Abstract
The phytohormone abscisic acid (ABA) has been shown to be effective in ameliorating chronic and acute inflammation. The objective of this study was to investigate whether ABA’s anti-inflammatory efficacy in the gut is dependent on peroxisome proliferator activated receptor γ (PPAR γ) in T cells. PPAR γ-expressing and T cell-specific PPAR γ null mice were fed diets with or without ABA (100 mg/kg) for 35 days prior to challenge with 2.5% dextran sodium sulfate (DSS). The severity of clinical disease was assessed daily, and mice were euthanized on day 7 of the DSS challenge. Colonic inflammation was assessed through macroscopic and histopathological examination of inflammatory lesions and real-time quantitative RT-PCR-based quantification of inflammatory genes. Flow cytometry was used to phenotypically characterize leukocyte populations in the blood and mesenteric lymph nodes (MLN). Colonic sections were stained immunohistochemically to determine the effect of ABA on colonic regulatory T (Treg) cells. ABA’s beneficial effects on disease activity were completely abrogated in T cell-specific PPAR γ null mice. Additionally, ABA improved colon histopathology, reduced blood F4/80+CD11b+ monocytes, increased the percentage of CD4+ T cells expressing the inhibitory molecule cytotoxic T lymphocyte antigen 4 (CTLA4) in blood, and enhanced the number of Treg cells in the MLN and colons of PPAR γ expressing but not T cell-specific PPAR γ null mice. We conclude that dietary ABA ameliorates experimental IBD by enhancing Treg accumulation in the colonic lamina propria through a PPAR γ-dependent mechanism.
Keywords: abscisic acid, PPAR γ, regulatory T cells, inflammatory bowel disease
1. Introduction
Inflammatory bowel disease (IBD), with its two clinical manifestations Crohn’s Disease (CD) and Ulcerative Colitis (UC), is characterized by significant inflammation and immune cell infiltration into the gastrointestinal (GI) tract [1]. There is a need for novel safer and more effective therapies, as the current IBD medications, including aminosalicylates, corticosteroids, immunosuppressors, and anti-tumor necrosis factor α (TNF-α) therapy, are modestly effective often have significant side effects [2]. Activation of PPAR γ by the thiazolidinedione (TZD), rosiglitazone, has shown efficacy in the treatment of human patients with UC [3,4]. However, TZDs are unlikely to be adopted for the treatment of IBD due to their significant side effects, including fluid retention, hepatotoxicity, weight gain and congestive heart failure [5].
Recent work in our laboratory has focused on the phytohormone abscisic acid (ABA) and its effects on inflammation in animal models of inflammation. For instance, we have demonstrated that ABA intake improves glucose tolerance and reduces white adipose tissue inflammation without discernable side effects in mouse models of obesity and diabetes [6,7]. The nuclear receptor peroxisome proliferator-activated receptor γ (PPAR γ), which is highly expressed in adipose tissue, colonic epithelial cells, and immune cells, was key in this response, as the deficiency of PPAR γ in immune cells resulted in a significantly diminished response to ABA’s antidiabetic effects [7]. PPAR γ has been shown to be influential in diminishing the inflammatory response and promoting an alternative M2 macrophage phenotype [8]. In lymphocytes it has been shown to be important in the function of CD4+CD25+FoxP3+ regulatory T cells (Treg) [9,10], and the regulation of colonic expression of adhesion molecules as well as recruitment of Treg into the inductive mucosal sites [11].
Recently we demonstrated that dietary ABA significantly decreases gut inflammation and colonic expression of cellular adhesion cell molecules. We also demonstrated that ABA treatment increases the expression of the immunosuppressive protein CTLA4 on the surface of PPAR γ-expressing CD4+ T cells. Based on these findings, we sought to determine whether T cell PPAR γ expression is required for ABA’s anti-inflammatory protective actions against experimental IBD [12]. This study demonstrates for the first time that ABA-induced inhibition of DSS colitis is abolished in mice lacking PPAR γ in T cells. ABA increases blood CTLA4+CD4+ T cells and Treg numbers in mesenteric lymph nodes (MLN) and colons of mice expressing PPAR γ, whereas in mice that lack T cell PPAR γ, the ABA-mediated modulation of regulatory CD4+ T cell subsets is impaired and ABA modestly exacerbates colitis. These findings demonstrate that T cell PPAR γ is critical for ABA’s anti-inflammatory effects in experimental IBD.
