Abstract
Objective
Interleukin 17A (IL17A) is involved in many inflammatory processes but its role in atherosclerosis remains controversial. We examined the role of IL17A in mouse and human atherosclerosis.
Methods and Results
Atherosclerosis was induced in ApoE−/− and IL17A/ApoE−/− mice using high fat feeding, angiotensin II infusion, or partial carotid ligation. In ApoE−/− mice, 3 months of high fat diet induced interferon gamma production by splenic lymphocytes, and this was abrogated in IL17A/ApoE−/− mice. IL17A/ApoE−/− mice had reduced aortic superoxide production, increased aortic nitric oxide levels, decreased aortic leukocyte and dendritic cell infiltration, and reduced weight gain after high fat diet compared to ApoE−/− mice. Despite these favorable effects, IL17A deficiency did not affect aortic plaque burden after high fat diet or angiotensin II infusion. In a partial carotid ligation model, IL17A deficiency did not affect percent stenosis but reduced outward remodeling. In this model, neutralization of the related isoform, IL17F, in IL17A/ApoE−/− mice did not alter atherosclerosis. Finally, there was no correlation between IL17A levels and carotid intima-media thickness in humans.
Conclusion
IL17 contributes to vascular and systemic inflammation in experimental atherosclerosis but does not alter plaque burden. The changes in plaque composition caused by IL17 might modulate plaque stability.
Keywords: Interleukin 17, Atherosclerosis, Interferon-gamma, Apolipoprotein E, Reactive oxygen species
INTRODUCTION
Atherosclerosis is a complex inflammatory disease characterized by derangements in the vascular, metabolic, and immune systems. Activated T cells, particularly CD4+ T helper cells, are found in atherosclerotic lesions and their role in plaque development varies depending on the subset. In 2005, a novel T helper subset that produces the unique cytokine, IL17, designated Th17 cells, was described1. There are 6 known isoforms of IL17, designated A–F, of which IL17A and IL17F are produced by Th17 cells2. IL17A is the most widely studied and mediates many autoimmune and inflammatory diseases (reviewed in Tesmer et al2). Other sources of IL17 include CD8+ T cells3, γδ T cells, NKT cells, NK cells4, and neutrophils5.
IL17 has been detected in human atherosclerotic lesions6, and Eid et al.7 found that human coronary artery infiltrating T cells produce IL17, IFN-γ, or both, and that IL17 and IFN-γ act synergistically to induce proinflammatory responses in vascular smooth muscle cells. Patients with acute myocardial infarction and unstable angina have increased peripheral Th17 cells and IL17 levels8. We previously showed that IL17A is required for the maintenance of angiotensin II-induced hypertension and vascular dysfunction, both of which are risk factors for atherosclerosis9. One might therefore predict that IL17A is proatherogenic. In prior studies, IL17 seems to reduce, increase, or have no effect on atherosclerosis depending on the experimental model and method of inhibition10–16. Unfortunately, most of the approaches used in these prior papers could be complicated by non-specific/off-target effects of the interventions and/or incomplete IL17 suppression.
We therefore sought to accurately examine the role of IL17 in atherosclerosis by generating IL17A/ApoE−/− double deficient mice and to induce lesions using three separate models (high fat diet, angiotensin II infusion, and partial carotid ligation). We also compared carotid intima-media thickness, a surrogate marker of early atherosclerosis, to serum levels of IL17A in a population of relatively healthy humans aged 50–69 years. Our results indicate that while IL17A modulates some aspects of systemic and vascular inflammation and vascular function, inhibition of IL17A is insufficient to decrease atherosclerotic plaque burden.
MATERIALS AND METHODS
Animals and induction of atherosclerosis
The Institutional Animal Care and Use Committee at Emory University approved all animal protocols. IL17A−/− mice were generated as described in Nakae et al17 and back-crossed to the C57BL/6J background. ApoE−/− mice on a C57BL/6J background were obtained from Jackson Laboratories and crossed to IL17A−/− mice to generate homozygous IL17/ApoE−/− mice. At 8–11 weeks of age, male mice were started on a diet composed of 35 kcal% fat, 1.25% cholesterol, and 0.5% cholate (OpenSource Diets; Cat no D12336) for 12 weeks. Other mice underwent implantation of osmotic minipumps (Alzet Model 2004, Alzet Corp) for infusion of angiotensin II (Sigma A2900) at a dose of 1000 ng/kg/min for 4 weeks. In separate mice, partial left carotid ligation was performed as previously described18. These animals were fed a high fat diet for the next two weeks. To examine a potential role of IL-17F, IL17A/ApoE−/− were treated with anti-IL-17F neutralization polyclonal antibody (100 μg/mouse/time, Catalog # AF2057; R&D Systems) or its isotype control (Goat IgG, 100 μg/mouse/time, Catalog # AB-108-C; R&D Systems) once a week for 3 weeks starting one week before carotid ligation.
