Abstract
Background
Metabolically benign obese individuals have a 10-year cardiovascular disease (CVD) risk comparable to healthy normal weight individuals. However, the burden of subclinical CVD among metabolically benign obese is not well known.
Methods
In cross-sectional analyses of 475 mid-life women, we compared common carotid artery intima media thickness (CCA-IMT), aortic pulse wave velocity (aPWV) and coronary (CAC) and aortic calcification (AC) among three groups: healthy normal weight, metabolically benign overweight/obese (<3 metabolic syndrome components/elevated CRP), and at-risk overweight/obese (≥3 metabolic syndrome components/elevated CRP).
Results
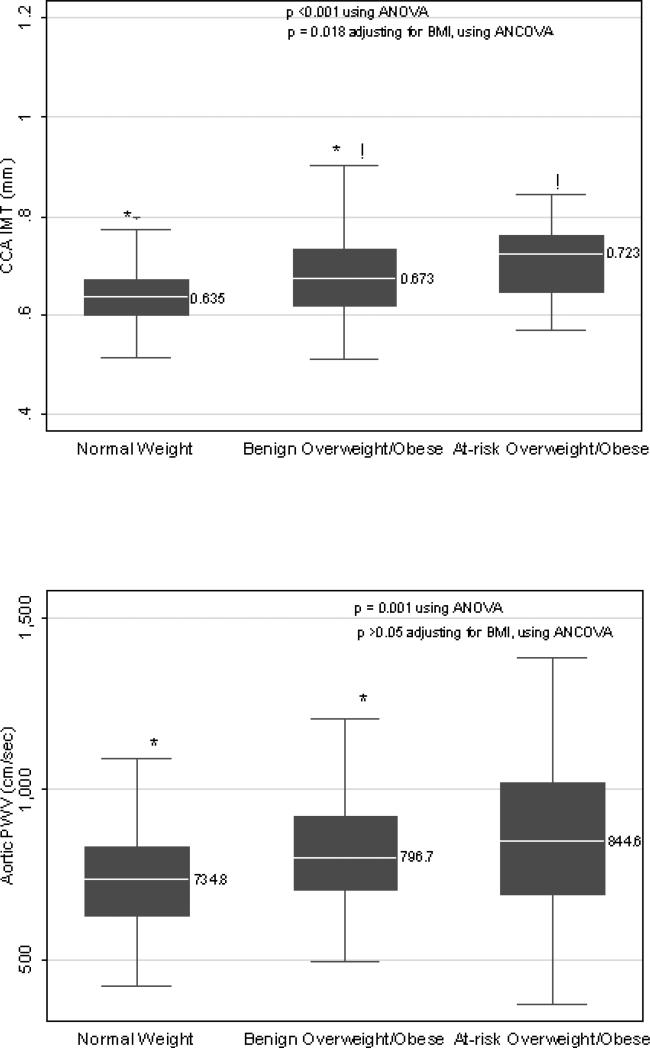
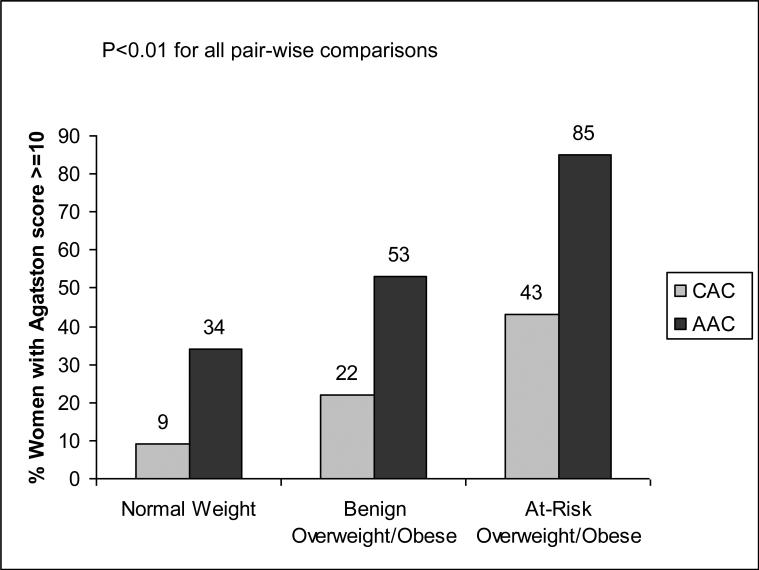
The mean (SD) CCA-IMT and aPWV were lowest in the normal weight group (n=145), followed by the benign overweight/obese (n=260) and at-risk overweight/obese (n=70) groups [CCA-IMT: 0.64 (0.08) vs. 0.68 (0.09) vs. 0.73 (0.13) mm, p<0.001; aPWV: 731.0 (176.4) vs. 809.9 (182.3) vs. 875.7 (228.8) cm/s, p<0.001]. Similar results were found for the frequency (%) of women with increased CAC and AC [CAC: 13 (9%) vs. 53(20%) vs. 28(40%), p<0.001; AC: 47(32%) vs. 130 (50%) vs. 55(79%), p<0.001]. These differences remained significant after multivariable adjustment. Further adjustment for BMI attenuated the statistical significance of differences in aPWV and calcification between benign and at-risk overweight/obese women, but had little effect on the magnitude of these differences.
