Abstract
Background
Animal models that explore differential sensitivity to the effects of acute and repeated exposure of alcohol (ethanol) may be influenced by both the developmental and genetic profile of the population. Therefore we sought to compare the influence of ontogeny on sensitivity to ethanol-induced locomotor stimulation and on the induction of locomotor sensitization to this effect across two inbred strains of mice; the ethanol consuming C57BL/6J and the ethanol avoiding DBA/2J strains.
Methods
C57BL/6J and DBA/2J adults (PD 60–80) and adolescents (PD 30 ± 2) were assessed for basal activity, acute response to 2.0g/kg ethanol, and the expression of locomotor sensitization following repeated administration of 2.5, 3.0 or 3.5 g/kg ethanol.
Results
Basal activity was different across development for the C57BL/6J, but not DBA/2J, with adult B6 mice showing persistently greater baseline activity. Adolescents of both strains were more sensitive than adults to acute ethanol-induced locomotor stimulation; adults exhibited a decrease in their acute response across the testing session. Adolescent DBA/2J mice developed less ethanol sensitization compared to adults, with significant sensitization observed only following repeated administration of the lowest ethanol dose (2.5g/kg), whereas DBA/2J adults sensitized to all doses. Age did not influence the development of ethanol sensitization for the C57BL/6J strain, as both adults and adolescents displayed a sensitized response following all ethanol doses.
Conclusions
These results suggest that the developmental pattern of locomotor sensitivity to ethanol is unique to the genotypic profile of the animal model.
Keywords: sensitization, stimulation, ethanol, adolescence, inbred mouse strain
Introduction
Adolescence is a unique period in mammalian development, characterized by a general shift from immaturity and parental dependence to maturity and parental independence. Physiologically, this transition is marked by changes in brain structure, systems, and connectivity; including synaptic pruning, myelination and shifts in neurotransmitter activity (Giedd, 2004; Kellogg, 1998; Spear, 2004). The dramatic changes that occur at this time may underlie the high rates of alcohol use among the members of this age group (Johnston et al., 2007; Miller et al., 2007). A comparison of dependence progression across ontogeny suggests that not only are adolescents more vulnerable to the development of addiction, but they may also exhibit unique responsivity to alcohol. Indeed, a lower level of response per drink (as assessed by self reports and physiological factors) has been associated with greater prevalence of alcohol use disorders in a late adolescent/young adult sample (Schuckit et al., 2004). Although there are no such data in younger/ early adolescent human populations, a number of studies have shown adolescent rats and mice (P21–P59; see Spear 2000) to be less sensitive to a variety of alcohol’s behavioral effects, including the hypnotic effects of high doses of ethanol (Linsenbardt et al., 2008; Silveri and Spear, 1998). The onset of this hypnotic effect may act as a limiting factor for continued alcohol use. Adolescents, therefore, may be physically capable of a unique level of over-consumption; needing more alcohol to achieve a specific drug response due to their lowered sensitivity to some drug effects. On the other hand, adolescents have been shown to be more sensitive to still other alcohol-effects, such as alcohol-induced facilitation of social interaction and stimulant effects of the drug (Varlinskaya and Spear, 2002; Stevenson et al., 2008;). Although both of these effects are thought to represent the positive or reinforcing properties of the drug, other studies do show adolescents may be more sensitive to aversive ethanol effects, like hypothermia (Ristuccia et al. 2007). Thus, much research is still needed to fully characterize alcohol’s behavioral effects during this distinctive developmental period.
In addition to the ontogenetic profile of alcohol responsivity and dependence potential, there exists strong evidence of a genetic component. A number of studies have shown a significant correlation between family history of alcohol abuse and individual dependence in a variety of human populations (Araujo and Monteiro, 1995; Schuckit and Smith, 1997). Animal models of alcohol abuse and consumption elegantly support the human evidence of a genetic component. Data from inbred mouse strains demonstrate the importance of genotype, with some strains readily consuming alcohol and others avoiding it. The C57BL/6J (B6) strain, for instance, shows a strong preference for alcohol in various classic two-bottle choice paradigms, while the DBA/2J (D2) strain avoids the substance (Rodgers and McClearn, 1959; Yoneyama et al., 2008).
The B6 and D2 strains not only show differences in their readiness to consume alcohol, but also in their acute and chronic responses to the drug. The alcohol avoiding DBA/2J strain exhibits a stronger withdrawal response, for example, than the alcohol consuming C57BL/6J strain (Crabbe and Belknap, 1993). The DBA/2J strain also shows a stronger acute and sensitized locomotor stimulant response to alcohol, a particularly interesting genotypic variation due to the relationship between locomotor sensitization and the development of addiction (Crabbe et al., 1982; Tabakoff and Kiianmaa, 1982; Phillips et al., 1994). Sensitization is the augmentation of a drug response, following repeated exposures. Locomotor sensitization has been evidenced for many psychostimulant drugs of abuse, and for a restricted dose range of ethanol (Masur and Boerngen, 1980). This phenomenon might be a manifestation of the neuroadaptations that occur following repeated drug use and may play an important role in the development of drug dependence (Robinson and Berridge, 1993). Specifically, locomotor sensitization is significantly correlated with neurochemical changes in the mesocorticolimbic reward pathway (Phillips and Shen, 1996). For example, repeated alcohol administration has been shown to augment electrically evoked dopamine release in the nucleus accumbens (Nestby et al., 1997). Locomotor sensitization may therefore provide a useful approach for exploring the neurobiological adaptations that occur in response to repeated alcohol exposure.
Recent investigations support a role of ontogeny in the expression of a stimulant response to alcohol as well as the development of sensitization to that response (Hefner and Holmes, 2007; Stevenson et al., 2008). Given the relationship between response to alcohol and family history of alcoholism (Schukit et al., 2006) as well as other clinical evidence of an effect of genetics on age of first alcohol use (McGue et al., 2001; Sartor et al., 2009), one may expect the developmental profile for the induction of sensitization following chronic ethanol exposure to vary based on genetic background. Thus, the present study aimed to investigate the role of ontogenetic and genetic factors in the induction of locomotor sensitization to alcohol. Specifically, we were interested in comparing the development of locomotor sensitization in adult and adolescent C57BL/6J and DBA/2J mice. Given the differences in alcohol response sensitivity noted across adults and adolescents as well as between C57BL/6J and DBA/2J mice, we expected unique alcohol dose requirements for the induction of locomotor sensitization across the groups, reflecting a significant effect of both genotype and ontogeny in this alcohol-induced behavioral phenomenon.
