Abstract
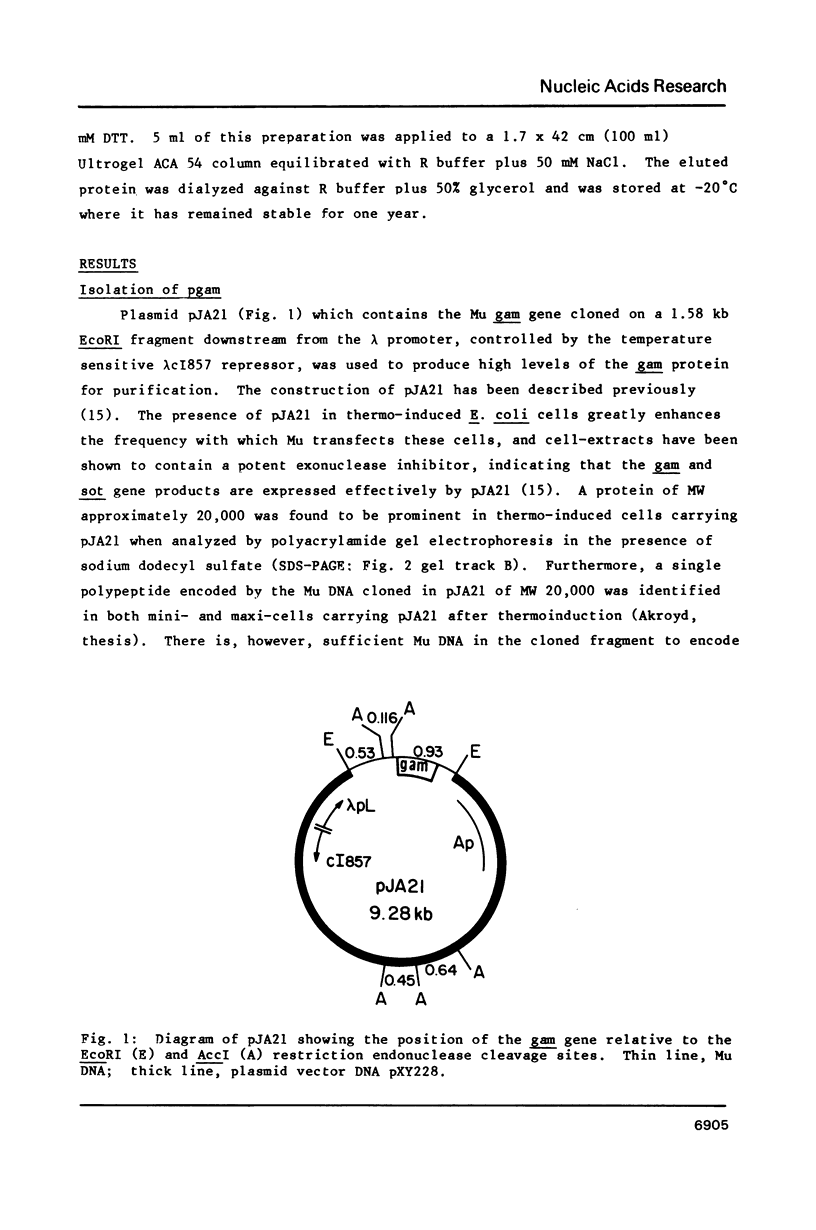
The gam gene of bacteriophage Mu encodes a protein which protects linear double stranded DNA from exonuclease degradation in vitro and in vivo. We purified the Mu gam gene product to apparent homogeneity from cells in which it is over-produced from a plasmid clone. The purified protein is a dimer of identical subunits of 18.9 kd. It can aggregate DNA into large, rapidly sedimenting complexes and is a potent exonuclease inhibitor when bound to DNA. The N-terminal amino acid sequence of the purified protein was determined by automated degradation and the nucleotide sequence of the Mu gam gene is presented to accurately map its position in the Mu genome.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akroyd J., Barton B., Lund P., Maynard Smith S., Sultana K., Symonds N. Mapping and properties of the gam and sot genes of phage mu: their possible roles in recombination. Cold Spring Harb Symp Quant Biol. 1984;49:261–266. doi: 10.1101/sqb.1984.049.01.030. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase C. D., Benzinger R. H. Transfection of Escherichia coli spheroplasts with a bacteriophage Mu DNA-protein complex. J Virol. 1982 Apr;42(1):176–185. doi: 10.1128/jvi.42.1.176-185.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie R., Arndt-Jovin D. J., Mizuuchi K. A defined system for the DNA strand-transfer reaction at the initiation of bacteriophage Mu transposition: protein and DNA substrate requirements. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7570–7574. doi: 10.1073/pnas.82.22.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie R., Mizuuchi K. Mechanism of transposition of bacteriophage Mu: structure of a transposition intermediate. Cell. 1985 Jul;41(3):867–876. doi: 10.1016/s0092-8674(85)80067-2. [DOI] [PubMed] [Google Scholar]
- Ghelardini P., Liebart J. C., Marchelli C., Pedrini A. M., Paolozzi L. Escherichia coli K-12 gyrB gene product is involved in the lethal effect of the ligts2 mutant of bacteriophage Mu. J Bacteriol. 1984 Feb;157(2):665–668. doi: 10.1128/jb.157.2.665-668.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelardini P., Paolozzi L., Liebart J. C. Restoration of ligase activity in E. coli K12 lig ts7 strain by bacteriophage Mu and cloning of a DNA fragment harbouring the Mu 'lig' gene. Nucleic Acids Res. 1980 Jul 25;8(14):3157–3173. doi: 10.1093/nar/8.14.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giphart-Gassler M., Reeve J., van de Putte P. Polypeptides encoded by the early region of bacteriophage Mu synthesized in minicells of Escherichia coli. J Mol Biol. 1981 Jan 5;145(1):165–191. doi: 10.1016/0022-2836(81)90339-9. [DOI] [PubMed] [Google Scholar]