Human Carotid Intima-Media Thickness evaluation and IL17A determinations
Carotid intima-media thickness was measured by B mode ultrasound was compared to IL17A levels (measured using reagents from R&D Systems and a Luminex platform) in 16 healthy subjects aged 50–69 years.
Statistics
Data are expressed as mean ± standard error of the mean. A p value ≤0.05 was considered significant.
See online supplement for additional/expanded Materials and Methods section.
RESULTS
IL17A is upregulated in response to diet induced atherosclerosis and promotes interferon gamma (INFγ production
To determine the role of IL17A in atherosclerosis, we first examined the effect of high fat feeding on T cell production of IL-17A. Splenic lymphocytes were isolated from ApoE−/− mice fed high fat diet or normal chow (regular) diet for 3 months and cultured on anti-CD3 plates for 48 hrs. As shown in Figure 1A, high fat diet markedly increased release of IL17A by these cells.
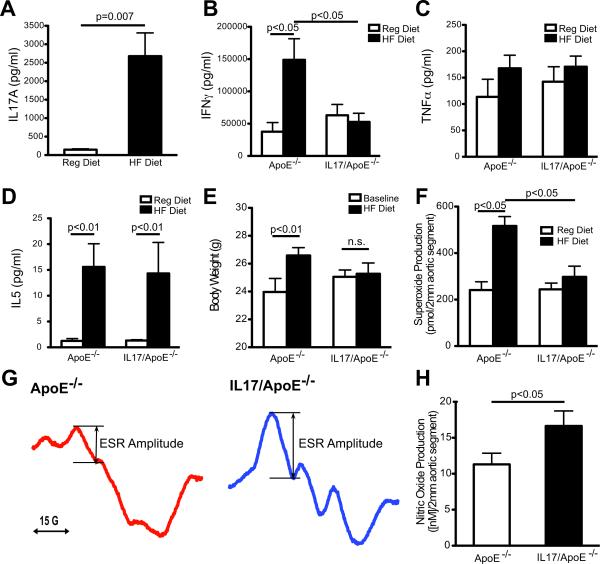
Figure 1.
Cytokine production, body weight and vascular reactive oxygen species in ApoE−/− and IL17/ApoE−/− mice in response to high fat diet. Splenic lymphocytes from ApoE−/− and IL17/ApoE−/− mice fed regular (Reg) diet or high fat (HF) diet for 3 months were cultured on anti-CD3 plates and the culture supernatants were analyzed for IL17A using ELISA (A), or cytokine bead array (B–D) [n=4–7 per group]. Body weight (in grams) was measured at baseline and after 3 months of HF diet in ApoE−/− and IL17/ApoE−/− mice (panel E, n=12–15 per group). Aortic superoxide production was measured by dihydroethidium-HPLC (panel F, n=5–7 per group). Aortic nitric oxide levels after 3 months of high fat diet feeding were measured by electron spin resonance (ESR). Example ESR spectra are shown in panel G and summary data are shown in panel H [n=4–5 per group]. Data in panels A and H were analyzed using Student's t test. Data in panel E were analyzed using two way repeated measures ANOVA. Other statistical data were analyzed using one way ANOVA with Neuman-Keuls post-hoc test.