Conclusions
Metabolically benign overweight/obese women have a significantly greater subclinical CVD burden than normal weight women, despite published data finding similar CVD event rates between the two groups. Prospective studies tracking the progression of subclinical atherosclerosis to clinical CVD in these women are needed.
Keywords: Obesity phenotypes, subclinical atherosclerosis, carotid intima media thickness, pulse wave velocity, coronary calcification, aortic calcification
INTRODUCTION
There is growing recognition of the heterogeneity in cardiovascular disease (CVD) risk in obese individuals. Multiple studies have identified a metabolically benign obese phenotype that fulfills the criteria of clinical obesity by BMI or waist circumference, but does not have the burden of adiposity-associated cardiometabolic risk factors found among those with the “at-risk” obese phenotype [1-4]. Longitudinal studies have found a similar prevalence of CVD events over 3-11 years between those with metabolically benign obesity (defined in these publications as the absence of metabolic syndrome and diabetes) and normal weight individuals, in contrast to the elevated CVD risk found in at-risk obese individuals [1, 2, 5]. However, recent analyses from the Health Professional's Follow-Up Study of men and the Nurse's Health Study of women demonstrate a 40% to 200% increased risk of CVD in overweight and obese men and women followed for 16 years [6].These most recent findings raise the possibility that individuals with metabolically benign obesity do have an increased risk of incident CVD compared to normal weight individuals, but experience a delay in the onset of clinical CVD compared to their at-risk counterparts. If this hypothesis were true, it might be expected that metabolically benign obese individuals would have a greater burden of subclinical CVD compared to healthy normal weight individuals, but a lesser subclinical CVD burden compared to at-risk obese individuals. Few studies have examined the burden of subclinical CVD in different body size phenotypes, and have used insulin resistance to define benign vs. at-risk phenotypes [3, 7] rather than definitions comparable to those used in the clinical CVD event literature.
The purpose of the current study was to compare the subclinical CVD burden in metabolically benign overweight/obese women compared to normal weight women and at-risk overweight/obese women participating in the Study of Women's Health Across the Nation Heart ancillary study using phenotype definitions similar to those used in the clinical CVD event literature.
METHODS
Study Population
The current study included participants from the Study of Women's Health Across the Nation (SWAN). SWAN is a multicenter, multiethnic longitudinal study designed to characterize the biological and psychosocial changes that occur during the menopausal transition in a community-based sample. Details of the study design and recruitment have been previously published [8]. Briefly, SWAN is being conducted at seven sites: Boston; Chicago; the Detroit area; Los Angeles; Newark, NJ; Pittsburgh, PA; and Oakland, CA. A total of 3,302 women aged 42 to 52 years were enrolled from 1996 to 1997.
The current data are derived from the SWAN Heart study, an ancillary study designed to characterize the natural history of subclinical atherosclerosis during the menopausal transition. SWAN Heart was conducted at the Pittsburgh and Chicago sites and was initiated approximately 4 years after the SWAN baseline enrollment. Thus, participants were between 45 and 58 years of age at entry into the SWAN Heart study. To be eligible for SWAN Heart, participants could not have evidence of clinical atherosclerosis (myocardial infarction, angina, intermittent claudication, cerebral ischemia, or revascularization). All measures were obtained concurrent to participation in SWAN Heart. A total of 608 participants (382 Caucasian, 226 African American) were enrolled in the ancillary study.
The institutional review boards of the participating institutions approved this study, and all women signed informed consent prior to participation.
Common Carotid Artery Intima-Media Thickness (CCA-IMT) Measurement
CCA-IMT was assessed at the time of the ancillary study visit with a Toshiba SSA-270A scanner at the Pittsburgh site and a Hewlett- Packard 5500 scanner at the Chicago site. The duplex scanners for the two sites were comparable in image quality. B-mode images were obtained from the near and far walls of the distal common carotid artery, one-cm proximal to the carotid bulb. IMT measurements were performed by electronically tracing the lumen-intima interface and the media-adventitia interface across a 1-cm segment. A computer-assisted measurement for each pixel over this area was generated, for a total of approximately 140 data points. The mean of the average IMT readings obtained at the near and far walls was calculated. All readings were conducted at the University of Pittsburgh Ultrasound Research Laboratory. An intra class correlation of 0.98 was obtained upon replicate readings of 20 IMT scans.
Aortic Pulse Wave Velocity (aPWV) Measurement
Arterial stiffness was assessed by carotid-to-femoral aPWV as described in previous reports.[9] In brief, arterial flow waves were simultaneously and non-invasively recorded at the carotid and femoral arteries of supine participants, using unidirectional transcutaneous Doppler flow probes (model 810-a, 10 MHz, Parks Medical Electronics, Aloha, OR). Aortic PWV is calculated as distance/transit time (cm/sec). The transit time of the pulse wave was calculated as the foot-to-foot delay between the averaged carotid and femoral waveforms. The distance traveled by the pulse waveform was estimated by measurement over the participant's torso. The distance from the carotid to aortic site was subtracted from the sum of the aortic to umbilicus and umbilicus to femoral site to adjust for the opposite direction of the blood flow in that arterial branch. A higher aPWV indicates a stiffer vessel. This measure has demonstrated good reproducibility with an intra-class correlation of 0.84 using replicate assessments on 20 individuals.
