Abstract
Background and Objective
Genetic linkage and association studies in late-onset Alzheimer’s disease (LOAD) or LOAD endophenotypes have pointed to several candidate regions on chromosome 10q, among these the ~250kb LD block harboring the three genes IDE, KIF11 and HHEX. We explored the association between variants in the genomic region harboring the IDE-KIF11-HHEX complex with plasma Aβ40 and Aβ42 levels in a case-control cohort of Caribbean Hispanics.
Methods
First, we performed single marker multivariate linear regression analysis relating the individual SNPs with plasma Aβ40 and Aβ42 levels. Then we performed 3-SNP sliding window haplotype analyses, correcting all analyses for multiple testing
Results
Out of 32 SNPs in this region, three SNPs in IDE (rs2421943, rs12264682, rs11187060) were significantly associated with plasma Aß40 or Aß42 levels in single marker and haplotype analyses after correction for multiple testing. As described above, all these SNPs lie within the same linkage disequilibrium block, and are in linkage disequilibrium with the previously reported haplotypes.
Conclusion
Our findings provide modest support for an association in the IDE harboring region on chromosome 10q with Aβ 40 and 42 levels.
Keywords: amyloid beta, Alzheimer’s disease, genetics, insulin-degrading enzyme
INTRODUCTION
Risk loci for late-onset Alzheimer’s disease (LOAD) may be present on chromosome 10q having been identified using case-control status, age-of onset, or plasma Aβ levels as the phenotype. One of the functionally plausible candidate genes lying within the genetic region showing evidence for association or linkage reported by these studies is IDE occurring in a ~250kb haplotype block with KIFF11 and HHEX. Following the initial report on linkage and association of markers around IDE with LOAD (Bertram et al., 2000), some studies that used LOAD as the phenotype did not find an association (Cousin et al., 2009; Reiman et al., 2007) but other independent studies identified haplotypes spanning the IDE-KIFF-HHEX complex that show association with LOAD risk or intermediate LOAD phenotypes (Ertekin-Taner et al., 2004; Prince et al., 2003), including CSF tau levels, MMSE scores, senile plaque and neurofibrillary tangle density, and age-at-onset (Prince et al., 2003). The same haplotypes were associated with plasma Aβ levels in 24 extended Caucasian LOAD families, and with LOAD status in two independent case control series (Ertekin-Taner et al., 2004). Three SNPs in IDE, that are in linkage disequilibrium (LD) with these haplotypes, have been shown to influence IDE expression in LOAD brains (Zou et al., 2010). The objective in the present paper was to confirm or refute a role of genetic variation in the IDE-KIF11-HHEX complex on chromosome 10q in variation of plasma Aβ40 and Aβ42 levels, the main putative culprits in LOAD.
METHODS
Participants
We selected unrelated affected and unaffected individuals from Caribbean Hispanics participating in a population-based study in northern Manhattan (Tang et al., 1998) and single individuals from Caribbean Hispanic families multiply affected by LOAD (Romas et al., 2002). The final analytic sample consisted of 454 Caribbean Hispanic subjects (160 cases, 294 controls) with information on plasma Aβ40 and 42 levels. Dementia diagnosis was based on DSM-IV criteria and NINCDS/ADRDA criteria.
Plasma Aß40 and Aß42 levels
Plasma was obtained at baseline and follow-up, and was stored within 2 hours after collection at −70 °C. Aß40 and Aß42 levels were measured using a combination of monoclonal antibody 6E10 (specific to an epitope present on 1 to 16 amino acid residues of Aß) and rabbit antibodies specific for Aß40 (R162) and Aß42 (R165) in a double-antibody sandwich ELISA. The detection limit for this assay was 5pg/mL for Aß40 and 10pg/mL for Aß42.
Genotyping
Genotyping was performed using the HumanHap650Y BeadChip from Illumina, Inc. Included in the present analyses were 32 SNPs spanning the IDE-KIF11-HHEX region (Supplemental table 1).
