Abstract
Background and Aim
Dietary n-3 polyunsaturated fatty acids (PUFA) are associated with decreased plasma homocysteine (Hcy), an important biomarker for cardiovascular disease. The S-adenosylmethionine synthetase type-1 (MAT1A), an essential enzyme in the conversion of methionine to S-adenosylmethionine, plays a key role in homocysteine metabolism. This study investigated the interaction between dietary fatty acids and MAT1A genotypes on plasma Hcy concentrations among Boston Puerto Ricans.
Methods and Design
Plasma Hcy and MAT1A genotypes were determined in 994 subjects of the Boston Puerto Rican Health Study. Dietary fatty acid intakes were assessed by interviews using a questionnaire adapted from the NCI/Block food frequency form.
Result
In the cross-sectional analysis, genetic variant MAT1A 3U1510 displayed a significant interaction with dietary n-3:n-6 PUFA ratio in determining plasma Hcy (p-value for interaction = 0.025). 3U1510G homozygotes had significantly lower plasma Hcy concentration than major allele homozygotes and heterozygotes (AA+AG) (p-value for trend = 0.019) when the n-3:n-6 ratio was >0.09. Two other MAT1A variants, d18777 and i15752, also showed significant interactions with different constituents of dietary fat influencing Hcy concentrations. Furthermore, haplotypes consisting of three variants displayed a strong interaction with n3:n6 ratio influencing Hcy concentrations.
Conclusions
Our results suggest that MAT1A genotypes appear to modulate effects of dietary fat on plasma Hcy.
Keywords: methionine adenosyltransferase, dietary fatty acids, plasma homocysteine
Introduction
Elevated plasma homocysteine (Hcy), a thiol-containing amino acid byproduct of methionine metabolism [1], has been demonstrated to be an independent risk factor for cardiovascular disease (CVD) [1]. In addition to pathophysiological conditions, including menopause, renal disease, and hypothyroidism [2], the etiology of hyperhomocysteinemia (HHcy) is known to be multifactorial, including genetic and environmental factors, such as diet and lifestyle [2–3]. The genetic causes of elevated plasma Hcy include rare inborn errors of Hcy metabolism, such as cystathionine beta-synthase (CBS) and methylenetetrahydrofolate reductase (MTHFR) [4]. Recently, studies of polymorphisms from the critical genes involved in Hcy metabolic pathways demonstrated that MTHFR 677C>T [5], MTHFR 1298A>C [6], MTRR 66A>G [7] and MTR 2756A>G [8] were associated with elevated plasma Hcy concentration. For environmental factors, lifestyle and diet play an important role in Hcy metabolism. Two-thirds of HHcy subjects in an elderly US population were associated with low plasma/serum concentrations of one or more of B group vitamins [9]. Smoking, drinking alcohol and physical activity have also been associated with elevated plasma Hcy [10].
Importantly, n-3 polyunsaturated fatty acids (n-3 PUFA), which have a protective effect on the cardiovascular system [11], were shown to improve Hcy metabolism [12]. Previously, we reported that plasma Hcy was significantly negatively correlated with the plasma/platelet phospholipids (PL) n-3 PUFA and n-3:n-6 PUFA ratio [12–13]. Subsequent intervention studies have demonstrated that n-3 PUFA supplementation decreases plasma Hcy [14]. However, the results from studies evaluating the relationship between fatty acids and plasma Hcy are inconclusive [15]. Whether genetic variation may account for such inconsistent results is unknown. The relationship between n- 3 PUFA and plasma Hcy is not yet fully understood. Methionine adenosyltransferase (MAT), an essential enzyme in methionine metabolism, catalyzes the conversion of methionine to S-adenosylmethionine (SAM). SAM is subsequently converted to S-adenosyl homocysteine and then homocysteine in separate reactions [16]. We previously demonstrated that MAT1A variants were associated with stroke and hypertension [16]. Therefore, we hypothesize that MAT1A variants, single nucleotide polymorphisms (SNPs), modulate the effect of dietary fatty acids on plasma Hcy.
In the present study, we conducted a population-based evaluation to investigate the combined contributions of MAT1A genotype and dietary fatty acids to HHcy in the Boston Puerto Rican population. This population has experienced severe health disparity, including high rates of hypertension, diabetes, obesity, and CVD (16, 17). We examined the effects of MAT1A variants and dietary fatty acids on plasma Hcy concentration and assessed their potential interactions in modifying plasma Hcy.
