Abstract
Glia have been, in many ways, the proverbial elephant in the room. Although glia are as numerous as neurons in vertebrate nervous systems, technical and other concerns had left research on these cells languishing, while research on neurons marched on. Importantly, model systems to study glia had lagged considerably behind. A concerted effort in recent years to develop the canonical invertebrate model animals, Drosophila melanogaster and Caenorhabditis elegans, as settings to understand glial roles in nervous system development and function has begun to bear fruit. In this review we summarize our current understanding of glia and their roles in the nervous system of the nematode C. elegans. The recent studies we describe highlight the similarities and differences between C. elegans and vertebrate glia, and focus on novel insights that are likely to have general relevance to all nervous systems.
Keywords: C. elegans, glia, ensheathment, sensory receptive endings, synapses, amphid
Introduction
Animal nervous systems promote a spectacular range of functions, from simple reflexive escape behaviors, to complex cognitive tasks. The diversity of animal behavior is executed through signals speeding down neurons connected in complex ways, and tasked with sensing stimuli, analyzing sensory input, and generating responses. Other complex multicellular assemblies, such as the immune system, are generally composed of multiple cell types, each providing important contributions towards the activity of the system as a whole. It would be, therefore, surprising if nervous systems comprised only one relevant cell type. Nature seems to agree. Neurons have also been assigned dedicated partners: the glia.
Whether cells functioning as glia exist in cnidarians such as hydra, whose nervous systems are simple nerve nets, is not yet clear. However, as organisms increase in complexity, an explosive expansion in glia number and morphological complexity is evident. A recent study, for example, suggests that the human brain consists of roughly equal numbers of neuronal and glial cells (Azevedo et al., 2009). Furthermore, each human astrocyte can contact thousands of neurons (Spacek, 1985; Ventura and Harris, 1999; Oberheim et al., 2009). These observations suggest that nervous systems benefit greatly from having an additional cellular component. Indeed, in vertebrates, there are few if any aspects of neuron development and function in which glia have not been implicated. Neuronal differentiation, survival, proliferation, migration, morphogenesis, circuit formation, synaptogenesis, establishment and maintenance of ionic balance, and neurotransmission all seem to involve glia (Barres, 2008).
Although an ultimate understanding of nervous systems must eventually explain the detailed action of the human brain, a basic understanding of neurons and glia at the level of cell biology and simple circuitry is more easily achieved by studying relevant model systems. Despite the differences between invertebrate model systems and humans, these systems have been invaluable in deciphering important aspects of human biology. Studies in Drosophila, for example, have led to major insights into animal development and human innate immunity, while research in the nematode Caenorhabditis elegans has provided a deep understanding of the underpinnings of cell polarity, cell death, and RNA interference processes in humans. There is every reason, therefore, to expect similar discoveries in the nervous system.
Progress towards understanding the glia of Drosophila is described elsewhere in this issue; here we summarize our understanding of glia development and function in C. elegans.
The anatomy of C. elegans glia
The nervous system of C. elegans consists of 302 neurons, 50 glial cells derived from neuronal/epithelial progenitors, and six glial cells that are mesodermally derived. The nervous system is essentially identical anatomically between individuals, even at the level of the positions of individual synapses, and has been reconstructed in its entirety using electron microscopy (Ward et al., 1975; White et al., 1986; Hall and Russell, 1991). Sensory cues are detected by specialized sensory neurons, most of which reside in the head of the animal. Information from sensory neurons is relayed to a central processing neuropil called the nerve ring, which encircles the pharynx and which can be thought of as the animal's brain. The nerve ring is densely packed with en passant synapses between sensory neurons and interneurons, and between interneurons. Some interneurons connect to motor neurons that control the musculature of the head and body of the animal (Ware et al., 1975). Glial processes are found at neuronal junctions at every level: at sensory receptive endings, at neuron-neuron synapses, and at neuromuscular junctions, raising the intriguing notion that glia in C. elegans may be primarily involved in intercellular information transfer, an idea that is supported by several lines of evidence (see following sections).