METHODS
Subjects
Male and female DBA/2J (D2) and C57BL/6J (B6) adult (PD 60–80) and adolescent (PD 30± 2) mice bred and housed at the Binghamton University animal facility were used (N= 219 mice). Animals in the follow-up assessment on the temporal pattern of sensitized response (Fig. 6) were of the same age range and genotype, but were bred and housed at Indiana University-Purdue University Indianapolis (N=96 mice). In both cases, breeders were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were weaned at PD 21 and housed with same sex conspecifics, 2–4 per cage. Both vivariums were maintained at 21 ± 1 degrees Celsius and approximately 50% humidity. Food and water were available ad libitum, except during locomotor activity testing. Alcohol (ethanol) was administered intraperitoneally (I.P). All procedures were approved by either the Binghamton University Institutional Animal Care and Use Committee or Indiana University-Purdue University Indianapolis School of Science Institutional Animal Care and Use Committee and were consistent with the Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
Behavioral apparatus
Locomotor activity was monitored in an automated system made of Plexiglass chambers (40 × 40 cm) equipped with eight pairs of photocell beams located 2cm above the chamber floor (Accuscan Instruments, Columbus, OH, USA). Each individual monitoring system was housed in a sound attenuating chamber (53 × 58 × 43cm) and furnished with a house light (50 lux; mounted above) and a fan (mounted on the rear wall) for ventilation. Animals were placed in the chambers for fifteen minutes and consecutive beam breaks were transformed to total horizontal movement (in centimeters), collected in 15 one-minute time-bins. After each test, the chamber was cleaned using a 10% ethanol solution.
Drug
95% Ethanol (Ethanol; Pharmco Products Inc., Brookfield, CT) was diluted with 0.9% physiological saline to a 20% v/v solution that was intraperitoneally administered to animals at varying doses. Alternate dosing was achieved by varying the volume of ethanol solution administered (Linakis and Cunningham 1979).
Procedure
The general sensitization procedure followed Boehm et al. (2008) and is summarized in Table 1. All drug administration and behavioral testing occurred during the animals’ light cycle. Each day, including non-test days, mice were moved to the behavioral testing room and allowed to acclimate for at least 30 minutes. On the first two days of the experiment, mice were intraperitoneally administered 0.9% NaCl (in a volume equivalent to that of a 2.0g/kg ethanol dose) and placed in the activity monitoring system for 15 minutes. These two sessions allowed habituation to the testing environment and injection procedure. Horizontal activity from the second habituation session was used to balance experimental groups. On day 3 (acute test day), animals received an injection of either 20% v/v ethanol at 2.0g/kg or an equivalent volume of physiological saline. Immediately following drug or saline administration, mice were placed in the locomotor activity testing chambers for 15 minutes for assessment of the acute response to ethanol. On days 4–13, mice received daily administrations of physiological saline or 2.5, 3.0 or 3.5 g/kg of 20% ethanol and were immediately returned to their home-cage (not tested in the chambers). These repeated doses were chosen in order to span both the stimulant and sedative ethanol dose ranges. Given the differences in sensitivity to sedation across the strains and ages used, we hoped to take advantage of the development of tolerance to the hypolocomotor producing effects of ethanol, in order to reveal a sensitized stimulant response, following the low challenge dose. On day 14, all mice received a challenge of 2.0g/kg ethanol and were placed in the activity monitoring system for 15 minutes to assess the locomotor sensitized response. Retro-orbital sinus bloods (25µL) were collected immediately following this 15-minute test. Blood samples were centrifuged and plasma supernatant was stored at −20°C. Plasma was later analyzed using an Analox Ethanol Analyzer (Analox Instruments, Lunenburg, MA) and blood ethanol concentration recorded as mg/dL.
Table 1.
Locomotor Sensitization Paradigm
| Day | 1–2 | 3 | 4–13 | 14 |
|---|---|---|---|---|
| Treatment | Habituation | Acute Response | Repeated Administration | Sensitized Response |
| Drug | Saline | EtOH (2.0 g/kg) or Saline |
EtOH (2.5, 3 or 3.5 g/kg) or Saline |
EtOH (2.0 g/kg) |
| Chamber? | Yes | Yes | No | Yes |
Statistical Analyses
Data were compared using Analysis of Variance (ANOVA). Each ANOVA included sex as a factor (n=5–10 per sex/age/dose/genotype). This variable was subsequently collapsed upon, as there were no significant interactions between sex and any other factors across analyses. A three-way mixed factor ANOVA was used to analyze baseline activity across the two habituation sessions, with age (adolescent vs. adult), genotype (B6 vs. D2) and day (day 1 vs. day 2) as independent variables. Acute locomotor response to ethanol was analyzed using a three-way ANOVA (age X genotype X treatment). Acute response to ethanol was also assessed as a simple change from baseline activity (day 2 vs. day 3) using a four-way mixed factor ANOVA (age X genotype X treatment X day). Sensitization was defined as a significant increase in ethanol response following repeated administration of the drug when compared to initial ethanol response (within group) as well as compared to acute ethanol controls (between group). These sensitization data were analyzed using four-way mixed factor ANOVA with drug dose (0, 2.5, 3 or 3.5 g/kg), age (adolescent vs. adult), genotype (B6 vs. D2) and day (Day 3 vs Day 14), as factors. Given the temporal restriction of the expression of ethanol induced stimulation in the B6 strain noted here and elsewhere (Middaugh et al., 1992; Hefner and Holmes 2007), sensitization was also reassessed using only the first five minutes of activity in the chamber, following the same statistical procedures used for the total 15 minutes of testing, described earlier. Tukey’s post hoc test was used, when applicable, to explore significant interactions. All statistical analyses were carried out using the Statistica Version 7 statistical package (Tulsa, OK, USA). Results were considered significant at p<0.05.
RESULTS
Effect of age on baseline activity
Average total distance traveled for adolescent and adult B6 and D2 strains can be seen in Table 2. A repeated measures ANOVA of total 15 minute activity across the 2 habituation sessions revealed a main effect of day [F(1,215)=264.8 p<0.0001], as all mice decreased activity across habituation sessions. A main effect of age was also detected [F(1,215) = 28.5 p < 0.0001], with adults showing a greater pattern of activity than adolescents. The three-way interaction of habitation session, age and genotype [F(1,215)=22.0 p< 0.0001], clarified that the main effect of age was driven mainly by differences in the B6 strain. Specifically, while D2 adults and adolescents showed no statistical difference in baseline activity across the two habituation sessions, B6 adults had greater activity on the first (p<0.0001) and second (p<0.05) sessions, than B6 adolescents. In order to clarify the level at which this developmental difference in baseline may confound analysis of the acute response to ethanol (Day 3), activity of all mice receiving saline on this day were compared. This analysis did not reveal any developmental effect on activity for either genotype.
Table 2.