Methods
Study design and subjects
This study was conducted within the ongoing Boston Puerto Rican Health Study (BPRHS) as described previously [17]. The analysis included 994 subjects who participated in the BPRHS study and had complete data on dietary intake, anthropometry, biochemical parameters, and MAT1A genotype. Interviews were conducted in volunteers’ homes to collect demographic and anthropometric data, and detailed data were collected on dietary intake using a questionnaire previously adapted from the NCI/Block food frequency form and validated for this population [18]. Physical activity was estimated as a physical activity score, based on the Paffenbarger questionnaire [19]. Smoking status was described in three categories: current, former, and never smoking. Alcohol consumption was defined as current drinkers and nondrinkers.
Fasting blood samples were collected the morning following health interviews in the volunteer’s home [17]. Approval for the BPRHS was obtained from the Institutional Review Boards of the Tufts Medical Center and Tufts University.
Population admixture
Population admixture was calculated using STRUCTURE 2.2 based on 100 SNPs selected as ancestry informative markers specifically for Puerto Rican populations [16]. Using the estimated admixture of each subject, we adjusted for population admixture for all genotype-associated analyses.
MAT1A SNP selection and genetic analysis
A panel of eight SNPs mapping in/near the MAT1A gene were selected for genotyping based primarily on linkage disequilibrium analysis of HapMap data for the CEU population and the characteristics of these eight SNPs have been described [16]. Results of TAGGER [20] run with the parameters of pair-wise option, CEU population, r2 > 0.80, minor allele frequency > 0.00, placed most SNPs into one of eleven blocks. Each of the SNPs chosen for genotyping falls into a different LD block within MAT1A, and these blocks overall span approximately 10 kbp to either side of the gene. DNA was isolated from blood samples using QIAamp DNA Blood Mini kits according to the manufacture’s instructions (Qiagen, Valenic, CA). Eight SNPs were genotyped using the TaqMan SNP genotyping system (Applied Biosystems, Foster City, CA).
Linkage disequilibrium and haplotype analysis
Pair-wise linkage disequilibria among SNPs were estimated as correlation coefficients (r2) using the haploview program. For haplotype analysis, the global association between haplotypes and plasma Hcy, and estimated haplotype frequencies we used the R software (haplo.stats package).
Measurement of anthropometric and plasma biochemical parameters
Anthropometric variables, including height and weight were measured with standard techniques. Body mass index (BMI) was calculated as weight (kg)/height (m)2. Fasting blood samples were collected by venipuncture in all participants. Total plasma Hcy was measured using HPLC with fluorescence detection [21] with coefficient of variation of 6%. Plasma pyridoxal phosphate (PLP) was determined using the radio-enzymatic method of Camp, et al. [22] with coefficient of variation of 7%. Plasma folate and vitamin B12 were measured using Immulite chemiluminescent kits according to manufacturer’s instructions (Diagnostic Products Corporation/Siemens, Los Angeles, CA) with coefficient of variation of 4% and 5% respectively. Plasma creatinine was assessed using a modified ‘Jaffe’ method [23] with coefficient of variation of 2%.
Dietary Assessment
Dietary intake was assessed using a questionnaire (FFQ) that was designed for and validated in this population [18]. Dietary data were linked to the Minnesota Nutrient Data system (1999, version 25) for nutrient analysis. Intakes of fatty acids were expressed as percentages of total energy intake and were included in analyses as both continuous and categorical variables. Intakes were classified into two groups according to the median intake of the population, to construct categorical variables.
Statistical analyses
Data analyses were performed using SAS for Windows, version 9.1 (SAS Institute). All continuous and dependent variables were examined for normal distribution. Dependent variables with abnormal distribution were BOX-COX- transformed to achieve normality before fitting statistical models [16]. Men and women were analyzed together to ensure adequate statistical power. Chi-square tests were conducted to examine whether the genotype frequencies of the selected SNPs were in Hardy-Weinberg equilibrium. Relationships between dietary fatty acids, MAT1A genotypes and plasma Hcy were evaluated using linear regression models. To examine interactions between dietary fatty acid intake and genotype on Hcy, the population medians for total saturated fatty acids (SFA), monounsaturated fatty acid (MUFA), and total PUFA, n-3 PUFA, and n-6 PUFA intakes were used as cutoffs to dichotomize these variables. Then interactions between dietary fatty acids (as dichotomized variables) and MAT1A genotypes/haplotypes were tested in a general linear model while controlling the main effects of fatty acid and genotypes, and adjustment for potential confounding factors (age, sex, physical activity, alcohol use, smoking, population admixture, plasma folate, vitamin B6, vitamin B12, and genotypes of MTHFR 1298 A>C and MTHFR 677 C>T). All results were expressed as mean ± SD. Differences between groups were considered to be statistically significant at p-value <0.05.