We then examined how IL17A affects production of the Th1 cytokines, interferon gamma (IFNγ and tumor necrosis factor alpha (TNFα), and the Th2 cytokines, IL4 and IL5. IL17A deficient mice were crossed with ApoE deficient mice to generate homozygous IL17/ApoE−/− mice. IFNγ and IL5 production by splenic lymphocytes, as detected by cytokine bead array, were markedly increased by high fat diet in ApoE−/− mice (Figures 1B and D). The increase in IFNγ, but not IL5, was significantly abrogated in IL17/ApoE−/− mice, indicating that IL17A is necessary for IFNγ but not IL5 production in response to high fat diet (Figures 1B and D). TNFα production was not altered by high-fat diet and was similar between ApoE−/− mice and IL17/ApoE−/− mice (Figure 1C). IL4 production was undetectable in mice fed a regular diet and was increased in response to high fat diet to a similar degree in ApoE−/− and IL17/ApoE−/− mice (data not shown). Thus, production of both Th1 (IFNγ) and Th2 (IL4 and IL5) cytokines increased in response to high fat diet, but only the increase in IFNγ was IL17A-dependent.
Effect of IL17A on weight gain and plasma lipids in response to high fat diet
Body weight and plasma lipids were not significantly different between ApoE−/− and IL17/ApoE−/− mice at baseline (Figure 1E and Table). High fat feeding increased weight by 2.6g in ApoE−/− mice but not in IL17/ApoE−/−, suggesting that IL17A may play a role in weight gain in response to high fat diet (Figure 1E).
Table.
Plasma Lipid Profile in Response to High Fat Diet
| Parameter | ApoE−/− (n=12) [mg/dL] | IL17/ApoE−/−) (n=11) [mg/dL] | p-value |
|---|---|---|---|
| Total Cholesterol | 1185±83 | 1593±241 | 0.11 |
| Triglycerides | 56±6.6 | 57±9.6 | 0.93 |
Data are expressed as mean ± standard error of the mean.
IL17A promotes superoxide production and decreases nitric oxide (NO) levels in response to high fat diet
Atherosclerosis increases vascular production of reactive oxygen species and decreases NO production. We determined the effect of IL17A on aortic superoxide production using ApoE−/− and IL17/ApoE−/− mice fed regular diet or high fat diet for 3 months. Baseline levels of superoxide were similar between ApoE−/− and IL17/ApoE−/− mice. High fat diet, however, increased aortic superoxide production in ApoE−/− mice by approximately 2-fold, and this increase was significantly blunted in IL17/ApoE−/− mice (Figure 1F). Thus, IL17 is necessary for high fat diet induced elevations in vascular superoxide.
Aortic nitric oxide levels correlate with endothelium-dependent relaxation and improved vascular function. Aortic nitric oxide levels in ApoE−/− and IL17/ApoE−/− mice fed a high fat diet were measured using electron spin resonance (ESR). Nitric oxide production by aortic rings of IL17/ApoE−/− mice were increased compared to vessels of ApoE−/− mice (Figures 1G–H).
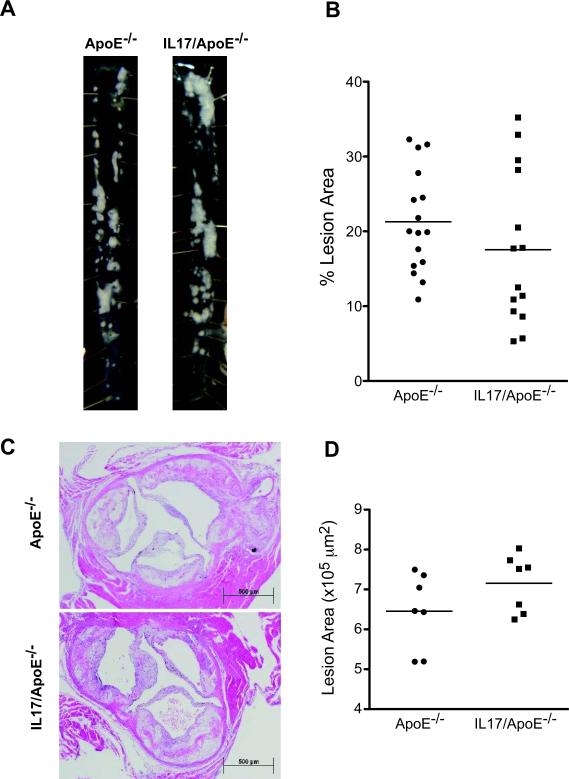
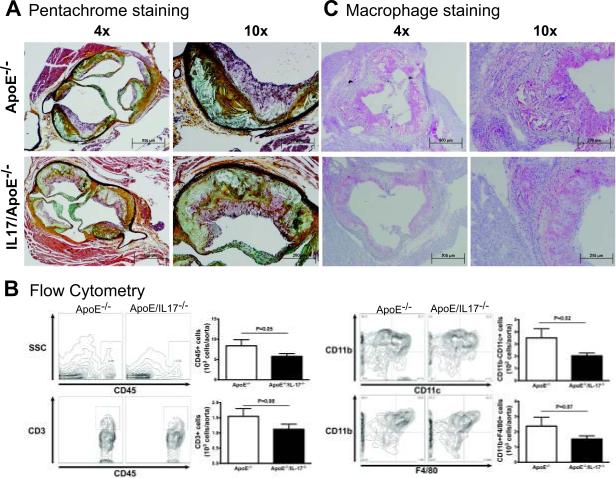
