Abstract
Chronic intestinal inflammation culminates in cancer and a link to TLR4 has been suggested by our observation that TLR4 deficiency prevents colitis-associated neoplasia. In the current study, we address the effect of the aberrant activation of epithelial TLR4 on induction of colitis and colitis-associated tumor development. We take a translational approach to address the consequences of increased TLR signaling in the intestinal mucosa. Mice transgenic for a constitutively-active TLR4 under the intestine-specific villin promoter (villin-TLR4 mice) were treated with DSS for acute colitis and azoxymethane-dextran sulfate sodium. TLR4 expression was analyzed by immunohistochemistry in colonic tissue from patients with ulcerative colitis and ulcerative colitis associated cancer. The effect of an antagonist TLR4 Ab was tested in prevention of colitis-associated neoplasia in the AOM-DSS model. Villin-TLR4 mice were highly susceptible to both acute colitis and colitis-associated neoplasia. Villin-TLR4 mice had increased epithelial expression of COX-2 and mucosal PGE2 production at baseline. Increased severity of colitis in villin-TLR4 mice was characterized by enhanced expression of inflammatory mediators and increased neutrophilic infiltration. In human UC samples, TLR4 expression was upregulated in almost all CAC and progressively increases with grade of dysplasia. As a proof of principle, a TLR4/MD-2 antagonist antibody inhibited colitis-associated neoplasia in the mouse model. Our results show that regulation of TLR's can affect the outcome of both acute colitis and its consequences—cancer. Targeting TLR4 and other TLR's may ultimately play a role in prevention or treatment of colitis-associated cancer.
Keywords: Toll-like receptor, Cancer, Innate immunity, Ulcerative colitis, villin
Introduction
Patients with ulcerative colitis and Crohn's disease develop chronic intestinal inflammation in the absence of a pathogen. Research using genetic models of inflammatory bowel disease has revealed that Th1 and Th17 cytokines are required for inflammation (1)(2). This understanding has resulted in the use of biologic agents that target TNF-α and, more recently, IL-12 and IL-23 (anti-p40). On the other hand, genetic research in patients with inflammatory bowel disease substantiates a role for innate immunity playing a role in inflammatory bowel disease (3)(4)(5).
We became interested in how the innate immune response to luminal bacteria might play a role in inflammatory bowel disease. Using the dextran sodium sulfate (DSS) model of colitis, we found that animals deficient in TLR4 or MyD88 experienced severe epithelial injury but a relative lack of an inflammatory response (6). In spite of epithelial ulcers, the lamina propria infiltrate was nearly devoid of neutrophils and bacteria could be seen infiltrating the mucosa—a finding not present in wild-type (WT) mice. At least part of the reason for greater epithelial injury was due to defective proliferation of epithelial cells (6, 7).
The observation that TLR4-/- mice had decreased acute inflammation led us to test whether the same inability to mount a robust inflammatory response to injury could protect the mice from inflammation-induced neoplasia. We found that TLR4-/- mice given the mutagen azoxymethane (AOM) followed by DSS were significantly protected from polyposis (8). Further studies using bone marrow chimeras found that epithelial TLR4 was associated with more neoplastic lesions than hematopoietic expression of TLR4 in the AOM-DSS model, suggesting that epithelial TLR4 was required for inflammatory neoplasia (9). Moreover, in AOM-DSS-treated WT mice, TLR4 staining revealed that the epithelial component of tumors had high expression of TLR4 compared with the surrounding tissue (6).
With that as a backdrop, we chose to mimic the findings of increased epithelial expression of TLR4 in an animal model by generating a transgenic mouse that expresses TLR4 under the control of the villin promoter, villin-TLR4 mice. The advantage of this approach is that the remainder of the animal expresses normal levels of TLR4, unlike the knock-out mice which might have immunological or developmental defects due to TLR4 deficiency in all cell types. The other characteristic of villin-TLR4 mice is that the TLR4 construct is constitutively active and does not require LPS for its activation (10). The transgene is highly expressed in both small bowel and colon.
In the current study, we wished to take a translational approach to understanding the role of TLR4 in colitis and colitis-induced neoplasia. We now show that villin-TLR4 mice develop severe acute inflammation in response to DSS accompanied by a high rate of mortality. Villin-TLR4 mice also are more prone to colitis-associated neoplasia. Mechanistically, we show that TLR4 increases mucosal expression of TNF-α, increases epithelial COX-2 protein expression, and increases mucosal PGE2 production, a known colonic tumor promoter. To determine whether TLR4 over-expression occurs in ulcerative colitis associated neoplasia, we have performed immunostaining for TLR4 in ulcerative colitis associated dysplasia and cancer and found that TLR4 expression increases with the grade of dysplasia. As a proof of principle, we used a TLR4/MD-2 antagonist antibody to inhibit TLR4 signaling in WT mice treated with AOM-DSS. This treatment significantly decreased the development of colonic tumors. Our results show an important contribution of epithelial TLR4 in the induction of acute colitis and intestinal tumorigenesis in response to mucosal injury. Understanding the mechanisms by which innate immune signaling contributes to intestinal homeostasis and cancer may result in improved prevention and treatment of patients at risk for inflammation-induced neoplasia.