Results
Information about demographic, biochemical, and dietary intake data are provided in Table 1. Men and women had similar mean ages. No significant differences were observed across sex for vitamins B6, and B12. However, mean body mass index (BMI, kg/m2) was significantly higher in women than in men, whereas smoking and alcohol consumption were more prevalent in men than in women. Plasma Hcy concentration for all subjects ranged from 3.90-30.4 µmol/L. Men had significantly higher mean Hcy. Genotype frequencies of eight MAT1A SNPs did not deviate from Hardy-Weinberg equilibrium (data not shown).
Table 1.
Demographic, anthropometric, biochemical data in the Boston Puerto Rican Health Study population
| Characteristics of Participants | Men | Women |
|---|---|---|
| n=292 | n=702 | |
| Age, y | 57.7 ± 7.6 | 57.9 ± 7.2 |
| BMI, kg/m2 | 29.7 ± 5.1 | 33.1 ± 7.0* |
| Smoker, n (%) | 80 (31.12) | 126 (19.84)* |
| Drinker, n (%) | 132 (51.36) | 219 (34.48)* |
| Alcohol, g/d | 9.22 ± 30.43 | 1.55 ± 6.55* |
| Energy intake, kcal/d | 2696 ± 1321 | 2175 ± 1115* |
| Total fat, % of energy | 31.9 ± 5.4 | 30.8 ± 5.2* |
| Total SFA, % of energy | 9.8 ± 2.5 | 9.3 ± 2.2 |
| Total MUFA, % of energy | 11.7 ± 2.1 | 11.2 ± 2.1 |
| Total PUFA, % of energy | 7.8 ± 1.7 | 7.6 ± 1.8 |
| n-3 PUFA, % of energy | 0.67 ± 0.16 | 0.68 ± 0.17 |
| n-6 PUFA, % of energy | 7.16 ± 1.61 | 6.93 ± 1.73 |
| n-3:n-6 ratio | 0.09 ± 0.02 | 0.10 ± 0.02 |
| Plasma Folate, ng/mL | 17.7 ± 8.7 | 20.1 ± 9.4* |
| Plasma Vitamin B12, pg/mL | 527 ± 276 | 550 ± 284 |
| Plasma Vitamin B6, nmol/L | 61.4 ± 60.3 | 59.2 ± 63.3 |
| Plasma Hcy, µmol/L | 10.7 ± 6.2 | 8.8 ± 4.2* |
Values are Mean ± SD or n (%).
Significantly different from men (p<0.01).
SFA: saturated fatty acid, MUFA: monounsaturated fatty acid, PUFA: polyunsaturated fatty acid
Intake of n-6 PUFA was significantly positively associated with plasma Hcy (p=0.025), in a dose independent manner (Table 2). In addition, we found that the ratio of dietary n-3:n-6 PUFA was significantly negatively associated with plasma Hcy (p=0.048, Table 2), also in a dose independent manner. However, we did not find significant associations between other dietary fatty acids and plasma Hcy concentration.
Table 2.
Association between dietary fatty acids and plasma homocysteine by quartile of dietary fatty acids.
| plasma homocysteine level1 (µmol/L) | |||||
|---|---|---|---|---|---|
| % of energy | Q1 | Q2 | Q3 | Q4 | p-value (trend) |
| Total Fat | 9.30 ± 3.73 | 9.14 ± 3.89 | 8.84 ± 3.20 | 9.64 ± 4.59 | 0.183 |
| Total SFA | 9.44 ± 3.92 | 9.38 ± 3.92 | 8.87 ± 4.00 | 9.22 ± 3.69 | 0.404 |
| Total MUFA | 8.91 ± 3.41 | 9.46 ± 4.15 | 9.18 ± 3.66 | 9.38 ± 4.27 | 0.444 |
| Total PUFA | 9.01 ± 3.44 | 9.56 ± 4.21 | 8.84 ± 3.72 | 9.51 ± 4.11 | 0.127 |
| n-3 PUFA | 9.31 ± 3.84 | 9.18 ± 3.97 | 9.65 ± 4.33 | 8.74 ± 3.27 | 0.094 |
| n-6 PUFA | 8.94 ± 3.30 | 9.45 ± 4.13 | 8.77 ± 3.43 | 9.77 ± 4.52 | 0.025 |
| n-3:n-6 ratio | 9.65 ± 4.11 | 9.41 ± 4.51 | 9.12 ± 3.94 | 8.74 ± 2.77 | 0.048 |
The association was tested using general linear models.