2. Materials and Methods
2.1 Animal Procedures
Six to eight week old PPAR γ flfl; CD4Cre− (wild-type; WT) and PPAR γ flfl; CD4Cre+ (T cell-specific PPAR γ null mice) in a C57BL/6J background (n=35) were housed at the animal facilities at Virginia Polytechnic Institute and State University in a room maintained at 75° F, with a 12:12 h light-dark cycle starting from 6:00 AM. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Virginia Polytechnic Institute and State University and met or exceeded requirements of the Public Health Service/National Institutes of Health and the Animal Welfare Act. Mice undergoing in vivo experimental treatments were fed AIN-93G-based purified diets with or without 100 mg/kg of a racemic ABA mixture for 35 days prior to induction of colitis with water containing 2.5% dextran sodium sulfate (DSS), 36,000–44,000 mol wt (ICN Biomedicals, Aurora, OH). Following the DSS challenge mice were weighed on a daily basis and examined for clinical signs of disease associated with colitis (i.e., perianal soiling, rectal bleeding, diarrhea, and piloerection). For the DSS challenge, the disease activity indices and rectal bleeding scores were calculated using a modification of a previously published compounded clinical score [13]. Mice in the DSS study were euthanized on day 7 of the DSS challenge by carbon dioxide narcosis followed by secondary thoracotomy and blood was withdrawn from the heart. Spleen and mesenteric lymph nodes (MLN) were scored based on size (splenomegaly or adenopathy) and macroscopic inflammatory lesions (0-3), excised, and single-cell suspensions were generated as previously described [9].
2.2 Histopathology
Colonic sections were fixed in 10% buffered neutral formalin, later embedded in paraffin, and then sectioned (5 μm) and stained with H&E stain for histologic examination. Colons were graded with a compounded histologic score including the extent of (1) leukocyte infiltration, (2) mucosal thickening, and (3) epithelial cell erosion. The sections were graded with a score of 0–4 for each of the previous categories and data were analyzed as a normalized compounded score.
2.3 Immunophenoptying of blood and mesenteric lymph nodes
MLN were excised, crushed with frosted slides, and were resuspended in PBS and enumerated with a Coulter Counter (Beckman Coulter, Fullerton, CA). MLN-derived cells (2 × 105 cells/well) or whole blood (10 μL/well) were seeded onto 96-well plates, centrifuged at 4°C at 3000 rpm for 4 minutes, and washed with PBS containing 5% serum and 0.09% sodium azide (FACS buffer). To assess differential monocyte/macrophage subsets, the cells were then incubated in the dark at 4°C for 20 minutes in FcBlock (20 μg/ml; BD Pharmingen), and then for an additional 20 minutes with fluorochrome-conjugated primary antibodies anti-F4/80, anti-Ly6c and anti-CD11b as previously shown [14]. For lymphocyte assessment, cells were incubated with anti-CD4, anti-CD8, CD3, anti-FoxP3-PE, anti-CD152 (CTLA4) as previously shown [9,14]. Flow results were computed with a BD LSR II flow cytometer and data analyses was performed with FACS Diva software (BD).
2.4 Immunohistochemistry
Immunohistochemistry was performed as previously described [15]. Regulatory T cells (Treg) were detected with anti-CD4 conjugated with FITC (1:100; eBioscience) and co-localized with anti-FoxP3 conjugated with biotin (1:100; eBioscience). Immunodetection of FoxP3 was performed using streptavidin conjugated with Alexa Fluor 546 (1 mg/L; Invitrogen). All images were captured with a Nikon Eclipse TE2000-U and analyzed with NIS-Elements (Nikon Instruments Inc., Melvile, NY).
2.5 Quantitative Real-Time RT-PCR
Total RNA was isolated from colons using the RNA isolation Minikit (Qiagen) according to the manufacturer’s instructions. Total RNA (1 μg) was used to generate complementary DNA (cDNA) template using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). The total reaction volume was 20 μL with the reaction incubated as follows in an MJ MiniCycler: 5 minutes at 25°C, 30 minutes at 52°, 5 minutes at 85°C, and hold at 4°C. PCR was performed on the cDNA using Taq DNA polymerase (Invitrogen, Carlsbad, CA) and using previously described conditions [13]. Each gene amplicon was purified with the MiniElute PCR Purification Kit (Qiagen) and quantitated on an agarose gel by using a DNA mass ladder (Promega). These purified amplicons were used to optimize real-time PCR conditions and to generate standard curves in the real-time PCR assay. Primer concentrations and annealing temperatures were optimized for the iCycler iQ system (Bio-Rad) for each set of primers using the system’s gradient protocol. PCR efficiencies were maintained between 92 and 105% and correlation coefficients above 0.98 for each primer set during optimization and also during the real-time PCR of sample DNA.
Complementary DNA (cDNA) concentrations for genes of interest were examined by real-time quantitative PCR using an iCycler IQ System and the iQ SYBR green supermix (Bio-Rad). A standard curve was generated for each gene using 10-fold dilutions of purified amplicons starting at 5 pg of cDNA and used later to calculate the starting amount of target cDNA in the unknown samples. SYBR green I is a general double-stranded DNA intercalating dye and may therefore detect nonspecific products and primer/dimers in addition to the amplicon of interest. In order to determine the number of products synthesized during the real-time PCR, a melting curve analysis was performed on each product. Real-time PCR was used to measure the starting amount of nucleic acid of each unknown sample of cDNA on the same 96-well plate. Results were normalized to the housekeeping gene β-actin for each sample, and accession numbers for each gene examined are outlined in Table 1.