Materials and Methods
Animal studies
The villin-TLR4 transgenic mice (villin-TLR4 mice) were generated using plasmids containing the mouse villin promoter (pBS-Villin) and the mCD4-hTLR4 fusion gene (11) as previously described (10). These mice express a constitutively active form of TLR4 on IECs under the villin promoter. Mice were kept in specific-pathogen free (SPF) conditions and fed under free access to a standard diet and water. All experiments were done according to the Mount Sinai and University of Miami Miller School of Medicine Animal Care and Use Committees' guidelines.
Acute colitis was induced by giving mice 3% DSS (MW 36-50 kDa: ICN, Aurora, Ohio) for 7 days. Mice were assessed daily with disease activity index (DAI) determined as previously described (12). To induce colitis-associated neoplasia, first we followed previously established methods (13): six to ten-week old gender-matched mice were injected with 7.4mg/kg of azoxymethane (AOM) (Sigma, St. Louis, MO) intraperitoneally (i.p.) at the beginning of the experiment (day 0). After 14 days, mice were treated with 3% DSS for 7 days. This was followed by 14 days of normal water, 7 days of 3% DSS treatment, and finally, normal water for an additional 7 days. A modified protocol was used after the initial set of experiments because of the high mortality in villin-TLR4 mice with AOM-DSS. All mice completed the modified protocol with three cycles of 2% DSS treatment (seven days each followed by 14 days recovery) 14 days after AOM injection (14.8mg/kg i.p. on day 0, n=4 each).
For TLR4 inhibiton, a mouse IgG2b monoclonal antibody against the mouse TLR4/MD-2 complex called 1A6 or an isotype-matched control was injected i.p. at a dose of 20mg/kg only during the recovery period of the AOM-DSS model (twice a week, 20 μg/g: n=9). The control group received an isotype-matched antibody (n=8). Tumors were examined under a dissecting microscope, and the number of lesions was counted. After macroscopic assessment, the samples were fixed in formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histological analysis. In some experiments, 1A6 was labeled with fluorescein using a Lynx rapid fluorescein antibody conjugation kit (Serotec Ltd., Raleigh, NC). WT mice treated with AOM-DSS were injected with fluorescein labeled Ab and sacrificed for immune-fluorescent studies.
Histopathological assessment was performed by two independent gastrointestinal pathologists (N.H., H.C.) and a veterinary pathologist (J.Z.) who were masked to the mouse genotype and treatment. Severity of mucosal inflammation was graded using a standard scoring system described previously (8). Acute inflammatory score was calculated by counting neutrophils and eosinophils in three independent high magnification fields (×400) and graded as (0-1=0, 2-9=0.5, 10-20=1, 21-30=1.5, 31-40=2, 41-50=2.5, 51-65=3, 66-80=3.5, >80=4). Dysplastic lesions were determined by previously established criteria (14).
RNA extraction and real-time PCR analysis
Total RNA from IECs was extracted using the RNeasy mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Reverse transcription was performed using 2 μg of total RNA. Quantitative PCR was conducted with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Primer sequences used in this study are as follows: (5′→3′ direction), for mouse Cox-2: sense primer, AAG GAA CTC AGC ACT GCA TCC, anti-sense primer, ACA GGG ATT GGA ACA GCA AGG A; for mouse TNF-α: sense primer, CGT GGA ACT GGC AGA AGA GG, anti-sense primer, GGA ATG AGA AGA GGC TGA GAC AT; for mouse IL-12: sense primer, CCC ATC ACA TCT CAT CTC CCC A, anti-sense primer, AAG CGA TGG AGG GGA CCA T; for mouse beta-actin: sense primer, ATG ACC CAG ATC ATG TTT G, anti-sense primer, TAC GAC CAG AGG CAT ACA. PCR cycling conditions were: 50°C for 2 min, 95°C for 10 min and 40 cycles of 95°C for 15 sec, 60°C for 1 min. Relative expression levels were calculated as 2^(Ct ubiquitin minus Ct gene) (for details see ABI PRISM 7700 - User Bulletin #2) using ubiquitin RNA as the endogenous control. Primers were designed using primer express 2.0 software (Applied Biosystems, Foster City, CA).
Enzyme linked immunosorbent assay (ELISA)
For the measurement of mucosal TNF-α production, 100 mg of duodenum or colonic mucosa (not including the polypoid lesions) were washed in cold PBS containing penicillin, streptomycin, and fungizone (100U/ml each) and cultured for 24 hours in 12 well flat bottom plates in serum free RPMI 1640 with penicillin and streptomycin. Supernatants were harvested for measurement of TNF-α. Mucosal production of TNF-α was measured by ELISA kit following manufacturer's instructions (R&D Systems, Minneapolis, MN).