All the data was presented in mean ± SD.
Adjusted for age, sex, BMI, smoking, drinking, population admixture, diabetes, dietary energy, plasma folate, plasma vitamin B12 and plasma vitamin B6, MTHFR 1298 A>C, MTHFR 677 C>T.
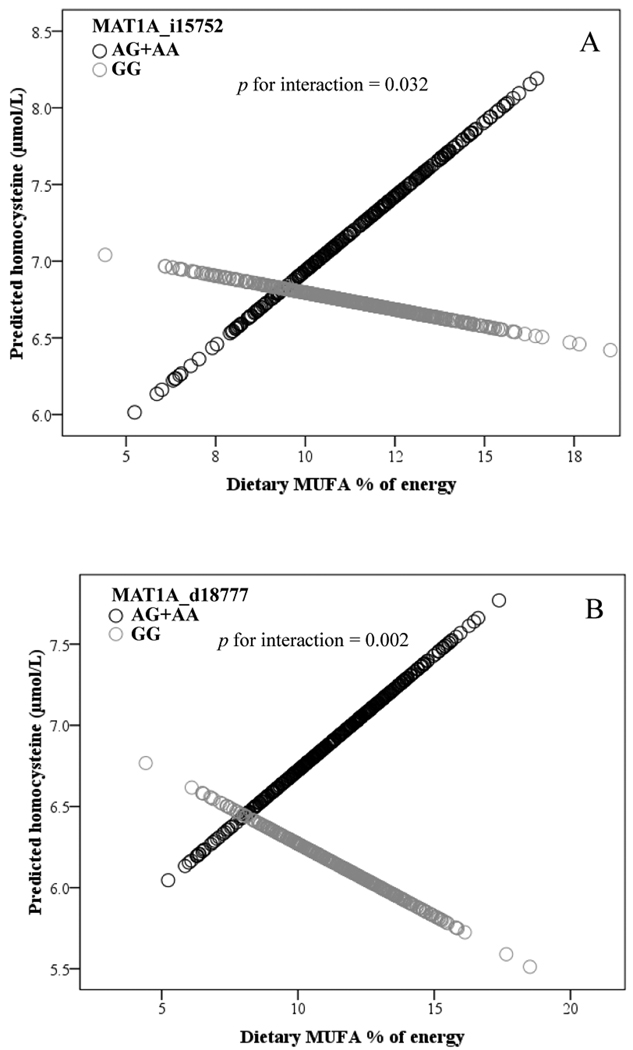
To examine the interaction between fatty acids and MAT1A genotypes, subjects were divided into two groups, based on the median of dietary fatty acid (Table 3). We observed a significant interaction between dietary total MUFA as a categorical variable and MAT1A i15752 for plasma Hcy (p=0.030). When dietary MUFA intake was low (<11.4 % energy/d), plasma Hcy was lower in carriers of the A allele compared to GG subjects (p=0.033). Consistent with the results for the categorical variable, dietary total MUFA, as a continuous variable, appears to interact with MAT1A i15752 by influencing plasma Hcy (p=0.032, Fig. 1A). With greater MUFA intake, plasma Hcy was higher in carriers of the A allele. We also observed that dietary total fat, as a categorized variable, interacted significantly with MAT1A i15752 with respect to plasma Hcy (p=0.015). When total fat intake was low (<31.3% energy/d), A carriers had significantly lower plasma Hcy than G homozygotes (p=0.047), while there was no significant difference between A carriers and non-carriers (p=0.158) when total fat intake was high (Table 3).
Table 3.
Interaction between MAT1A variants and dietary fatty acids on plasma homocysteine.