Table 1.
| Primer | Sequence | Length | Accession Number |
|---|---|---|---|
| βactinF | 5′CCCAGGCATTGCTGACAGG3′ | 141 | X03672 |
| βactinR | 5′TGGAAGGTGGACAGTGAGGC3′ | ||
| PPAR γF | 5′CAGGCTTGCTGAACGTGAAG3′ | 117 | NM_011146 |
| PPAR γR | 5′GGAGCACCTTGGCGAACA3′ | ||
| E-selectinF | 5′CAGCTTTGCATGATGGCGTCT3′ | 83 | NM_011345 |
| E-selectinR | 5′GAAGGGTACAGGCGAGTTGG3′ | ||
| VCAM-1F | 5′TCTCCCAGGAATACAACGAT3′ | 75 | NM_011693 |
| VCAM-1R | 5′ACAGGTCATTGTCACAGCAC3′ | ||
| MAdCAM-1F | 5′CCCATGGCCACAGCTACCTCA3′ | 85 | NM_013591 |
| MAdCAM-1R | 5′CCCTGGCCCTAGTACCCTAC3′ | ||
| MMP-9F | 5′GGTGGCAGCGCACGAGTT3′ | 128 | NM_013599 |
| MMP-9R | 5′GGATGCCGTCTATGTCGTCTT3′ | ||
| IL-6F | 5′TTTCCTCTGGTCTTCTGGAG3′ | 92 | NM_031168 |
| IL6-R | 5′CTGAAGGACTCTGGCTTTGT3′ | ||
| IFNγ F | 5′GGGGGTGGGGGACAGC3′ | 140 | K00083 |
| IFNγ R | TGGCCCGGAGTGTAGACATC3′ |
F, forward; R, reverse. PCR primer pairs were designed for an optimal annealing temperature of 57°C and product lengths between 78 and 157 base pairs.
When plotting threshold cycle versus log starting quantity (pg), standard curves had slopes between −3.1 and −3.7; PCR efficiencies between 92 and 105 and R2 above 0.98.
2.6 Statistics
Data were analyzed as a 2 × 2 factorial arrangement within a completely randomized design. To determine the statistical significance of the model, analysis of variance (ANOVA) was performed using the general linear model procedure of Statistical Analysis Software (SAS), and probability value (P) < 0.05 was considered to be significant. When the model was significant, ANOVA was followed by Fisher’s Protected Least Significant Difference multiple comparison method.
3. Results
3.1 The deficiency of PPAR γ in T cells abrogates the beneficial effects of ABA on the severity of experimental IBD
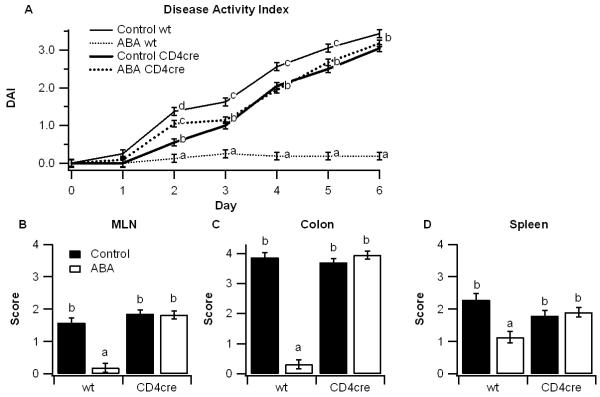
We have previously demonstrated that dietary supplementation with ABA significantly reduces obesity-related inflammation in adipose tissue, T cell and macrophage infiltration in aortic atherosclerotic plaques, and colonic inflammation [6,16,17]. Our objective in this study was to investigate whether T cell PPAR γ is an essential mediator of ABA’s anti-inflammatory mechanism. PPAR γ-expressing and T cell-specific PPAR γ-deficient mice (CD4cre) mice were fed diets with or without ABA (100 mg/kg) for 35 days prior to a 7-day challenge with DSS. Throughout the challenge period, disease severity was significantly reduced by dietary ABA in PPAR γ expressing mice, though not in T cell-specific PPAR γ null mice (Figure 1A). At day 7 post-DSS challenge colons, spleens, and mesenteric lymph nodes (MLN) also showed significantly fewer macroscopic gross lesions (i.e, splenomegaly and adenopathy) in PPAR γ-expressing mice administered ABA-supplemented diets in comparison to the other treatment groups (Figures 1B-1D).
Figure 1.
Effect of dietary abscisic acid (ABA)-supplementation on disease severity in PPAR γ-expressing and T cell-specific PPAR γ null mice. PPAR γ flfl;CD4 Cre− (wt) or PPAR γ flfl; CD4 Cre+ (CD4cre) mice were fed ABA-supplemented (100 mg/kg) or control diets for 35 days and challenged with 2.5% dextran sodium sulfate (DSS) for 7 days. The disease activity index (DAI), a composite score reflecting clinical signs of the disease (i.e. perianal soiling, rectal bleeding, diarrhea, and piloerection) was assessed daily (A). On day 7 mice were euthanized and the colon, spleen, and mesenteric lymph nodes (MLN) (B-D) were macroscopically scored for inflammation. Statistically significant differences (P<0.05) between groups are indicated with different letter subscripts (n=8 mice/group) in three independent experiments.