Measurement of PGE2
Production of PGE2 in the tissue culture supernatant was determined using a monoclonal EIA kit (Cayman, Ann Arbor, MI) according to the manufacturer's instructions and as described previously (8). Briefly, colonic samples from villin-TLR4 and WT mice were washed in cold PBS containing penicillin, streptomycin, and fungizone (100U/ml each). 100 mg tissue fragments from the distal part of the colon were cultured for 24 hours in 12 well flat bottom plates in serum free RPMI 1640 with penicillin and streptomycin. Culture supernatants were harvested for PGE2 measurement.
Hematologic and blood biochemistry measurement
Blood samples were taken from the orbital sinus of the eye. Hematocrit was measured in a heparinized tube using a microcapillary reader (IEC, Needham Heights, MA). Routine clinical enzymes were determined on an Ortho Vitros 250 automated chemistry analyzer (Ortho Diagnostics, Rochester, NY). All blood analyses were done by the Division of Comparative Pathology, Department of Veterinary Medicine at the University of Miami, Miller School of Medicine.
TLR4 staining of human colonic tissues
De-identified human colon, formalin-fixed paraffin-embedded, specimens from patients with ulcerative colitis were obtained from the Mount Sinai Department of Pathology under the auspices of the Mount Sinai Medical Center Institutional Review Board. TLR4 expression in epithelial cells was evaluated by immunohistochemistry using a tyramide amplification method as described (15). Thirteen ulcerative colitis cancers were compared to nine high-grade dysplasia samples, 17 low grade dysplasia samples, and 29 inflamed tissues from patients with ulcerative colitis. Grading of TLR4 positivity was performed by multiplying staining intensity (0=none, 1=small, 2=moderate, 3=high) by the percentage of epithelial cells staining positive (scored as follows: 0 = <1%, 1 = 1-10%, 2 = 11-50%, 3 = ≥50%). The degree of positivity (0-3) was multiplied by the degree of epithelial staining (0-3) for a maximum obtainable score of 9 (range 0-9).
Immunofluorescent studies
Paraffin-embedded intestinal sections were stained. The primary antibodies used in this study was rabbit anti-murine COX-2 (Cayman, Ann Arbor, MI) at a dilution of 1:200. Heat epitope retrieval was done in a citrate buffer. Alexa fluor 488 conjugated goat anti-rabbit IgG (1:200) was used as secondary antibody. Sections were counterstained with 4′, 6-diamidino-2-phenylindole. Non-immune rabbit IgG was substituted as negative controls.
Statistical analysis
Graphical analyses, statistical analysis and nonlinear regression analysis of the data were performed using Prism 2.0c (GraphPad Software, San Diego, Calif.). Data in the text are given as means ± SD unless otherwise stated. Unpaired Student's t test was used to determine statistical significance. Comparison of more than three subjects was performed by nonparametric ANOVA (Kruskal-Wallis) followed by Mann–Whitney U test. Differences were considered significant when P < 0.05.
Results
Villin-TLR4 mice express increased inflammatory mediators
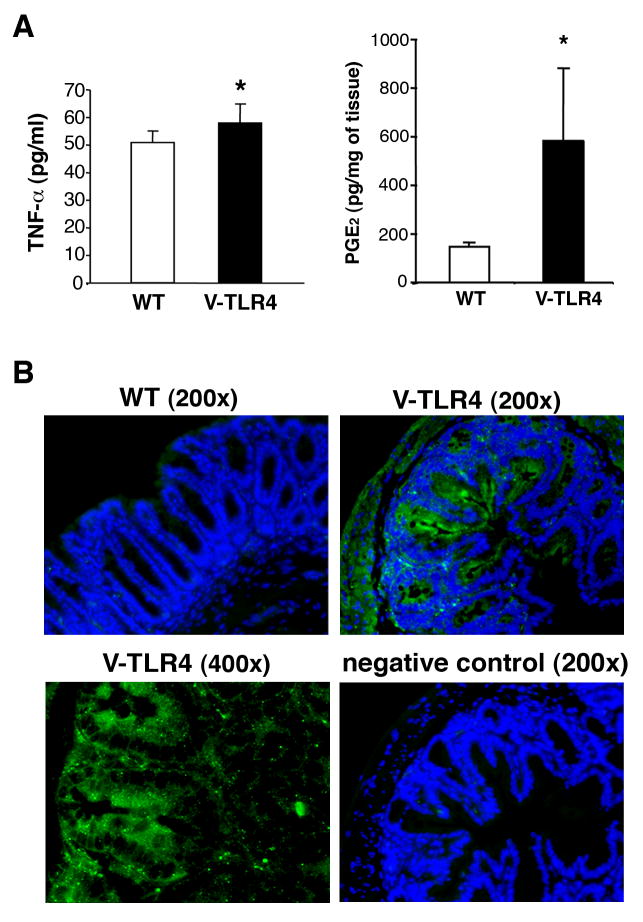
The intestinal mucosa of villin-TLR4 mice is characterized by increased epithelial activation of NF-κB but we do not see a neutrophilic infiltrate at baseline (10). We have previously shown that TLR4-/- mice have decreased expression of COX-2 and PGE2 is regulated by TLR4 signaling in the intestine (16). We have also described an increase in TNF-α mRNA expression in the small intestine of villin-TLR4 mice (10). To address whether epithelial TLR4 could drive expression of these mediators, we examined baseline production of PGE2 and TNF-α by the colonic mucosa of villin-TLR4 mice and WT littermates. Villin-TLR4 mice produced significantly higher amount of both PGE2 and TNF-α in the colonic mucosa when compared to WT mice (Figure 2A). To examine mucosal COX-2 expression, sections of the colon from villin-TLR4 and WT mice were stained for COX-2 protein (Figure 2B). We did not detect COX-2 protein expression in normal WT mucosa. However, a strong COX-2 signal was detected in epithelial cells from villin-TLR4 mice. These results indicate that epithelial expression of TLR4 results in expression of inflammatory mediators implicated in neoplasia.