| MAT1A i15752 | MAT1A d18777 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| % of total energy | GG (n=360) | AG+AA (n=532) |
p-value (trend) |
p-value1 (interaction) |
GG (n=430) | AG+AA(n=390) |
p-value (trend) |
p-value1 (interaction) |
|
| Total Fat | ≤ 31.312 | 10.35 ± 0.273 | 9.59 ± 0.34 | 0.047 | 0.015 | 10.73 ± 0.30 | 9.54 ± 0.29 | 0.001 | <0.0001 |
| >31.31 | 9.23 ± 0.25 | 9.81 ± 0.34 | 0.158 | 8.89 ± 0.28 | 9.95 ± 0.28 | 0.006 | |||
| Total SFA | ≤ 9.27 | 10.40 ± 0.27 | 9.82 ± 0.35 | 0.146 | 0.064 | 10.54 ± 0.30 | 9.85 ± 0.30 | 0.077 | 0.022 |
| >9.27 | 9.13 ± 0.25 | 9.58 ± 0.33 | 0.256 | 9.01 ± 0.28 | 9.54 ± 0.27 | 0.163 | |||
| Total MUFA | ≤ 11.35 | 10.16 ± 0.26 | 9.47 ± 0.34 | 0.033 | 0.03 | 10.41 ± 0.30 | 9.51 ± 0.28 | 0.015 | 0.001 |
| >11.35 | 9.34 ± 0.26 | 9.87 ± 0.34 | 0.201 | 9.08 ± 0.28 | 9.89 ± 0.29 | 0.041 | |||
| Total PUFA | ≤ 7.76 | 9.97 ± 0.25 | 9.53 ± 0.32 | 0.246 | 0.196 | 10.16 ± 0.29 | 9.53 ± 0.27 | 0.087 | 0.035 |
| >7.76 | 9.50 ± 0.26 | 9.70 ± 0.35 | 0.642 | 9.27 ± 0.29 | 9.75 ± 0.29 | 0.222 | |||
Adjusted for age, sex, BMI, population admixture, smoking, alcohol use, dietary energy, plasma folate, plasma vitamin B12, plasma vitamin B6, and genotypes of MTHFR 1298 A>C and MTHFR 677 C>T.
Dichotomized values for fatty acids are adjusted for energy using the residuals method.
Data are expressed as mean ± SE.
Figure 1.
A. Interaction between dietary MUFA and MAT1A i15752 on plasma homocysteine.
Adjusted for age, sex, BMI, population admixture, smoking, alcohol use, dietary energy, plasma folate, plasma vitamin B12 and plasma vitamin B6, and genotypes of MTHFR 1298 A>C and MTHFR 677 C>T. Sample size: GG (n=360); AG+AA (n=532)
B. Interaction between dietary MUFA and MAT1A d18777 on plasma homocysteine: Adjusted for age, sex, BMI, population admixture, smoking, alcohol consumption, dietary energy, plasma folate, plasma vitamin B12 and plasma vitamin B6, and genotypes of MTHFR 1298 A>C and MTHFR 677 C>T. Sample size: GG (n=430); AG+AA(n=390).
We observed that SNP d18777 displayed significant interactions with dietary total fat in association with plasma Hcy concentration (p<0.0001). Concentrations of plasma Hcy in participants carrying the d18777A allele, the stroke-associated allele [16], were significantly lower than G homozygotes (p=0.001) when consuming lower (<31.3% energy/d) total fat (Table 3). On the other hand, those carriers (of d18777A) with higher total fat intake (>31.3% energy/d) had a significantly higher Hcy (p=0.006). We also observed interactions between intakes of SFA (p=0.022), MUFA (p=0.001), total PUFA (p=0.035) and d18777 genotype for plasma Hcy concentration (Table 3). Consistent results were observed in that dietary MUFA as a continuous variable significantly interacted with d18777 in relation to plasma Hcy (p=0.002, Fig. 1B). With higher MUFA, the level of plasma Hcy was higher in carriers of the A allele. However, we did not observe interactions between other fatty acids (n-3 PUFA, n-6 PUFA and n-3:n-6) and MAT1A genotypes (d18777, i15752) for Hcy (data not shown). Further examination whether MAT1A 3U1510 modulated the effect of dietary fatty acid intake on Hcy revealed a significant interaction between the ratio of dietary n-3:n-6 and 3U1510 for plasma Hcy (p=0.025) (Suppl. Fig. 1). When dietary n-3:n-6 ratio was low (<0.09), there was no significant difference between the genotypes in plasma Hcy (p=0.996), but when dietary n-3:n-6 ratio intake was high (>0.09), homozygotes for the G allele had significantly lower plasma Hcy than did A carriers (p=0.019).
To examine the combined effect of MAT1A genetic variants on plasma Hcy, we performed haplotype analysis based on three MAT1A variants (i15172, 3U1510, and d18777). For these three SNPs, we observed four haplotypes: GGG, GAG, AGA, GGA, with frequencies ranging from 0.09 to 0.38, accounting for 87% of all haplotypes in the population (Supplementary Table 1). MAT1A haplotypes displayed a significant global association with plasma Hcy (p=0.003). A strong interaction between AGA haplotype and dietary n-3:n-6 ratio on plasma Hcy was observed (Table 4, p=0.002), whereas other haplotypes did not display significant interactions with dietary n-3:n-6 ratio for plasma Hcy (Table 4). We also found a significant interaction between dietary MUFA and haplotype AGA for plasma Hcy (p=0.027, Supplementary Fig. 2). When daily intake of total MUFA was high (≥11.35% energy/d), those carrying the AGA haplotype had significantly higher plasma Hcy than non-carriers (p=0.031, Supplementary Fig. 2). We did not find an interaction between other fatty acids and MAT1A haplotypes for plasma Hcy (data not shown).
