Abstract
Cortical interneurons play a crucial role in the functioning of cortical microcircuitry as they provide inhibitory input to projection (pyramidal) neurons. Despite their involvement in various neurological and psychiatric disorders, our knowledge about their development in human cerebral cortex is still incomplete. Here we demonstrate that at the beginning of corticogenesis, at embryonic 5 gestation weeks (gw, Carnegie stage 16) in human, early neurons could be labeled with calretinin, calbindin and GABA antibodies. These immunolabeled cells show a gradient from the ganglionic eminences (GE) towards the neocortex, suggesting that GE is a well conserved source of early born cortical interneurons from rodents to human. At mid-term (20 gw), however, a subset of calretinin+ cells proliferates in the cortical subventricular zone (SVZ), suggesting a second set of interneuron progenitors that have neocortical origin. Neuropeptide Y, somatostatin or parvalbumin cells are sparse in mid-term cerebral cortex. In addition to the early source of cortical interneurons in the GE and later in the neocortical SVZ, other regions, such as the subpial granular layer, may also contribute to the population of human cortical interneurons. In conclusion, our findings from cryosections and previous in vitro results suggest that cortical interneuron progenitor population is more complex in humans relative to rodents. The increased complexity of progenitors is probably evolutionary adaptation necessary for development of the higher brain functions characteristic to humans.
Keywords: cortical interneurons, human fetal brain, immunohistochemistry, cerebral cortex
INTRODUCTION
Two types of neurons: projection and interneurons are both necessary to establish complex neural network and proper function of the cerebral cortex. Projection neurons are pyramidal in shape, contain glutamate and are excitatory in action, whereas interneurons are small local circuit neurons, mostly GABAergic and inhibitory. Cortical GABAergic interneurons are highly diverse and classified according to a variety of morphological, antigenic and electrophysiological properties (e.g. Markram et al., 2004; Wonders and Anderson, 2006, Butt et al., 2008). On the basis of their calcium-binding proteins and neuropeptide expression, interneurons can be subdivided into partially overlapping categories of cells that express calbindin (CB), calretinin (CalR), parvalbumin (PV), vasoactive intestinal peptide (VIP), neuropeptide Y (NPY), cholecystokinin (CCK), somatostatin (Sst) and choline acetyltransferase (ChAT) (e.g., Xu et al., 2004; Markram et al., 2004; Wonders and Anderson, 2006; Butt et al. 2008; Gonchar et al., 2008). Understanding how interneuron diversity emerges during development is crucial for a better understanding of normal cortical function as well as numerous neurodevelopmental disorders. Cortical interneurons have a role in normal development through their influence on cell proliferation and migration (Haydar et al., 2000; Owens and Kriegstein, 2002). Importantly, cortical interneurons are implicated in neuropsychiatric disorders that range from cortical ectopias with epilepsy (DeFelipe, 1999; Gleeson and Walsh, 2000) to schizophrenia, autism and bipolar disorder (Akbarian et al., 1995; Knable, 1999; Lewis and Levitt, 2002; Levitt 2003; Baraban and Tallent, 2004; Lewis et al., 2005).
In rodents most, if not all of the cortical interneurons, originate in the ganglionic eminence (GE) of the ventral telencephalon, and migrate tangentially to the dorsally located cerebral cortex (Tamamaki et al., 1997; Anderson et al., 1997; Parnavelas et al., 2000; Anderson et al., 2001; Marin and Rubenstein, 2001). Different subpopulations of interneurons have distinct ventral origins: PV-expressing (PV+) and Sst+ cells originate from the Nkx2.1 lineage in the medial GE (MGE), CalR+ cells originate mainly in the caudal GE (CGE), whereas CB+ cells originate throughout the GE (Xu et al., 2004; Gonchar et al., 2008).
In contrast to rodents, the long developmental period and a larger human brain with new cortical areas and expanded upper cortical layers (Hill and Walsh, 2005; Molnár et al., 2006; Rakic 2009) is likely to need additional sources of interneurons in late corticogenesis. The neocortical subventricular zone (SVZ) evolutionary expands in primates (Smart et al., 2002; Lukaszewicz et al., 2005) and even more in the human brain (Zecevic et al., 2005, Bayatti et al., 2007). This additional proliferative zone is a source of late born cortical neurons, including interneurons. Although not conclusive, several lines of evidence support the dual origin of cortical interneuron in primates (Letinic et al., 2002; Rakic and Zecevic, 2003; Petanjek et al., 2009; Fertuzinhos et al., 2009). The idea that primates have a more diverse interneuronal population relative to other mammals including rodents, came from reports on various interneuronal cell types, such as well developed double-bouquet cells, their possible ventral and dorsal origin and molecular characteristics in the human brain (Gabbott et al., 1997; Letinic et al., 2002; Rakic and Zecevic, 2003; Preuss and Coleman 2002; Yanez et al., 2005; DeFelipe 2002, DeFelipe et al., 2006; Jones 2009; Zaitsev et al., 2009). In this study we provide additional results that support dual origin of cortical interneurons. The terms “ventral” and “dorsal” for GE and cortex, respectively, are commonly used referring to rodent brains in which GE is indeed positioned ventrally to the cerebral cortex. In the large human brain, the newly developed temporal lobe, with amygdala, hippocampus and the abutting neocortical SVZ, is however, also ventrally positioned. This part of the brain may be supplied with interneurons from the inferior part of the GE (Ulfig, 2002), not present in rodents. This is why throughout this paper we use terms GE and neocortical VZ/SVZ, instead of ventral telencephalon (pallidum) and dorsal telencephalon (pallium), which are commonly used in reports on rodents.
Another specificity of the human fetal brain is the transient cellular layer, the subpial granular layer (SGL), below the pia, which may generate an inward migrating subtype of cortical interneurons in primates (Zecevic and Rakic, 2001; Rakic and Zecevic, 2003).
Our findings in the human fetal brain argue for a more complex interneuronal progenitor population and various sites of origin of cortical interneurons relative to rodents. Although rodent brain is an excellent model and continues to provide important data with recent advances in genetics and molecular biology, it needs to be complemented with studies of the human brain. More knowledge about human cortical interneurons is necessary for understanding how the initial cortical circuitry is established as well as to devise future therapeutic approaches to disorders characterized by malfunction of GABAergic signaling (e.g. epilepsy, psychiatric disorders).
METHODS
Human fetal CNS tissue
Human fetal brain tissue was obtained from the Human Fetal Tissue Repository at the Albert Einstein College of Medicine (Bronx, New York, USA), after legal abortions, with proper consent from parents. Handling of human material was done with special care following all necessary requirements and regulations set by the Ethics Committee of the University of Connecticut and the Helsinki Convention. In all studied cases, ultrasound and gross neuropathological examination confirmed that the brain tissue was normal. Brain tissue was placed in medium containing modified Hank’s Balanced Salt Solution (Sigma, St. Louis, MO), 2 mM glutamine (Invitrogen, Carlsbad, CA), 10 mM HEPES buffer (Sigma), and 20 µg/ml gentamycine sulfate and transported to our institution, where it was immediately dissected into coronal blocks for freezing and subsequent immunohistochemistry, or for cell cultures. Blocks of tissue were fixed in 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) overnight, cryoprotected in 30% sucrose, frozen in pre-cooled 2-metylbutane, and stored at −70°C until sectioned (15µm thick) in the frontal or sagittal plane and processed for immunohistochemistry.
Immunohistochemistry
Various primary and secondary antibodies (Table 1) were used at optimal dilutions. Cryosections were incubated in blocking solution [1% BSA (Sigma), 5% normal goat serum (Vector Laboratories, Burlingame, CA), and 0.5% Tween 20 in phosphate buffered saline (PBS)] for 30 min at room temperature. Primary antibodies were applied overnight at 4°C, whereas corresponding fluorescein- or rhodamine-tagged secondary antibodies were subsequently applied for 2 h at room temperature. For double and triple staining, the primary antibodies were mixed at optimal dilutions and subsequently detected using mixtures of appropriate secondary antibodies (multiple-labeling grade antibodies, Jackson ImmunoResearch, West Grove, PA). Nuclei were counterstained with short (5 min) incubation in 1% bisbenzamide (Polysciences, Warrington, PA). For enzyme-substrate immunohistochemistry the procedure was essentially identical, except that at the beginning the endogenous tissue peroxidase activity was blocked for 20 minutes by 0.3% H2O2 solution in methanol, and the incubation with biotin-conjugated secondary antibody was for only 30 min. at room temperature. Immunoreactivity was revealed with the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA) by using 0.05% diaminobenzidine (DAB) as a substrate, according to manufacturer’s instructions. For each antigen, specificity of staining was tested by omitting the first antibody, or replacing it with corresponding normal serum at the same concentration. In addition, analysis was performed only on those sections in which the morphology of the labeled cells appeared to be characteristic of the cell type expected to be labeled, and the staining pattern was consistent with the sub-cellular distribution of the antigen (e.g. transcription factors within nucleus, receptors on the membrane).