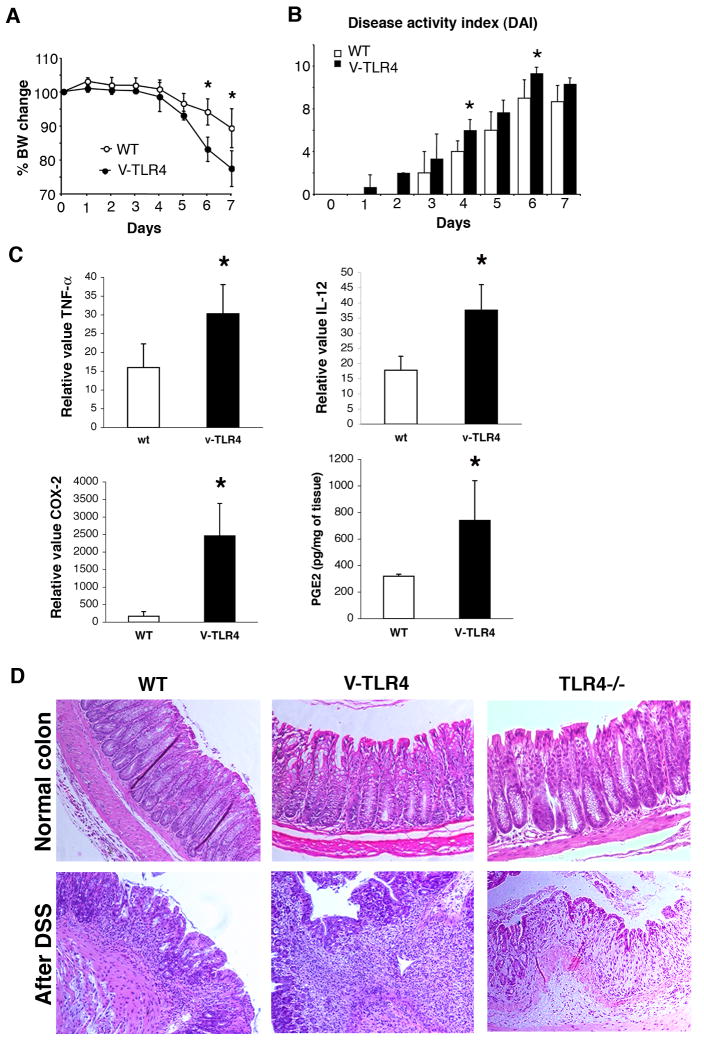
Figure 2. Villin-TLR4 mice are more susceptible to acute DSS-induced colitis.
A. Villin-TLR4 mice have significantly greater body weight loss than WT littermates (P < 0.05,
B. ANOVA) during the course of colitis. B. Villin-TLR4 mice show more severe colitis as measured by Disease Activity Index (DAI) than WT littermates (P < 0.05, ANOVA). C. Enhanced induction of inflammatory mediators in villin-TLR4 mice during acute DSS colitis. Significantly higher expression of colonic TNF-α, IL-12, and COX-2 as well as PGE2 is seen in villin-TLR4 mice compared to WT mice after DSS. D. H&E staining of DSS colitis. At baseline, villin-TLR4 and TLR4-/- colons are similar to WT littermates. After DSS treatment, villin-TLR4 mice have greater inflammatory cell infiltration in the colon compared to WT and TLR4-/- mice.
Villin-TLR4 mice are highly susceptible to acute intestinal inflammation
Given the inflammatory milieu in villin-TLR4 mice, we asked whether villin-TLR4 mice were more susceptible to DSS-induced colitis. We found that villin-TLR4 mice had greater weight loss and bleeding (hematocrit (%) WT 42.5 ± 3.7; villin-TLR4 36.3 ± 3.5, p=0.025) than WT littermates (Figure 2A and B). After DSS treatment, villin-TLR4 mice demonstrated a further increase in expression of pro-inflammatory cytokines TNF-α and IL-12, as well as COX-2 and PGE2 in the colonic mucosa (Figure 2C). Histological examination of the colon showed significantly greater acute inflammation in villin-TLR4 compared to WT mice (acute inflammatory score WT= 0.7 ± 0.65, villin-TLR4=1.6 ± 0.4 n=5 each, p=0.03) (Figure 2D). For comparison, we show the mucosa of a TLR4-/- mouse treated with DSS in parallel demonstrating a paucity of acute inflammation as we had reported previously (6). These data indicate that TLR4 signaling by IECs is important for recruitment of acute inflammatory cells to the injured epithelium. We concluded that increased epithelial TLR4 signaling results in more severe biochemical and histological inflammation and more severe clinical manifestations of colitis.