IL17A deficiency has no effect on atherosclerotic plaque area or aneurysm formation but decreases aortic total leukocyte and dendritic cell infiltration
To determine the effect of IL17A on atherosclerotic plaque development, we quantified plaque area in the descending aorta (Figures 2A–B) and aortic root (Figures 2C–D) in ApoE−/− and IL17/ApoE−/− mice fed a high fat diet for 3 months. Surprisingly, plaque area in these regions was similar between IL17/ApoE−/− mice and age-matched ApoE−/− controls. We then investigated whether plaque morphology was different using Russell-Movat pentachrome staining (Figure 3A). No major differences were noted among extracellular matrix components such as elastin (black), collagen (yellow), and mucins (blue-green). Flow cytometry of whole aortas, however, revealed a modest decrease in total leukocytes (CD45+ cells) and dendritic (CD11b−11c+) cells and a trend toward reduction of aortic macrophages (CD11b+F4/80+ cells) and T cells (CD3+ cells) in the IL17A deficient mice (Figure 3B). Immunostaining of atherosclerotic plaques demonstrated a decrease in total macrophage content within lesions (Figure 3C). Thus, although plaque size and extracellular matrix content are similar between ApoE−/− and IL17/ApoE−/− mice, the plaques do appear to be less inflammatory in IL17/ApoE−/− mice.
Figure 2. Effect of IL17 on atherosclerotic lesion development.
ApoE−/− and IL17/ApoE−/− mice were fed a high fat diet for 3 months. Atherosclerotic plaque burden in the descending aortas was quantified using planimetry. Example aortas are shown in panel A and mean values are shown in panel B [n=14–16 per group; p=n.s.]. Atherosclerotic plaque area in the aortic root was analyzed by paraffin sectioning, hematoxylin and eosin staining, and planimetry using ImageJ software. Example sections are shown in panel C, and mean data are shown in panel D [n=7 per group; p=n.s.]. Data were analyzed using Student's t test.
Figure 3.
Effect of IL17 on Plaque Composition. ApoE−/− and IL17/ApoE−/− mice were fed 3 months of high fat diet. Panel A shows Russell-Movat pentachrome staining in the aortic root at 4× and 10× magnification. Black=elastic fibers, yellow=collagen, blue/green=mucins, red=muscle, intense red=fibrinoid. Figures are representative of 4 per group. To analyze inflammatory cell content, flow cytometry of single cell suspensions of whole aortas were performed as shown in panel B. Representative scatter plots are shown on the left, and summary data of n=9–12 mice per group are shown on the right. Data were analyzed using Student's t test. CD45+ cells represent total leukocytes, CD3+ cells represent T cells, CD11b-11c+ cells represent dendritic cells, and CD11b+F4/80+ cells represent macrophages. Panel C shows plaque macrophage content in the aortic root as determined by immunostaining with a Mac3 antibody. Alkaline phosphatase (pink) was used to detect the secondary antibody. Slides were counterstained with hematoxylin. A representative of n=3 per group is shown at 4× and 10× magnification.