Table 1.
Primary antibodies used in this study.
| Antigen | Host | Clone | Dilution | Manufacturer | Catalog # |
|---|---|---|---|---|---|
| Somatostatin | Rat IgG | 1:100 | Millipore Chemicon | MAB354 | |
| Parvalbumin | Mouse IgG1 | PARV-19 | 1:100 | Sigma | P3088 |
| Calretinin | Mouse IgG1 | 1:100 | Chemicon | MAB1568 | |
| Calretinin | Rabbit IgG | 1:2000 | Swant, Belliziona, Switzerland | 7696 | |
| Calbindin | Mouse IgG | CB-955 | 1:2000 | Sigma | C9848 |
| Calbindin | Rabbit IgG | 1:5000 | Sigma | C7254 | |
| GABA | Mouse IgG | GB-69 | 1:1000 | Sigma | A 0310 |
| GAD65 | Mouse IgG | 1:100 | Millipore Chemicon | MAB351 | |
| GAD67 | Rabbit IgG | 1:1000 | Chemicon | AB108 | |
| Nkx2.1 (TTF1) | Rabbit IgG | EP1584Y | 1:1000 | Epitomics | 2044-1 |
| panDlx (Dll) | Rabbit IgG | 1:1000 | Gift from Dr. Y. Morozov, Yale Univ., New Haven, CT. | ||
| NPY | Rabbit IgG | 1: 5000 | ImmunoStar, Hudson, WI | 22940 | |
| Mash1 | Mouse IgG | 1:500 | BD Pharmingen | 556604 | |
| Phospho-histone-H3 | Rabbit IgG | 1:500 | Upstate Biotechnology | 06-570 | |
| Ki67 | Mouse IgG | 1:500 | Gift from Dr. Noctor, Columbia University, NY, NY | ||
| Ki67 | Rabbit IgG | 171018 | 1:500 | Abcam | ab833 |
Cell quantification
The counts were performed on an Axioskop microscope (Zeiss, Oberkochen, Germany) equipped with a motorized stage and Neurolucida software-controlled computer system (MicroBrightField, Colchester, VT). Sections were observed and delineated under low-power magnification (10 × objective) with a 365/420nm excitation/emission filter set (Zeiss, blue fluorescence). The nuclear staining allowed delineation of areas of interest (e.g. ganglionic eminence, cortical plate etc). For various transcription factors, calretinin and Ki67, two-dimensional counts of the labeled cells / nuclear profiles were performed under 40 × objective in each delineated field and profile number was normalized to area. In addition, the number of immunolabeled cell profiles normalized to overall cell number (number of nuclear profiles) was calculated, and will be further referred to as a “relative density”. In large midgestational brains a grid (60×60 µm) was placed over area of interest and random samples 120 µm apart were counted and averaged for overall area. At least three sections per case were counted. We would like to stress that in some cases when we doubted the specificity of immunostaining, or when parts of sections were missing and/or appeared damaged during tissue processing we did not include results in our analysis. Due to a very limited sample size we did not consider applying stereological methods, and we offer our quantifications just as an estimate and look forward to further studies on more cases to confirm our results.