Villin-TLR4 mice have increased susceptibility to inflammation-induced neoplasia
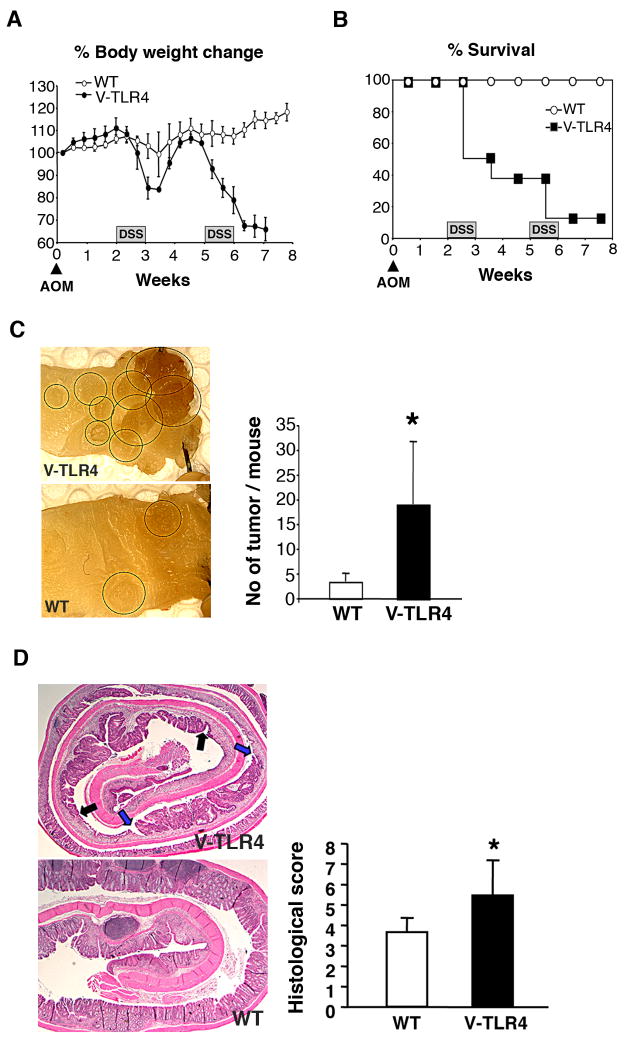
Chronic colonic inflammation results in increased dysplasia and colon cancer in patients with inflammatory bowel disease (17). Up-regulation of mucosal COX-2 and PGE2 production and the number of intraepithelial neutrophils have been associated with the risk of colitis-associated tumorigenesis in mouse and human (18)(19). Given our observation that DSS induces severe acute inflammation in villin-TLR4 mice, we hypothesized that villin-TLR4 mice would demonstrate increased susceptibility to inflammation-associated neoplasia. To answer this question, we induced colitis-associated neoplasia in the villin-TLR4 mice with azoxymethane (AOM) and DSS. After the second cycle of DSS, the villin-TLR4 mice had significant weight loss and mortality compared with WT mice (Figure 3A and B). The cause of the greater mortality was due to severe dehydration and anemia; villin-TLR4 mice had significantly increased serum BUN and decreased hematocrit compared with WT mice (Table 1). Animals that survived treatment with AOM-DSS were evaluated for colonic tumor development. Surviving villin-TLR4 mice had significantly higher numbers of colonic tumors compared to WT mice (Figure 3C). Histological assessment showed that the colonic mucosa from villin-TLR4 mice was replaced by adenomas and surrounded by ulcerated tissue, whereas few ulcers were seen in the WT mice (Figure 3D). The histological severity of the colitis was significantly higher in villin-TLR4 mice (5.5 ± 1.7) compared to WT littermates (3.6 ± 0.6, p=0.008) (Figure 3D). Therefore, we conclude that upregulated epithelial TLR4 signaling promotes the development of colitis-associated neoplasia.
Figure 3. Villin-TLR4 mice are more susceptible to chronic colitis and colitis-associated neoplasia.
A. Villin-TLR4 mice have significantly greater body weight loss than WT littermates (P < 0.05, ANOVA) during the course of colitis. B. Increased mortality of villin-TLR4 mice after DSS treatment (n=8). C. Left panel: A Colonic mucosa from villin-TLR4 versus WT littermates (polypoid lesions are circled). The graph shows increased numbers of colon tumors in villin-TLR4 compared to WT littermates (n=5 each, p=0.012). D. The graph shows histological severity of the chronic colitis.