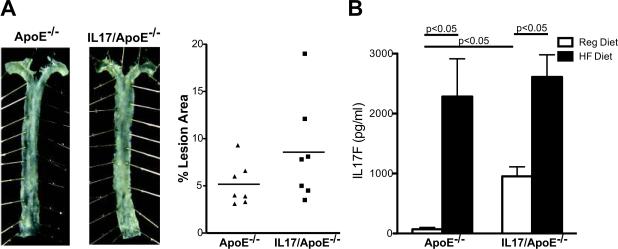
Angiotensin II enhances atherosclerosis in ApoE−/− mice19. Since we previously showed that angiotensin II stimulates T cell production of IL17A9, we examined the role of IL17A in angiotensin II induced atherosclerosis. Angiotensin II (1000 ng/kg/min) was infused via osmotic minipump for four weeks in ApoE−/− and age-matched IL17/ApoE−/− mice. Angiotensin II induced atherosclerosis in ApoE−/− and IL17/ApoE−/− mice to a similar extent indicating that IL17A does not contribute to plaque development in an angiotensin II-induced model of atherosclerosis (Figures 4A). Plasma lipids following 4 weeks of angiotensin II were similar between ApoE−/− and IL17/ApoE−/− mice (data not shown).
Figure 4.
Effect of IL17 on Angiotensin II Induced Atherosclerosis and Examination of IL17F Levels. ApoE−/− and IL17/ApoE−/− mice were infused with angiotensin II for 4 weeks via osmotic minipump. Atherosclerotic lesions in the thoracic aortas were quantified by planimetry (A). Example aortas are shown on the left, and summary data are shown on the right [n=7 per group; p=n.s.]. Splenic lymphocytes from ApoE−/− and IL17/ApoE−/− mice fed regular (Reg) diet or high fat (HF) diet for 3 months were cultured on anti-CD3 plates, and IL17F released into the media was measured by ELISA [panel B, n=5–7 per group]. Data were analyzed using Student's t test. The statistical values in panel B represent a Bonferonni correction for 4 comparisons.
Infusion of angiotensin II at this dose, 1000 ng/kg/min, induces aneurysm formation in ApoE−/− mice19. We found that aneurysm formation was similar in ApoE−/− mice and IL17/ApoE−/− mice (18% and 20%, respectively) suggesting that IL17A does not influence aneurysm development.
IL17F is upregulated in IL17A deficient mice and is increased by high fat diet
IL17F is closely related to IL17A, can dimerize with IL17A, and binds to the same receptor. Ley and colleagues20 showed that IL17F was upregulated in IL17A deficient mice. Therefore, we examined whether IL17F was also upregulated in our IL17/ApoE−/− mice and whether IL17F production was influenced by high fat diet. Interestingly, IL17F production at baseline (regular diet) was markedly increased in IL17/ApoE−/− mice. High fat diet further increased IL17F production, and IL17F levels were similar in ApoE−/− and IL17/ApoE−/− mice after 3 months of high fat diet (Figure 4B). This elevation in IL17F in our model raised the possibility that IL17F could be assuming a compensatory/redundant role in plaque formation in the absence of IL17A.
Neutralization of IL17F in IL17A deficient mice has no effect on partial carotid ligation induced atherosclerosis
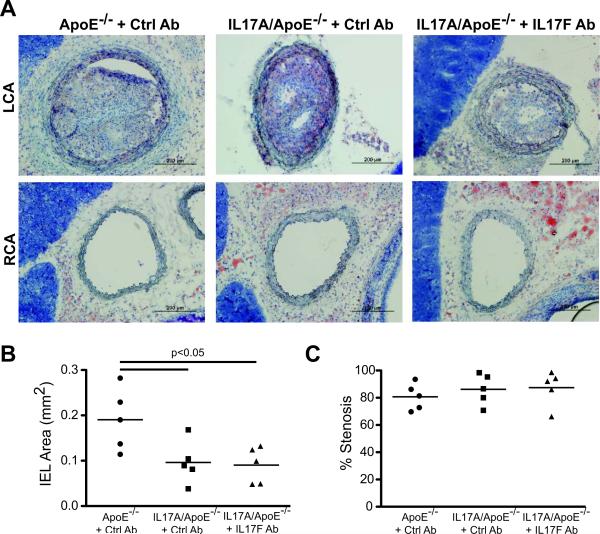
We further examined the effect of IL17A with and without concomitant IL17F neutralization in a rapid (2-week) partial carotid ligation model of atherosclerosis described by Nam et al18. Interestingly, we found that percent stenosis of the ligated carotid artery was not affected by the absence of IL17A, but vessel diameter (as measured by internal elastic lamina (IEL) area) was significantly decreased in the IL17A deficient mice indicating that IL17A may play an important role in outward remodeling of vessels under conditions of disturbed flow (Figures 5A and B). To determine if IL17F compensates for the loss of IL-17A during the development of atherosclerosis, we administered an IL17F neutralizing antibody weekly beginning one week before carotid ligation. IL17F neutralization had no effect on IEL area or percent stenosis (Figures 5A and C) arguing against a significant role of IL17F in atherosclerosis development.