Glia at sensory receptive sites
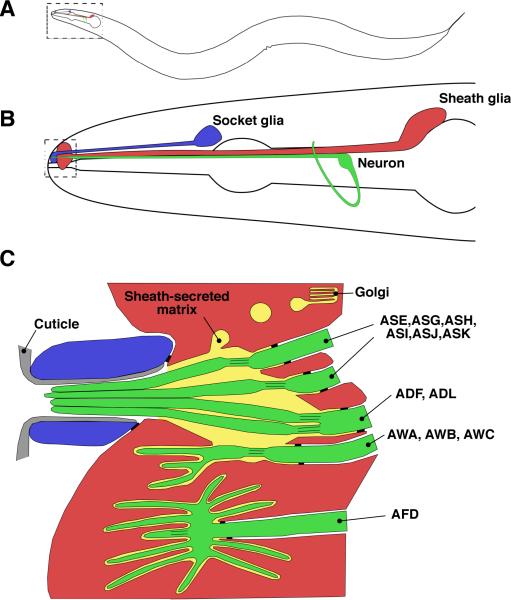
Glial functions at sensory receptive endings of C. elegans have been primarily examined in the context of the amphid, a bilateral sensory organ located in the head of the animal (Fig. 1A), and consisting of twelve sensory neurons and two glial cells, also referred to as sheath and socket cells (Ward et al., 1975). All cells of the amphid extend processes to the tip of the nose (Fig. 1B). At the tip, the sheath and socket glia come together to form a tube known as the amphid channel (Fig. 1C). The amphid channel contains a material called matrix, at least some components of which are secreted by the sheath glia (Perens and Shaham, 2005).
Figure 1.
The amphid sensory organ is a model system for neuron-glia interactions. A: Schematic of an adult C. elegans hermaphrodite. The amphids are located in the head. B: Each amphid consists of twelve neurons (in green; only one is drawn here for simplicity) and two glial cells, the sheath (AMsh, in red) and the socket (in blue). C: Processes from the neurons and glia come together at the tip of the nose. The glial cells align to form a tube, the amphid channel, through which some amphid neuron dendrites extend sensory cilia that adopt distinct morphologies: single cilia (neurons ASE, ASG, ASH, ASI, ASJ, ASK), double cilia (neurons ADF, ADL), wing cilia (neurons AWA, AWB, AWC) and the finger cilia of AFD. Adherens junctions between the dendrites and the sheath glia, as well as between the sheath and socket glia establish a niche for the sensory cilia. This niche is filled with matrix material secreted by the sheath glia; thus, the site where the nervous system of the animal meets the environment is under the direct control of glia. Adapted from Perkins et al., 1986.
Of the twelve neurons comprising the amphid (Fig. 1C), eight extend non-motile, microtubule-based sensory cilia that travel the length of the amphid channel to become exposed to the environment. These specialized receptive endings are sites of localization for receptors that recognize chemical and mechanosensory stimuli (Bargmann, 2006). The receptive endings of three other amphid sensory neurons enter the amphid channel, but then divert and expand elaborately branched cilia into blind pockets within the sheath glial cell where the detection of volatile chemicals takes place. The remaining amphid sensory neuron, which senses temperature, is also embedded within the sheath glia, but its dendritic terminus does not track through the amphid channel.
In addition to seven classes of hermaphrodite sensory organs, all of which have an architecture resembling that of the amphid, C. elegans males possess four more classes of sensilla, located in the male tail, that facilitate mating with hermaphrodites (Sulston et al., 1980). Although all of these male-specific sensilla include glial cells, studies of glia in the male have focused on the ray sensilla. Each ray consists of two sensory neurons that extend dendrites through a channel formed by a single glial cell known as the structural cell.
In all sensory organs, a key role for the sheath glia seems to be the formation of an isolated extracellular compartment in which to house neuronal sensory receptive endings. Such an arrangement has been conserved in sensory organs of other invertebrates and vertebrates.