Coronary Artery Calcification (CAC) and Aortic Calcification (AC) Measurement
Methods of assessing calcification have been described previously. [10] Briefly, images were obtained using electron beam tomography with an Imatron C-150 Ultrafast scanner (Imatron, South San Francisco, CA). ECG triggering was used to ensure that all images were obtained during the same phase of the cardiac cycle. For CAC, 30 to 40 contiguous 3-mm-thick transverse images of the coronary arteries were obtained from the level of the aortic root to the apex of the heart during maximal breath holding. For AC, cross-sectional 6-mm images from the aortic arch to the iliac bifurcation were obtained. All scans were scored at the University of Pittsburgh site with a DICOM work station and AcuImage, Inc software (South San Francisco, CA) using the method established by Agatston et al. [11] Calcification was considered present if at least three contiguous pixels with a density of >130 Hounsfield units were detected. CAC score was obtained as the sum of scores for each of the four major epicardial coronary arteries and AC as a single score. An Agatston score of ≥ 10 was used to define mild-moderate calcification. [12]
Body Fat Distribution Measures
Central adiposity was measured using an electron beam CT scan, as described elsewhere [13]. Briefly, a 6-mm transverse image was obtained between L4 and L5, with a c-150 Ultrafast CT Scanner (GE Imatron, San Francisco, CA). Scans were read by a single reader at the University of Pittsburgh. A pixel range of -30 to -190 Hounsfield units was used to define fat. The area of adipose tissue was defined using image analysis (AcuImage software, South San Francisco, CA). A region of interest line was drawn at the interior of abdominal musculature along the fascial plane. Fat within this area was considered visceral fat area. Subcutaneous fat was calculated as the difference between the whole image and visceral fat area. Inter observer reliability was determined by repeat reads on 10 scans, with intra class coefficient values of 0.97 and 0.94 for total and visceral fat area, respectively.
Blood Assays
Standard cardiovascular risk factors were assayed at the Medical Research Laboratories (Lexington, KY), which is certified by the National Heart Lung and Blood Institute, Centers for Disease Control and Prevention Part II program, as previously described [14]. Low-density lipoprotein (LDL) was calculated using the Friedewald equation [15], excluding women with values of triglycerides ≥ 400 mg/dL. The homeostasis model assessment insulin resistance index (HOMA-IR) was calculated from fasting insulin and glucose as (fasting insulin x fasting glucose)/22.5 [16]. High sensitivity C-reactive protein (CRP) levels were measured using an ultra-sensitive rate immunonephelometric method (BN 100, Dade-Behring, Marburg, Germany).
Subject Data
Race, current smoking habits and education status (≤ high school/ post high school/ ≥ college) were obtained from a self-reported questionnaire. Women were also asked about their menstrual bleeding patterns in the 12 months prior to recruitment and divided into categories: 1) Pre menopausal: menstrual periods in the past three months with no irregularity in the past 12 months; 2) Peri menopausal: no menstrual periods in the last three months with some irregularity in the past 12 months; 3) Post menopausal: those with no menstrual periods within the last 12 months; 4.) Menopausal hormone therapy (MHT) use within the past 12 months.
Physical Measures
Blood pressure was measured in the right arm with the participant seated, after at least 5 minutes of rest. Two sequential blood pressure values were obtained and averaged. Height and weight were measured in light clothing and without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters. Waist circumference was measured with the participant in nonrestrictive undergarments, at the level of the natural waist, defined as the narrowest part of the torso as seen from the anterior aspect. For cases in which waist narrowing was difficult to determine, the measure was taken at the smallest horizontal circumference in the area between the ribs and the iliac crest.
Definitions of Body Size Phenotypes
Women were categorized as normal weight (BMI<25 kg/m2) or overweight/obese (BMI≥25 kg/m2) [17]. To characterize the cardiometabolic risk burden, we used the Adult Treatment Panel (ATP III) definition of metabolic syndrome, excluding the waist circumference criterion (due to its co variability with BMI).[18] To better assess the inflammatory component of cardiovascular risk, we added CRP > 3.0 mg/dL to our criteria of metabolic abnormalities.[19] Thus the following five characteristics were included: 1) systolic/diastolic blood pressure ≥ 130/85 mmHg or antihypertensive medication use; 2) fasting triglycerides of ≥ 150 mg/dL; 3) fasting HDL cholesterol levels ≤ 50 mg/dL or lipid lowering medication use; 4) fasting glucose ≥ 100 mg/dL or self-reported use of antidiabetic medications, and 5) CRP levels ≥ 3.0 mg/dL. Women who had <3 abnormalities were defined as “benign” and those with ≥ 3 abnormalities were defined as being “at-risk”. Thus, there were four defined phenotypes of women: (1) Metabolically benign normal weight (2) At-risk normal weight (3) Metabolically benign overweight/obese (4) At-risk overweight/obese. As no women fit the criteria of “at-risk normal weight”, results will include the three remaining body size phenotypes. The metabolically benign normal weight group will be referred to as normal weight.
Statistical Analysis
Of the 608 women enrolled in the ancillary study, 106 had incomplete CCA-IMT or PWV measurements and were excluded. One woman had vascular surgery and one had history of stroke/TIA and their data were removed from the analyses. 50 women had more than one physical measurement or blood assays missing that were required for phenotype categorization and for these women missing data was imputed. Using longitudinal data from the SWAN parent study, an estimator using one time-interval after the missing value was picked as the best estimator. hs-CRP values were not obtained on 145 women at any time during the parent SWAN study and, therefore, could not be imputed. However, 120 of these women were able to be retained in analyses as knowledge of their hs-CRP value would not have changed their body size phenotype categorization. Thus final analyses were performed on 475 women. All analyses were performed with and without imputed data (n=50). Women excluded from the analyses were not different in age, ethnicity, or menopause status, but had higher education compared to women who were included in the analyses.