Image analysis and photographic documentation
Photographic documentation was done using a confocal laser-scanning microscope (Carl Zeiss, LSM 510), Axioplan fluorescent microscope (Carl Zeiss), and Axioskop brightfield microscope (Carl Zeiss). Image processing was done by Adobe Photoshop CS2 software (Adobe Systems Inc, San Jose, CA). Images were modified with regard to brightness/contrast in order to get the nearest representation of the image seen through the microscope oculars.
RESULTS
Interneuronal markers in the human embryonic brain (5 to 8 gw)
Several established interneuronal markers are expressed in the human cerebral cortex from early embryonic stages (see Zecevic et al., 1999; Zecevic and Milosevic, 1997; Rakic and Zecevic, 2003). Cells expressing GABA, CalR and CB at 5 gw (Carnegie stage, CS 16) are reported in the primordial plexiform layer (PPL), an early structure that is divided by the emerging cortical plate around the 8th gw into layer I (upper part) and the subplate layer (SP, lower part).
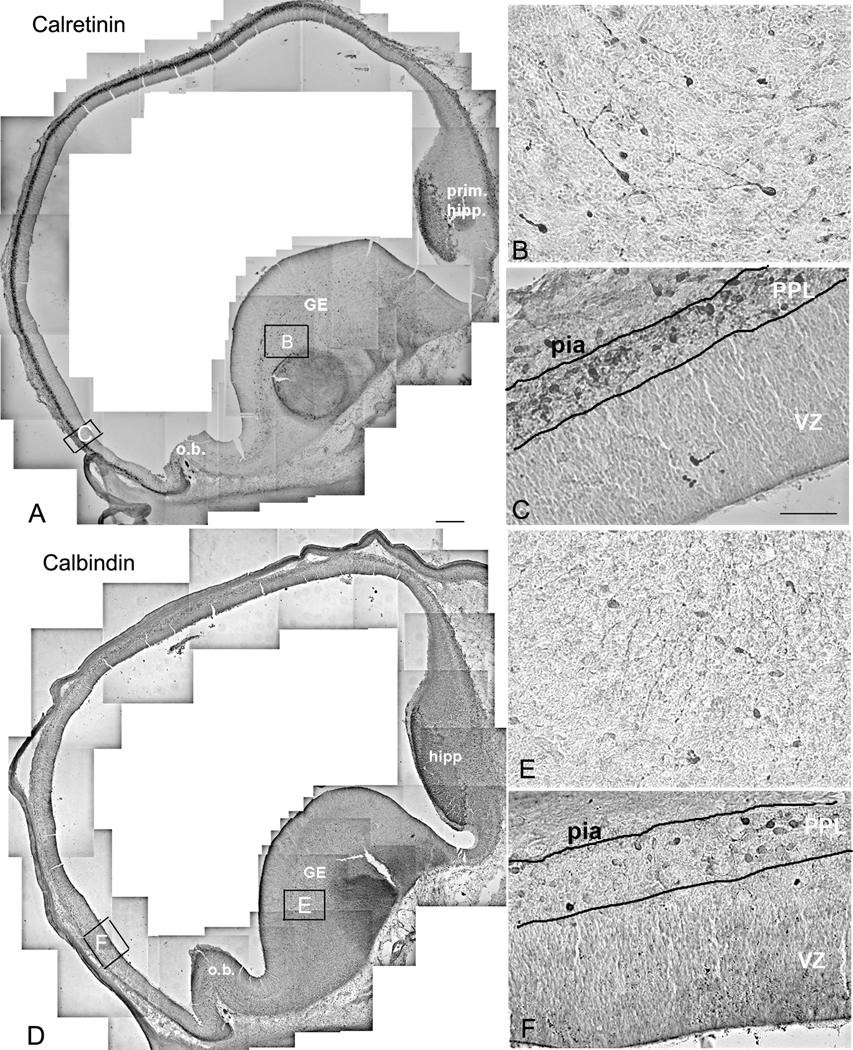
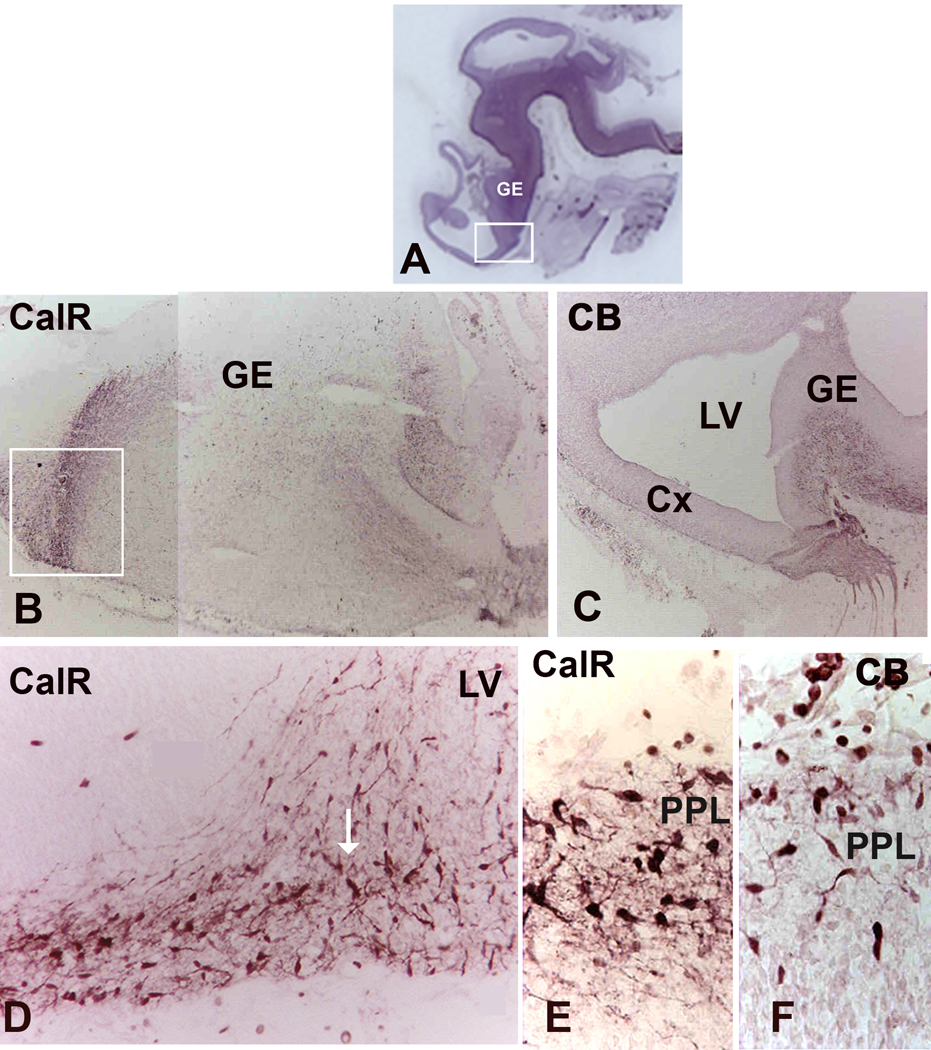
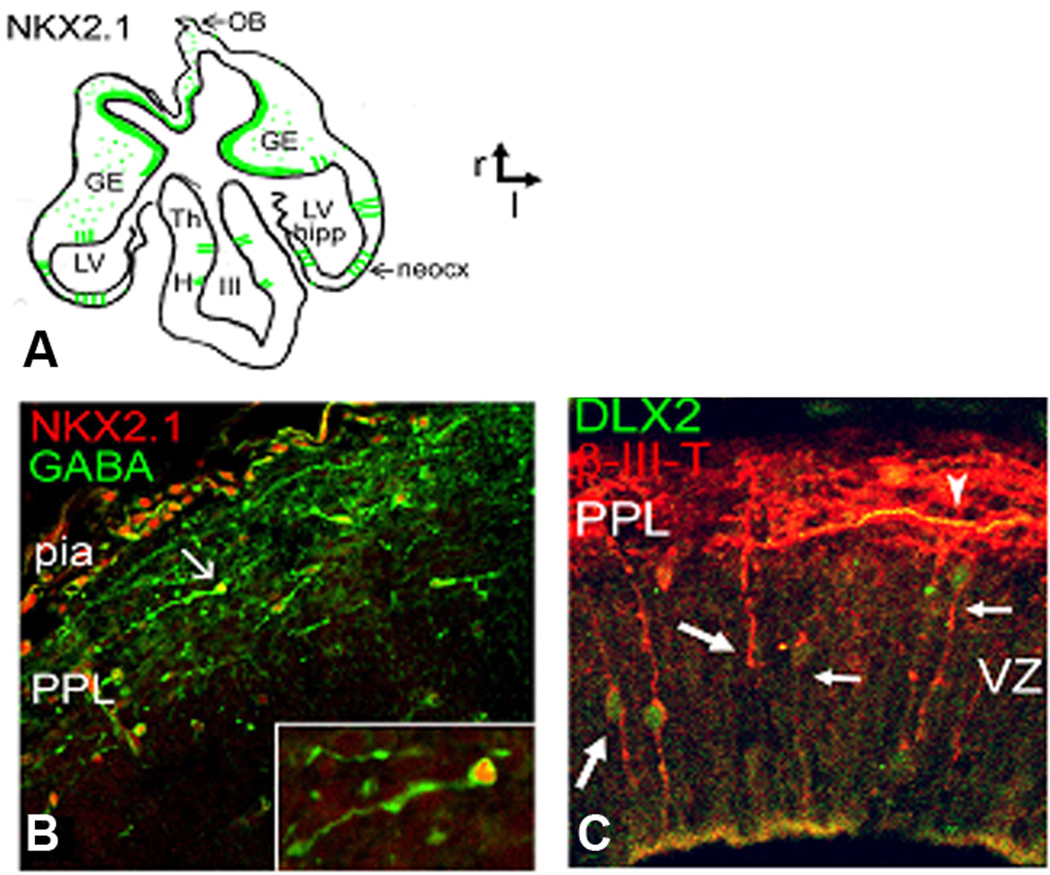
At 6 gw (CS 17) neurons in the PPL and the GE express two calcium-binding proteins, CalR and CB, but not PV or Sst (Figs. 1, 2). The density of immunolabeled cells with either of these two markers is the greatest in the matrix of the GE, which at this stage is not yet divided into LGE and MGE. There is a clear ventro-dorsal gradient of immunoreactivity for both CalR and CB from the GE towards the emerging neocortex (Figs. 1, 2). Importantly, all immunolabeled cells were demonstrated outside of the proliferative ventricular zone, consistent with labeling of young interneurons, and not their progenitors. Immunoreactive cells had the appearance suggestive of tangentional migration to the neocortex, based on the orientation of their processes and their ventro-dorsal gradient (Fig. 2). Notably, a stream of migrating cells appeared to come into close contact with ventral brain surface, before “bouncing off” to more dorsally located cortical primordium (Fig. 2D). At a similar embryonic