Table 1. Blood test results.
| Hct (%) | BUN (mg/dL) | |
|---|---|---|
| WT | 42.5 ± 3.7 | 20.25 ± 2.2 |
| Villin-TLR4 | 36.3 ± 3.5 | 26.25 ± 2.2 |
| p value | 0.025 | 0.0043 |
Dysplasia and CAC in ulcerative colitis patients show increased epithelial expression of TLR4
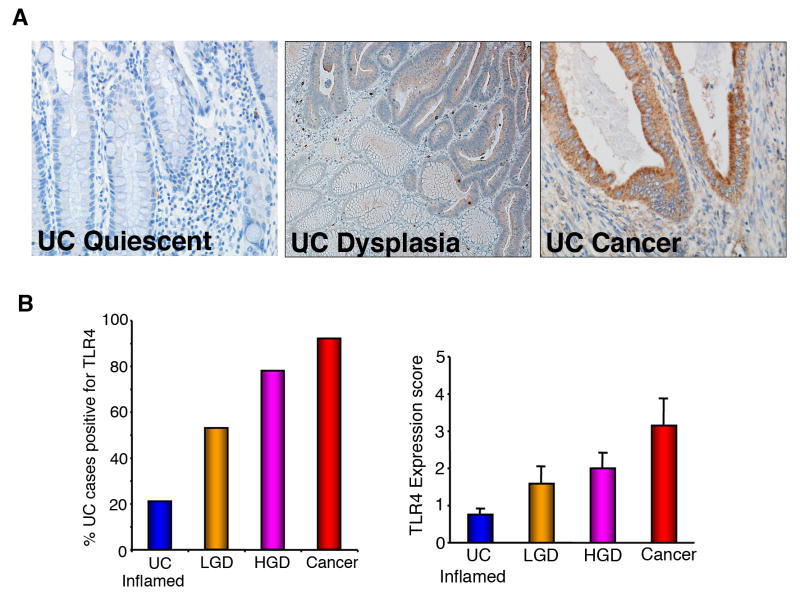
Patients with chronic ulcerative colitis are at increased risk for dysplasia and CAC (20, 21). The finding of dysplasia often leads to a total proctocolectomy in patients with ulcerative colitis (22). We previously analyzed a limited number of CAC and found that they had increased expression of TLR4 (8). Our next question was to determine whether increased TLR4 expression occurs prior to the development of CAC. To that end, we examined the expression of TLR4 in ulcerative colitis cancers, high-grade dysplasia, and low-grade dysplasia and compared these to active, inflamed ulcerative colitis. We found that the majority of CACs, 92%, expressed high levels of TLR4, followed by high-grade dysplasia, 78%, and low grade dysplasia, 53%, compared with only 21% of inflamed, non-dysplastic ulcerative colitis (Figure 4A, B). Not only did a higher percentage of ulcerative colitis cancers express TLR4, but also the intensity of staining and the percentage of the neoplastic lesion expressing TLR4 were higher in ulcerative colitis cancers compared with dysplasia. When TLR4 was expressed in active UC it tended to be in lamina propria macrophages rather than in the epithelial cells in dysplasia and cancer. These data suggest that increased epithelial TLR4 expression occurs prior to the development of cancer in a subset of patients with ulcerative colitis and may play a role in the transition from inflammation to neoplasia.
Figure 4. TLR4 expression in human colitis-associated dysplasia and cancer.
A. Immunohistochemistry of TLR4 expression. (×400) (UC: normal mucosa in UC specimen, UC Dysplasia: high grade dysplasia specimen, CAC: cancer). B. Percentage and intensity of TLR4 staining. Higher-grade dysplastic lesions are more likely to be positive for TLR4. (UC Inflam: inflamed mucosa without dysplasia, LGD: low grade dysplasia, HGD: high grade dysplasia, Ca: cancer)
Antibody blockade of TLR4 decreases incidence of colitis-associated tumors
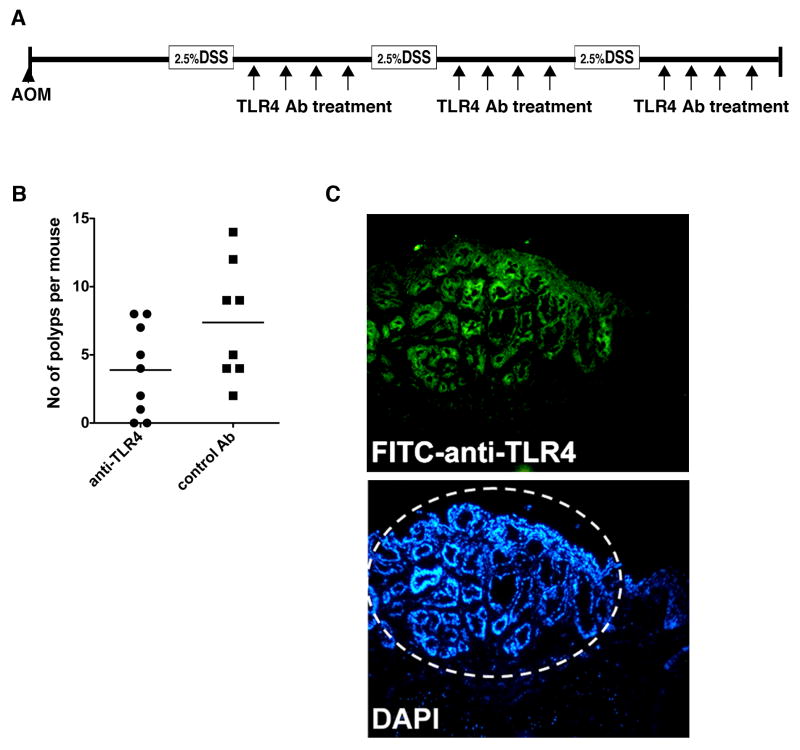
Given our results that TLR4 signaling promotes neoplasia, we wished to test whether inhibiting TLR4 would prevent the development of neoplasia. In previous studies we found that inhibition of TLR4 during the acute phase of colitis worsened bleeding (15). Therefore, we asked whether inhibition of TLR4 during the recovery phase of colitis, prevented the development of neoplastic colonic lesions (Figure 5A). We reasoned this was equivalent to patients in remission from their ulcerative colitis. We used an antibody that recognizes the TLR4/MD-2 complex and has been shown to inhibit LPS-induced septic shock (23). Our data demonstrate that inhibition of TLR4 significantly decreased development of polyps (Figure 5B). In established colonic tumors from WT mice treated with AOM-DSS, the TLR4 antibody recognized the epithelial compartment of the tumors when given parenterally (Figure 5C). These data support a role for inhibition of TLR4 in prevention of dysplasia in the setting of colonic inflammation and provide a proof of principle for future clinical interventions.
Figure 5. Inhibition of TLR4 decreases colitis-associated tumors.