Synaptic glia
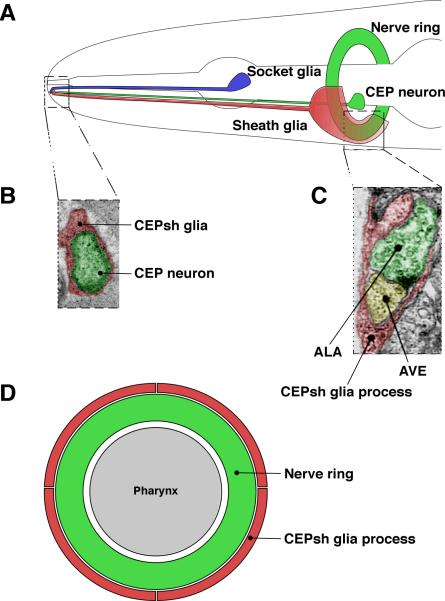
All glia in C. elegans, including the sheath glia of the cephalic sensilla (CEPsh glia), are associated with sensory organs (Fig. 2A and 2B). However, the four CEPsh glia are unique in that they also extend sheet-like processes that wrap around the nerve ring (Fig. 2A). Anatomically, these sheets may serve to isolate the nerve ring in the same way the blood-brain barrier isolates neurons in vertebrate brains. Consistent with this idea, the ancestral vertebrate has been postulated to have had a glial blood-brain barrier, with the endothelial barrier arising only later in evolution (Bundgaard and Abbott, 2008). However, no functional evidence supporting a barrier role for CEPsh glia has been described so far.
Figure 2.
The cephalic sheath glia (CEPsh glia) physically interact with both the sensory receptive endings of the CEP neurons, and with the nerve ring. A: The CEP sensillum displays the same basic architecture as the amphid. A thin anterior process from the CEPsh glia runs parallel to the CEP neuron, while a sheet-like posterior process wraps around the “brain” of the animal. B: The anterior process establishes a channel similar to, but smaller than the amphid channel. C: Thin projections from the posterior process enter the nerve ring and can ensheath synapses, similar to astrocytes of vertebrate systems. Adapted from White et al., 1986. D: Schematic of a cross-section through the nerve ring. Each animal has four CEPsh glia that wrap around the nerve ring without overlapping with each other. Thus, CEPsh glia establish independent domains, in a fashion reminiscent of astrocytic domains.
The sheet-like processes of the CEPsh glia also send processes that penetrate deep within the nerve ring and contact a small number of synapses (Fig. 2D), an architecture reminiscent of the fine processes of vertebrate astrocytes that surround synapses. As with vertebrate astrocytes (Bushong et al., 2002; Ogata and Kosaka, 2002; Halassa et al., 2007), CEPsh glia processes are organized in sharply defined, non-overlapping domains with respect to the neuronal processes they contact (Fig. 2D), suggesting that tiling is a fundamental property of synapse-associated glial cells. The anatomy of CEPsh glia suggests the hypothesis that in evolution, glia first appeared in the periphery in association with sensory receptive endings, and were then co-opted by the central nervous system to ensheath axons and neuron-neuron synapses (Heiman and Shaham, 2007).
Glia at neuromuscular junctions
C. elegans possesses three pairs of GLR cells (dorsal, ventral and lateral) that are arranged in a six-fold symmetrical fashion around the pharynx, just posterior to the nerve ring (White et al., 1986). From each GLR cell body emanates an anteriorly-directed sheet-like process that wraps around the inside aspect of the nerve ring (Fig. 3A). Here, GLR cells make gap junctions to head muscle cells, as well as to the RME motor neurons that innervate these muscles (Fig. 3B). More anteriorly, the sheet-like processes narrow into thin extensions (Fig. 3A). Unlike other glial cells, GLR cells express the C. elegans homolog of myoD (Krause et al., 1994) and are of mesodermal origin. Although GLR glia are situated in an ideal position to affect neuron-muscle communication, their functions have not been studied. It is intriguing to speculate that they may serve functions analogous (but probably not homologous) to those of perisynaptic Schwann cells. It is striking that only C. elegans head muscles form partnerships with glia. These muscles mediate fine motor behaviors that are less stereotypical than the undulations produced by body wall muscles, perhaps explaining the need for glial companionship.
Figure 3.