Baseline Activity
| DAY | 1 | 2 | 3 | |
|---|---|---|---|---|
| D2 | Adolescent | 3617.6 ± 169.4 | 2837.8 ± 119.2 | 2660.9 ± 243.4 |
| Adult | 3427.7 ± 167.8 | 3000.1 ± 135.3 | 2534.7 ± 155.6 | |
| B6 | Adolescent | 3256.2 ± 129.8 | 2150.3 ± 82.3 | 2095.3 ± 157.0 |
| Adult | 4934.3 ± 129.3* | 2948.8 ± 86.4* | 2232.3 ± 133.9 | |
Asterisk indicates greater activity (p<0.05) as compared to respective adolescents.
Acute response to 2.0g/kg EtOH
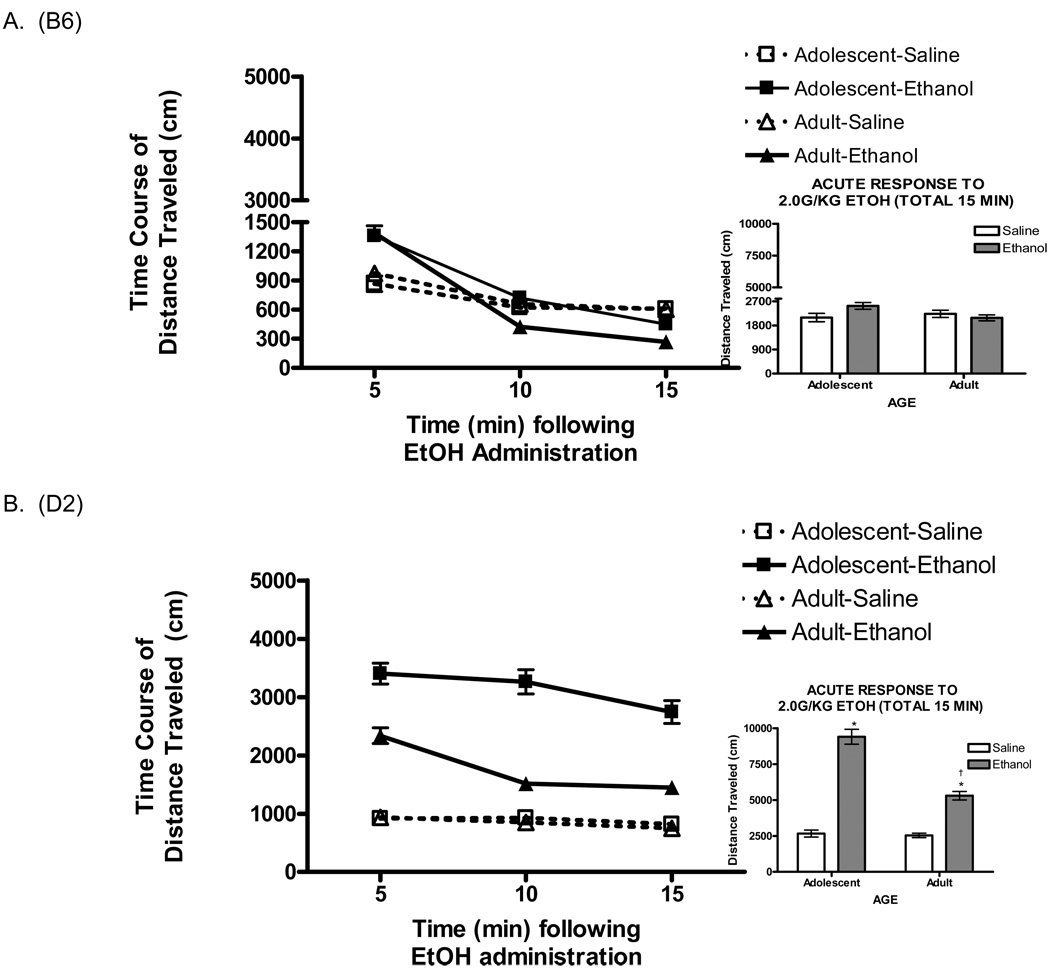
Acute response to ethanol was defined as a significant difference in activity for animals administered 2.0g/kg ethanol, compared to animals administered saline (Fig. 1a and b, insets). A 3-way ANOVA revealed a significant main effect of acute drug [F (1,211) = 116.9 p<0.0001], reflecting the stimulant property of the 2.0g/kg dose of ethanol. There was a main effect of genotype [F(1,211)=141.8 p<0.0001], as D2’s showed a greater locomotor response than B6’s, and age [F(1,211) = 24.1 p <0.0001], with adolescents showing greater activity than adults. Additionally, there was a significant 3 way interaction of age × genotype × treatment [F (1,211) = 11.8 p<0.001]. Post hoc tests confirmed that only D2 mice (both adolescents and adults) exhibited a significant increase in locomotion following ethanol administration. Furthermore, consistent with the literature, D2 adolescents displayed a greater ethanol response than D2 adults.
Fig. 1.
Temporal pattern of locomotor activity shown as 5 minute time bins ± SEM for B6 and D2 adults and adolescents. All animals displayed significant stimulant response in the initial five minutes of testing following ethanol administration. a) Average locomotor response fell below saline level in the final ten minutes for B6 adults and in the final five minutes for B6 adolescents. Total activity across the 15 minute test (inset) show that B6 mice do not show an overall stimulant response when activity is collapsed across the 15 minutes. b) D2 adults also show a decrease in activity five minutes after ethanol administration, but both D2 adults and adolescents stay above saline response for the entirety of the test. When total activity is collapsed (inset), D2 adolescents show a greater stimulant response than D2 adults.
Previous work by Hefner and Holmes (2007) demonstrated a complex interaction between age and the temporal pattern of ethanol-induced locomotion in adolescent and adult B6 mice. We therefore plotted the acute response to ethanol or saline on day 3, as 5-minute time bins, and analyzed it by a 4-way mixed factor ANOVA (Fig. 1a and b). We found a main effect of time [F (2,422) = 100.2 p< 0.0001] as well as a significant interaction between time and treatment [F (2, 422) = 36.1 p<0.0001], reflective of the change in ethanol-induced activity across time. Furthermore, there was a significant interaction of time, treatment and age [F (2, 422) = 3.4 p<0.01]. Surprisingly, there was no time × age × treatment × genotype interaction, despite the apparent differences in acute ethanol responses across age, genotype, and time. Post hoc analyses established that, whereas adults exhibited ethanol-induced increases in activity only during the first five minutes (p<0.0001), adolescents displayed a significant stimulant response during the first (p<0.0001), second (p<0.0001) and third (p<0.01) five minute bins.