age (7gw, CS 19), among tangentially migrating cells, occasional radially oriented cells within the telencephalic wall could be double-labeled with neuronal markers (β-III tubulin or GABA) and ventral transcription factors, Nkx2.1 or Dlx2 (Rakic and Zecevic, 2003, Fig. 3). The early presence of these double-labeled cells extending from the VZ to the pia, is suggestive of their local, cortical origin.
Figure 1.
Figure 2.
Figure 3.
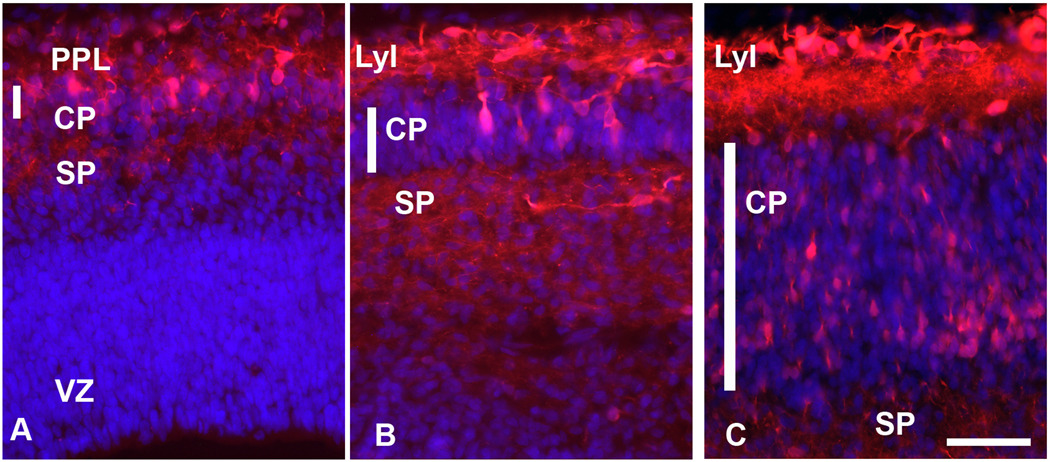
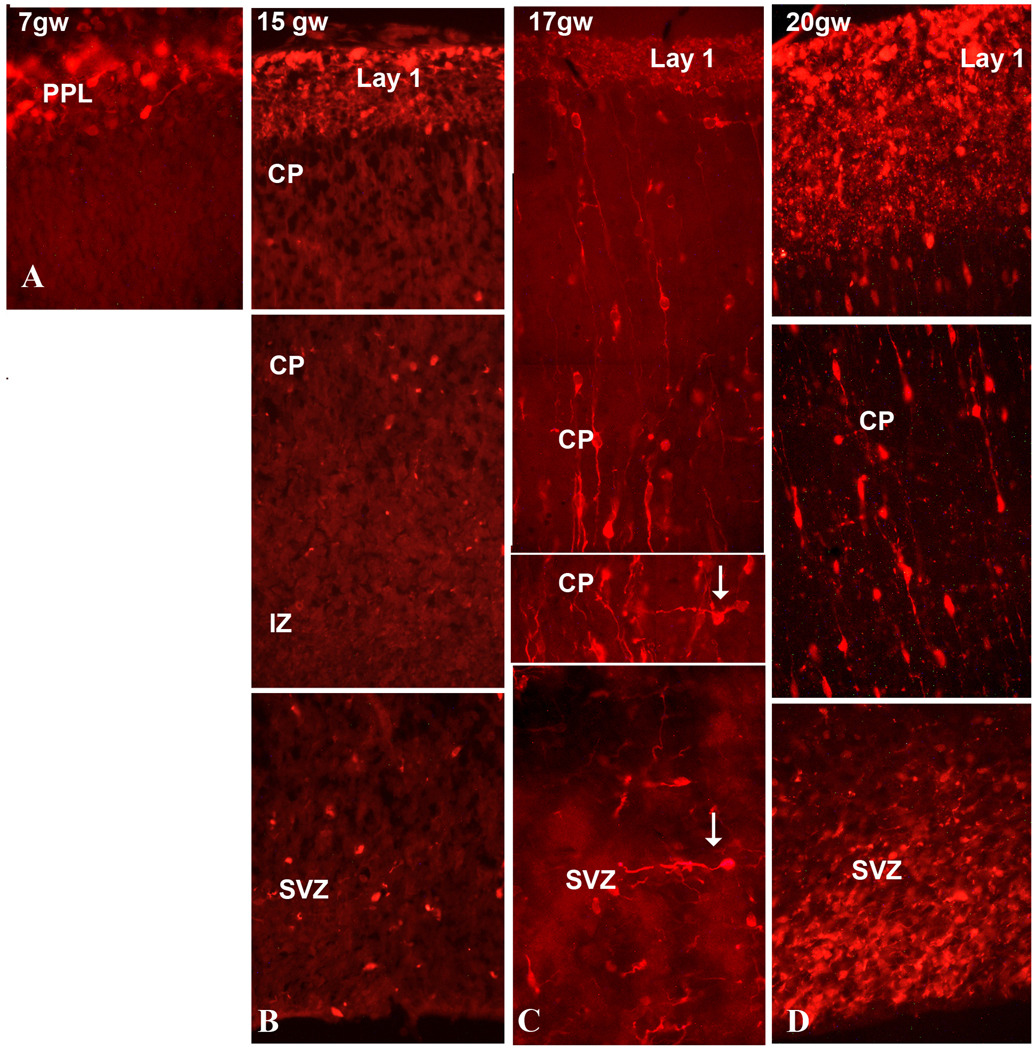
At 8 gw (CS 20), the cortical plate (CP), emerges as initially only 1–2 cells rows thick layer dividing the PPL into upper and lower parts. From the very beginning GABA+ or CalR+ neurons are present in the newly formed CP (Fig. 4A). These early immunolabeled cells will eventually become deep layers V–VI interneurons, as the inside-out formation of the CP proceeds. On coronal sections from an 8-week-old embryo the width of the CP is seven times larger in the lateral (140 µm) than in the medial (20 µm) regions of the telencephalic wall. CalR+ cells are observed to follow the inside-out developmental gradient, as described for projection neurons (Fig. 4).
Figure 4.
Cortical interneurons at 15–20 gw
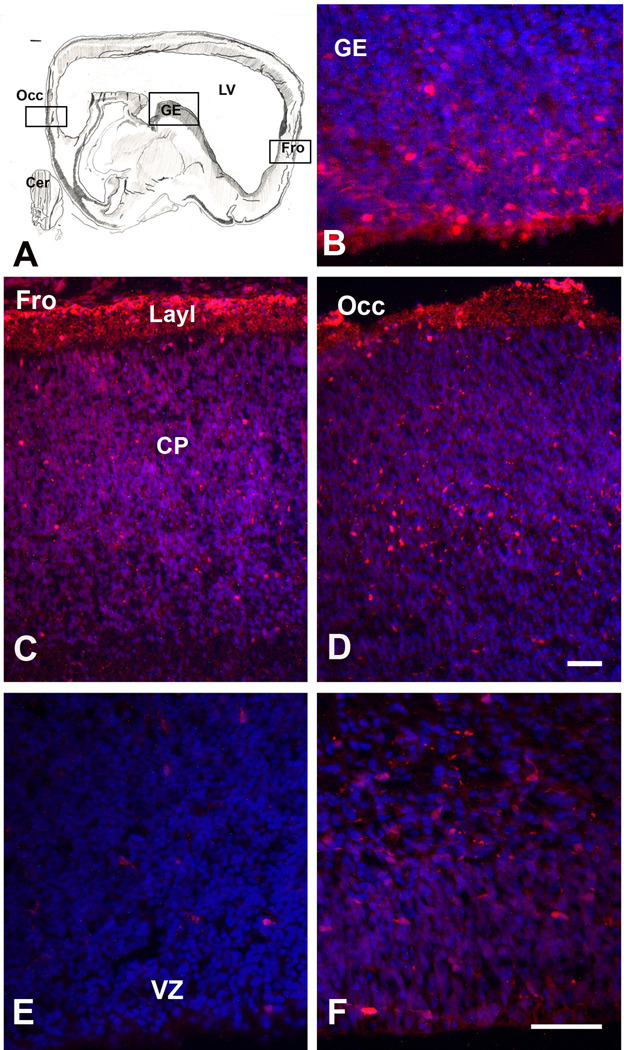
During the next two months, from 8 gw to 15 gw, the number of CalR+ cells increases throughout the developing cerebral cortex. In sagittally cut sections through the 15 gw brain, the density of CalR+ cells is higher in occipital than in frontal cortical regions (Fig. 5), demonstrating a caudo-rostral gradient of CalR expression. A similar caudo-rostral developmental gradient has been reported for calcium-binding protein expression in embryonic (Zecevic et al., 1999) and fetal human cortex (Bayatti et al., 2007), as well as for general cellular and functional maturation of the CNS (e.g., Yan et al., 1995; Del Rio et al., 1995).
Figure 5.
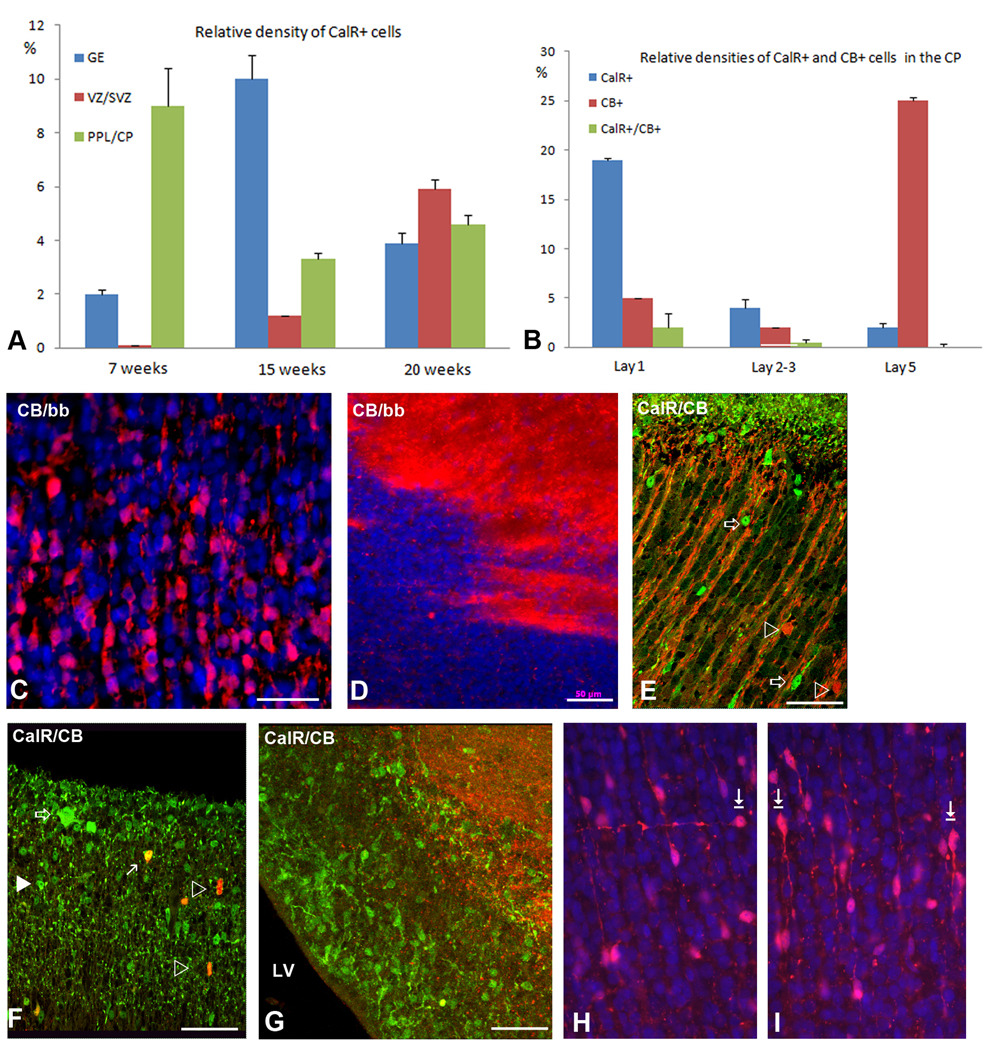
At 15 gw, relative density of CalR+ cells is still the highest in the GE with much lower density calculated in the cortical VZ/SVZ (Fig. 6A). This distribution suggests that at 15 gw the GE is still the main source of cortical CalR+ cells. At 15 gw GE is divided into LGE and MGE, with CGE at more caudal telencephalic levels. From the immunolabeling studies it is not clear where exactly in the GE do CalR+ or CB+ cells originate, since they are seen as postmitotic, probably migrating cells.