Demographics, metabolic risk factors, Framingham Risk Score and subclinical CVD measurements were compared between the three body size phenotypes using the chi- square test for categorical variables and one-way analysis of variance (ANOVA) for normally distributed continuous variables. Pair-wise comparisons were tested when significant differences were found. For skewed continuous data, the Wilcoxon rank-sum test was used.
To estimate the unit increase in CCA-IMT and aPWV measures associated with being in the benign overweight/obese phenotype vs. either the normal weight phenotype or the at-risk overweight/obese phenotype, separate linear regression models were run for CCA- IMT and aPWV, adjusting for age, race, site of recruitment (Pittsburgh or Chicago), education level, and smoking status. Regression models comparing benign overweight/obese women to at-risk overweight/obese women were additionally adjusted for BMI. To estimate the odds of coronary and aortic calcification associated with being in the benign overweight/obese phenotype vs. either the normal weight phenotype or the at-risk overweight/obese phenotype, separate logistic regression models were run for coronary and aortic calcification, adjusting for the factors named above. Multiplicative race-by-body size phenotype interactions were examined for each measure of subclinical atherosclerosis.
To further examine the role of obesity independent of its associated cardiometabolic risk factors in the development of subclinical atherosclerosis, regression analyses were repeated using more stringent criteria to define metabolically benign overweight/obesity. Thus, overweight/obese women were re-categorized as benign overweight/obese if they had no cardiometabolic abnormalities and the at-risk overweight/obese group as having ≥ 1 cardiometabolic abnormalities.
Additional analyses adjusted for visceral adipose tissue levels, as well as replaced BMI with waist circumference (≥88 cm. vs. < 88 cm.) to define obesity [18]. Additionally, the glucose/diabetes medication criterion was substituted with HOMA-IR (≥75th percentile vs. <75th percentile), and analyses were repeated excluding the 58 menopausal hormone therapy (MHT) users.
RESULTS
Of the 475 women with complete data on both CCA-IMT and aPWV, 31% were normal weight, 55% were in the benign overweight/obese group and 15% were in the at-risk overweight/obese group. Comparing the three body size phenotypes (Table 1), there were no statistically significant differences in education or menopause status. The at-risk overweight/obese was significantly older than the normal weight and the benign overweight/obese phenotypes. A lower percentage of African American women were in the normal weight phenotype compared to the overweight/obese phenotypes. Compared to the normal weight and at-risk overweight/obese groups, the benign overweight/obese group had intermediate levels of adiposity such as BMI, waist circumference, abdominal visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (SAT), as well as intermediate values of most cardiometabolic risk factors. The difference in lipid markers varied. Whereas the metabolically benign overweight/obese group had intermediate cholesterol levels, total cholesterol was not different between the three groups. In addition, LDL cholesterol was significantly higher in the overweight/obese groups compared to the normal weight group but was similar between the two overweight/obese groups. Triglyceride levels were significantly lower in the metabolically benign overweight/obese group than the at-risk overweight/obese group, but did not differ compared to the normal weight phenotype. Despite that certain lipid markers did not differ between the metabolically benign overweight/obese and the other groups, the aggregate 10- year risk of cardiovascular disease as calculated by Framingham risk score (FRS) in the benign overweight/obese group was intermediate compared to the normal weight and the at-risk overweight/obese phenotypes.
Table 1.
Demographic and Metabolic Characteristics of the Study Population by Body Size Phenotypes◆
| Metabolically Benign Normal Weight (N=145; 30%) | Metabolically Benign Overweight/Obese (N=260; 55%) | At-Risk Overweight/Obese (N=70; 15%) | P-Value* | |
|---|---|---|---|---|
| Age, yrs (mean ± sd) | 50.5 ± 2.8 | 50.9 ± 2.8 | 51.7 ± 3.2† | 0.012 |
| Education, n (%) | 0.104 | |||
| ≤ High School | 26 (19) | 33 (13) | 14 (21) | |