Figure 5.
Effect of IL17A and F Isoforms on Partial Carotid Ligation Induced Atherosclerosis. The left carotid artery (LCA) of ApoE−/− and IL17A/ApoE−/− mice was partially ligated followed by 2 weeks of high fat feeding to induce accelerated atherosclerosis in the ligated artery. Mice were injected weekly with IL17F or isotype control antibody for 3 weeks starting one week before ligation. Frozen sections of carotid arteries were stained with Oil Red O, hematoxylin, and eosin. A representative LCA and right carotid artery (RCA) from each group is shown in (A). Quantification of internal elastic lamina (IEL) area of the LCA is shown in (B), and percent stenosis of the LCA is shown in (C) [n=5 per group]. Data were analyzed using one way ANOVA.
Interleukin 17A levels do not correlate with carotid intima-media thickness in humans
Increased carotid intima-media thickness (IMT) is an early subclinical marker of atherosclerosis and a predictor of future cardiovascular events21. We examined serum IL17A levels and carotid IMT in a small population of healthy humans aged 50–69 years. As shown in the Supplemental Figure, there was no correlation between IL17A levels and either mean or far wall carotid IMT, suggesting that IL17A does not participate in human atherosclerotic plaque formation.
DISCUSSION
In the present study, we found that IL17A modulates systemic and vascular inflammation by stimulating IFNγ production, increasing vascular reactive oxygen species and vascular leukocyte infiltration, and contributes to weight gain in response to high fat diet. Despite these favorable effects, IL17A had no effect on gross lesion content in either mouse or human atherosclerosis. Furthermore, neutralization of the related cytokine, IL17F, had no effect on atherosclerosis in a rapid partial carotid ligation model.
To our knowledge this is the first study to use a genetic deletion of IL17A and ApoE to study the role of IL17A in atherosclerosis. Several recent studies on this topic have provided conflicting results showing that IL17A reduces, increases, or has no effect on atherosclerotic plaque development10–16. Differences in these results may be due to the methods used for eliminating IL17A, non-specific effects of the interventions, and/or lack of complete elimination in some studies.
Despite no effect on plaque burden, we did find decreased vascular inflammation in the IL17A deficient mice. We employed two independent methods to assess vascular infiltration of inflammatory cells. We first used flow cytometry of whole aortas and found that total leukocytes and dendritic cells were significantly reduced in IL17A deficient mice with a trend toward reduced macrophages and T cells. Flow cytometry has the limitation that the entire aorta is analyzed, which could underestimate differences that could be present if only lesions were analyzed. We then performed immunostaining on atherosclerotic plaques and found that plaque macrophage content appeared to be reduced in IL17A deficient mice. These results are consistent with reports by Erbel et al12 and Smith et al13, who also found that IL17A blockade reduced plaque macrophage content. A potential mechanism for this finding is that IL17A can act on vascular wall cells to induce the production of numerous cytokines and chemokines such as IL6, CXCL8, CCL5, CXCL1, GCSF3, and monocyte chemoattractant protein 2(MCP2)9, 13. MCP2 binds to cell surface receptors such as CCR1 and CCR5 expressed on leukocytes22, and CXCL1 is important in monocyte recruitment into the artery wall. In addition, monocytes express the IL17RA receptor, and IL17A directly influences monocyte chemotaxis23, 24. We and others have shown that IL17A promotes inflammation in a manner similar to that observed in the present study. Shahrara et al.25 demonstrated that in rheumatoid arthritis, IL17A promotes monocyte migration into synovial tissue via upregulation of chemokine ligand 2(CCL2)/monocyte chemoattractant protein-1 (MCP1) expression. We previously found that IL17A promotes aortic infiltration of CD45+ leukocytes and CD3+ T lymphocytes in response to angiotensin II-induced hypertension9.
