042–3047. doi: 10.1161/01.CIR.0000101924.04515.2E. [DOI] [PubMed] [Google Scholar]
- 29.Schwedhelm E, Maas R, Tan-Andresen J, Schulze F, Riederer U, Böger RH. High-throughput liquid chromatographic-tandem mass spectrometric determination of arginine and dimethylated arginine derivatives in human and mouse plasma. Journal of Chromatography B. 2007;851:211–219. doi: 10.1016/j.jchromb.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 30.Maas R, Tan-Andreesen J, Schwedhelm E, Schulze F, Böger RH. A stable-isotope based technique for the determination of dimethylarginine dimethylaminohydrolase (DDAH) activity in mouse tissue. Journal of Chromatography B. 2007;851:220–228. doi: 10.1016/j.jchromb.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Schulze F, Wesemann R, Schwedhelm E, Sydow K, Albsmeier J, Cooke JP, Böger RH. Determination of asymmetric dimethylarginine (ADMA) using a novel ELISA assay. Clinical Chemistry and Laboratory Medicine. 2004;42:1377–1383. doi: 10.1515/CCLM.2004.257. [DOI] [PubMed] [Google Scholar]
- 32.Jacobi J, Sydow K, von Degenfeld G, Zhang Y, Dayoub H, Wang B, Patterson AJ, Kimoto M, Blau HM, Cooke JP. Overexpression of Dimethylarginine Dimethylaminohydrolase Reduces Tissue Asymmetric Dimethylarginine Levels and Enhances Angiogenesis. Circulation. 2005;111:1431–1438. doi: 10.1161/01.CIR.0000158487.80483.09. [DOI] [PubMed] [Google Scholar]
- 33.Stühlinger MC, Conci E, Haubner BJ, Stocker E-M, Schwaighofer J, Cooke JP, Tsao PS, Pachinger O, Metzler B. Asymmetric Dimethyl l-Arginine (ADMA) is a critical regulator of myocardial reperfusion injury. Cardiovascular Research. 2007;75:417–425. doi: 10.1016/j.cardiores.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 34.Lundberg JO, Weitzberg E. NO-synthase independent NO generation in mammals. Biochemical and Biophysical Research Communications. 2010;396:39–45. doi: 10.1016/j.bbrc.2010.02.136. [DOI] [PubMed] [Google Scholar]
- 35.Lu Z, Fassett J, Xu X, Hu X, Zhu G, French J, Zhang P, Schnermann J, Bache RJ, Chen Y. Adenosine A3 Receptor Deficiency Exerts Unanticipated Protective Effects on the Pressure-Overloaded Left Ventricle. Circulation. 2008;118:1713–1721. doi: 10.1161/CIRCULATIONAHA.108.788307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, Fassett J, Tao Y, Zhang P, Remedios Cd, Pritzker M, Hall JL, Garry DJ, Chen Y. Oxidative Stress Regulates Left Ventricular PDE5 Expression in the Failing Heart. Circulation. 2010;121:1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundblad C, Bentzer P. Effects of l-arginine on cerebral blood flow, microvascular permeability, number of perfused capillaries, and brain water content in the traumatized mouse brain. Microvascular Research. 2007;74:1–8. doi: 10.1016/j.mvr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 39.Pope AJ, Karrupiah K, Kearns PN, Xia Y, Cardounel AJ. Role of Dimethylarginine Dimethylaminohydrolases in the Regulation of Endothelial Nitric Oxide Production. Journal of Biological Chemistry. 2009;284:35338–35347. doi: 10.1074/jbc.M109.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang D, Gill PS, Chabrashvili T, Onozato ML, Raggio J, Mendonca M, Dennehy K, Li M, Modlinger P, Leiper J, Vallance P, Adler O, Leone A, Tojo A, Welch WJ, Wilcox CS. Isoform-Specific Regulation by NG,NG-Dimethylarginine Dimethylaminohydrolase of Rat Serum Asymmetric Dimethylarginine and Vascular Endothelium-Derived Relaxing Factor/NO. Circ Res. 2007;101:627–635. doi: 10.1161/CIRCRESAHA.107.158915. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa T, Kimoto M, Sasaoka K. Dimethylarginine:pyruvate aminotransferase in rats. Purification, properties, and identity with alanine:glyoxylate aminotransferase 2. Journal of Biological Chemistry. 1990;265:20938–20945. [PubMed] [Google Scholar]
- 42.Rodionov RN, Murry DJ, Vaulman SF, Stevens JW, Lentz SR. Human Alanine-Glyoxylate Aminotransferase 2 Lowers Asymmetric Dimethylarginine and Protects from Inhibition of Nitric Oxide Production. Journal of Biological Chemistry. 2010;285:5385–5391. doi: 10.1074/jbc.M109.091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogawa T, Kimoto M, Watanabe H, Sasaoka K. Metabolism of NG,NG- and NG,NG-dimethylarginine in rats. Archives of Biochemistry and Biophysics. 1987;252:526–537. doi: 10.1016/0003-9861(87)90060-9. [DOI] [PubMed] [Google Scholar]
- 44.Breckenridge R, Kelly P, Nandi M, Vallance P, Ohun T, Leiper J. A role for Dimethylarginine Dimethylaminohydrolase 1 (DDAH1) in mammalian development. Int J Dev Biol. 2010;54:215–220. doi: 10.1387/ijdb.072356rb. [DOI] [PubMed] [Google Scholar]
- 45.Morishita T, Tsutsui M, Shimokawa H, Sabanai K, Tasaki H, Suda O, Nakata S, Tanimoto A, Wang K-Y, Ueta Y, Sasaguri Y, Nakashima Y, Yanagihara N. Nephrogenic diabetes insipidus in mice lacking all nitric oxide synthase isoforms. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10616–10621. doi: 10.1073/pnas.0502236102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.