Statistical Methods
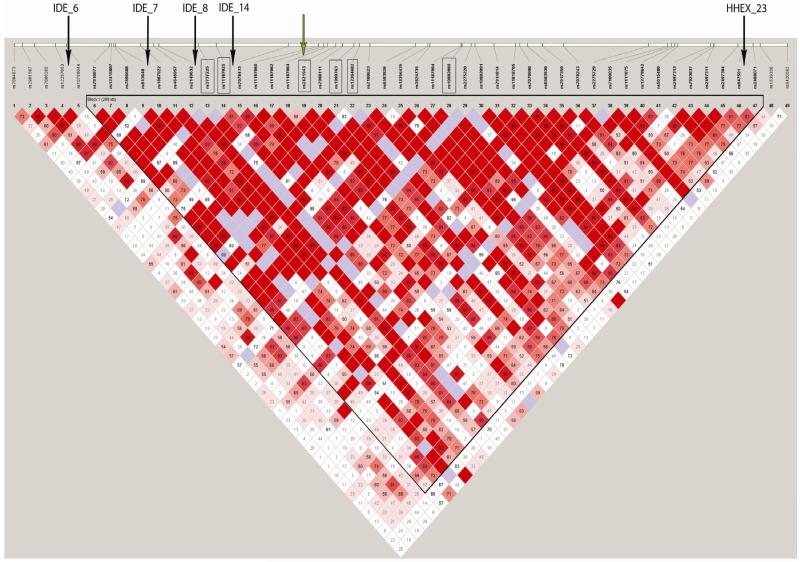
Regression analyses were performed individually relating the 32 SNPs with Aβ40 and Aβ42 levels, adjusting for sex, age-at-onset or age-at-examination, APOE genotype, education, and population stratification. Finally, we performed three-SNP sliding window haplotype analyses for Aβ40 and 42 levels. We did not correct for multiple testing as all explored SNPs are in strong LD (Figure) and marker-phenotype associations are therefore not independent (Nyholt, 2001).
Figure.
LD pattern of the 10q region spanning IDE, KIF11 and HHEX in the present sample. The boxes indicate SNPs significant in the present analyses, the black arrows indicate SNPs significant in the study by Ertekin-Taner (2004).(Ertekin-Taner et al., 2004) The green arrow indicates the SNPs that have been shown to influence IDEexpression in LOAD brain samples inthe study by Zou et al.(Zou et al., 2010)
RESULTS
All SNPs were in HWE. The man age of the sample was 82.2±6.3 years. The A-allele of IDE SNP rs2421943 was associated with significantly higher Aß40 levels and the A allele of IDE SNP rs12264682 was associated with significantly lower Aß40 levels (Table 1, Supplemental table 2). While the association of rs2421943 with Aß40 was driven by LOAD cases, the association of rs12264682 was driven by the unaffected persons.
Table 1.
Association between SNPs in the IDE-KIF11-HHEX complex and plasma Aβ40 and Aβ42 levels
| ALL (n=454) | Cases (n=160) | Controls (n=294) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | SNP | BP | A1 | Aβ (mean) by genotype | BETA | SE | P | Aβ (mean) by genotype | BETA | SE | P | Aβ (mean) by genotype | BETA | SE | P |
| Aβ40 | |||||||||||||||
| IDE | rs11187060 | 94294112 | T | T/T, T/C, C/C (88.7, 86.8, 94.5) | −4.5 | 3.9 | 0.3 | T/T, T/C, C/C (100.7, 90.1, 108) | −8.1 | 6.3 | 0.2 | T/T, T/C, C/C (79.9, 85.1, 86.7) | −2.4 | 5.0 | 0.6 |
| IDE | rs2421943 | 94301795 | A | A/A, A/G, G/G ( 92.62 , 98.1, 83) | 9.4 | 3.8 | 0.01 | A/A, A/G, G/G ( 112.2, 105.8, 91.43) | 12.3 | 6.5 | 0.05 | A/A, A/G, G/G (83.58 , 93.53, 78.66 ) | 6.8 | 4.6 | 0.14 |
| IDE | rs12264682 | 94312407 | A | A/A, A/C, C/C (60.55, 71.1, 92) | −21.8 | 9.1 | 0.02 | A/A, A/C, C/C (--, 82.75, 101.5) | −21.9 | 14.9 | 0.15 | A/A, A/C, C/C (60.55, 61.91, 87.02) | −22.7 | 11.7 | 0.05 |
| Aβ42 | |||||||||||||||
| IDE | rs11187060 | 94294112 | T | T/T, T/C, C/C (36.23, 36.39, 39.83) | −2.3 | 1.8 | 0.2 | T/T, T/C, C/C ( 37.02, 38.65, 46.19) | −5.6 | 2.9 | 0.05 | T/T, T/C, C/C (79.93, 85.1, 86.66) | −0.5 | 2.3 | 0.82 |
| IDE | rs2421943 | 94301795 | A | A/A, A/G, G/G ( 40.4, 37.7, 37.4) | 1.3 | 1.7 | 0.4 | A/A, A/G, G/G ( 46.7, 43.6, 39.7) | 3.9 | 2.9 | 0.1 | A/A, A/G, G/G ( 37.4, 34.3, 36.2) | 0.2 | 2.1 | 0.9 |
| IDE | rs12264682 | 94312407 | A | A/A, A/C, C/C (39.1, 30.9, 41.4) | −7.1 | 4.2 | 0.09 | A/A, A/C, C/C (-, 32.2, 42.9) | −11.5 | 6.8 | 0.09 | A/A, A/C, C/C (39.1, 30.0, 40.1) | −4.6 | 5.3 | 0.3 |
For all 3 models, gender, age-at-onset/examination, education, population stratification and APOE genotype (presence vs. absence) were included as covariates. For the model combining cases and controls, affection status was included as an additional covariate.