The GLR glia establish gap junctions that bridge neurons with muscles. A: Each animal has six GLR glia. Only one is depicted here. The cell body lies posterior to the nerve ring. An anterior, sheet-like process lines the inside of the nerve ring and then tapers to a thinner process. B: The sheet-like processes of the GLR glia make gap junctions to head muscle cells and the RME motor neurons that innervate them. Adapted from White et al., 1986.
Specification of glia in C. elegans
An important regulator of glial determination in C. elegans is the zinc-finger transcription factor LIN-26. Mutations in the lin-26 gene were initially reported to transform epithelial (hypodermal) cells into neurons (Ferguson et al., 1987). Later, Labouesse et al. (1996) realized that lin-26 is also important for glial specification: mutations in lin-26 lead either to glial cell death or, more rarely, to glial transformation into neurons (Labouesse et al., 1996). The function of lin-26 is reminiscent of the function of the glial-cells-missing (gcm) gene in Drosophila (Hosoya et al., 1995; Jones et al., 1995; Vincent et al., 1996). GCM protein contains a Zn-finger domain, and gcm mutations transform glia into neurons. However, LIN-26 and GCM proteins are not obviously homologous.
More recent studies of CEPsh glia development demonstrate similarities with aspects of mammalian glial differentiation. hlh-17, the C. elegans gene most closely related to the mammalian oligodendrocyte differentiation gene Olig2, is specifically expressed in CEPsh glia (McMiller and Johnson, 2005; Yoshimura et al., 2008). hlh-17 expression in and differentiation of the two dorsal CEPsh glial cells depends on the Paired-domain of the transcriptional regulator VAB-3. Expression of hlh-17 in ventral CEPsh glia depends on the homeodomain of VAB-3, as well as on its downstream target, the MLS-2 transcriptional regulator. Strikingly, VAB-3 is the C. elegans protein most similar to vertebrate Pax6 and Pax7 transcription factors, which regulate ventral and dorsal expression, respectively, of Olig2 in the developing spinal cord. Likewise, MLS-2 belongs to the Nkx/Hmx superfamily of transcriptional regulators and is distantly related to Nkx6, which promotes Olig2 expression in the ventral pMN region of the developing vertebrate neural tube. Thus, despite the evolutionary distance between nematodes and vertebrates, it seems that aspects of glia specification are related.
Glia and nervous system morphogenesis
Sensory channel development
Sheath glia
The amphid sensory channel allows sensory neurons to communicate with the outside environment. The sheath glial cell contributes the posterior end of this channel, and a number of recent studies have provided insight into the mechanism by which the sheath glia channel is formed. These studies were initially enabled by the identification of DAF-6 (Riddle et al., 1981), a Patched-related protein that acts within glia to regulate amphid channel shape (Herman, 1987; Perens and Shaham, 2005). In animals carrying mutations in the daf-6 gene, the sheath glia channel is bloated and malformed. Furthermore, sensory cilia appear trapped within the channel and are unable to reach the outside environment. daf-6 mutants are defective in behaviors that depend on the direct contact of sensory neurons with the animal's surrounding (Albert et al., 1981; Perkins et al., 1986), suggesting that channel integrity is required for amphid functionality (Perens and Shaham, 2005).
DAF-6 lines the amphid channel and also localizes to apical surfaces of other luminal structures in C. elegans. Furthermore, animals mutant for both daf-6 and che-14, a Dispatched homolog that is also important for amphid morphogenesis (Michaux et al., 2000), display defectes in many tubular systems. These results suggest that the machinery for channel formation overlaps, at least to some extent, with that controlling tube formation, raising the possibility that glial ensheathment of neurons in other animals may also be related to tubulogenesis.