| Post High School | 35 (25) | 84 (33) | 25 (37) | |
| ≥ College | 78 (56) | 137 (54) | 28 (42) | |
| African American, n (%) | 31 (22) | 98 (41)* | 29 (39) | 0.001 |
| Current Smokers, n (%) | 28 (19) | 31 (12)* | 15 (21)† | 0.050 |
| Menopause Status, n (%) | 0.308 | |||
| Pre menopausal | 15 (10) | 20 (8) | 10 (14) | |
| Early/Late menopausal | 82 (57) | 154 (59) | 33 (47) | |
| Post menopausal/surgery | 38 (26) | 72 (28) | 25 (36) | |
| Menopausal Hormone Therapy Users | 21 (14) | 28 (11) | 9 (13) | |
| Systolic Blood Pressure, mmHg (mean ± sd) | 110 ± 13) | 119 ± 14* | 130 ± 21† | < 0.001 |
| Diastolic Blood Pressure, mmHg (mean ± sd) | 71 ± 9 | 76 ± 9* | 81 ± 10† | < 0.001 |
| Elevated Blood Pressure, n (%) | 7 (5) | 43 (17)* | 31 (44)† | < 0.001 |
| Total Cholesterol, mg/dL (mean ± sd) | 194.3 (36.6) | 202.1 (35.2) | 206.0 (38.2) | 0.042 |
| LDL Cholesterol, mg/dL (mean ± sd) | 113.0 (32.3) | 121.5 (32.0)* | 123.9 (32.3) | 0.018 |
| HDL Cholesterol, mg/dL (mean ± sd) | 63 ± 15 | 59 ± 13* | 45 ± 10† | < 0.001 |
| HDL<50 mg/dL, n (%) | 25 (17) | 53 (20) | 58 (83)† | < 0.001 |
| Triglycerides, mg/dL (median, IQR) | 87.0 (68.0, 107.0) | 96.0 (72.0, 124.0) | 173.0 (106.0, 222.0)† | < 0.001 |
| Triglycerides ≥ 150 mg/dL, n (%) | 11 (8) | 28 (11)* | 45 (64)† | < 0.001 |
| Glucose, mg/dL (median, IQR) | 84.0 (79.0, 89.0) | 88.0 (83.0, 93.0)* | 102.5 (93.0, 110.0)† | < 0.001 |
| Glucose ≥100 mg/dL (or medication use), n (%) | 9 (6) | 19 (7) | 45 (64)† | < 0.001 |
| Insulin, uU/mL (median, IQR) | 6.9 (5.5, 8.3) | 9.1 (7.2, 12.7)* | 14.6 (11.9, 20.5) f | < 0.001 |
| HOMA Insulin resistance (median, IQR) | 1.4 (1.1, 1.9) | 1.9 (1.5, 2.8)* | 4.1 (2.8, 5.5)† | < 0.001 |
| BMI, kg/m2 (mean ± sd) | 22.7 ± 1.6 | 30.8 ± 5.1* | 34.6 ± 5.7† | < 0.001 |
| Waist Circumference, cm (mean ± sd) | 74.9 ± 6.1 | 92.0 ± 12.1* | 102.3 ± 12.5† | < 0.001 |
| Visceral Adipose Tissue, cm2 (mean ± sd) | 70.4 ± 35.4 | 127.3 ± 58.4* | 186.2 ± 60.2† | < 0.001 |
| Subcutaneous Adipose Tissue, cm2 (mean ± sd) | 189.9 ± 81 | 379.9 ± 136.4* | 440.0 ± 119.9† | < 0.001 |
| C Reactive Protein, mg/dL(median, IQR) | 0.8 (0.4, 1.7) | 2.1 (1.0, 5.5)* | 6.1 (4.0, 9.4)† | < 0.001 |
| CRP >3.0 mg/dL, n (%) [N= 355] | 16 (15) | 68 (35)* | 47 (84)† | < 0.001 |
| Framingham Risk Score (mean± sd) | 8.61 (3.48) | 9.50 (3.25)* | 12.87 (3.55)† | < 0.001 |
| Family History of CHD, n (%) | 91 (63) | 178 (68) | 45 (64) | 0.479 |
Body Size Phenotypes: Normal Weight: BMI <25 and <3 cardiometabolic risk factors; Metabolically Benign Overweight/Obese: BMI ≥25 and <3 cardiometabolic risk factors; At-risk Overweight/Obese: BMI ≥25 and ≥ 3 cardiometabolic risk factors
p<0.05 comparing Normal Weight and Metabolically Benign Overweight/Obese phenotypes
p< 0.05 comparing Metabolically Benign Overweight/Obese and At-risk Overweight/Obese phenotypes
Compared to the normal weight and at-risk overweight/obese phenotypes, the metabolically benign overweight/obese phenotype had intermediate values of mean CCA-IMT (Figure 1), and intermediate prevalence of coronary and aortic calcification (Agatston score ≥ 10) (Figure 2). The benign overweight/obese group also appeared to have intermediate values of aortic PWV (Figure 1). However, this difference achieved statistical significance only for the comparison between benign overweight/obese and normal weight groups.
Figure 1.
Box Plots of Median CCA IMT and aPWV Levels by Body Size Phenotypes
Upper and lower lines of the box represent the 25th and 75th percentiles and the middle line represents the median (given next to the box plot). Whiskers represent 1.5 times the inter quartile range.
*!: Similar symbols represent significant differences (p< 0.001) between the two groups. Differences between the three body size phenotypes were compared using ANOVA. Differences between metabolically benign and At-risk Overweight/Obese groups after adjusting for BMI were compared using ANCOVA.
Figure 2.
Prevalence of Coronary and Aortic Calcification (Agatston Score ≥10) by Body Size Phenotypes
After multivariable adjustment, significantly higher CCA-IMT and aPWV values were observed in the metabolically benign overweight/obese women compared to normal weight women (Table 2). Similar results were found for calcification measures. No significant effect modification was observed by race for comparisons between metabolically benign overweight/obese and normal weight women for any of the subclinical atherosclerosis measures.
Table 2.