Effect of Dose on development of sensitization
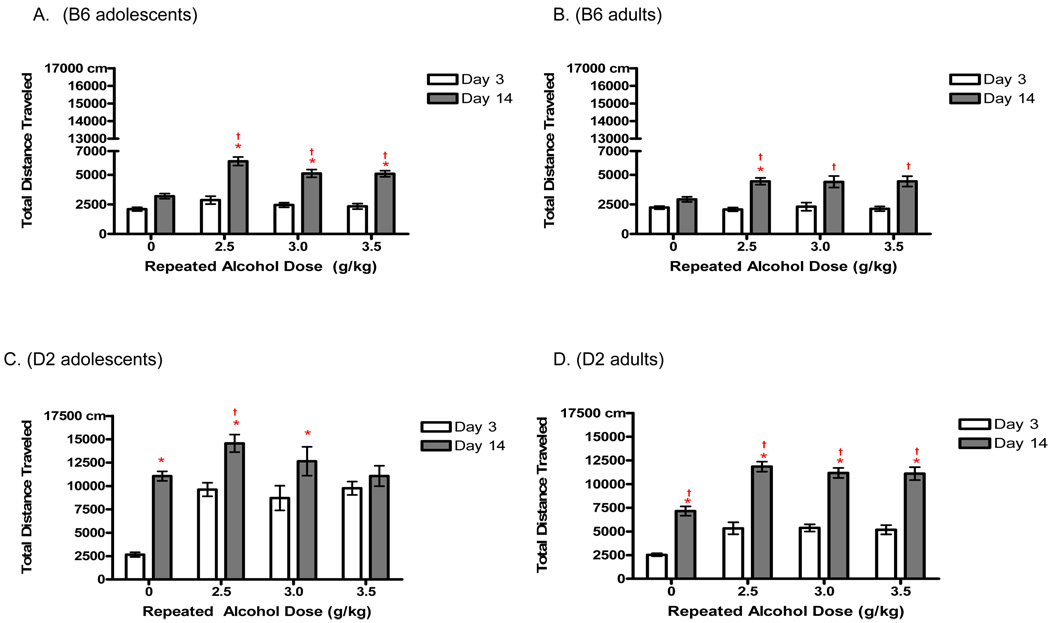
Locomotor sensitization is classically defined as a significant enhancement in ethanol-induced locomotion following repeated administration of the drug. Therefore, our initial assessment compared activity on Day 14 to activity on Day 3 in a 4-way mixed factor ANOVA (Fig. 2). This analysis revealed main effects of age [F(1, 203)= 51.7 p< 0.0001], genotype F(1, 203)= 620.1 p< 0.0001], dose F(1, 203)= 51.7 p< 0.0001], and day [F(1, 203)= 551.7 p <0.0001], as well as a four-way interaction of these factors [F(3, 203) = 9.5 p < 0.0001]. Post hoc tests revealed different dose × age interactions across the strains. Specifically, B6 adults appeared to exhibit a sensitized response (day 14 vs. day 3) only following repeated administration of the lowest dose (2.5g/kg, p<0.05), whereas B6 adolescents developed a significantly greater response following repeated administration of all doses (p’s < 0.01). Although D2 adults developed a sensitized response following repeated administration of all doses (p<0.0001), D2 adolescents demonstrated a sensitized locomotor response following repeated administration of all but the highest ethanol dose (3.5g/kg).
Fig. 2.
Development of locomotor sensitization following repeated administration of ethanol (2.5, 3.0 or 3.5 g/kg; n’s=10–15) or saline (n’s=18–22) ± SEM in D2 and B6 mice. a-b) B6 adults showed an effect of day only following repeated administration of 2.5g/kg (*, p<0.05), whereas B6 adolescents showed this within-subjects sensitization effect following all doses (*, p’s<0.05). c-d) D2 adults showed within-subjects (*) and between-group (†) sensitization following all doses (p’s<0.001), whereas D2 adolescents exhibited an effect of day following 2.5g/kg and 3.0g/kg (p’s<0.001) and between group sensitization following 2.5g/kg only (p<0.01).
Although a within-subjects analysis is classically used to statistically demonstrate sensitization, confounds specific to a developmental analysis of the phenomenon make it more difficult to interpret such an analysis. Specifically, the adolescents are aging during the 14-day sensitization procedure, maturing through a large portion of adolescence before the conclusion of the repeated exposure procedure. Therefore, the between subjects analysis of day 14 data may provide a more valid assessment of our developmental questions. The between subjects analysis did not support the same effect of development for both genotypes. For B6 mice there was a significant effect of dose [F(3,102)=24.8 p<0.0001] and no interaction of dose and development, as both adolescents and adults displayed a sensitized response at all ethanol doses (p’s<0.05), even when maturation occurring during the sensitization process is accounted for. In contrast, D2 adolescents no longer exhibited an increase in activity at the 3.0g/kg dose; there was only a significant difference between the repeated-2.5g/kg group and repeated-saline controls (p<0.01). D2 adults continued to display a sensitized locomotor response to ethanol at all the repeated doses tested.
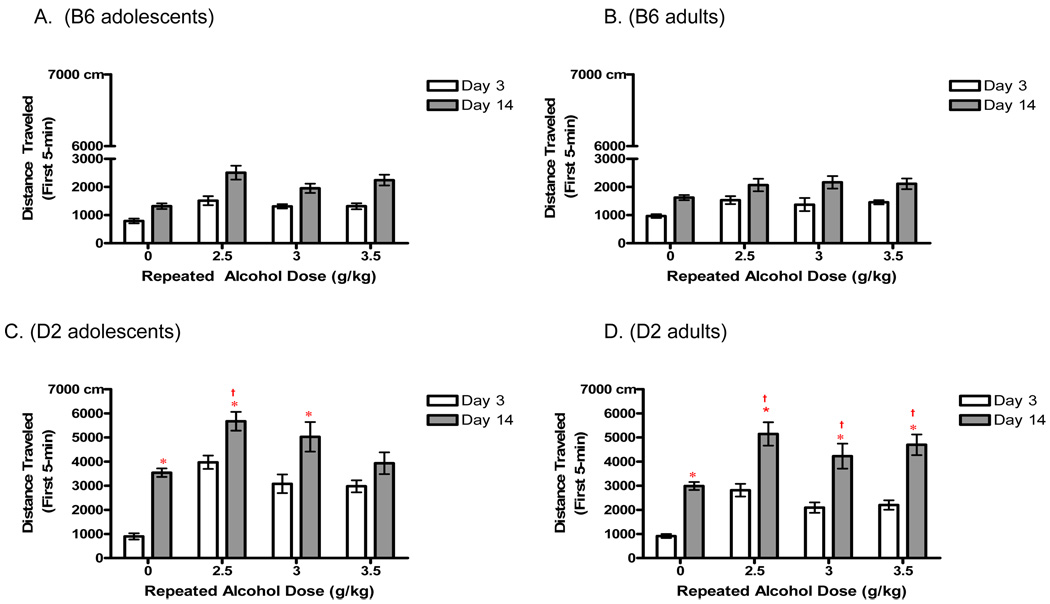
During the acute administration of 2.0 g/kg Ethanol (Day 3), the initial five minutes of activity was the only time period during which we consistently saw a stimulant response across both ages and genotypes. Therefore, we re-assessed between groups sensitization using only the initial five minute activity bin on day 14 (Fig. 3). This analysis revealed main effects of genotype [F (1, 203) = 288.0 p < 0.0001], dose [F (3,203) = 21.7.0 p <0.0001] and a dose × genotype interaction [F(3,203) = 6.2 p<0.001], with the D2 strain displaying a sensitized response at all doses of ethanol (p<0.01; compared to saline). B6 mice did not develop a significant sensitized response to any dose in this analysis. Due to the significant difference in the response to 2.0 g/kg ethanol challenge for B6 mice as compared to D2 mice, a separate analysis was carried out, for each genotype. This simple 2-way ANOVA (age × dose) clarified that B6 mice indeed exhibit a significant sensitized response following all doses of repeated ethanol [main effect of dose, F(3,102) = 8.2 p<0.0001] when compared to ethanol challenge in the repeated-saline animals (p’s<0.0001). The D2 mice again show a significant main effect of dose [F(3,101) = 15 p<0.0001], with all repeated-ethanol dosing groups exhibiting greater ethanol-stimulated locomotion compared to repeated-saline mice (p’s <0.01). Neither assessment (for either inbred strain) revealed an effect of development on the effectiveness of any of the doses in inducing sensitization, when only the first five minutes of activity is analyzed.