Figure 6.
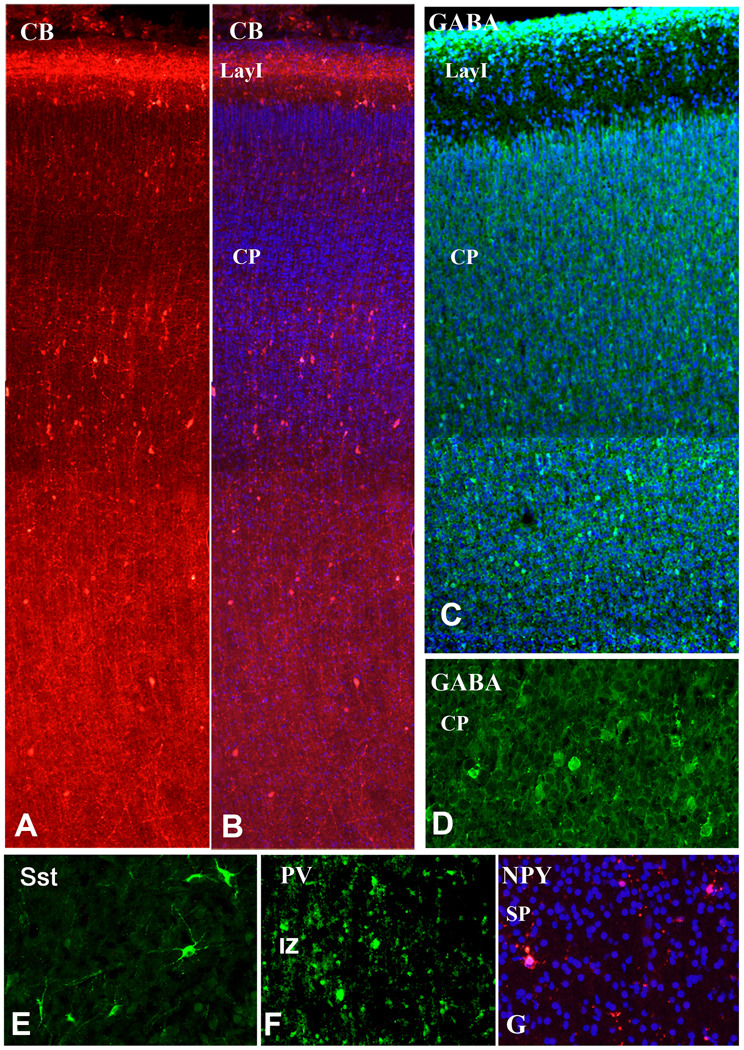
In the neocortical VZ/SVZ the percentage of CalR+ cells from all cells increases approximately six times from only 1% at 15 gw to 5.9% at 20gw (Fig. 6A). In layer I, both small cells and much larger Cajal-Retzius cells are strongly reactive for CalR at this stage (Fig. 6E, 7D), similar to earlier fetal stages (Figs. 1, 2). Relative density of CalR+ cells in different forebrain regions also changes and for the first time at mid-term becomes higher in the cortical VZ/SVZ relative to the GE (Fig. 6A). In respect to the ratio of two Ca-binding proteins, CalR+ cells at mid-term outnumber CB+ cells in all cortical regions except in deep layers V–VI (Fig. 6B, C). Some of the deep CB+ cells may represent a subpopulation of pyramidal cells. This observation is corroborated by the strong labeling of axonal tracts, including the internal capsule which contains corticospinal axons (Fig. 6D). Additionally, in the upper cortical layers (layers II–III) single CB+ cells with large round cell bodies were labeled, as well as numerous radially spreading processes (Fig. 6E). CalR+ cells are closely apposed to these CB+ processes, as if migrating along them. CB did not label CalR+ Cajal-Retzius cells, but in layer I, 30% of small CB neurons co-expressed CalR+; single labeled cells for CalR and CB were also present (Fig. 6B, F). It is interesting to note that in all cortical regions studied at mid-term, layer I was filled with CalR+ puncta, reminiscent of axonal terminals and/or dendrites (Fig. 5F). In contrast to numerous CalR+ cells in the cortical VZ/SVZ only rare CB+ cells could be detected in that region (Fig. 5G, 8), in contrast to the GE, where both single CalR+, CB+, and double-labeled cells were present (not shown).
Figure 7.
Figure 8.
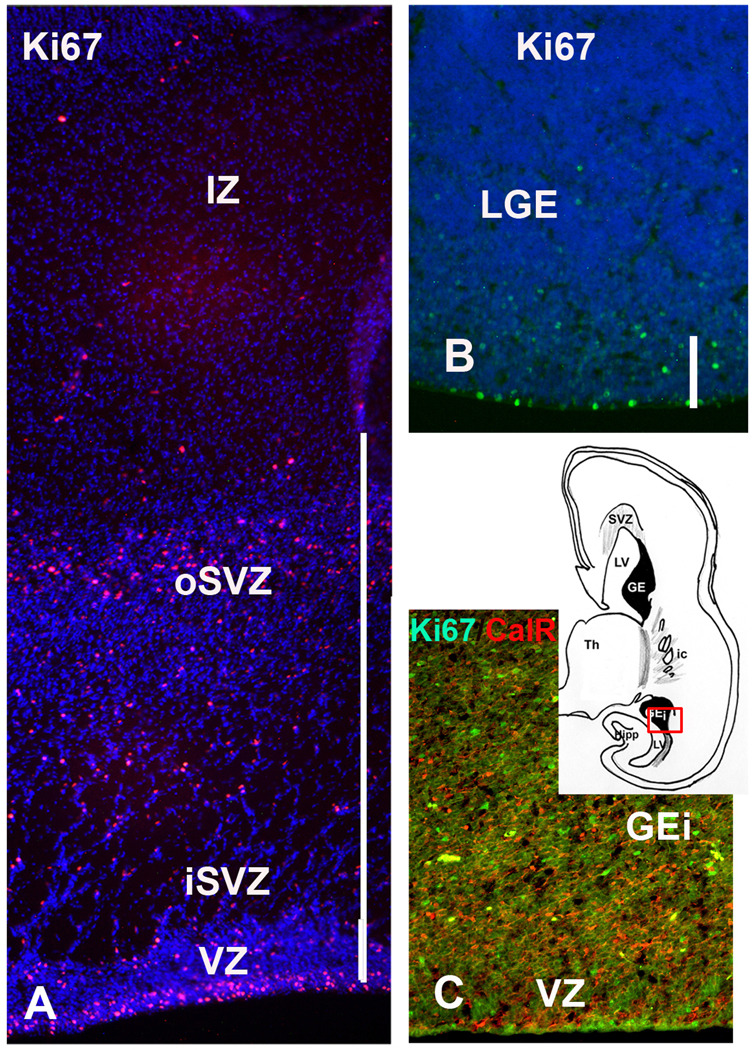
Numerous CalR+ and CB+ cells were present in a well developed GE positioned caudally and inferiorly, around the temporal extension of the lateral ventricle (Fig. 9C). A number of Ki67+ cells in the inferior GE indicates cell proliferation in this region at mid-term. This part of GE would correspond by its position to the caudal GE (CGE) described in mice (Nery et al, 2002; Kanatani et al., 2008).
Figure 9.
Morphology of cells labeled with interneuronal markers
The morphology of CalR+ and CB+ cells in the first half of gestation in all cortical layers is mainly bipolar, with one process on each cell poles. In the intermediate zone (IZ), the subplate (SP) and CP their orientation is mostly radial, consistent with their migration towards upper cortical layers (Fig. 6, 7). Occasional transversally crossing cells can be seen at all cortical levels, similar to what was described in our previous report (Zecevic et al., 1999). The tangentally oriented CalR+ cells at mid-term are demonstrated in the SVZ (Fig. 7C), and occasionally even in the CP (Fig. 6H, arrow). The majority of immunolabeled cells in the CP are, however, radially oriented with a leading process oriented either towards pia, or less often towards the SVZ (Fig. 6I, arrows). Immunolabeled cells with randomly oriented processes or with two processes branching from the upper side of the cell could be seen in the CP (Fig. 7C, arrow). A number of CalR+ cells could also be seen in close proximity to the ventricular surface (Fig. 7D, 10A).