3.2 ABA improves colonic histopathology PPAR γ-expressing but not in T cell-specific PPAR γ null mice
We next examined the effect of ABA and T cell-specific PPAR γ deficiency on histopathological colonic inflammatory lesions. In line with our observations of disease severity and gross lesions, ABA’s anti-inflammatory effects were significantly attenuated in mice lacking PPAR γ in T cells (Figure 2). Histopathological scores of epithelial erosion, leukocyte infiltration, and mucosal thickening were significantly reduced in ABA-fed PPAR γ-expressing mice though this effect was abrogated in mice lacking PPAR γ in T cells (Figure 2).
Figure 2.
Effect of dietary abscisic acid (ABA) supplementation on colon histopathology in PPAR γ-expressing and T cell-specific PPAR γ null mice. PPAR γ flfl;CD4 Cre− (wt) and PPAR γ flfl; CD4 Cre+ (CD4cre) were fed ABA-supplemented (100 mg/kg) or control diets for 35 days and challenged with 2.5% dextran sodium sulfate (DSS) or water (no DSS) for 7 days. Representative photomicrographs from wt, control (A), wt, ABA (B), CD4cre, control (C), and CD4cre, ABA (D) groups are illustrated (Original magnification, 40×). All specimens underwent blinded histological examination and were scored (1-4) on epithelial erosion (E), mucosal wall thickening (F), and leukocyte infiltration (G). Data are represented as mean ± standard error. Statistically significant differences (P<0.05) between groups are indicated with different letter subscripts (n=8 mice/group) in three independent experiments.
3.3 Dietary ABA reduces blood monocytes and enhances the numbers of CTLA4-expressing and regulatory CD4+ T cells in blood and MLN of PPAR γ-expressing mice
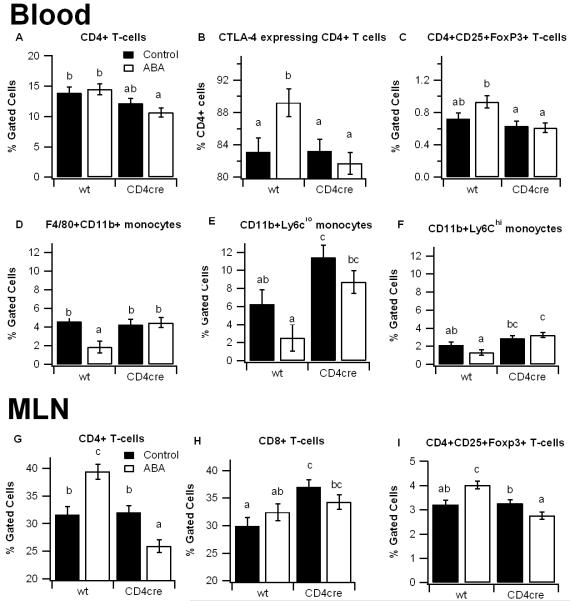
To more closely investigate the effect of ABA on immune cells we phenotypically characterized monocyte/macrophage and lymphocyte subsets in blood and mesenteric lymph nodes (MLN). In blood, ABA modestly increased CD4+CD25+FoxP3+ Treg cells and significantly enhanced the percent of CD4+ T cells expressing the inhibitory surface CTLA4 (CD152) in PPAR γ-expressing mice. The percentage of CD4+ T cells and Tregs were both significantly reduced in the T cell-specific PPAR γ null mice in comparison to PPAR γ-expressing mice.
Dietary ABA-supplementation also significantly reduced the percent of F4/80+CD11b+ blood monocytes in PPAR γ-expressing mice, however this effect was impaired in the T cell-specific PPAR γ-deficient mice. We also examined the percent of CD11b+Ly6clo (Ly6clo) and CD11b+Ly6chi (Ly6chi) monocytes. Ly6Chi monocytes have been noted for high expression of CCR2 and a propensity for differentiating in to M1 pro-inflammatory macrophages, whereas Ly6clo monocytes, which highly express the fractalkine receptor CX3CR1, represent a more mature phenotype with greater phagocytic capacity [18]. Here, both Ly6chi and Ly6clo monocytes were significantly upregulated in T cell-specific PPAR γ null mice compared to PPAR γ-expressing mice. In addition, dietary ABA caused a decrease in numbers of both monocyte subsets. In MLN, dietary ABA supplementation significantly enhanced the percentage of CD4+ T cells and Tregs in PPAR γ-expressing mice and it exerted the reverse effect in tissue-specific PPAR γ null mice (Figures 3G-3I).
Figure 3.