Fig. 3.
Initial five minutes of activity was isolated in order to re-assess sensitization to the locomotor stimulating effects of ethanol (as this was the only time period during which adults and adolescents both showed ethanol-induced hyperlocomotion following acute administration: see Fig. 3). a-b) There was no evidence of sensitization for B6 adolescents or adults. c-d) D2 adults showed increased activity when comparing Day 14 to Day 3 for all repeated doses (* ; p’s <0.0001), and adolescents for all doses (p’s<0.001) except 3.5g/kg. D2 adults showed a significant difference between repeated saline and repeated ethanol administration at all doses († ; p’s<0.001), whereas D2 adolescents only show a significance following the lowest dose of repeated ethanol administration (2.5g/kg; p<0.0001).
Blood Ethanol Concentrations
Three way analysis of blood ethanol concentrations (Table 2) following administration of 2.0g/kg ethanol on the final day of the sensitization procedure revealed main effects of genotype [F(1, 200) = 4.7 p <0.05] and dose [F(3, 200) = 7.6 p<0.0001]. Specifically, the repeated-saline group exhibited higher BECs than the repeated-3.0g/kg and −3.5g/kg groups, and D2 mice exhibited higher BECs than B6 mice. There was, however, no effect of age nor were there any interactions.
Temporal Pattern of Sensitized Response
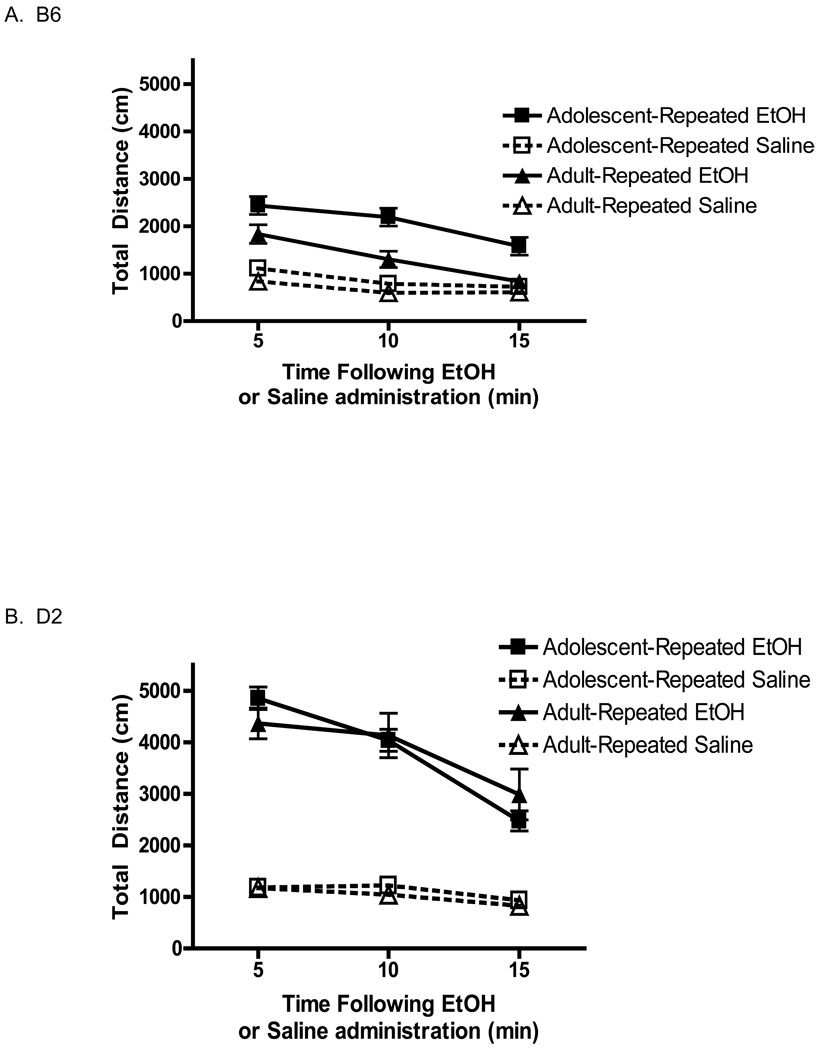
The acute response (see Fig. 1) indicated a different temporal pattern of locomotion in response to ethanol between adolescent and adult mice, with the adult response waning after the first 5 min of the locomotor activity test. Indeed, this waning acute locomotor stimulant response manifests as sedation or hyperlocomotion in the B6 adults (activity significantly below baseline levels). This effect was not seen for B6 adolescents, however. Therefore, we ran a separate study to determine the pattern of the day 14 locomotor sensitized responses in adolescent and adult B6 and D2 mice. A separate cohort of mice was repeatedly administered either saline or 2.5 g/kg ethanol (a dose that resulted in a sensitized response to 2.0g/kg in all groups; see Fig. 3). Acute response for this cohort (data not shown) was comparable to the pattern described earlier (Fig. 1). Analysis of activity following repeated administration of 2.5g/kg or saline revealed an interaction of Time × Genotype × Age × Treatment [F(2,176) = 5.5 p<0.01], indicating a change in time course of activity for both adult and adolescent B6 mice. Similar to their response during the previous acute test, adult B6 mice only displayed a significant stimulant response during the initial five minutes (Fig. 4A). However, unlike their acute response, these animals (having received repeated administrations of 2.5g/kg) did not show a hypolocomotor response, never falling below baseline at any time-bin. A similar pattern was noted for D2 adults (Fig. 4B), who demonstrated a significant stimulant response at each time-bin; no longer exhibiting a latent decrease in their stimulant response following the first five minutes of testing. D2 adolescents continued to show a stable increase in activity across the testing period (at each 5-min time bin). BEC’s analyzed following this experiment replicated the lack of developmental effect on blood ethanol concentration following repeated 2.5g/kg and challenge dose of 2.0g/kg noted above (data not shown).