At mid-term, the three interneuronal markers, CalR, CB and GABA were strongly expressed in the CP neurons, mostly in deeper layers, as well as in the proliferative SVZ (Fig. 8A–D). Other interneuronal markers such as PV, Sst and NPY were expressed by sparse cells in the subplate or intermediate zone, and more often in the hippocampal region than in the neocortical areas (Fig. 8E–G). Few NPY+ cells were present also in layer I (not shown).
Progenitors of cortical interneurons in the neocortical SVZ at mid-term
Neocortical SVZ at mid-term is still a very active proliferative zone that extends 800–1200µm from the ventricular surface, and can be divided into inner and outer SVZ (Fig. 9A). Labeling with proliferation marker Ki67 demonstrated that more cells are proliferating in the SVZ than in the VZ/SVZ of the GE, where cell proliferation is almost exhausted (Fig. 9B), suggesting that the main proliferative zone for late born cortical neurons is the outer neocortical SVZ. Notably, among various cell types that proliferate in the SVZ at mid-term (Zecevic et al., 2005), is a subpopulation of CalR+ cells, as demonstrated by their co-labeling with a proliferation marker Ki67 (Fig. 10, Yu et al., 2009). We estimated that at 20 gw 17% of CalR+ cells in the cortical VZ/SVZ were proliferating, as they were co-labeled with Ki67. On the other hand, CalR+ cells represent 10% of the total proliferating population in the SVZ at that time. This finding obtained on cryosections complements earlier reports in vitro (Zecevic et al., 2005). Combined, these results are consistent with the local origin of a subpopulation of late born cortical CalR+ cells in the human brain.
Subpial granular layer
Cells of the transitory subpial granular layer (SGL) in primates spread over the entire neocortex (Brun, 1965; Meyer and Wahle, 1999; Rakic and Zecevic, 2003). At 13 gw, a gradient of CalR+ cells extends from temporal lobe, where it consists of several rows of immunolabeled neurons, to dorsolateral Cx, where it is only one cell thick (Zecevic and Milosevic, 1997). At mid-term numerous CalR+ cells, Dll+, and a much smaller population of Nkx2.1+ and CB+ cells can be seen in the SGL (Zecevic et al., 1999; Zecevic and Rakic, 2001; Rakic and Zecevic, 2003). Numerous Dll+ small neurons are GABAergic (Rakic and Zecevic, 2003), whereas a different population of much larger cells is reelin+ Cajal-Retzius neurons (Fig. 6F).
DISCUSSION
In this study we summarize our results on antigen characteristics, morphology and distribution of several classes of cortical interneurons and their progenitors in human fetal cerebral cortex during the first half of gestation. Using immunolabeling with a battery of interneuronal markers, we extended our previous results on the initial appearance of the GABA and calcium-binding proteins in the human cortical primordium (Zecevic and Milosevic, 1997; Rakic and Zecevic, 2003) with the results in fetal brains up to mid-term (20 gw). An important new finding shown in this study is the presence of proliferating CalR+ cells at mid-term as demonstrated on cryosections by their double-labeling with the proliferation marker, Ki67. These double-labeled cells represent, in our opinion, a novel type of cortical progenitor cells previously unreported in either rodents or primates (see below).
The initial appearance of CalR+ and CB+ cells
In the human fetal brain CalR+ and CB+ cells are present continuously from early embryonic stages, before the appearance of the CP, to mid-term. On the basis of their gradient in distribution, CalR+ and CB+ cells seem to be generated first in the MGE. In later fetal stages, however, CalR+ cells are also generated in the CGE, the inferior GE, and cortical SVZ. From the GE first CalR+ and CB+ neurons in the embryonic forebrain (5–6gw) migrate tangentially to the PPL, where they are present about a week prior to appearance of reelin+ Cajal-Retzius cells (Zecevic et al., 1999; Meyer et al., 2000). Thus, some of these cells, including early GABAergic neurons (Zecevic and Milosevic, 1997) probably represent pioneer neurons reported previously (Meyer et al., 2000). In following weeks CalR+ cells are a part of the emerging CP from the very beginning. They continue to populate cortical layers as they form in the inside-out manner, migrating radially similar to what has been described for projection neurons (Rakic, 1974).
Tangentially migrating interneurons at early embryonic stages come from the GE, confirming results reported in rodents (e.g. Anderson et al., 1997; Tamamaki et al., 1997; Parnavelas et al., 2000). The early existence of GABAergic system, even before the emergence of the CP, indicates that secreted GABA can influence proliferation of cortical progenitor cells from the very beginning of neurogenesis. Release of GABA by neurons has differential effect on VZ and SVZ progenitors, promoting their proliferation in the VZ and inhibiting it in the SVZ (Haydar et al., 2000). Electromicroscopic studies have shown that the initial synapses in the human layer I and subplate appear at around 12 gw (Kostovic and Rakic, 1990; Zecevic 1998). These results are temporally matched by the expression of molecular markers of synaptogenesis, such as VGAT (vesicular transporter for GABA) and synpatophysin at the same fetal ages (Bayatti et al., 2007). Thus, it is likely that spontaneous activity of GABAergic system could be the initial driving force for cortical development (e.g. Ben-Ari, 2002). This is particularly interesting since at early stages of cortical development GABA acting through GABAA receptors has an excitatory action, before becoming an inhibitory transmitter later in life (e.g., Cherubini et al., 1991; Ben-Ari, 2002; Khazipov and Luhmann, 2006). The switch from excitation to inhibition in GABA action is attributed to the changing intracellular concentration of chloride [Cl−]i, which depends on the expression of two chloride transporters, NKCC1, expressed early, and KCC2 expressed in more mature neurons. As judged by the expression of KCC2, in the human fetal cortex this switch has started but is not completed by 16 gw, when only some neurons in the subplate express KCC2 (Bayatti et al., 2007).
Cortical interneurons at fetal stages
Around mid-term, the numbers of both CalR+ and CB+ cells increase in the human cerebral cortex. Interestingly their co-localization was only occasionally seen in layer I. This is in contrast to early fetal stages (11–17 weeks) when 35% of CalR+ cells in the cortical plate were colabeled with CB (Zecevic et al., 1999). Both CalR and CB label neurons in deep CP occasionally resemble pyramidal cells, with triangular cell bodies and an apical dendrite. This transient labeling of pyramidal cells by calcium-binding proteins is in accord with previous reports in animal models and human fetal cortex (Yan et al., 1997; Zecevic et al., 1999).
By mid-term other subgroups of cortical interneurons such as Sst+, PV+ and NPY+ cells are detectable, but are sparse and located mainly in the subplate layer. These interneurons become more numerous in humans during the second half of gestation, not studied here. Indeed, Sst+, PV+ and NPY+ neurons were reported in later stages of gestation in human cerebral cortex (Kostovic et al., 1991; Cao et al., 1996; Yan et al., 1997; Delalle et al., 1997; Bayatti et al., 2007). Similarly, in mice these markers were expressed only after birth (Gonchar et al., 2007).
Consequently we did not observe cells co-labeled with CalR and either Sst or PV, cell types previously reported in mice (Wonders and Anderson, 2006; Xu et al., 2006; Miyoshi et al., 2007). In the mouse, 70% of cortical CalR+ interneurons develop after E14.5, they do not co-express either PV or Sst, and they are destined for deep cortical layers. This population of CalR+ neurons is bitufted/bipolar, vertically oriented and derived from the CGE (Xu et al., 2004; Butt et al., 2005; Wonders and Anderson, 2006). A smaller CalR+ cell population (30%) can be co-labeled with Sst and are derived either from the MGE (Butt et al., 2005; Xu et al., 2006) or dorsal CGE (Xu et al., 2004; Wonders and Anderson, 2006). They were reported to migrate in the inside-out manner and connect with their targets in all cortical layers (e.g. Xu et al., 2004). Contrasting this view, it has been recently proposed that cortical interneurons from CGE, regardless of the time of their origin, target pyramidal neurons in superficial cortical layers (Miyoshi et al., 2009). Interneurons from CGE in the mouse migrate caudally to hippocampus and caudal cerebral cortex (Nery et al., 2002; Yozu et al., 2005) under COUP-TFII control (Kanatani et al., 2008). In the human brain clear molecular evidence for the existence of the well-defined CGE is not yet present.