Effect of dietary abscisic acid (ABA) supplementation on lymphocyte subsets in PPAR γ-expressing and T cell-specific PPAR γ null mice. PPAR γ flfl;CD4 Cre− (wt) and PPAR γ flfl; CD4 Cre+ (CD4cre) mice were fed ABA-supplemented (100 mg/kg) or control diets for 35 days and challenged with 2.5% dextran sodium sulfate (DSS) for 7 days. Blood (A-C) and MLN (D-E) were immunophenotyped with anti-CD3, anti-CD4, anti-CD8, anti-CTLA4, anti-FoxP3, anti-F4/80, and anti-CD11b mouse monoclonal antibodies and the immune cell populations were analyzed by flow cytometry with FACS diva software. Data are represented as mean ± standard error. Statistically significant differences (P<0.05) between groups are indicated with an asterisk (n=8 mice/group) in three independent experiments.
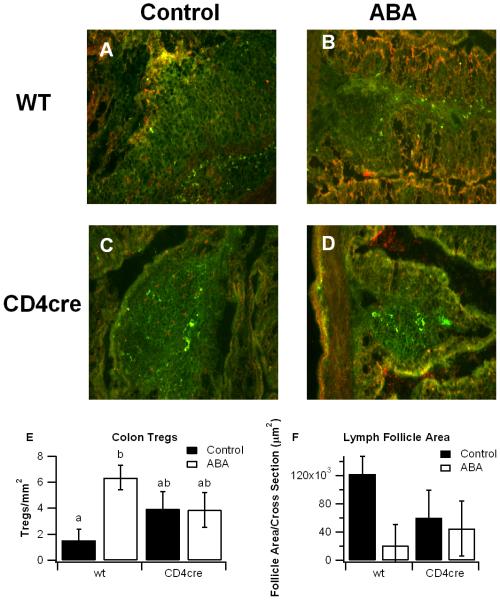
3.4 Dietary ABA increases Treg numbers in the colonic mucosa
Our data indicates that ABA’s anti-inflammatory effects are significantly mitigated in mice deficient in T cell PPAR γ. PPAR γ has been shown to be important in the function of Treg [9,19], which serve as down-regulators of inflammation in the gastrointestinal tract [20,21]. We find here that ABA significantly increases the number of Treg in the colonic lamina propria of PPAR γ-expressing mice, an effect that is attenuated in the colons T cell-specific PPAR γ null mice (Figure 4). ABA also caused a numerical reduction in the average size of lymphoid follicles in PPAR γ-expressing mice (Figure 4).
Figure 4.
Effect of dietary abscisic acid (ABA) supplementation on colonic regulatory T (Treg) cells in PPAR γ-expressing and T cell-specific PPAR γ null mice. PPAR γ flfl;CD4 Cre− (wt) and PPAR γ flfl; CD4 Cre+ (CD4cre) mice were fed ABA-supplemented (100 mg/kg) or control diets for 35 days and challenged with 2.5% dextran sodium sulfate (DSS) for 7 days. Treg cells were detected with anti-CD4 conjugated with FITC (1:100) and co-localized with anti-FoxP3 conjugated with biotin (1:100). Immunodetection of FoxP3 was performed using streptavidin conjugated with Alexa Fluor 546 (1 mg/L). Representative images from wt, control (A), wt, ABA (B), CD4cre, control (C), and CD4cre, ABA (D) groups are depicted (Original magnification, 200×). The number of colonic T-regs (E) and the lymphoid follicle areas (F) were assessed for each group. Data are represented as mean ± standard error. Statistically significant differences (P<0.05) between groups are indicated with different letter subscripts (n=8 mice/group) in three independent experiments.
3.5 Dietary ABA down-regulates expression of adhesion molecules and pro-inflammatory cytokines in PPAR γ-expressing but not in T cell-specific PPAR γ-deficient mice
We have previously shown that dietary ABA-supplementation reduces expression of adhesion molecules VCAM-1, MAdCAM-1, and E-selectin and pro-inflammatory cytokines IL-6 and matrix metalloprotease-9 (MMP-9) in colons of DSS treated mice [11,17]. Here, we used real-time RT-PCR to investigate the differential effect of ABA on gene expressions in colons of PPAR γ-expressing and T cell-specific PPAR γ null mice. ABA inhibited mRNA expression of adhesion molecules and pro-inflammatory cytokines in wild-type mice. In contrast, dietary ABA-supplementation resulted in a net increase of inflammatory markers in colons of T cell-specific PPAR γ null mice (Figure 5). Specifically, mRNA concentrations of MAdCAM-1, VCAM-1, E-selectin, IL-6, and MMP-9 were all elevated in ABA-fed T cell-specific PPAR γ null mice in comparison to control-fed mice (Figure 5).
Figure 5.