A. Colitis-associated neoplasia was induced by AOM injection (10 mg/kg) followed by three cycles of 3% DSS treatment in WT mice. The anti-mouse TLR4 mAb was administered during the recovery period (twice a week, 20 μg/g:, n=9). The control group received an isotype-matched mAb (n=8). B. The number of polyps per mouse decreased significantly. C. Fluorescein-labeled anti-TLR4 mAb detects intestinal tumor cells in WT mice treated with AOM-DSS 3 hours after i.p injection in vivo.
Discussion
In patients with inflammatory bowel disease, the host perceives a chronic pathogenic infection. Patients with ulcerative colitis or Crohn's disease generate adaptive immune responses to commensal bacteria and have an increased risk for colorectal cancer (17, 24). We found that dysplasia and colon cancer specimens from patients with ulcerative colitis show increased TLR4 expression (8) and that TLR4 expression correlates with the degree of dysplasia. We chose to mimic this by forcing epithelial expression of TLR4. Some of the results were surprising. In spite of constitutive TLR4 activation, there was no spontaneous, overt inflammation histologically. Biochemically, however, villin-TLR4 mice spontaneously made higher levels of TNF-α and expressed COX-2 leading to PGE2 secretion. We believe this heightened state of inflammation at baseline participates in an over-exuberant TLR-mediated response to epithelial injury which sows the seeds for colitis associated cancer. Indeed, COX-2 and TNF-α have both been implicated in colitis-associated neoplasia in humans and mouse models (25)(18). It is worth noting that the TLR4 transgene we have used in the current study is constitutively-active and contains the intracellular domain of TLR4. It is likely that its expression activates TLR signaling broadly. Therefore, our results suggest that TLR signaling by TLR4 and other TLRs is pro-inflammatory and tumorigenic. Like many things in biology, extremes are deleterious. Too little TLR4 signaling (as in the knock-out situation) results in poor mucosal healing and bacterial translocation; too much TLR4 signaling (villin-TLR4 mice) results in severe inflammation and cancer.
We have gone further in this study to determine the potential for TLR manipulation as a clinical intervention. Antagonizing TLR4 during the recovery phase of colitis, after administration of DSS, significantly protected mice from colitis-associated cancer. We chose this strategy because we reasoned that this most closely resembled using a TLR4 antagonist when an ulcerative colitis patient was in remission, as opposed to during a flare. Our own work found that antagonizing TLR4 during acute colitis led to more bleeding (15). Obviously, the murine and human situations are quite different. It is intriguing that the Ab we used against TLR4 and MD-2 targeted the tumor and specifically recognized the epithelial compartment of the tumor in WT mice—again corroborating that high level TLR4 expression by epithelial cells identifies neoplasia. These data suggest that TLR staining may have a role as a biomarker as well. Our data combined with the human specimens that demonstrate a progression of TLR4 expression from quiescent UC to dysplasia suggest that TLR inhibition may someday play a role in the prevention of dysplasia in ulcerative colitis. We believe the results of our studies will permit rational therapies to be developed that combine blockade of TLR4 and other signaling pathways in the prevention or treatment of inflammation-induced cancers of the gastrointestinal tract.