Multivariable-adjusted Regression Coefficients of CCA-IMT and aPWV and Odds Ratios of CAC and AC associated with the Metabolically Benign Overweight/Obese Phenotype vs. Normal Weight
| CCA-IMT | aPWV | Coronary Calcification | Aortic Calcification | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
||||||||||||
| β estimate (mm) | 95% CI | p-value | β estimate (cm/sec) | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| |
||||||||||||
| Normal weight | reference | ---- | ---- | reference | ----- | ---- | reference | ----- | ---- | reference | ---- | ---- |
| Metabolically Benign Overweight/Obese | 0.034 | 0.017, 0.051 | <0.001 | 59.7 | 23.7, 95.7 | 0.001 | 2.38 | 1.20, 4.70 | 0.013 | 2.37 | 1.49, 3.80 | <0.001 |
After multivariable adjustment, the at-risk overweight/obese women had significantly higher CCA-IMT and aPWV values compared to the metabolically benign overweight/obese women (Table 3; Model 1). After further adjustment for BMI, the difference in the CCA-IMT values remained statistically significant. However, the aPWV difference between the metabolically benign and at-risk overweight/obese women, though only slightly attenuated in magnitude, did not remain statistically significant (Table 3; Model 2). Similar results were found for calcification measures. No significant effect modification was observed by race for comparisons between metabolically benign and at-risk overweight/obese women for aPWV or calcification outcomes. For CCA-IMT, African American at-risk overweight/obese women had higher values of CCA-IMT compared to their metabolically benign counterparts (β estimate [95% CI] 0.077 [0.032, 0.122] comparing at-risk to metabolically benign overweight/obese) while in Caucasian women, the CCA-IMT values were similar between the two body size phenotypes (-0.009 [-0.040, 0.022] ) (p for interaction: 0.002). Race stratified results of measures of subclinical atherosclerosis between normal weight and metabolically benign overweight/obese women and metabolically benign and at-risk overweight/obese women are presented in supplementary Tables 2a and 3a respectively.
Table 3.
Multivariable-adjusted Regression Coefficients of CCA-IMT and aPWV and Odds Ratios of CAC and AC Associated with the At-Risk Overweight/Obese vs. the Metabolically Benign Overweight/Obese Phenotype
| CCA-IMT | aPWV | Coronary Calcification | Aortic Calcification | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
||||||||||||
| β estimate (mm) | 95% CI | p-value | β estimate (cm/sec) | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| |
||||||||||||
| Model 1: | ||||||||||||
| Metabolically Benign | reference | ---- | ---- | reference | ----- | ---- | reference | ----- | ---- | reference | ---- | ---- |
| At-Risk | 0.041 | 0.016, 0.066 | 0.001 | 58.2 | 7.3, 109.1 | 0.025 | 2.48 | 1.33, 4.63 | 0.004 | 4.56 | 2.16, 9.64 | <0.001 |
| Model 2: | ||||||||||||
| Metabolically Benign | reference | ---- | ---- | reference | ----- | ---- | reference | ----- | ---- | reference | ---- | ---- |
| At-Risk | 0.030 | 0.004, 0.056 | 0.022 | 44.7 | -8.5, 97.9 | 0.099 | 1.28 | 0.63, 2.61 | 0.492 | 2.22 | 0.91, 5.41 | 0.081 |
Model 1 adjusted for age, site of recruitment, education, race, and smoking status.
Model 2 adjusted for Model 1 variables plus BMI
CCA-IMT: common carotid artery intima media thickness; aPWV: aortic pulse wave velocity; Coronary and Aortic Calcification: Agatston score ≥ 10
Using a more stringent definition of metabolically benign overweight/obesity where the metabolically benign overweight/obese group was defined as having zero cardiometabolic risk factors, the differences in both CCA-IMT and aPWV between metabolically benign overweight/obese and normal weight women were only slightly attenuated in magnitude (CCA-IMT beta: 0.029 mm; aPWV beta: 53.2 cm/s) but no longer achieved statistical significance. Similarly for CAC and AC, the odds of having coronary or aortic calcification were only slightly attenuated, but no longer achieved statistical significance (CAC OR: 1.97, 95%CI: 0.56, 6.98; AC OR: 1.57, 95% CI: 0.71, 3.46).
In additional linear regression models, neither adjustment for visceral adipose tissue levels, nor categorizing body size phenotypes using either waist circumference as the measure of adiposity or HOMA-IR as the measure of glucose metabolism materially altered the associations between the phenotypes and CCA-IMT or aPWV measurements or aortic or coronary artery calcification scores (data not shown). Results were also similar when the 58 MHT users and women with imputed data (N=50) were removed from analyses (data not shown).
DISCUSSION
Reports of CVD events have indicated that compared to normal weight individuals, metabolically benign overweight/obese individuals are not at increased 3-11 year risk of CVD, [1, 2] but are at increased 16-year risk of CVD [6]. In our midlife women, we found that compared to normal weight women, women with metabolically benign overweight/obesity had a greater burden of subclinical CVD. This was reflected by higher levels of CCA-IMT and aPWV and a greater prevalence of coronary and aortic calcification. In addition, we found metabolically benign overweight/obese women to have lower levels of subclinical disease compared to at-risk overweight/obese women. Our results held true not only when we used a clinical definition of metabolically benign overweight/obesity (<3 cardiometabolic risk factors), but also when we used a more stringent definition (0 cardiometabolic risk factors), though in the latter case statistical significance was not attained likely due to the smaller sample size of those analyses.