Differential effect of abscisic acid (ABA) on colon gene expression in PPAR γ-expressing and T cell-specific PPAR γ deficient mice. PPAR γ flfl;CD4 Cre− (wt) and PPAR γ flfl; CD4 Cre+ (CD4cre) mice were fed ABA-supplemented (100 mg/kg) or control diets for 35 days and challenged with 2.5% dextran sodium sulfate (DSS) for 7 days. Expression of (A) peroxisome proliferator-activated receptor γ (PPAR γ), (B) mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1), (C) vascular adhesion molecule 1 (VCAM-1), (D) E-selectin, (E) interleukin 6 (IL-6), (F) matrix metalloproteinase 9 (MMP-9), and (G) interferon γ (IFN-γ) was quantified by real-time quantitative RT-PCR. Statistically significant differences (P<0.05) between groups are indicated with different letter subscripts (n=8 mice/group) in three independent experiments. (H) The effect of ABA treatment on colonic gene expression for each genotype was calculated as a relative expression value with respect to the control treatment. Data are represented as mean ± standard error. Points with an asterisk are significantly different (P<0.05). Statistically significant differences (P<0.05) between groups are indicated with an asterisk (n=8 mice/group) in three independent experiments.
4. Discussion
ABA is a phytohormone discovered and chemically synthesized in 1965 involved in plant growth, response to stress dormancy and life cycle [22]. Interestingly, recent evidence demonstrates that ABA exerts pleiotropic effects in a number of animal models of inflammatory diseases [23]. For instance, dietary ABA has shown efficacy in suppressing inflammatory macrophage accumulation into abdominal white adipose tissue, thereby ameliorating peripheral insulin resistance and inflammation in mouse models of type II diabetes and obesity [6,7,24]. ABA at nanomolar concentrations acts locally on human pancreatic β-cells and enhances their insulin-secreting ability [25]. In addition, dietary ABA ameliorates atherosclerotic plaque formation in the aortas of ApoE−/− mice by suppressing macrophage and CD4+ T cell infiltration into the intima and media layers of the aortic root walls [26]. In line with these findings indicating anti-inflammatory efficacy of ABA disease models, more recently we have demonstrated that dietary ABA prevents or ameliorates colonic inflammation in a mouse model of experimental IBD [17]. This study demonstrated that ABA treatment significantly reduces adhesion molecule expression and colitis, and in vitro examination of cultured splenocytes indicated that PPAR γ-dependent upregulation of CTLA4 expression on T cells may be involved in ABA’s immunomodulatory mechanism [17]. Thus, the objective of this study was to examine whether the deficiency of T cell PPAR γ significantly impairs ABA’s effect on DSS colitis severity.
PPAR γ-expressing or T cell-specific PPAR γ-deficient mice were fed diets with or without ABA (100 mg/kg) for 35 days prior to a 7-day induction of colitis. The results from the DSS challenge demonstrated that the absence of PPAR γ in T cells essentially abolishes the ability of ABA to decrease experimental IBD, including the anti-inflammatory action of ABA in the colon. Of note, ABA increased the numbers of Treg in MLN and the colonic lamina propria. Interestingly, this effect of ABA was abrogated in T cell-specific PPAR γ null mice, indicating that T cell PPAR γ was required for the regulation of Treg number in the mucosal inductive (MLN) and effector sites (colonic lamina propria). The finding that ABA’s anti-inflammatory effects are dependent on functional PPAR γ in immune cells is in line with our previous findings in a model of diet-induced obesity, in which the absence of PPAR γ in hematopoietic and epithelial cells significantly impaired ABA-induced improvements in glucose tolerance and adipose tissue macrophage infiltration [7].
One factor possibly contributing to the improvements by ABA was the enhancement of CTLA4 expression on CD4+ T-cells. We had previously reported that ABA increases CTLA4 expression in vitro in CD3/CD28-stimulated CD4+ T cells in a PPAR γ-dependent manner [17]. CTLA4 is a molecule involved in down-regulation of T cell responses that is highly expressed by naturally occurring CD4+CD25+ Treg cells [27]. The importance of PPAR γ in Treg function is well established [9,10], as too is the importance of CTLA4 for the in vivo functional effects of Tregs [28,29]. Thus, ABA may enhance Treg numbers and their function by activating PPAR γ. Further studies are warranted to dissect ABA’s effect on effector versus regulatory T cells during chronic intestinal inflammation in adoptive co-transfer models of T cell-induced colitis.
Dietary ABA worsened colonic inflammation and enhances cellular adhesion molecule expression in T cell-specific PPAR γ null mice. The finding that ABA can worsen inflammation in mammalian cells is not surprising since studies by Bruzzone et al and Magnone et al have shown that ABA exerts pro-inflammatory effects in human granulocytes and monocytes in vitro [30,31], and these authors have suggested that ABA may act as an endogenously generated pro-inflammatory signaling molecule. Specifically, Magnone et al posited that ABA evokes an intracellular calcium rise through the second messenger cyclic ADP-ribose, leading to nuclear factor-κB (NF-κB) activation in monocytes [31]. Our findings in several studies [6,7,12,24,26] do not support their conclusion but suggest rather that T cell PPAR γ is important in ABA’s systemic and mucosal anti-inflammatory effects in vivo. Nonetheless, since ABA treatment worsened the colitis severity in mice lacking PPAR γ in T cells, it is tempting to speculate that ABA-mediated activation of anti-inflammatory pathways predominates when PPAR γ is expressed in T cells, whereas pro-inflammatory pathways such as NF-κB become ABA’s targets PPAR γ is deleted from T cells. Interestingly, T cell-specific PPAR γ null mice did not respond to oral ABA administration despite expressing PPAR γ in macrophages, dendritic cells, neutrophils, endothelial cells and epithelial cells; all of which contribute to the pathogenesis of DSS colitis. Together these findings indicate that T cell PPAR γ is a crucial mediator of ABA’s anti-inflammatory responses. More investigation is needed to characterize the cell specificity ABA’s immune modulatory mechanisms.