Fig. 4.
B6 (a and b) and D2 (c and d) mice were repeatedly administered ethanol (2.5g/kg) or saline and then challenged with ethanol or saline, respectively. This analysis shows an attenuation of the transient hypolocomotion noted for B6 adults during the final ten minutes of their acute response.
DISCUSSION
Neurobiological changes associated with adolescence are suggested to be responsible for the variation in addiction potential during this developmental period, with adolescent rodents and humans exhibiting unique physiological responses to drugs of abuse when compared to adult conspecifics. With respect to ethanol, studies have been inconclusive; adolescents have been shown to be more sensitive to certain effects of the drug, and less sensitive to other effects. The present results suggest that these ontogenic effects can be influenced by genetic factors, with the B6 and D2 inbred mouse strains exhibiting unique patterns of developmental differences in the effects of acute and repeated administration of ethanol on locomotor stimulation.
Consistent with the results of previous findings (Stevenson et al., 2008), D2 adolescents were more sensitive than adults to the acute locomotor stimulant effects of 2.0g/kg ethanol. This was not the case with the ethanol consuming B6 strain. Although B6 adolescents displayed a significantly greater ethanol response when compared to that of B6 adults, this activity was not significantly greater than their saline controls. Therefore, greater sensitivity to acute ethanol stimulation in adolescents was unique to the D2 strain.
Previous work on ethanol-induced motor activity in the B6 strain suggested a stimulant effect should be noted in the first few minutes following drug administration (Crabbe et al., 1982; Hefner and Holmes 2007; Lessov et al., 2001; Middaugh et al., 1992). Indeed, examination of the time course for the acute ethanol response clarified that B6 adults and adolescents displayed a similar stimulant response in the initial five minutes of behavioral testing. The D2 inbred strain on the other hand, maintained the effect of age, with adolescent activity appearing greater than that of adults in this same time period.
The above analysis also revealed an interesting temporal pattern of activity in the final two 5-minute time bins, with adults failing to show significantly greater ethanol response during this 10-minute period. As shown in Fig. 1, locomotion following 2.0 g/kg for B6 adults actually fell 217 and 335 cm below that of the saline controls during the 2nd and 3rd five minutes of the activity test respectively, suggesting what could be considered a transient sedative, or hypolocomotor response. Locomotor activity for B6 adolescents following 2.0g/kg ethanol fell below baseline (activity for saline group) only for the final five minutes of testing. This is again consistent with work by Hefner and Holmes (2007), where adolescent B6 mice exhibited an ethanol-induced decrease in activity (9–12 minutes following the 1.5 g/kg dose used by those investigators), but to a lesser degree than adults. In that study, adult B6 mice also displayed a sedative response that began earlier (6 minutes following intraperitoneal administration) and lasted for the entire duration of activity monitoring. An overall assessment of the total activity following ethanol administration (described earlier), and this temporal pattern of ethanol-induced activational changes suggests that, while the D2 adolescents were unique in their heightened sensitivity to ethanol-induced stimulation, adolescents of both genotypes were less sensitive to ethanol-induced sedation, or hypolocomotion. This could be reflective of genotypic differences in the maturation of neural substrates underlying the stimulant response to 2.0g/kg ethanol (with B6 mice exhibiting a more precocious pattern of development) not seen for the maturation of neural substrates underlying reduced activity following this dose.
In as much as an increase in locomotor activity can be associated with hedonic reward (Wise and Bozarth, 1987), our acute locomotor data suggests that D2 adolescents are more sensitive to the rewarding effects of ethanol administration than their adult counterparts. B6 inbred mice do not show this age effect. Instead, B6 adolescents may be less sensitive to the sedative effects of ethanol than B6 adults. Although the result of different mechanisms, one would expect both these developmental differences to increase consumption in adolescent B6 and D2 mice. Indeed, we have previously shown B6 adolescents to consume more ethanol than their adult counterparts (Moore et al., 2010). However, we were unable to consistently find that pattern for D2 adolescents. Furthermore, a recent study by Dickinson and colleagues showed that D2 adolescents were unable to form a conditioned place preference for an environment paired with 2.0g/kg ethanol (Dickinson et al., 2009). Therefore, the consistent finding of greater sensitivity to acute locomotor effect of ethanol in D2 adolescents compared to adults may not necessarily be reflective of developmental differences in the rewarding effects of ethanol in the adolescent members of this strain.
Sensitization, the augmentation of a drug response following repeated exposures, is suggested to be a behavioral manifestation of the neural adaptations associated with multiple administrations of drugs of abuse, including ethanol (Phillips and Shen, 1996; Robinson and Berridge, 1993). Therefore, variations in sensitivity to the development of locomotor sensitization may be indicative of variations in the addictive potential of ethanol. Furthermore, as previous work suggests a positive relationship between the development of sensitization and later voluntary drinking of ethanol in the strains used (Lessov et al., 2001), the interaction of genetic and ontogenetic differences in the development of this phenomenon could underlie individual differences in the vulnerability to develop maladaptive drinking problems, following adolescent exposure.