The amphid channel lining fits snuggly around the neuronal cilia it ensheathes, suggesting the possibility that a neuronal signal may be involved in controlling channel diameter and shape. Interestingly, the shape of the sheath glia channel and localization of DAF-6 along the lenght of this channel depends on the presence of neuronal sensory cilia. In animals carrying mutations that stunt or block cilia development, DAF-6 protein is no longer localized to the channel surface and accumulates instead in a punctate structure, which may be the sheath/socket junction. In addition, the channel acquires an abnormal expanded morphology (Perens and Shaham, 2005)
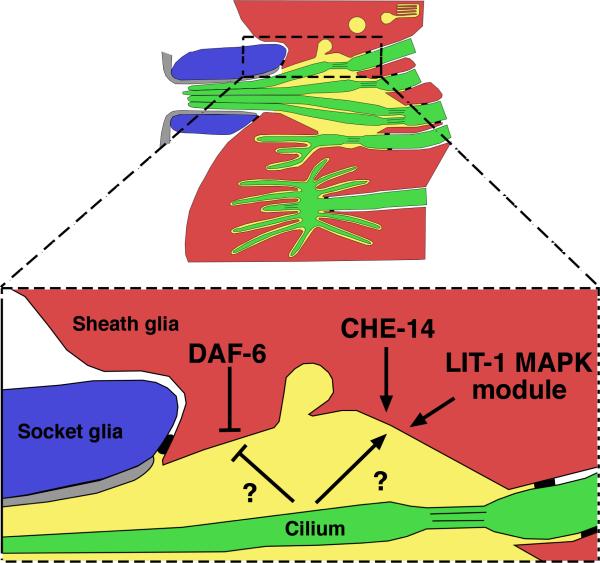
Both daf-6 and neuronal signals seem to limit the size of the surrounding glial channel (G.O., Yun Lu and S.S., unpublished observations), suggesting the possibility that a counteracting mechanism exists to promote channel expansion. To identify components of such a pathway, we used random mutagenesis to screen for daf-6 mutant animals possessing patent amphid channels. We have shown that one such suppressed strain harbors a mutation in the gene lit-1, a NEMO/MAP kinase homolog (G.O., Elliot Perens, Yun Lu and S.S., unpublished observations). Strikingly, some lit-1 single mutants have channels that are either narrower than wild-type channels, failing to accommodate all amphid sensory cilia, or that fail to form altogether. lit-1 is expressed in many glial cells and the LIT-1 protein localized to the luminal membranes of the amphid channel. This localization also depends on the presence of cilia and seems to be important for lit-1 function. It seems, therefore, that two opposing forces, one promoting lumen expansion, the other limiting lumen growth, act in balance to form the properly proportioned gllia channel of wild-type animals (Fig. 4, G.O., Elliot Perens, Yun Lu and S.S., unpublished observations).
Figure 4.
The size of the amphid glia channel is regulated by two opposing forces. DAF-6/Patched-related acts within the glia to reduce channel girth, while CHE-14/Dispatched and the LIT-1/Nemo-like kinase MAPK module act (also within glia) to increase channel cross-section. Unidentified neuronal signals are required for the correct localization of DAF-6 and LIT-1, as well as normal channel morphogenesis.
Socket glia
Socket glia have been less intensively explored than their sheath glia counterparts, however, studies of the C. elegans alr-1 gene support an important role for these glia in amphid function. alr-1 (Tucker et al., 2005) encodes the C. elegans homolog ot the Paired class homeobox transcription factor Aristaless (Galliot et al., 1999), first characterized in Drosophila (Schneitz et al., 1993). In humans, mutations of the Aristaless homolog ARX cause a range of neurological defects (Strømme et al., 2002). In C. elegans, alr-1 mutants hatch with no detectable abnormalities, but progressively lose amphid functionality as the animal grows. Ultrastructural studies revealed that in older alr-1 mutants, the tight junctions between sheath and socket glia cannot be detected, while matrix material and sensory cilia can now be found throughout the tip of the head (Tucker et al., 2005).
The alr-1 mutant defects reflect the importance of a precise and stable connection between the sheath and socket glial channels for sensory organ function. Embryonic ablation studies of socket glia progenitor cells reveal that long-range signaling may be involved in directing this connection. Specifically, Sulston et al. (Sulston et al., 1980) used a laser microbeam to ablate progenitor cells of specific sensory organ socket glia and found that the cognate neuron and sheath glia now associated with socket glia of a different sensory organ. This observation suggests an active attraction between socket glia and neurons/sheath glia. The molecular basis of this attractive signal has not yet been determined.