There are few published reports regarding the presence of subclinical atherosclerosis in metabolically benign overweight or obese individuals. Using a cross-classification of BMI and insulin resistance to identify metabolically benign obese women, Marini et al. found insulin-sensitive obese women to have IMT measurements that were intermediate between normal weight and insulin-resistant obese women [3]. Although not statistically significant, Stefan et al. also reported insulin-sensitive obese individuals to have higher IMT values compared to normal weight individuals, and the difference was of similar magnitude to the 0.04 mm difference in CCA-IMT we found between metabolically benign overweight/obese women and normal weight women [7]. Therefore, our findings are in accordance with the few existing reports, and suggest that overweight/obesity may be associated with subclinical CVD independent of the metabolic abnormalities which frequently accompany it.
Recent prospective studies using a definition of body size phenotypes similar to ours have reported that metabolically benign overweight/obese individuals are not at increased risk of CVD events compared to normal weight individuals across 3-11 years of follow-up, [1, 2, 5] but report an increased risk over more extended follow-up [6]. Our finding of a greater burden of subclinical atherosclerosis in metabolically benign overweight/obese women compared to healthy normal weight women but less than at-risk overweight/obese women suggests that metabolically benign overweight/obese individuals do have enhanced CVD risk compared to healthy normal weight individuals, but likely develop overt disease more slowly than their at-risk counterparts. It is also possible that the combination of excess adipose tissue and cardiometabolic risk factors is required for progression of subclinical CVD to clinical CVD events. It may be that in the absence of this combination, individuals with metabolically benign overweight/obesity continue to be at a lower risk of CVD events compared to at-risk overweight/obese individuals even over extended follow-up. The Nurses Health Study and Health Professionals Follow-up Study data did not account for changes in risk factor status over the 16-year follow-up period. It may have been that obese individuals who remained metabolically benign may not have experienced any increased CVD risk compared to normal weight individuals, even across the extended follow-up. Further study of the progression of subclinical atherosclerosis to clinical CVD events among these phenotypes is needed.
Insulin resistance resulting from visceral adiposity is proposed to be the driving force behind CVD development in subjects with the metabolic syndrome [20-24]. We found that women with metabolically benign overweight/obesity had intermediate levels of visceral adiposity compared to the at-risk overweight/obese and normal weight phenotypes. However, additional analyses adjusting for VAT, redefining obesity based on waist circumference rather than BMI, or adding HOMA-IR as a marker of insulin resistance to the cardiometabolic abnormalities to define the phenotypes did not alter the differences in subclinical disease measures seen between the three groups. This suggests that the conglomeration of cardiometabolic risk factors is more important in determining the subclinical CVD burden among phenotypes than presence of visceral adiposity or its resulting insulin resistance alone.
Although African Americans have been shown to have higher levels of cIMT and aPWV compared to Caucasians, they have been found to have lower CAC scores.[25] [26] We did not find significant effect modification of body size phenotype associations by race for any of the measures of subclinical atherosclerosis except CCA-IMT, where-by metabolically benign overweight/obese African American women had intermediate CCA-IMT values between normal weight and at-risk overweight/obese, while metabolically benign overweight/obese Caucasian women had CCA-IMT values as high as at-risk overweight/obese women. Given the number of interaction terms which were tested and the sample size, it is quite possible that this finding resulted from chance, and should be interpreted with great caution. Future studies are warranted to examine potential race differences in the subclinical atherosclerotic burden among metabolically benign overweight and obese women.
Our results need to be interpreted in light of certain limitations. Our cross-sectional analysis cannot be used to determine causation. The lack of normal weight women with clustering of cardiometabolic risk factors led to the exclusion of this group from our analyses, limiting our knowledge about this important phenotype. In addition, using BMI as a measure of overweight and obesity has limitations, as it cannot distinguish between fat and lean tissue. However, additional analyses using waist circumference as a surrogate measure of abdominal adiposity and accounting for VAT, associations between the body size phenotypes and subclinical disease measures were similar. As our cohort consisted of women in midlife, the results may not be generalizable to younger women or men. The predictive strength of family history of early heart disease in CHD risk is well known.[27] Although family history of early heart disease was not collected in the SWAN cohort, we did not find a significant difference in family history of overall heart disease between the three body-size phenotypes.
Despite these limitations, our study has several strengths. Unlike previous studies, we present data on both structural and functional markers of subclinical disease, and the consistency of our results across these multiple markers ensures the plausibility of our results. In addition, we have used a definition similar to that utilized in examinations of the clinical CVD event rate in metabolically benign obese individuals allowing for comparison of results. Further, by defining the phenotypes based on the metabolic syndrome criteria with the addition of a marker of systemic inflammation, we offer a more complete and clinically relevant model to identify those likely to have a greater subclinical CVD burden. Finally, unlike most of the few previous studies, we include comparisons between metabolically benign overweight/obese individuals with both normal weight and at-risk overweight/obese individuals.
In summary, it appears that despite published data indicating a similar 3-11 year CVD event rate among metabolically benign obese individuals and normal weight individuals, women in midlife with metabolically benign overweight/obesity participating in SWAN have an intermediate burden of subclinical CVD, with significantly higher CCA-IMT and aPWV levels as well as greater prevalence of calcification compared to normal weight women without risk factors and significantly or borderline significantly lower levels of these factors compared to at-risk overweight/obese women. Whether the presence of subclinical disease among individuals with the metabolically benign overweight/obese phenotype leads to an actual increase in risk of clinical endpoints over extended follow-up remains unclear and will require additional prospective studies with collection of both subclinical disease and CVD event data.