Although D2 adolescents (compared to D2 adults) demonstrated greater initial response to an acute dose of ethanol in this and other studies (Stevenson et al., 2008), they displayed a more restricted dose range requirement for the induction of locomotor sensitization. In the present study, a robust sensitized response was observed in D2 adults at each of the 2.5, 3.0 and 3.5 g/kg doses, whereas D2 adolescents demonstrated a sensitized response at the 2.5 and only a modestly greater response following the 3.0 g/kg dose. This could be due to increased sensitivity to the sedative-hypnotic effects associated with the higher dose (3.5g/kg) of ethanol that was repeatedly administered for eleven days. This may appear contrary to previous work in rats (Silveri and Spear, 1998) that show adolescents are less sensitive to such effects of ethanol. However, recent work from our laboratory has shown that adolescent B6 and D2 mice develop chronic tolerance to ethanol-induced loss of righting reflex to a lesser degree than adults (Linsenbardt et al., 2009). Therefore, adolescents may be less sensitive to the sedative effects of acute ethanol, as well as less apt to develop tolerance to this effect following repeated exposures. The difference between D2 adults and adolescents in the development of sensitization at various doses could be due to the very nature of the experimental procedure; adolescents are being administered ethanol for almost the entire duration of the adolescent developmental period (as conservatively defined, see Spear, 2000). Therefore, it may be the case that the higher doses of ethanol perturbed, for the D2 adolescents, the development of one or multiple neurobiological systems associated with ethanol’s psychomotor effects. For example, blockade of the GABAB system has been associated with disruption of the induction of locomotor sensitization (Boehm et al., 2002; Broadbent and Harless, 1999). Although postsynaptic GABAB have been shown to become responsive to GABA early in postnatal life (Cherubini et al., 1991), during adolescence, the system may only be functional in certain regions (Lopéz-Bendito et al., 2004), while still undergoing dramatic changes (Cunningham et al., 2007). Our design repeatedly exposed the mice to ethanol early through late adolescence (from PD 30± 2 to PD 44± 2); therefore, our results could be related to genetic differences in the maturation of multiple systems. Additionally, there could be genetic and ontogenetic differences in the effect of context on the development of sensitization. For example, work by Faria and colleagues (2008), suggests that adolescents may display tolerance to ethanol induced stimulation following contextual pairings, while adults in their paradigm have shown the opposite effect. In our study, the context was not paired with repeated administrations (on days 4 through13, mice were injected and immediately returned to their home-cages). Furthermore, the animals experienced two drug-free sessions in the chamber, prior to ethanol exposure. Thus, we hope that the design used in our experiments should result in reduced drug-context conditioning, given the possibility of developmental differences to that effect. Finally, although we did not find any interaction of our variables (age, genotype and dose) on the blood ethanol concentration at the end of the experiment, we cannot dismiss the possibility that genotypic and age differences in the pharmacokinetic response at any time before the end of our sensitization paradigm may underlie some of our results, such as the lack of sensitization seen for D2 adolescents in the higher repeated dose group.
It is important to note that, although B6 adolescents also appear more sensitive to the development of sensitization given the initial statistical analysis presented (4 way ANOVA), separating the data by genotype clarifies that B6 adults also show a similar increase in activity following all doses (in fact, there is no interaction of dose and age; we find a significant increase in activity for both B6 adults and adolescents, at all doses tested). Furthermore, a between subjects analysis of the data (arguably a more valid statistical assessment for use in long-term/developmental studies) strongly supports the development of sensitization following all doses in B6 adults and adolescents. We therefore conclude that, unlike their D2 counterparts, B6 mice did not exhibit an effect of development on the induction of sensitization.
To determine the extent to which the expression of sensitization on day 14 was a direct consequence of enhanced sensitivity to ethanol’s locomotor stimulant actions (and not tolerance to the ethanol induced hypolocomotion seen in the final ten minutes of testing), a separate analysis of the time course of ethanol-induced locomotion on day 14 was performed. We were particularly interested in whether B6 adults developed significant sensitization during this time frame as it was the only period during which this group displayed locomotor stimulation following acute challenge on day 3. Of note, the B6 adolescent locomotor response also fell below basal levels during the final five minutes of testing on day 3, so we were also interested in determining the extent of actual increases in locomotor stimulant sensitivity among the members of this group. Interestingly, B6 adults and adolescents exhibited no significant evidence of sensitization following this analysis. These results suggest that the change in activity reflected by the overall analysis (total fifteen-minute testing period) might be due, at least in part, to shifts in the locomotor response curve during the final ten minutes of the behavioral test. These data may call into question whether appreciable sensitization develops at all in this strain; B6 adults and (to a lesser extent) adolescents may instead be developing tolerance to ethanol’s sedative effects. Indeed, the data shown in Figure 4 (where a separate cohort of mice received saline for the entire procedure, including the challenge test) supports this interpretation; the transient hypolocomotor response that appears in the final ten minutes of activity testing for B6 adults and adolescents, respectively, is no longer observed.
In essence, the work presented here supports a dynamic interaction between genetic and ontogenic factors in the development (or lack of development) of sensitization to the locomotor stimulating effects of ethanol. Our results support similar developmental profiles for acute ethanol responsivity across the strains, but dissimilar developmental profiles for the effects of repeated administration. Acutely, we observed evidence (albeit marginal for the B6 adolescents) of greater sensitivity to ethanol-induced locomotion for adolescents of both strains. Conversely, following repeated administration of the varied doses of ethanol, D2 adolescents displayed a more restricted dose range effective in the induction of sensitization than D2 adults (perhaps reflective of increased sensitivity to the aversive effects of the high doses for the D2 adolescents). Whereas age played a role in both the acute stimulant response to ethanol and the dose required to develop sensitization to this drug effect, the specifics of this role (i.e. increased vs. decreased sensitivity) was different between the two strains. This suggests that genetic variation may be responsible for different rates of neurobiological development (such as maturation of reward pathway neurocircuitry), or dissimilarities in the systems that interact with ethanol. Future work will use genetic differences in the development of sensitization during adolescence or adulthood to explore the role that genotype plays in the effect of adolescent ethanol pre-exposure on adult drinking. To this end, it will be important to include a complete assessment of genotype as well as sex. Because sex did not interact with any of our initial variables of interest, this factor was collapsed upon. However, we recognize that, in doing so, we were ignoring an important mediator of individual variability to drug response. Finally, we hope to also take advantage of the effect of genotype on the ontogenetic profile of ethanol-induced locomotor sensitization to explore the mechanisms behind this phenomenon.
Table 3.
Blood Ethanol Concentration
| 0 g/kg | 2.5 g/kg | 3.0 g/kg | 3.5 g/kg | |||
|---|---|---|---|---|---|---|
| ADULT | D2 | BEC (mg/dL) | 222.5 ± 15.3 | 186.6 ± 14.3 | 185.4 ± 3.9 | 172.4 ± 14.8 |
| n | 21 | 14 | 12 | 12 | ||
| B6 | BEC | 186.9 ± 15.9 | 186.3 ± 29.3 | 159.4 ± 28.5 | 174.6 ± 14.8 | |
| n | 19 | 15 | 9 | 9 | ||
| ADOLESCENT | D2 | BEC | 235.9 ± 11.6 | 228.7 ± 14.1 | 166.0 ± 17.6 | 173.7 ± 11.9 |
| n | 13 | 15 | 10 | 10 | ||
| B6 | BEC | 189.2 ± 13.8 | 208.3 ± 9.2 | 171.9 ± 11.9 | 169.2 ± 14.5 | |
| n | 18 | 12 | 15 | 12 | ||
Although analysis of the blood ethanol concentration following the challenge dose of 2.0g/kg revealed no interaction of age, genotype and dose, there was a main effect of genotype and dose (p’s <0.05). Specifically, the repeated-saline group demonstrated a greater BEC than the 3.0g/kg and 3.5g/kg groups, and D2 mice exhibited greater BEC’s than B6 mice.
Acknowledgments
This work was supported in part by grants from The National Institute on Alcohol Abuse and Alcoholism (AA015434, AA016789, and AA017849) and the Center for Development and Behavioral Neuroscience at Binghamton University.