The role of glia in neurite extension and nerve ring structure
The sensory neurons of the amphid extend dendrites over a length of about 100 microns towards the tip of the nose. These dendrites fasciculate into stereotyped bundles that run in parallel and in close proximity to the process of the amphid sheath glial cell. Recently, aspects of the mechanism underlying amphid process extension and the coordination of glia and neuron process lengths have been described (Heiman and Shaham, 2009).
During embryogenesis, amphid sensory neurons are born near the tip of the developing head (Sulston et al., 1983). There they extrude a short projection that is attached to the tip of the nose. Posterior migration of the neuronal cell bodies then results in dendrite extension- a mechanism that has been coined retrograde extension (Heiman and Shaham, 2009). The tip anchor is composed of at least two proteins (Heiman and Shaham, 2009): the zona pellucida (ZP) domain protein DYF-7, which is secreted by the neurons, and DEX-1, a zonadhesin domain containing protein secreted by non-neuronal neighboring cells. DEX-1 and DYF-7 are similar to α and β tectorins, proteins that compose the tectorial membrane, an extracellular matrix that anchors hair cell cilia in the inner ear (Legan et al., 1997). Like the tectorins and other ZP domain proteins, DYF-7 can multimerize, and its multimeric state seems to be required for proper dendrite anchoring. These parallels suggest a common, evolutionary conserved module utilized in sensory organ development.
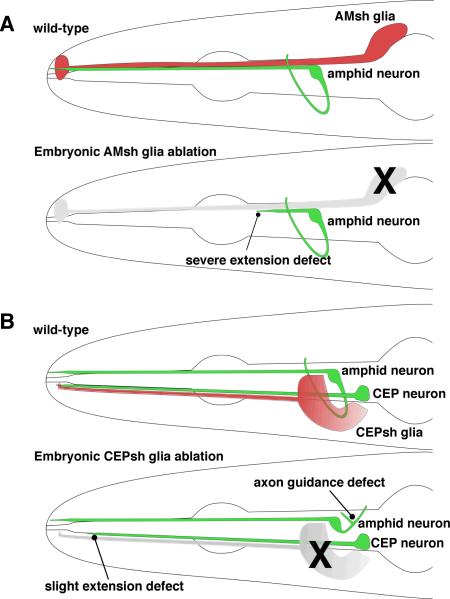
dyf-7 and dex-1 mutants are defective not only in the lengths of their amphid sensory dendrites, but also in the length of the sheath glia process, suggesting that the morphogenesis of neurons and glia in this sensory organ is tightly linked (Heiman and Shaham, 2009). Although it is not yet clear whether DEX-1 is expressed in the amphid sheath glia, other observations suggest a direct role for glia in amphid dendrite extension. First, ablation of the amphid sheath glia during embryogenesis results in short dendrites (Fig. 5A), similar to the ones seen in dyf-7 and dex-1 animals (T. Bacaj and S.S., unpublished observations). A similar result is obtained in the cephalic sensory organ where ablation of the CEPsh glia results in dendrite extension defects of the CEP neurons (Fig. 5B) (Yoshimura et al., 2008). Second, expression of either dex-1 or dyf-7 cDNA by the amphid glia can rescue the dendrite extension defect of each respective mutant, suggesting that glia are positioned to establish or modify the extracellular matrix required for dendritic tip anchoring. Third, a study of amphid sheath glia gene expression using gene microarrays revealed that of the 298 glia-enriched gene transcripts, 159 encoded secreted/transmembrane proteins, including a number of proteins predicted to contain ZP domains that could, perhaps, interact with DYF-7 and DEX-1 (Bacaj et al., 2008).
Figure 5.
Embryonic ablation of glia results in dendrite extension and axonal guidance defects. A: Ablation of the amphid sheath glia during embryogenesis results in amphid neurons with dendrites that are much shorter than normal, reminiscent of dex-1 and dyf-7 mutants (see text). B: Ablation of the CEPsh glia results in slightly shorter CEP dendrites, and axon guidance defects.
The gene ram-5 also encodes a ZP domain protein and is expressed in many glial cells, including the structural cell of the ray sensilla in the male tail. Interestingly, in ram-5 mutants, the overall architecture of the ray sensillum is grossly malformed due to defects in the shape of the glial structural cell (Yu et al., 2000), supporting the notion that ZP domain proteins secreted by both neurons and glia play important roles in shaping sensory organs.