- Goosen T., Giphart-Gassler M., Van de Putte P. Bacteriophage Mu DNA replication is stimulated by non-essential early functions. Mol Gen Genet. 1982;186(1):135–139. doi: 10.1007/BF00422925. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Higgins N. P., Geballe A. P., Snopek T. J., Sugino A., Cozzarelli N. R. Bacteriophage T4 RNA ligase: preparation of a physically homogeneous, nuclease-free enzyme from hyperproducing infected cells. Nucleic Acids Res. 1977 Sep;4(9):3175–3186. doi: 10.1093/nar/4.9.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Krasnow M. A., Cozzarelli N. R. Catenation of DNA rings by topoisomerases. Mechanism of control by spermidine. J Biol Chem. 1982 Mar 10;257(5):2687–2693. [PubMed] [Google Scholar]
- Krause H. M., Higgins N. P. On the mu repressor and early DNA intermediates of transposition. Cold Spring Harb Symp Quant Biol. 1984;49:827–834. doi: 10.1101/sqb.1984.049.01.093. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K. Mechanism of transposition of bacteriophage Mu: polarity of the strand transfer reaction at the initiation of transposition. Cell. 1984 Dec;39(2 Pt 1):395–404. doi: 10.1016/0092-8674(84)90018-7. [DOI] [PubMed] [Google Scholar]
- Pheiffer B. H., Zimmerman S. B. Polymer-stimulated ligation: enhanced blunt- or cohesive-end ligation of DNA or deoxyribooligonucleotides by T4 DNA ligase in polymer solutions. Nucleic Acids Res. 1983 Nov 25;11(22):7853–7871. doi: 10.1093/nar/11.22.7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y., Karu A. E., Linn S., Echols H. Purification and properties of the gamma-protein specified by bacteriophage lambda: an inhibitor of the host RecBC recombination enzyme. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2215–2219. doi: 10.1073/pnas.70.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaus N. A., Wright A. Inhibition of Escherichia coli exonuclease V by bacteriophage Mu. Virology. 1980 Apr 15;102(1):214–217. doi: 10.1016/0042-6822(80)90083-5. [DOI] [PubMed] [Google Scholar]
- Schumann W., Bade E. G., Lögl C. Three phage-coded functions involved in the expression of bacteriophage Mu immunity. Virology. 1982 Feb;117(1):1–10. doi: 10.1016/0042-6822(82)90501-3. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1933–1937. doi: 10.1073/pnas.76.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein J. L., Goldberg E. B. T4 DNA injection. II. Protection of entering DNA from host exonuclease V. Virology. 1976 Jul 1;72(1):212–223. doi: 10.1016/0042-6822(76)90324-x. [DOI] [PubMed] [Google Scholar]
- Van Vliet F., Couturier M., De Lafonteyne J., Jedlicki E. Mu-1 directed inhibition of DNA breakdown in Escherichia coli, recA cells. Mol Gen Genet. 1978 Aug 4;164(1):109–112. doi: 10.1007/BF00267606. [DOI] [PubMed] [Google Scholar]
- Waggoner B., Pato M., Toussaint A., Faelen M. Replication of mini-Mu prophage DNA. Virology. 1981 Aug;113(1):379–387. doi: 10.1016/0042-6822(81)90163-x. [DOI] [PubMed] [Google Scholar]
- van de Putte P., Westmaas G. C., Wijffelman C. Transfection with Mu-DNA. Virology. 1977 Aug;81(1):152–159. doi: 10.1016/0042-6822(77)90067-8. [DOI] [PubMed] [Google Scholar]