Table 4.
Interactions between MAT1A haplotypes and dietary n-3:n-6 ratio for plasma homocysteine
| MAT1A haplotype | |||||
|---|---|---|---|---|---|
|
MAT1A haplotypes1 |
n-3:n-6 ratio | Carrier (n) | Non-Carrier (n) | p-value (trend) |
p-value (interaction)4 |
| GGG | ≤ 0.092 | 9.93 ± 0.293 (254) | 9.89 ± 0.32 (187) | 0.904 | 0.191 |
| >0.09 | 9.26 ± 0.22 (267) | 9.23 ± 0.28 (175) | 0.933 | ||
| GAG | ≤ 0.09 | 9.63 ± 0.44 (259) | 9.98 ± 0.27 (182) | 0.439 | 0.131 |
| >0.09 | 9.20 ± 0.42 (263) | 9.47 ± 0.27 (179) | 0.458 | ||
| AGA | ≤ 0.09 | 10.09 ± 0.36 (301) | 9.67 ± 0.24 (141) | 0.285 | 0.002 |
| >0.09 | 9.19 ± 0.37 (301) | 9.47 ± 0.24 (140) | 0.472 | ||
| GGA | ≤ 0.09 | 9.91 ± 0.51 (371) | 9.80 ± 0.21 (70) | 0.572 | 0.081 |
| >0.09 | 9.52 ± 0.53 (359) | 9.33 ± 0.21 (83) | 0.892 | ||
MAT1A haplotypes were estimated based on three SNPs in the order: i15172, 3U1510, and d18777.
Dichotomized values for n-3:n-6 ratio are adjusted for energy using the residuals method.
Data are expressed as mean ± SE.
Adjusted for age, sex, physical activity, drinking, smoking, population admixture, plasma folate, vitamin B6, vitamin B12, MTHFR 1298 A>C, MTHFR 677 C>T
Discussion
In the present study we report that dietary n-6 PUFA and n-3:n-6 PUFA ratio were significantly associated with plasma Hcy concentration, although no difference in plasma Hcy based on MAT1A genotype was detected. We also identified interactions of dietary n-3:n-6 ratio and MUFA with MAT1A variants (SNP or haplotypes) in relation to plasma Hcy concentration. While these interactions have not been reported previously, several other studies provide evidence to support our findings (below).
Based on cardioprotective effects ascribed to n-3 PUFA and vascular damage attributed to plasma Hcy [3], a growing number of recent studies have investigated the relationship between n-3 PUFA and Hcy metabolism. In the present study, we demonstrated that a high dietary n-3:n-6 ratio was significantly associated with lower plasma Hcy, which is consistent with our previous report showing that plasma Hcy concentration was significantly negatively correlated with plasma phospholipid (PL) concentration (mg/100 mL) of total n-3 (r=−0.270, p=0.002) and with the n-3:n-6 ratio (r =−0.265, p=0.002) [12]. In sex-, age- and BMI-controlled partial correlations, the plasma Hcy concentration was significantly negatively correlated with platelet PL 22:6 n-3 and with n-3:n-6 ratio (p<0.01), and positively correlated with 22:4 n-6 (p<0.05) [13]. These results suggest that increased consumption of dietary n-3 PUFA may be associated with lower plasma Hcy. A subsequent intervention study supported this hypothesis by demonstrating that n-3 PUFA decreased plasma Hcy concentrations in participants with diabetes and dyslipidemia who were treated with a statin-fibrate combination [24]. Furthermore, plasma Hcy was significantly decreased in acute myocardial infarction survivors after one year of n-3 PUFA treatment [14]. In a third study, consumption of n-3 PUFA supplements (3 g/day) over a two-month period decreased Hcy in participants with diabetes with no change in fasting blood sugar, malondialdehyde, or C-reactive protein [25].