Glia in C. elegans also play important roles in organizing and directing axon outgrowth. In addition to affecting the lengths of CEP neuron dendrites, embryonic ablation of CEPsh glia precursor cells has profound effects on nerve ring structure and axon guidance (Yoshimura et al., 2008). In 20% of animals in which two of the four CEPsh glial cells are eliminated, the nerve ring is not formed, and instead neuronal processes are splayed over the entire head region of the animal. In the remaining animals, a distinct nerve ring is formed, however neuronal axons within the ring are disorganized (Fig. 5B), with individual axons often displaying extensive guidance and branching defects. At least part of the axon guidance defect is due to the lack of UNC-6/Netrin secretion from the ventral CEPsh glia, which normally guides axons within the nerve ring (Wadsworth et al., 1996; Yoshimura et al., 2008), consistent with the observation that UNC-6/Netrin is required for proper guidance of the axon of the nerve ring interneuron RIA (Colón-Ramos et al., 2007) Thus, glia play key roles in both dendrite and axon extension in C. elegans.
Glia and synaptogenesis
In vertebrates, glia seem to play an important role in synaptogenesis by secreting proteins that establish postsynaptic competence (Christopherson et al., 2005; Eroglu et al., 2009). In C. elegans, non-neuronal epithelial cells have been shown to play direct roles in synaptogenesis (Shen and Bargmann, 2003; Shen et al., 2004), suggesting that glia may also have synaptogenic roles in this animal. Studies of CEPsh glia, which envelop the syanpse-rich nerve ring suggest the possibility that these glia might participate in synapse formation. Colón-Ramos et al. (2007) demonstrated that synapse formation between the AIY and RIA neurons requires activity of the netrin receptor unc-40/DCC in AIY (Colón-Ramos et al., 2007), raising the possibility that glial UNC-6/Netrin might direct RIA-AIY synaptogenesis. This idea was supported by the demonstration that unc-6 mutants exhibit defects similar to unc-40 in RIA-AIY synapse formation. Although UNC-6/Netrin may play a direct role in the formation of this synapse, EM serial reconstruction of the synaptic site in adult animals suggests that the CEPsh glial process does not closely abut this synapse (White et al., 1986), raising the possibility that UNC-6 may affect synaptogenesis indirectly, by affecting the fine-tuning of axon guidance. Higher resolution studies of the early development of this synapse are likely to shed more light on the role of glia in establishing this synaptic contact.
Phagocytosis
Vertebrate and Drosophila glia engulf neuronal cell corpses and neurite debris during development and following neuronal injury (Aldskogius and Kozlova, 1998; Logan and Freeman, 2007). Glia in C. elegans are also able to phagocytose dying cells during embryogenesis (Sulston et al., 1983). Whether injury to glia-associated neurons promotes glial phagocytotic activity and whether phagocytosis could contribute to regulation of synapse formation as has recently been shown in Drosophila (Fuentes-Medel et al., 2009), remains to be determined.
The roles of glia in nervous system function
Glial control of sensory neuron function
C. elegans responds to a variety of stimuli including temperature, taste, smell, touch, high osmolarity, oxygen, UV light, and pheromones (Bargmann, 2006). Many of these responses are well characterized, robust, and quantifiable, and begin with the detection of the stimulus by specific sensory neurons of the amphid. The amphid, therefore, provides a suitable setting for investigating the role of glia in sensory neuron function.