Cortical origin of a subtype of interneurons
The most intriguing finding in the fetal cerebral cortex is the presence of proliferating CalR+ cells in the SVZ at mid-term. Although these cells are a small percent of all SVZ cells considering the overall size of the SVZ at mid-term, their total amount may be quite significant (Yu et al., 2009). Strengthening this finding, cells expressing Dlx2 and Nkx2.1 transcription factors characteristic to the MGE in rodents, are numerous in the neocortical SVZ (Rakic and Zecevic, 2003). Moreover, both Nkx2.1+ and Dlx2+ cells proliferate at mid-term at the same neocortical location as CalR+ cells (Jakovcevski et al., in preparation). These results on fixed fetal cryosections confirm our earlier findings in the organotypic SVZ slices from human brain that cells containing Nkx2.1 and Dlx2 take up BrdU, indicating their local proliferation (Zecevic et al., 2005). Moreover, on the human fetal slices from the SVZ it was demonstrated that retroviral labeled cells divide locally several times before migrating to the upper layers of the cortex (Letinic et al., 2002). It has been estimated that 65% of cortical GABAergic interneurons are derived from the Dlx1/2+/Mash1+ lineage located in the neocortical VZ/SVZ, whereas 35% have a GE origin (Letinic et al., 2002). In monkey, early interneuronal progenitors are initially GAD65+/Mash1+ cells in the GE, but later in gestation another population of GAD65+/Mash1+ cells proliferate in the neocortical VZ/SVZ, implying that later born interneurons have cortical origin (Petanjek et al., 2009). Furthermore, in human cases with severe holoprosencephaly, where substantial parts of the GE are missing, only CalR+ neurons were demonstrated in the cerebral cortex, while other subgroups of interneurons were absent. This result suggests a dorsal origin of cortical CalR+ cells (Fertuzinhos et al., 2009). In addition, we have shown double-labeled GABA+/Nkx2.1+ cells in the embryonic cortical primordium, suggestive of their origin in the cortical VZ (Rakic and Zecevic, 2003). The expansion of the cortical SVZ during evolution has undoubtedly had a great impact on the evolution of the human cerebral cortex allowing for the increase in diversity of neuronal progenitors. At mid-term proliferation is still very active in the large outer SVZ which has no equivalent in rodents (Smart et al., 2002; Zecevic et al., 2005; Bayatti et al., 2007). Prolonged proliferation in the primate-specific outer SVZ (Lukaszewicz et al., 2005; Dehay and Kennedy, 2007), is temporally matched with emergence of enlarged supragranular layers (layers II – IV) in primates (Hill and Walsh, 2005) These cortical layers are essential for forming cortico-cortical connections and development of higher brain functions, such as cognitive functions, abstract thinking, language, that characterize us as humans (Hill and Walsh, 2005; Rakic, 2009; Jones, 2009).
Subpial granular layer as an additional source of cortical interneuron
Subpial aggregation of cells form a subpial granular layer (SGL), first described as specific for primates (Brun 1965, Gadisseux et al., 1992, Zecevic and Milosevic 1997; Meyer and Wahle, 1999). Recently, however, this layer has been recognized also in rudimentary form in rodents (Wichterle et al., 2001). This transient layer emerges in human forebrain at 11 gw and disappears by 27 gw. In primates, SGL is characterized by the continuous addition of cells for several months. In the monkey, using tritiated thymidine labeling we have shown that cells are added to SGL and layer I for a period of almost 2 months, from E43–93, (Zecevic and Rakic, 2001). Thus, although SGL is not a proliferative zone, it serves as a conduit for neurons coming from the olfactory region and region around paleocortical ventricle (Meyer and Wahle, 1999). SGL disappears relatively quickly at 27 gw, not only by cell death but probably also by inward migration, thus contributing to a subpopulation of cortical interneurons (Zecevic and Rakic, 2001; Rakic and Zecevic, 2003).
In conclusion, several sites of origin for cortical interneurons, including the GE, neocortical VZ/SVZ, and the subpial granular layer contribute to the complexity of the interneuronal population in the human cerebral cortex. Importantly CalR+ cells seem to be a heterogeneous cell population which can be generated at different locations, depending on the stage of development. Early born CalR+ neurons seem to be derived exclusively from the GE and assume their final position in deep cortical layers. Late generated CalR+ neurons destined for superficial layers II/III generated by the end of corticogenesis appear to have cortical SVZ origin. The population of calretinin+ cells is spared in the cortex of patients with a severe holoprosencephaly (Fertuzinhos et al., 2009), which implies their cortical origin. In rodents dorsally derived interneurons seem to be a much smaller population, reported as calretinin+ cells for olfactory bulbs (Kohwi et al., 2007, Yang 2008), or neonatal GABAergic cells destined for various cortical areas in the mouse (Inta et al., 2008). Notably, vertically oriented CalR+ cells that are targeting other interneurons rather than pyramidal neurons are more numerous in upper cortical layers of primates relative to rodents (Gabbott et al., 1997, Zaitsev et al., 2005). The predominance of these slow-spiking CalR+ interneurons in monkey prefrontal cortex was suggested to be instrumental for formation of species specific neocortical circuits important for cognitive functions of primates (Zaitsev et al., 2005). In the absence of fate mapping and transgenic approaches in humans, we rely mainly on distribution of immunolabeled cells correlated with specific time of development. So far, however, all the studies performed in primates by different groups using diverse approaches agree that a subtype of cortical interneurons has local (cortical) origin. Most importantly, those studies complement each other. It is possible, even probable, that Dlx1/2+/Mash1+ cells in the human brain (Letinic et al., 2002), as well as GAD65+/Mash1+ cells in monkeys (Petanjek et al., 2009) represent the same population as the CalR+/Ki67+ cells that we report here. This cortically derived subpopulation of cortical interneurons may be selectively damaged in psychiatric pathologies (e.g., Lewis and Levitt, 2002; Levitt 2003; Baraban and Tallent, 2004; Lewis et al., 2005), and thus, better understanding of human interneuronal diversity and how it is created could help in devising targeted therapies for psychiatric diseases.
ACKNOWLEDGEMENT
(NIH NSO 41489-09))
Literature
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch. Gen. Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JLR. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Tallent MK. Interneuron diversity series: interneuronal neuropeptides—endogenous regulators of neuronal excitability. Trends Neurosci. 2004;27:135–142. doi: 10.1016/j.tins.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, Ambrose P, Ward JFH, Lindsay S, Clowry GJ. A Molecular Neuroanatomical Study of the Developing Human Neocortex from 8 to 17 Postconceptional Weeks Revealing the Early Differentiation of the Subplate and Subventricular Zone. Cer Cortex. 2008;18(7):1536–1548. doi: 10.1093/cercor/bhm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory Actions of GABA during Development : The Nature of the Nurture. Nature Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Brun A. The subpial granular layer of the fetal cerebral cortex in man. Its ontogeny and significance in congenital cortical malformations. Acta Pathol. Microbio.Scan.Suppl. 1965;179:1–98. [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: An Excitatory Transmitter in Early Postnatal Life. Trends in Neurosci. 1991;14(12):515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Butt SJB, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The Requirement of Nkx2-1 in the Temporal Specification of Cortical Interneuron Subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao QL, Yan XX, Luo XG, Garey LJ. Prenatal Development of Parvalbumin Immunoreactivity in the Human Striate Cortex. Cer Cortex. 1996;6(4):620–630. doi: 10.1093/cercor/6.4.620. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Chandelier cells and epilepsy. Brain. 1999;122:1807–1822. doi: 10.1093/brain/122.10.1807. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Cortical interneurons: from Cajal to 2001. Progr Brain Res. 2002;136:215–238. doi: 10.1016/s0079-6123(02)36019-9. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Ballesteros-Yanez I, Inda MC, Munoz A. Double-bouquet cells in the monkey and human cerebral cortex with special reference to areas 17 and 18. Prog Brain Res 2006. 2006;154:15–32. doi: 10.1016/S0079-6123(06)54002-6. [DOI] [PubMed] [Google Scholar]
- Dehay C, Kennedy H. Cell-cycle control and cortical development. Nature. 2007;8:438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- Del Rio JA, Martinez A, Fonseca M, Auladell C, Soriano E. Wide distribution, glutamate-like immunoreactivity and fate of Cajal-Retzius cells in the developing murine cortex as identified with calretinin immunocytochemistry. Cer. Cortex. 1995;5:13–21. doi: 10.1093/cercor/5.1.13. [DOI] [PubMed] [Google Scholar]