Figure 1. Villin-TLR4 mice express increased inflammatory mediators.
A. Mucosal production of TNF-α (left) and PGE2 (right) from the colon. Intestinal samples (100mg each) from the colon were incubated in serum free RPMI for 24 hours, and the supernatants were examined for TNF-α and PGE2 (n=4 mice each). Villin-TLR4 mice have significantly higher production of TNF-α and PGE2 than WT littermates. Data are shown as mean (±SD) (*P < 0.05). B. Epithelial expression of COX-2 is up-regulated in villin-TLR4 mice. WT mice do not have detectable COX-2. Epithelial cells are positive for COX-2 in the colonic epithelium of villin-TLR4 mice.
Acknowledgments
Supported by NIH grant AI05226607 (MTA), NCI grant CA137869 (MTA), and Career Development Award from the Crohn's and Colitis Foundation of America (MF).
Abbreviations
- TLR4
Toll-like receptor-4
- CAC
colitis associated cancer
- AOM
azoxymethane
- DSS
dextran sodium sulfate
Footnotes
Author contributions
Masayuki Fukata- designed and performed research, wrote paper
Limin Shang- performed research and wrote paper
Rebeca Santaolalla- performed research, wrote paper
John Sotolongo- performed research
Cristhine Pastorini- performed research
Cecilia España- performed research
Ryan Ungaro- performed research
Noam Harpaz- performed research
Harry S. Cooper- performed research
Greg Elson- performed research
Marie Kosco-Vilbois- performed research
Julia Zaias - performed research
Maria T. Perez- performed research, helped in study design
Lloyd Mayer- Designed and interpreted research
Arunan S. Vamadevan - performed research
Sergio A. Lira - designed and performed research and wrote paper
Maria T. Abreu – designed research and wrote paper
References
- 1.Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199–207. doi: 10.1016/j.molmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Ahern PP, Izcue A, Maloy KJ, et al. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 3.Annese V, Latiano A, Palmieri O, et al. Dissecting genetic predisposition to inflammatory bowel disease: current progress and prospective application. Expert Rev Clin Immunol. 2007;3:287–298. doi: 10.1586/1744666X.3.3.287. [DOI] [PubMed] [Google Scholar]
- 4.Henckaerts L, Pierik M, Joossens M, et al. Mutations in pattern recognition receptor genes modulate seroreactivity to microbial antigens in patients with inflammatory bowel disease. Gut. 2007;56:1536–1542. doi: 10.1136/gut.2007.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenstiel P, Sina C, Franke A, et al. Towards a molecular risk map--recent advances on the etiology of inflammatory bowel disease. Semin Immunol. 2009;21:334–345. doi: 10.1016/j.smim.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Fukata M, Michelsen KS, Eri R, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 7.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Fukata M, Chen A, Vamadevan AS, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukata M, Hernandez Y, Conduah D, et al. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm Bowel Dis. 2009;15:997–1006. doi: 10.1002/ibd.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang L, Fukata M, Thirunarayanan N, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 12.Murthy SN, Cooper HS, Shim H, et al. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Digestive Diseases & Sciences. 1993;38:1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 13.Okayasu I, Ohkusa T, Kajiura K, et al. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39:87–92. doi: 10.1136/gut.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper HS, Murthy S, Kido K, et al. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000;21:757–768. doi: 10.1093/carcin/21.4.757. [DOI] [PubMed] [Google Scholar]
- 15.Ungaro R, Fukata M, Hsu D, et al. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1167–1179. doi: 10.1152/ajpgi.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukata M, Chen A, Klepper A, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2009;29:781–788. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–105. doi: 10.1053/j.gastro.2007.08.001. quiz 1340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solem CA, Harmsen WS, Zinsmeister AR, et al. Small intestinal adenocarcinoma in Crohn's disease: a case-control study. Inflamm Bowel Dis. 2004;10:32–35. doi: 10.1097/00054725-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Jess T, Loftus EV, Jr, Velayos FS, et al. Risk factors for colorectal neoplasia in inflammatory bowel disease: a nested case-control study from Copenhagen county, Denmark and Olmsted county, Minnesota. Am J Gastroenterol. 2007;102:829–836. doi: 10.1111/j.1572-0241.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 22.Awais D, Siegel CA, Higgins PD. Modelling dysplasia detection in ulcerative colitis: clinical implications of surveillance intensity. Gut. 2009;58:1498–1503. doi: 10.1136/gut.2008.169714. [DOI] [PubMed] [Google Scholar]
- 23.Daubeuf B, Mathison J, Spiller S, et al. TLR4/MD-2 monoclonal antibody therapy affords protection in experimental models of septic shock. J Immunol. 2007;179:6107–6114. doi: 10.4049/jimmunol.179.9.6107. [DOI] [PubMed] [Google Scholar]
- 24.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn's disease. J Clin Invest. 2004 May;113:1296–306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popivanova BK, Kitamura K, Wu Y, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]