In addition to promoting inflammation, we found that IL17A is necessary for increased vascular superoxide (O2·−) production in response to high fat diet-induced atherosclerosis. This is similar to our previous finding that IL17A promotes vascular O2·− production in response to angiotensin II-induced hypertension9. The mechanisms by which IL17A induces vascular O2·− production remain unclear. In preliminary studies, we were unable to demonstrate a direct effect of IL-17A on O2·− production in either human endothelial or vascular smooth muscle cells in culture, however a recent report suggests that IL17A can activate the NADPH oxidase in murine vascular smooth muscle cells26. It is also possible that IL17A increases superoxide production via recruiting inflammatory cells which in turn can produce reactive oxygen species.
It is interesting that IL17A modulates a number of factors thought to be associated with plaque progression such as IFNγ production, vascular inflammation, and superoxide production, and yet we did not find any effect on aortic plaque burden or carotid stenosis. Our finding that atherosclerotic plaque burden was similar despite reduced O2·− production is in keeping with prior studies showing that mice overexpressing superoxide dismutase are not protected against atherosclerosis27, 28. Moreover, plaque size does not necessarily correlate with clinical outcomes in human coronary atherosclerosis. Plaque stability is more predictive of acute plaque rupture and coronary events. Our findings suggest that IL17A deficiency, through reduced leukocyte infiltration and decreased superoxide production, could stabilize plaques. Thus, IL17A might not be an important determinant of plaque size but rather a modulator of plaque composition and stability. This is in agreement with clinical data showing that Th17 cells and IL17 levels are elevated in patients with unstable angina and acute myocardial infarction compared to patients with stable angina and controls8.
In our carotid ligation model, we observed that IL17A deficiency had no effect on percent stenosis in the ligated vessel, but markedly decreased outward remodeling. Outward remodeling is a compensatory response to lesion development that preserves lumen diameter in the setting of increasing atherosclerosis burden. In this regard, IL17A−/− mice are similar to mice lacking matrix metalloproteinase-9 (MMP-9), in which outward remodeling in response to total carotid occlusion is significantly impaired29. Since reactive oxygen species are known to increase MMP-9 expression and activation30, 31, it is interesting to speculate that the absence of outward remodeling in the IL17/ApoE−/− might reflect the lower levels of O2·− observed in these animals as compared to ApoE−/− mice.
We also show that IL17/ApoE−/− mice are resistant to high fat diet-induced weight gain. A link between IL17A and obesity was first suggested by a small clinical study showing that plasma levels of IL17A were elevated in 26 obese women compared to 20 lean women32. Similar to our findings, Winer et al33 showed that diet induced-obesity increases IL17 from splenic lymphocytes. Moreover, IL17A stimulates pro-inflammatory responses in adipocytes34. Recently, Pini and Fantuzzi35 found that neutrophil-derived IL17A is increased in obese mice during acute inflammation and contributes to inflammatory responses. These findings are in keeping with a role of inflammation in the metabolic syndrome and suggest that IL17 might contribute to this disorder.
In summary, using a genetic approach that avoids potential confounding off-target and non-specific effects, we found that IL17A is involved in systemic and vascular inflammation and weight gain in response to high fat diet. Contrary to several prior reports, however, we showed that IL17A is unlikely to contribute significantly to plaque progression. Nevertheless, through its effects on reactive oxygen species and leukocyte infiltration, IL17A is an important potential mediator of plaque stability, and its effects on plaque stability and outward remodeling warrant further investigation.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING This research was supported by NIH Grants P01HL58000, R01HL39006, and a Department of Veterans Affairs merit grant. Dr. Madhur was supported by NIH NRSA F32 HL092738-01. SA Funt was supported by ACTSI grant numbers UL1 RR025008 and TL1 RR025010.