References
- Araujo N, Monteiro M. Family history of alcoholism and psychiatric co-morbidity in Brazilian male alcoholics and controls. Addiction. 1995;90:1205–1211. doi: 10.1046/j.1360-0443.1995.90912055.x. [DOI] [PubMed] [Google Scholar]
- Boehm SL, II, Goldfarb KJ, Serio KM, Moore EM, Linsenbardt DN. Does context influence the duration of locomotor sensitization to ethanol in female DBA/2J mice? Psychopharmacology (Berl) 2008;197:191–201. doi: 10.1007/s00213-007-1022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, II, Piercy M, Bergstrom H, Phillips TJ. Ventral tegmental area region governs GABAB receptor modulation of alcohol-stimulated activity in mice. Neuroscience. 2002;115:185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Bonomo YA, Bowes G, Coffey C, Carlin JB, Patton JC. Teenage drinking and the onset of alcohol dependence: a cohort study over seven years. Addiction. 2004;99:1520–1528. doi: 10.1111/j.1360-0443.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Harless W. Differential effects of GABA(A) and GABA(B) agonists on sensitization to the locomotor stimulant effects of alcohol in DBA/2 J mice. Psychopharmacology (Berl) 1999;141:197–205. doi: 10.1007/s002130050825. [DOI] [PubMed] [Google Scholar]
- Camarini R, Griffin WC, III, Yanke A, Rosalina dos Santos B, Olive MF. Effects of adolescent exposure to cocaine on locomotor activity and extracellular dopamine and glutamate levels in nucleus accumbens of DBA/2J mice. Brain Res. 2007;1193:34–42. doi: 10.1016/j.brainres.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. 12. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK. Behavior genetic analyses of drug withdrawal. Alcohol Alcohol Suppl. 1993;2:477–482. [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Increasing interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb Cortex. 2007;18:1529–1535. doi: 10.1093/cercor/bhm183. [DOI] [PubMed] [Google Scholar]
- Dickinson SD, Kashawny SK, Thiebes KP, Charles DY. Decreased Sensitivity to Ethanol Reward in Adolescent Mice as Measured by Conditioned Place Preference. Alcohol Clin Exp Res. 2009;33:1246–1251. doi: 10.1111/j.1530-0277.2009.00950.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of alcohol across adolescence in mice. Psychopharmacology (Berl) 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biol Psychiatry. 2000;48:265–275. doi: 10.1016/s0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. National Institute on Drug Abuse. Bethesda, MD: 2007. Monitoring the Future National Survey Results on Drug Use, 1975–2006, Volume 1, Secondary School. [Google Scholar]
- Kellog CK. Early developmental modulation of GABAA receptor function. Influence on adaptive responses. Perspect Dev Neurobiol. 1998;5:219–234. [PubMed] [Google Scholar]
- Lessov C, Palmer A, Quick E, Phillips T. Voluntary alcohol drinking in C57BL/6J and DBA/2J mice before and after sensitization to the locomotor stimulant effects of alcohol. Psychopharmacology (Berl) 2001;155:91–99. doi: 10.1007/s002130100699. [DOI] [PubMed] [Google Scholar]
- Linakis JG, Cunningham CL. Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology (Berl) 1979;64:61–65. doi: 10.1007/BF00427346. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., 2nd Sensitivity and Tolerance to the Hypnotic and Ataxic Effects of Alcohol in Adolescent and Adult C57BL/6J and DBA/2J Mice. Alcohol Clin Exp Res. 2008;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopéz-Bendito G, Shigemoto R, Kulik A, Vida I, Fairén A, Luján R. Distribution of metabotropic GABA receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus during prenatal and postnatal development. Hippocampus. 2004;14:836–848. doi: 10.1002/hipo.10221. [DOI] [PubMed] [Google Scholar]
- Masten A, Faden V, Zucker R, Spear L. Underage drinking: a developmental framework. Pediatrics. 2008;121:S235–S251. doi: 10.1542/peds.2007-2243A. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res. 2001;25:1166–1173. [PubMed] [Google Scholar]
- Middaugh LD, Bao K, Shepherd C. Comparative effects of alcohol on motor activity and operant behavior. Pharmacol Biochem Behav. 1992;43:625–629. doi: 10.1016/0091-3057(92)90202-q. [DOI] [PubMed] [Google Scholar]
- Miller J, Naimi T, Brewer R, Jones S. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- Moore E, Mariani J, Linsenbardt D, Melon L, Boehm S., 2nd AdolescentC57BL/ 6J(but not DBA / 2J) Mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol Clin Exp Res. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- Nestby P, Vanderschuren LJ, De Vries TJ, Hogenboom F, Wardeh G, Mulder AH, Schoffelmeer AN. Ethanol, like psychostimulants and morphine, causes long-lasting hyperreactivity of dopamine and acetylcholine neurons of rat nucleus accumbens: possible role of behavioral sensitization. Psycopharmacology (Berl) 1997;133:69–76. doi: 10.1007/s002130050373. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S, Burkhart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Shen EH. Neurochemical bases of locomotion and ethanol stimulant effects. Int Rev Neurobiol. 1996;1996:243–282. doi: 10.1016/s0074-7742(08)60669-8. [DOI] [PubMed] [Google Scholar]
- Rodgers DA, McClearn GE. Differences in alcohol preference among inbred strains of mice. Q J Stud Alcohol. 1959;20:691–695. [Google Scholar]
- Ristuccia R, Spear L. Autonomic responses to alcohol in adolescent and adult rats: a dose-response analysis. Alcohol. 2008;42:623–629. doi: 10.1016/j.alcohol.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Bucholz KK, Madden PAF, Martin NG, Heath AC. Timing of first alcohol use and alcohol dependence: evidence of common genetic influences. Addiction. 2009;104:1512–1518. doi: 10.1111/j.1360-0443.2009.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit M, Smith T. Assessing the risk for alcoholism among sons of alcoholics. J Stud Alcohol. 1997;58:141–145. doi: 10.15288/jsa.1997.58.141. [DOI] [PubMed] [Google Scholar]
- Schuckit M, Danko G, Smith T. Patterns of drug-related disorders in a prospective study of men chosen for their family history of alcoholism. J Stud Alcohol. 2004;65:613–620. doi: 10.15288/jsa.2004.65.613. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Pierson J, Danko GP, Beltran IA. Relationships among the level of response to alcohol and the number of alcoholic relatives in predicting alcohol-related outcomes. Alcohol Clin Exp Res. 2006;30:1308–1314. doi: 10.1111/j.1530-0277.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Silveri M, Spear L. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Spear L. Adolescent Brain Development and Animal Models. Ann NY Acad Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Stevenson R, Besheer J, Hodge C. Comparison of alcohol locomotor sensitization in adolescent and adult DBA/2J mice. Psychopharmacology (Berl) 2008;197:361–370. doi: 10.1007/s00213-007-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya E, Spear L. Acute effects of alcohol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Wise R, Bozarth M. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]