Haplotype analyses confirmed these findings. In 3-SNP sliding window haplotype analyses, haplotypes that included the A alleles of SNP rs2421943 (rs11187062|rs11187064|rs2421943: TTA, ß=7.8, p=0.04; rs11187064|rs2421943|rs7908111: TAG, ß=7.9, p=0.03; and rs2421943|rs7908111|rs1999763: AGG, ß=7.8, p=0.03) were associated with higher Aß40 levels, and halotypes that included the A allele of SNP rs12264682 (rs7908111|rs1999763|rs12264682: GGA, ß=−20.5, p=0.02; rs1999763|rs12264682|rs7100623: GAC, ß=−20.5, p=0.02; and rs12264682|rs7100623|rs6583826: ACG, ß=−22.6, p=0.01) were associated with lower Aβ40 levels.
The association with Aß42 levels differed (Table 1). In these analyses, the T allele of IDE SNP rs11187060 was associated with lower Aß42 levels in LOAD cases. Also this finding was confirmed by haplotype analyses (rs11187025|rs7078413|rs11187060: CCT, ß=-−9.5, p=0.02; rs7078413|rs11187060|rs11187062: CTT, p=0.07; ß=-−5.6, rs11187060|rs11187062|rs11187064: TTT, ß=-−8.1, p=0.01). Of note, the directions of effects for all three SNPs were similar for Aβ40 and 42 levels, and all three SNPs lie in the same haplotype block as the previously reported SNPs (Figure) (Ertekin-Taner et al., 2004; Prince et al., 2003; Zou et al., 2010). Adjustment for disease duration did not change the associations. When we used AD as the phenotype to explore whether any of the identified SNPs is also associated with the disease phenotype, the C allele of SNP rs11187062 was associated with significantly lower AD risk (OR=0.54±0.3, p=0.03). Of note, this SNP is adjacent and only 2kb apart from rs11187060 that is associated with Aβ 42 levels.
DISCUSSION
Three IDE SNPs (rs2421943, rs12264682, rs11187060) were significantly associated with changes in plasma Aß40 or Aß42 levels in single marker and haplotype analyses. We used nominal p-values as all assessed SNPs are in strong LD and marker-phenotype associations are therefore not independent (Nyholt, 2001). Of note, while these SNPs have not been assessed in previous studies, they also lie within the same LD block as the haplotypes reported in previous Caucasian studies (Ertekin-Taner et al., 2004; Prince et al., 2003). In addition, they are in strong LD (D’ >90) with the three SNPs that have previously been shown to influence IDE expression in brain samples of 200 LOAD cases (Figure) (Zou et al., 2010).
While SNPs rs2421943 and rs12264682 were associated with changes in Aβ 40 levels, rs11187060 was associated with changes in Aβ42 levels. The directions of associations of all three SNPs were consistent for Aβ 40 and Aβ 42 levels. A likely explanation for the differences in the strengths of the associations of the individual SNPs with Aβ 40 and 42 levels is, that Aβ 42 is a stronger surrogate of pathological changes underlying AD than Aβ 40 which is rather a marker of aging. This note is supported by the fact that the association of SNP rs11187060 with Aβ 42 levels is 10-fold stronger in cases than controls (β: −5.6 vs. −0.5), and that this SNP is in close proximity (~2kb) and strong LD with SNP rs11187062 that is associated with AD. Alternative explanations for differences in the strengths of the associations with Aβ 40 and 42 levels are differences in allele frequencies or power.
IDE binds and degrades Aβ40 and Aβ42 (Perez et al., 2000), and this Aβ degrading activity has been shown to be lower in AD brains than in controls (Perez et al., 2000). In IDE-knock–out mice, brain Aβ levels are elevated (Farris et al., 2003), suggesting that IDE activity is one of several factors determining the amount of brain Aβ in vivo. Enhanced IDE activity in IDE and APP double transgenic mice decreases their brain Aβ levels, and reduces the formation of AD pathology (Leissring et al., 2003). Finally, polymorphisms in IDE may also contribute to the risk of type 2 diabetes (Rudovich et al., 2009), which itself is associated with LOAD. Taken together the findings reported here support the possibility that the IDE-KIF11-HHEX region on chromosome 10q may contain genetic variants that modify Aβ40 and 42 levels.