Another aspect that remains unknown is how ABA interacts with PPAR γ to suppress inflammation. There is currently data showing that ABA may act through the lanthionine synthetase C-like protein (LANCL2) to increase intracellular levels of cAMP, CD38-produced cADP-ribose, and calcium [32]. Recent findings from our laboratory suggest that PPAR γ activation by ABA may occur downstream of the cAMP/PKA signaling axis [24]. We have also identified the binding site of PPAR γ agonists, including ABA, on LANCL2 [33]. Further insight into this response may be key to understanding the cell-specific response of ABA-induced PPAR γ activation and how it resembles and differs from the mechanism of PPAR γ activation of the TZD class of anti-diabetic drugs that is associated with significant adverse side effects described in the introduction.
In conclusion, we find that dietary ABA-supplementation significantly ameliorates disease activity and colonic inflammatory lesions during experimental IBD through a T cell PPAR γ-dependent mechanism. This study suggests that ABA may be effective as a therapeutic or prophylactic agent for IBD and other autoimmune disorders in which T cell-mediated immunoregulation exerts a beneficial effect.
Acknowledgments
Supported by award number 5R01AT4308 of the National Center for Complementary and Alternative Medicine at the National Institutes of Health awarded to J.B.-R., European Commission grant number 224836, the Ramon y Cajal Program and funds from the Nutritional Immunology and Molecular Nutrition Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Hanauer SB. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S3–9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 2.Mackner LM, Sisson DP, Crandall WV. Review: Psychosocial issues in pediatric inflammatory bowel disease. J Pediatr Psychol. 2004;29:243–57. doi: 10.1093/jpepsy/jsh027. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JD, Lichtenstein GR, Deren JJ, et al. Rosiglitazone for active ulcerative colitis: A randomized placebo-controlled trial. Gastroenterology. 2008;134:688–95. doi: 10.1053/j.gastro.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis JD, Lichtenstein GR, Stein RB, et al. An open-label trial of the ppar-gamma ligand rosiglitazone for active ulcerative colitis. Am J Gastroenterol. 2001;96:3323–8. doi: 10.1111/j.1572-0241.2001.05333.x. [DOI] [PubMed] [Google Scholar]
- 5.Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the american heart association and american diabetes association. Circulation. 2003 October 7;108:2941–8. doi: 10.1161/01.CIR.0000103683.99399.7E. 2003. [DOI] [PubMed] [Google Scholar]
- 6.Guri AJ, Hontecillas R, Si H, Liu D, Bassaganya-Riera J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin Nutr. 2007;26:107–16. doi: 10.1016/j.clnu.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Guri AJ, Hontecillas R, Ferrer G, et al. Loss of ppar gamma in immune cells impairs the ability of abscisic acid to improve insulin sensitivity by suppressing monocyte chemoattractant protein-1 expression and macrophage infiltration into white adipose tissue. J Nutr Biochem. 2008;19:216–28. doi: 10.1016/j.jnutbio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific ppargamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator-activated receptor gamma is required for regulatory cd4+ t cell-mediated protection against colitis. J Immunol. 2007;178:2940–9. doi: 10.4049/jimmunol.178.5.2940. [DOI] [PubMed] [Google Scholar]
- 10.Wohlfert EA, Nichols FC, Nevius E, Clark RB. Peroxisome proliferator-activated receptor gamma (ppargamma) and immunoregulation: Enhancement of regulatory t cells through ppargamma-dependent and -independent mechanisms. J Immunol. 2007;178:4129–35. doi: 10.4049/jimmunol.178.7.4129. [DOI] [PubMed] [Google Scholar]
- 11.Guri AJ, Mohapatra SK, Horne WT, Hontecillas R, Bassaganya-Riera J. The role of t cell ppar gamma in mice with experimental inflammatory bowel disease. BMC Gastroenterology. 2010 doi: 10.1186/1471-230X-10-60. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guri AJ, Hontecillas R, Bassaganya-Riera J. Abscisic acid ameliorates experimental ibd by downregulating cellular adhesion molecule expression and suppressing immune cell infiltration. Clinical Nutrition. 2010 doi: 10.1016/j.clnu.2010.02.009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassaganya-Riera J, Reynolds K, Martino-Catt S, et al. Activation of ppar gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127:777–91. doi: 10.1053/j.gastro.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 14.Bassaganya-Riera J, Misyak S, Guri AJ, Hontecillas R. Ppar gamma is highly expressed in f4/80(hi) adipose tissue macrophages and dampens adipose-tissue inflammation. Cell Immunol. 