Collectively, earlier studies provide strong support for n-3 PUFA in regulating plasma Hcy concentration, but results have not been entirely consistent [15]. Some intervention studies have observed lower plasma Hcy after n-3 PUFA supplementation [14, 24], while other studies did not show beneficial effects of n-3 PUFA on plasma Hcy [15]. We hypothesize that variation in genes which encode critical enzymes in the metabolism of methionine may underlie these inconsistent results, and that intake of fatty acids may modulate Hcy through its regulation of enzyme activity and gene expression. MAT catalyzes the formation of S-adenosylmethionine from methionine and ATP. Defects in MAT1A which partially inactivate MAT activity [26] are one cause of hypermethioninemia [26] and this may, therefore be expressed differentially through MAT1A genotypes by dietary fatty acid interactions. Dietary factors may also alter MAT function, as demonstrated in our earlier animal study which showed that n-3 PUFA up-regulated MAT gene expression and enzyme activity [27]. While we did not observe MAT1A 3U1510 genotype-associated differences in plasma Hcy in the current study, we did show that MAT1A 3U1510 significantly interacted with the dietary n-3:n-6 ratio in modulating plasma Hcy. Further, MAT1A haplotypes displayed strong interaction with n-3:n-6 ratio - carriers of the risk haplotype (AGA) tend to have higher plasma Hcy when the ratio is low compared to non-carriers. These results support our hypothesis that MAT1A genotype may modulate the regulatory effect of n-3 PUFA or n-6 PUFA on Hcy metabolism. Moreover, we observed that MAT1A i15752 interacted with total fat and total MUFA, and that MAT1A d18777 interacted with total fat, SFA, MUFA and total PUFA in modulating plasma Hcy. Although total fat, SFA, MUFA and total PUFA were not associated with altered Hcy metabolism independent of genotype, these dietary fat intakes displayed significant interaction with MAT1A i15752 and d18777 genotypes on plasma Hcy concentrations.
There are, however, some limitations to the present study. First, the sample size is small, which limits statistical power. Second, we calculated the dietary fatty acids using a questionnaire previously adapted from the NCI/Block food frequency form for this population. Compared with plasma fatty acids, dietary fatty acids may have some limitation. Lastly, it is debatable that elevated Hcy could be a consequence of vascular disease, instead of a cause. Higher n-3 PUFA intake may reduce vascular pathology and thus reduce plasma Hcy concentration. Therefore, the mechanisms by which n-3 PUFA regulates plasma Hcy level remains to be illustrated.
In summary, the present study confirms earlier studies reporting the relationship between PUFA intake and plasma Hcy. Our results further suggest that interactions between MAT1A variants and dietary fatty acids modulate plasma Hcy concentration. In light of strong evidence that elevated plasma Hcy is an important risk factor for cardiovascular disease, understanding the role of dietary factors in the potential amelioration of genetically based risk of hyperhomocystemia is critical. Confirmation of these interactions will require further investigation in additional populations.
Supplementary Material
Acknowledgement
This work is supported by the China Scholarship Council, the National Institutes of Health, National Institute on Aging, Grant Number 5P01AG023394-02, NIH/NHLBI grant number HL54776 and HL078885 and contracts 53-K06–5-10 and 58–1950-9–001 from the U.S. Department of Agriculture Research Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No potential conflicts of interest
Author’s contributions to manuscript
TH, YL, and JS carried out the studies, analyzed data and drafted the manuscript; CL, LP, YL, CS and JC participated in manuscript preparation; and CL, KT, DL and JO participated in the project design. All authors read and approved the final manuscript.