Footnotes
DISCLOSURES None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 2.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taleb S, Tedgui A, Mallat Z. Adaptive T cell immune responses and atherogenesis. Curr Opin Pharmacol. 2010;10:197–202. doi: 10.1016/j.coph.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, Idu MM, van Maldegem F, Aten J, van der Wal AC. Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J Pathol. 2010;220:499–508. doi: 10.1002/path.2667. [DOI] [PubMed] [Google Scholar]
- 7.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, Yao R, Chen Y, Liao YH. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Es T, van Puijvelde GH, Ramos OH, Segers FM, Joosten LA, van den Berg WB, Michon IM, de Vos P, van Berkel TJ, Kuiper J. Attenuated atherosclerosis upon IL-17R signaling disruption in LDLr deficient mice. Biochem Biophys Res Commun. 2009;388:261–265. doi: 10.1016/j.bbrc.2009.07.152. [DOI] [PubMed] [Google Scholar]
- 11.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, Yoshimura A, Tedgui A, Mallat Z. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Bockler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 13.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Shimada K, Zhang W, Huang G, Crother TR, Arditi M. IL-17A is proatherogenic in high-fat diet-induced and Chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J Immunol. 2010;185:5619–5627. doi: 10.4049/jimmunol.1001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng X, Taleb S, Wang J, Tang TT, Chen J, Gao XL, Yao R, Xie JJ, Yu X, Xia N, Yan XX, Nie SF, Liao MY, Cheng Y, Mallat Z, Liao YH. Inhibition of IL-17A in atherosclerosis. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, Guo C, Wang Q, Wang X, Ma C, Zhang Y, Chen W, Zhang L. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820–5827. doi: 10.4049/jimmunol.1000116. [DOI] [PubMed] [Google Scholar]
- 17.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 18.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Vietinghoff S, Ley K. IL-17A controls IL-17F production and maintains blood neutrophil counts in mice. J Immunol. 2009;183:865–873. doi: 10.4049/jimmunol.0804080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 22.Gong W, Howard OM, Turpin JA, Grimm MC, Ueda H, Gray PW, Raport CJ, Oppenheim JJ, Wang JM. Monocyte chemotactic protein-2 activates CCR5 and blocks CD4/CCR5-mediated HIV-1 entry/replication. J Biol Chem. 1998;273:4289–4292. doi: 10.1074/jbc.273.8.4289. [DOI] [PubMed] [Google Scholar]
- 23.Sergejeva S, Ivanov S, Lotvall J, Linden A. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am J Respir Cell Mol Biol. 2005;33:248–253. doi: 10.1165/rcmb.2004-0213OC. [DOI] [PubMed] [Google Scholar]
- 24.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182:3884–3891. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahrara S, Pickens SR, Mandelin AM, 2nd, Karpus WJ, Huang Q, Kolls JK, Pope RM. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol. 2010;184:4479–4487. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietrowski E, Bender B, Huppert J, White R, Luhmann HJ, Kuhlmann CR. Pro-inflammatory effects of interleukin-17A on vascular smooth muscle cells involve NAD(P)H-oxidase derived reactive oxygen species. J Vasc Res. 2010;48:52–58. doi: 10.1159/000317400. [DOI] [PubMed] [Google Scholar]
- 27.Tribble DL, Gong EL, Leeuwenburgh C, Heinecke JW, Carlson EL, Verstuyft JG, Epstein CJ. Fatty streak formation in fat-fed mice expressing human copper-zinc superoxide dismutase. Arterioscler Thromb Vasc Biol. 1997;17:1734–1740. doi: 10.1161/01.atv.17.9.1734. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95:1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 29.Lessner SM, Martinson DE, Galis ZS. Compensatory vascular remodeling during atherosclerotic lesion growth depends on matrix metalloproteinase-9 activity. Arterioscler Thromb Vasc Biol. 2004;24:2123–2129. doi: 10.1161/01.ATV.0000141840.27300.fd. [DOI] [PubMed] [Google Scholar]
- 30.Galis ZS, Asanuma K, Godin D, Meng X. N-acetyl-cysteine decreases the matrix-degrading capacity of macrophage-derived foam cells: new target for antioxidant therapy? Circulation. 1998;97:2445–2453. doi: 10.1161/01.cir.97.24.2445. [DOI] [PubMed] [Google Scholar]
- 31.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, Starcevic V, Trajkovic V, Micic D. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond) 2009;33:151–156. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 33.Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D, Dosch HM. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 34.Shin JH, Shin DW, Noh M. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem Pharmacol. 2009;77:1835–1844. doi: 10.1016/j.bcp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Pini M, Fantuzzi G. Enhanced production of IL-17A during zymosan-induced peritonitis in obese mice. J Leukoc Biol. 2010;87:51–58. doi: 10.1189/jlb.0309188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.