2009;258:138–46. doi: 10.1016/j.cellimm.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans NP, Misyak SA, Schmelz EM, Guri AJ, Hontecillas R, Bassaganya-Riera J. Conjugated linoleic acid ameliorates inflammation-induced colorectal cancer in mice through activation of ppargamma. J Nutr. 140:515–21. doi: 10.3945/jn.109.115642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guri AJ, Misyak SA, Hontecillas R, et al. Abscisic acid ameliorates atherosclerosis by suppressing macrophage and cd4(+) t cell recruitment into the aortic wall. J Nutr Biochem. 2010 doi: 10.1016/j.jnutbio.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guri AJ, Hontecillas R, Bassaganya-Riera J. Abscisic acid ameliorates experimental ibd by downregulating cellular adhesion molecule expression and suppressing immune cell infiltration. Clin Nutr. 2010 doi: 10.1016/j.clnu.2010.02.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee PY, Li Y, Kumagai Y, et al. Type i interferon modulates monocyte recruitment and maturation in chronic inflammation. Am J Pathol. 2009;175:2023–33. doi: 10.2353/ajpath.2009.090328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Straus DS, Glass CK. Anti-inflammatory actions of ppar ligands: New insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–8. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Aharoni R, Sonego H, Brenner O, Eilam R, Arnon R. The therapeutic effect of glatiramer acetate in a murine model of inflammatory bowel disease is mediated by anti-inflammatory t-cells. Immunol Lett. 2007;112:110–9. doi: 10.1016/j.imlet.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Horino J, Fujimoto M, Terabe F, et al. Suppressor of cytokine signaling-1 ameliorates dextran sulfate sodium-induced colitis in mice. Int Immunol. 2008;20:753–62. doi: 10.1093/intimm/dxn033. [DOI] [PubMed] [Google Scholar]
- 22.Cornforth JW, Milborrow BV, Ryback G. Synthesis of (+/−) abscisin ii. Nature. 1965;406:715. doi: 10.1038/211742b0. [DOI] [PubMed] [Google Scholar]
- 23.Bassaganya-Riera J, Skoneczka J, Kingston DGJ, et al. Mechanisms of action and medicinal applications of abscisic acid. Current Medicinal Chemistry. 2009 doi: 10.2174/092986710790226110. In press. [DOI] [PubMed] [Google Scholar]
- 24.Guri AJ, Hontecillas R, Bassaganya-Riera J. Abscisic acid synergizes with rosiglitazone to improve glucose tolerance and down-modulate macrophage accumulation in adipose tissue: Possible action of the camp/pka/ppar gamma axis. Clinical Nutrition. 2010 doi: 10.1016/j.clnu.2010.02.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruzzone S, Bodrato N, Usai C, et al. Abscisic acid is an endogenous stimulator of insulin release from human pancreatic islets with cyclic adp ribose as second messenger. J Biol Chem. 2008;283:32188–97. doi: 10.1074/jbc.M802603200. [DOI] [PubMed] [Google Scholar]
- 26.Guri AJ, Misyak S, Hontecillas R, et al. Abscisic acid ameliorates atherosclerosis by suppressing macrophage and cd4+ t cell recruitment into the aortic wall. Journal of Nutritional Biochemistry. 2010 doi: 10.1016/j.jnutbio.2009.10.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read S, Malmstrom V, Powrie F. Cytotoxic t lymphocyte-associated antigen 4 plays an essential role in the function of cd25(+)cd4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of ctla-4 and tgf-beta in cd4+cd25+ regulatory t cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 29.Kataoka H, Takahashi S, Takase K, et al. Cd25(+)cd4(+) regulatory t cells exert in vitro suppressive activity independent of ctla-4. Int Immunol. 2005;17:421–7. doi: 10.1093/intimm/dxh221. [DOI] [PubMed] [Google Scholar]
- 30.Bruzzone S, Moreschi I, Usai C, et al. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic adp-ribose as second messenger. Proc Natl Acad Sci U S A. 2007;104:5759–64. doi: 10.1073/pnas.0609379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnone M, Bruzzone S, Guida L, et al. Abscisic acid released by human monocytes activates monocytes and vascular smooth muscle cell responses involved in atherogenesis. J Biol Chem. 2009 doi: 10.1074/jbc.M809546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturla L, Fresia C, Guida L, et al. Lancl2 is necessary for abscisic acid binding and signaling in human granulocytes and in rat insulinoma cells. J Biol Chem. 2009;284:28045–57. doi: 10.1074/jbc.M109.035329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu P, Bevan DR, Lewis SN, Hontecillas R, Bassaganya-Riera J. Molecular modeling of lanthionine synthetase component c-like 2: A potential target for the discovery of novel type 2 diabetes prophylactics and therapeutics. Journal of Molecular Modeling. 2010 doi: 10.1007/s00894-010-0748-y. In press. [DOI] [PubMed] [Google Scholar]