Supplementary Material
ACKNOWLEDGMENTS
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The SWAN Heart Study is supported by the National Heart, Lung, and Blood Institute (Grants HL065581 and HL065591). The Chicago site of the SWAN Heart Study is also supported by the Charles J. and Margaret Roberts Trust. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Centers: University of Michigan, Ann Arbor – MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Rachel Wildman, PI 2010; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Marcia Ory 1994 – 2001; Sherry Sherman 1994 – present; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present.
Steering Committee: Chris Gallagher, Chair Susan Johnson, Chair
We thank the study staff at each site and all the women who participated in SWAN.
Conflicts of Interest: The authors have no conflicts of interest to report in relation to the work described in this manuscript.
REFERENCES
- 1.Song Y, Manson JE, Meigs JB, et al. Comparison of usefulness of body mass index versus metabolic risk factors in predicting 10-year risk of cardiovascular events in women. American Journal of Cardiology. 2007;100:1654–8. doi: 10.1016/j.amjcard.2007.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katzmarzyk PT, Janssen I, Ross R, et al. The importance of waist circumference in the definition of metabolic syndrome: prospective analyses of mortality in men. Diabetes Care. 2006;29:404–9. doi: 10.2337/diacare.29.02.06.dc05-1636. [DOI] [PubMed] [Google Scholar]
- 3.Marini MA, Succurro E, Frontoni S, et al. Metabolically healthy but obese women have an intermediate cardiovascular risk profile between healthy nonobese women and obese insulin-resistant women. Diabetes Care. 2007;30:2145–7. doi: 10.2337/dc07-0419. [DOI] [PubMed] [Google Scholar]
- 4.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Archives of Internal Medicine. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [see comment] [DOI] [PubMed] [Google Scholar]
- 5.Kip KE, Marroquin OC, Kelley DE, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109:706–13. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 6.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;2(6 Suppl):51S–209S. [PubMed] [Google Scholar]
- 7.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Archives of Internal Medicine. 2008;168:1609–16. doi: 10.1001/archinte.168.15.1609. [see comment] [DOI] [PubMed] [Google Scholar]
- 8.Sowers MF CS, Sternsfeld B, et al. Design, survey sampling and recruitment methods of SWAN: a multi-center, multi-ethnic community-based cohort of women and the menopausal transition. In: Lobos RMR, Kelsey JL, editors. Menopause: Biology and Pathobiology. Academic Press; San Diego: 2000. [Google Scholar]
- 9.Sutton-Tyrrell K, Mackey RH, Holubkov R, et al. Measurement variation of aortic pulse wave velocity in the elderly. American Journal of Hypertension. 2001;14:463–8. doi: 10.1016/s0895-7061(00)01289-9. [DOI] [PubMed] [Google Scholar]
- 10.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, et al. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women's Health Across the Nation Heart Study. Circulation. 2008;118:1234–40. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 12.Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74:243–52. doi: 10.4065/74.3.243. [DOI] [PubMed] [Google Scholar]
- 13.Thurston RC, Sowers MR, Sutton-Tyrrell K, et al. Abdominal adiposity and hot flashes among midlife women. Menopause. 2008;15:429–34. doi: 10.1097/gme.0b013e31815879cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation. 2005;111:1242–9. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . Obesity: Preventing and Managing a Global Epidemic. Report of a WHO Consultation on Obesity. World Health Org; Geneva: 1997. [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [see comment][erratum appears in Circulation. 2005 Oct 25;112(17):e297].
- 19.Smith SC, Jr., Greenland P, Grundy SM. AHA Conference Proceedings. Prevention conference V: Beyond secondary prevention: Identifying the high-risk patient for primary prevention: executive summary. American Heart Association. Circulation. 2000;101:111–6. doi: 10.1161/01.cir.101.1.111. [DOI] [PubMed] [Google Scholar]
- 20.St-Pierre AC, Cantin B, Mauriege P, et al. Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. Cmaj. 2005;172:1301–5. doi: 10.1503/cmaj.1040834. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rambaran C, Chowienczyk P, Ritter J, et al. The vascular effects of metabolic impairment clusters in subjects of different ethnicities. Atherosclerosis. 2007;192:354–62. doi: 10.1016/j.atherosclerosis.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Bacha F, Saad R, Gungor N, et al. Are obesity-related metabolic risk factors modulated by the degree of insulin resistance in adolescents? Diabetes Care. 2006;29:1599–604. doi: 10.2337/dc06-0581. [DOI] [PubMed] [Google Scholar]
- 23.Arsenault BJ, Lachance D, Lemieux I, et al. Visceral adipose tissue accumulation, cardiorespiratory fitness, and features of the metabolic syndrome. Archives of Internal Medicine. 2007;167:1518–25. doi: 10.1001/archinte.167.14.1518. [DOI] [PubMed] [Google Scholar]
- 24.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocrine Reviews. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 25.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 26.Newman AB, Naydeck BL, Whittle J, et al. Racial differences in coronary artery calcification in older adults. Arteriosclerosis, Thrombosis & Vascular Biology. 2002;22:424–30. doi: 10.1161/hq0302.105357. [DOI] [PubMed] [Google Scholar]
- 27.Barrett-Connor E, Khaw K. Family history of heart attack as an independent predictor of death due to cardiovascular disease. Circulation. 1984;69:1065–9. doi: 10.1161/01.cir.69.6.1065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.