- Delalle I, Evers P, Kostovic I, Uylings HBM. Laminar Distribution of Neuropeptide Y-Immunoreactive Neurons in Human Prefrontal Cortex During Development. J Comp Neurology. 1997;379:515–522. doi: 10.1002/(sici)1096-9861(19970324)379:4<515::aid-cne4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Fertuzinhos S, Krsnik Z, Kawasawa YI, Rasin MR, Kwan KY, Chen JG, Judas M, Hayashi M, Sestan N. Selective Depletion of Molecularly Defined Cortical Interneurons in Human Holoprosencephaly with Severe Striatal Hypoplasia. Cer Cortex. 2009;19(9):2196–2207. doi: 10.1093/cercor/bhp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PLA, Jays PRL, Bacon SJ. Calretinin Neurons in Human Medial Prefrontal Cortex (Areas 24a, b, c, 32, and 25) J Com Neurology. 1997;381:389–410. doi: 10.1002/(sici)1096-9861(19970519)381:4<389::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Gadisseux JF, Goffinet AM, Lyon G, Evrard P. The human transient subpial granular layer: an optical, immunohistochemical, and ultrastructural analysis. J Com Neurology. 1992;324:94–114. doi: 10.1002/cne.903240108. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Walsh CA. Neuronal migration disorders: from genetic diseases to developmental mechanisms. TINS. 2000;23:352–359. doi: 10.1016/s0166-2236(00)01607-6. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Wang Q, Burkhalter A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Frontiers in Neuroanatomy. 2008;1(3):1–11. doi: 10.3389/neuro.05.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J. Neurosci. 2000;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RS, Walsh CA. Molecular insights into human brain evolution. Nature. 2005;437(7055):64–67. doi: 10.1038/nature04103. [DOI] [PubMed] [Google Scholar]
- Inta D, Alfonso J, von Engelhardt J, Kreuzberg MM, Meyer AH, van Hooft JA, Monyer H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. PNAS. 2008;105(52):20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The origins of cortical interneurons: mouse vs. monkey and human. Cer Cortex. 2009;19:1953–1956. doi: 10.1093/cercor/bhp088. [DOI] [PubMed] [Google Scholar]
- Kanatani S, Yozu M, Tabata H, Nakajima K. COUP-TFII Is Preferentially Expressed in the Caudal Ganglionic Eminence and Is Involved in the Caudal Migratory Stream. J Neurosci. 2008;28(50):13582–13591. doi: 10.1523/JNEUROSCI.2132-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Luhmann H. Early patterns of Electrical Activity in the Developing Cerebral Cortex of Humans and Rodents. Trends in Neurosci. 2006;29(7):414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Knable MB. Schizophrenia and bipolar disorder: findings from studies of the Stanley Foundation Brain Collection. Schizophr Res. 1999;39:149–152. doi: 10.1016/s0920-9964(99)00114-0. [DOI] [PubMed] [Google Scholar]
- Kohwi M, Petryniak MA, Long JE, Ekker M, Obata K, Yanagawa Y, Rubenstein JL, Alvarez-Buylla A. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007;27:6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. Journal of Comparative Neurology. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Stefulj-Fucic A, Mrzljak L, Jukic S, Delalle I. Prenatal and perinatal development of the somatostatin-immunoreactive neurons in the human prefrontal cortex. Neurosci. Lett. 1991;124:153–156. doi: 10.1016/0304-3940(91)90082-5. [DOI] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type specific deficits, epilepsy, and behavioral dysfunction. J. Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Ann Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, Giroud P, Huissoud C, Berland M, Kennedy H, Dehay C. The concerted modulation of proliferation and migration contributes to the specification of the cytoarchitecture and dimensions of cortical areas. Cereb. Cortex. 2006;16 Suppl. 1:26–34. doi: 10.1093/cercor/bhk011. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the Neocortical Inhibitory System. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JLR. A long remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Meyer G, Schaaps J-P, Moreau L, Goffinet AM. Embryonic and early fetal development of the human neocortex. J.Neurosci. 2000;20(5):1858–1868. doi: 10.1523/JNEUROSCI.20-05-01858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Wahle P. The paleocortical ventricle is the origin of reelin-expressing neurons in the marginal zone of the foetal human neocortex. Eur J Neurosci. 1999;11:3937–3944. doi: 10.1046/j.1460-9568.1999.00818.x. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Leffler JH, Sousa V, Butt S, Fishell G. eMBO Workshop on cortical interneurons in Health and Disease. Spain: Palma de Mallorca; 2009. The integration of CGE-derived interneurons into the developing cortex: challenging the temporal matching hypothesis. [Google Scholar]
- Molnár Z, Métin C, Stoykova A, Tarabykin V, Price DJ, Francis F, Meyer G, Dehay C, Kennedy H. Comparative aspects of cerebral cortical development. Eur J Neurosci. 2006;23:921–934. doi: 10.1111/j.1460-9568.2006.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5(12):1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Olivier G, Pineau H. Horizons de streeter et age embrionnaire. Bull. Ass. Anat. 1962;47e:573–576. [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Parnavelas JG, Anderson SA, Lavdas AA, Grigoriou M, Pachnis V, Rubenstein JL. The contribution of the ganglionic eminence to the neuronal cell types of the cerebral cortex. Novartis Found Symp. 2000;228:129–139. doi: 10.1002/0470846631.ch10. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Berger B, Esclapez M. Origins of Cortical GABAergic Neurons in the Cynomolgus Monkey. Cer Cortex. 2009;19(2):249–262. doi: 10.1093/cercor/bhn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM, Coleman GQ. Human-specific organization of primary visual cortex: alternating compartments of dense Cat-301 and calbindin immunoreactivity in layer 4A. Cer Cortex. 2002;12(7):671–691. doi: 10.1093/cercor/12.7.671. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10(10):724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Emerging complexity of cortical layer I in humans. Cer.Cortex. 2003;13:1072–1083. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- Smart IHM, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extra striate cortex in the monkey. Cer Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Fujimori KE, Takauji R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J. Neurosci. 1997;17(21):8313–8323. doi: 10.1523/JNEUROSCI.17-21-08313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N. Ganglionic Eminence of the Human Fetal Brain-New Vistas. The Anat.Rec. 2002;267:191–195. doi: 10.1002/ar.10104. [DOI] [PubMed] [Google Scholar]
- Wonders C, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7(9):687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of Cortical Interneuron Subtypes. J Neuroscience. 2004;24(11):2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby K, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J. Com Neurol. 2006;499:144–160. doi: 10.1002/cne.21101. [DOI] [PubMed] [Google Scholar]
- Yan XX, Cao QL, Luo XG, Garey LJ. Prenatal Development of Calbindin D-28K in Human Visual Cortex. Cer Cortex. 1997;7:57–62. doi: 10.1093/cercor/7.1.57. [DOI] [PubMed] [Google Scholar]
- Yang Z. Postnatal Subventricular zone progenitors give rise not only to granular and periglomerular interneurons but also to interneurons in the external plexiform layer of the rat olfactory bulb. J Com Neurol. 2008;506:347–358. doi: 10.1002/cne.21557. [DOI] [PubMed] [Google Scholar]
- Yanez IB, Munoz A, Contreras J, Gonzalez J, Rodriguez-Veiga E, DeFelipe J. Double Bouquet Cell in the Human Cerebral Cortex and a Comparison with Other mammals. The J Com Neurology. 2005;486:344–360. doi: 10.1002/cne.20533. [DOI] [PubMed] [Google Scholar]
- Yozu M, Tabata H, Nakajima K. The caudal migratory stream: a novel migratory stream of interneurons derived from the caudal ganglionic eminence in the developing mouse forebrain. J Neurosci. 2005;25:7268–7277. doi: 10.1523/JNEUROSCI.2072-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Jakovcevski I, Mo Z, Zecevic N. Cortical origin of calretinin-expressing neurons in the human fetal forebrain. 2009 Abstract for SFN 2009 Oct. [Google Scholar]
- Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kroner S, Lewis DA, Krimer LS. Localization of Calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cer Cortex. 2005;15:1178–1186. doi: 10.1093/cercor/bhh218. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Gonzalez-Burgos G, Rotaru D, Fish KN, Krimer LS, Lewis DA. Interneuron Diversity in Layers 2–3 of Monkey Prefrontal Cortex. Cer Cortex. 2009;19:1597–1615. doi: 10.1093/cercor/bhn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N. Synaptogenesis in Layer I of the Human Cerebral Cortex in the First Half of Gestation. Cer Cortex. 1998;8(3):245–252. doi: 10.1093/cercor/8.3.245. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Chen Y, Filipovic R. Contributions of Cortical Subventricular Zone to the Development of the Human Cerebral Cortex. J.Com Neurol. 2005;491:109–122. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Milosevic A. Initial development of gamma-aminobutyric acid immunoreactivity in the human cerebral cortex. J Com Neurol. 1997;380:495–506. doi: 10.1002/(sici)1096-9861(19970421)380:4<495::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Milosevic A, Rakic S, Marin-Padilla M. The human neocortex: Early development and composition of the Primordial Plexiform Layer. An immunohistochemical study. J Com Neurol. 1999;412:241–254. [PubMed] [Google Scholar]
- Zecevic N, Rakic P. Development of Layer I neurons in the Primate Cerebral Cortex. J Neurosci. 2001;21(15):5607–5619. doi: 10.1523/JNEUROSCI.21-15-05607.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]