References
- 1.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 2.Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Huang T, Yuan G, Zhang Z, Zou Z, Li D. Cardiovascular pathogenesis in hyperhomocysteinemia. Asia Pac J Clin Nutr. 2008;17:8–16. [PubMed] [Google Scholar]
- 4.Blom HJ. Determinants of plasma homocysteine. Am J Clin Nutr. 1998;67:188–189. doi: 10.1093/ajcn/67.2.188. [DOI] [PubMed] [Google Scholar]
- 5.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 6.Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169–172. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 7.Wilson A, Platt R, Wu Q, Leclerc D, Christensen B, Yang H, et al. A common variant in methionine synthase reductase combined with low cobalamin (vitamin B12) increases risk for spina bifida. Mol Genet Metab. 1999;67:317–323. doi: 10.1006/mgme.1999.2879. [DOI] [PubMed] [Google Scholar]
- 8.Leclerc D, Campeau E, Goyette P, Adjalla CE, Christensen B, Ross M, et al. Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders. Hum Mol Genet. 1996;5:1867–1874. doi: 10.1093/hmg/5.12.1867. [DOI] [PubMed] [Google Scholar]
- 9.Selhub J, Jacques PF, Rosenberg IH, Rogers G, Bowman BA, Gunter EW, et al. Serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey (1991–1994): population reference ranges and contribution of vitamin status to high serum concentrations. Ann Intern Med. 1999;131:331–339. doi: 10.7326/0003-4819-131-5-199909070-00003. [DOI] [PubMed] [Google Scholar]
- 10.Schneede J, Refsum H, Ueland PM. Biological and environmental determinants of plasma homocysteine. Semin Thromb Hemost. 2000;26:263–279. doi: 10.1055/s-2000-8471. [DOI] [PubMed] [Google Scholar]
- 11.Erkkila AT, Lichtenstein AH, Mozaffarian D, Herrington DM. Fish intake is associated with a reduced progression of coronary artery atherosclerosis in postmenopausal women with coronary artery disease. Am J Clin Nutr. 2004;80:626–632. doi: 10.1093/ajcn/80.3.626. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Mann NJ, Sinclair AJ. A significant inverse relationship between concentrations of plasma homocysteine and phospholipid docosahexaenoic acid in healthy male subjects. Lipids. 2006;41:85–89. doi: 10.1007/s11745-006-5074-x. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Yu XM, Xie HB, Zhang YH, Wang Q, Zhou XQ, et al. Platelet phospholipid n-3 PUFA negatively associated with plasma homocysteine in middle-aged and geriatric hyperlipaemia patients. Prostaglandins Leukot Essent Fatty Acids. 2007;76:293–297. doi: 10.1016/j.plefa.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Grundt H, Nilsen DW, Mansoor MA, Hetland O, Nordoy A. Reduction in homocysteine by n-3 polyunsaturated fatty acids after 1 year in a randomised double-blind study following an acute myocardial infarction: no effect on endothelial adhesion properties. Pathophysiol Haemost Thromb. 2003;33:88–95. doi: 10.1159/000073852. [DOI] [PubMed] [Google Scholar]
- 15.Piolot A, Blache D, Boulet L, Fortin LJ, Dubreuil D, Marcoux C, et al. Effect of fish oil on LDL oxidation and plasma homocysteine concentrations in health. J Lab Clin Med. 2003;141:41–49. doi: 10.1067/mlc.2003.3. [DOI] [PubMed] [Google Scholar]
- 16.Lai CQ, Parnell LD, Troen AM, Shen J, Caouette H, Warodomwichit D, et al. MAT1A variants are associated with hypertension, stroke, and markers of DNA damage and are modulated by plasma vitamin B-6 and folate. Am J Clin Nutr. 2010;91:1377–1386. doi: 10.3945/ajcn.2009.28923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. doi: 10.1186/1471-2458-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148:507–518. doi: 10.1093/oxfordjournals.aje.a009676. [DOI] [PubMed] [Google Scholar]
- 19.Lee IM, Paffenbarger RS., Jr Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke. 1998;29:2049–2054. doi: 10.1161/01.str.29.10.2049. [DOI] [PubMed] [Google Scholar]
- 20.de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 21.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 22.Camp VM, Chipponi J, Faraj BA. Radioenzymatic assay for direct measurement of plasma pyridoxal 5'- phosphate. Clin Chem. 1983;29:642–644. [PubMed] [Google Scholar]
- 23.Soldin SJ, Wan BS, Cherian AG. The measurement of creatinine: a comparison between the Beckman Creatinine Analyzer II and the Selective Analyzer GSA IID. Clin Biochem. 1981;14:165–168. doi: 10.1016/s0009-9120(81)91168-1. [DOI] [PubMed] [Google Scholar]
- 24.Zeman M, Zak A, Vecka M, Tvrzicka E, Pisarikova A, Stankova B. N-3 fatty acid supplementation decreases plasma homocysteine in diabetic dyslipidemia treated with statin-fibrate combination. J Nutr Biochem. 2006;17:379–384. doi: 10.1016/j.jnutbio.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Pooya S, Jalali MD, Jazayery AD, Saedisomeolia A, Eshraghian MR, Toorang F. The efficacy of omega-3 fatty acid supplementation on plasma homocysteine and malondialdehyde levels of type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2009 doi: 10.1016/j.numecd.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Ubagai T, Lei KJ, Huang S, Mudd SH, Levy HL, Chou JY. Molecular mechanisms of an inborn error of methionine pathway. Methionine adenosyltransferase deficiency. J Clin Invest. 1995;96:1943–1947. doi: 10.1172/JCI118240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang T, Wahlqvist ML, Li D. Docosahexaenoic acid decreases plasma homocysteine via regulating enzyme activity and mRNA expression involved in methionine metabolism. Nutrition. 2008;26:112–119. doi: 10.1016/j.nut.2009.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.