Supplementary Material
Acknowledgments
FUNDING: This work was supported by grants from the National Institute of Health and the National Institute on Aging: R37-AG15473, P01-AG07232 (RM), Paul B. Beeson Career Development Award: K23AG034550 (CR), The Blanchette Hooker Rockefeller Foundation and The Charles S. Robertson Gift from the Banbury Fund (RM). The laboratory under the direction of Dr. St George-Hyslop received additional support from the Alzheimer Society of Canada, Japan-Canada and Canadian Institutes of Health Research Joint Health Research Program (ER), the Canadian Institutes of Health Research, Alzheimer Society of Ontario, Howard Hughes Medical Institute, The Wellcome Trust.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: None of the authors has any actual or potential conflicts of interest.
HUMAN SUBJECTS: Appropriate approval and procedures were used concerning human subjects and animals.
References
- Bertram L, Blacker D, Mullin K, Keeney D, Jones J, Basu S, Yhu S, McInnis MG, Go RC, Vekrellis K, Selkoe DJ, Saunders AJ, Tanzi RE. Evidence for genetic linkage of Alzheimer’s disease to chromosome 10q. Science. 2000;290(5500):2302–3. doi: 10.1126/science.290.5500.2302. [DOI] [PubMed] [Google Scholar]
- Cousin E, Mace S, Rocher C, Dib C, Muzard G, Hannequin D, Pradier L, Deleuze JF, Genin E, Brice A, Campion D. No replication of genetic association between candidate polymorphisms and Alzheimer’s disease. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Ertekin-Taner N, Allen M, Fadale D, Scanlin L, Younkin L, Petersen RC, Graff-Radford N, Younkin SG. Genetic variants in a haplotype block spanning IDE are significantly associated with plasma Abeta42 levels and risk for Alzheimer disease. Hum Mutat. 2004;23(4):334–42. doi: 10.1002/humu.20016. [DOI] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100(7):4162–7. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40(6):1087–93. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- Nyholt DR. Genetic case-control association studies--correcting for multiple testing. Hum Genet. 2001;109(5):564–7. doi: 10.1007/s00439-001-0611-4. [DOI] [PubMed] [Google Scholar]
- Perez A, Morelli L, Cresto JC, Castano EM. Degradation of soluble amyloid beta-peptides 1-40, 1-42, and the Dutch variant 1-40Q by insulin degrading enzyme from Alzheimer disease and control brains. Neurochem Res. 2000;25(2):247–55. doi: 10.1023/a:1007527721160. [DOI] [PubMed] [Google Scholar]
- Prince JA, Feuk L, Gu HF, Johansson B, Gatz M, Blennow K, Brookes AJ. Genetic variation in a haplotype block spanning IDE influences Alzheimer disease. Hum Mutat. 2003;22(5):363–71. doi: 10.1002/humu.10282. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Webster JA, Myers AJ, Hardy J, Dunckley T, Zismann VL, Joshipura KD, Pearson JV, Hu-Lince D, Huentelman MJ, Craig DW, Coon KD, Liang WS, Herbert RH, Beach T, Rohrer KC, Zhao AS, Leung D, Bryden L, Marlowe L, Kaleem M, Mastroeni D, Grover A, Heward CB, Ravid R, Rogers J, Hutton ML, Melquist S, Petersen RC, Alexander GE, Caselli RJ, Kukull W, Papassotiropoulos A, Stephan DA. GAB2 alleles modify Alzheimer’s risk in APOE epsilon4 carriers. Neuron. 2007;54(5):713–20. doi: 10.1016/j.neuron.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romas SN, Santana V, Williamson J, Ciappa A, Lee JH, Rondon HZ, Estevez P, Lantigua R, Medrano M, Torres M, Stern Y, Tycko B, Mayeux R. Familial Alzheimer disease among Caribbean Hispanics: a reexamination of its association with APOE. Arch Neurol. 2002;59(1):87–91. doi: 10.1001/archneur.59.1.87. [DOI] [PubMed] [Google Scholar]
- Rudovich N, Pivovarova O, Fisher E, Fischer-Rosinsky A, Spranger J, Mohlig M, Schulze MB, Boeing H, Pfeiffer AF. Polymorphisms within insulin-degrading enzyme (IDE) gene determine insulin metabolism and risk of type 2 diabetes. J Mol Med. 2009;87(11):1145–51. doi: 10.1007/s00109-009-0540-6. [DOI] [PubMed] [Google Scholar]
- Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. Jama. 1998;279(10):751–5. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- Zou F, Carrasquillo MM, Pankratz VS, Belbin O, Morgan K, Allen M, Wilcox SL, Ma L, Walker LP, Kouri N, Burgess JD, Younkin LH, Younkin SG, Younkin CS, Bisceglio GD, Crook JE, Dickson DW, Petersen RC, Graff-Radford N, Younkin SG, Ertekin-Taner N. Gene expression levels as endophenotypes in genome-wide association studies of Alzheimer disease. Neurology. 2010;74(6):480–6. doi: 10.1212/WNL.0b013e3181d07654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.