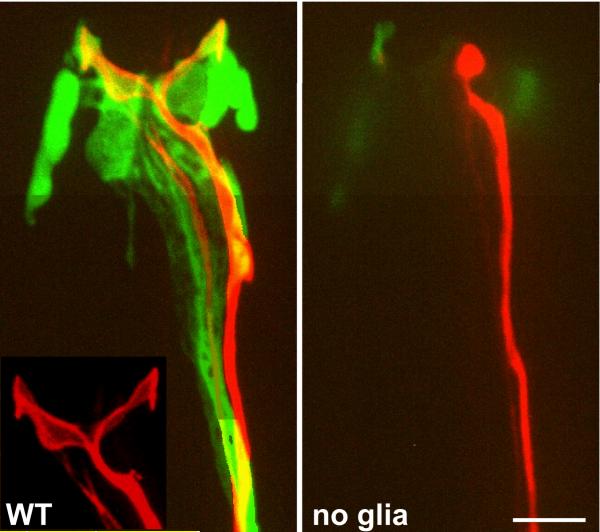
To determine whether amphid sheath glia are required for sensory neuron function, these glia were ablated (Bacaj et al., 2008) in larvae in which amphid morphogenesis had been completed. Animals lacking glia were then subjected to a battery of behavioral assays to test the functions of different amphid neurons. Morphological studies were also performed to examine effects of glia on neuronal receptive ending shapes. The results of these studies suggest that amphid neurons can be classified into three categories with respect to their interactions with the sheath glial cell. Upon glia removal, three of the four glia-embedded neurons (AWA, AWC, and AFD) display involution of their receptive endings (Fig. 6), accompanied by defects in the behaviors controlled by these neurons. A similar result can be elicited by transiently blocking the secretory pathway in glia (A. Singhvi and S.S., unpublished). These results demonstrate that amphid sheath glia are continuously required to maintain sensory neuron receptive ending morphology of a subset of sensory neurons.
Figure 6.
Ablation of the amphid sheath glia in adult animals can result in severe disruption of the morphology of neuronal receptive endings. Left panel, unablated amphid. Glia, green; AWC neuron, red. Right panel, glia-ablated amphid. Note loss of extended sensory receptive ending of AWC neuron. Scale bar 5μm. Adapted from Bacaj et al., 2008.
The remaining glia embedded neuron, AWB, makes up the second class of amphid neurons. AWB receptive ending morphology is less disrupted by glia ablation, and AWB seems to maintain its function. The third class of neurons comprises the channel neurons. Although these neurons retain their normal morphology after glia ablation, and properly localize ciliary components and odorant receptors, they are, nonetheless, unable to promote normal behavior. Calcium transients normally induced in one of these neurons, ASH, in response to a stimulus of high osmolarity are absent in glia-ablated animals. These results demonstrate that the maintenance of neuronal structure cannot be the sole function of amphid sheath glia.
A first step towards deciphering the mechanism by which glia promote channel neuron activity was provided by analysis of the gene fig-1. fig-1 is expressed specifically in amphid sheath glia and is predicted to encode a secreted protein contain two thrombospondin TSP1 domains, multiple C6 domains of unknown function, and a putative EGF-like type II motif. Mutations in fig-1 stunt the aversive response of C. elegans to 1-Octanol, a behavior mediated by amphid channel neurons. fig-1 mutants also display a curious defect in the ability of channel neurons to fill with the lipophilic dye DiI: wild-type animals soaked in DiI take up the dye through the amphid channel, resulting in labeling of some, but not all channel sensory neurons. Despite possessing amphid channels of normal morphology, amphid sensory neurons in fig-1 mutants are unable to take up dye. A similar defect is seen in animals in which amphid sheath glia are ablated. The basis of this defect is not yet known, however, the observation raises the possibility that glial FIG-1 protein may affect aspects of neuronal membrane architecture.
TSP1 and EGF-like type II domains are also found in the vertebrate protein thrombospondin, which is secreted by astrocytes, and which promotes synaptogenesis by acting on postsynaptic membranes (Christopherson et al., 2005; Eroglu et al., 2009), raising the possibility that FIG-1 may share common functions with thrombospondins. Many similarities exist between the “sensory synapses” at which environmental signals are detected and processed by sensory neurons, and neuron-neuron synapses. These similarities have been recently reviewed (Shaham, 2010), and raise the interesting possibility that sensory synapses may serve as useful models to understand glial functions at neuron-neuron synapses.
Glia have been postulated to control ion homeostasis in synaptic regions, and studies of the C. elegans amphid may support this notion. C. elegans can detect and avoid solutions of low pH, and a recent study suggests that ion channels of the DEG/ENaC variety may play a role in this behavior. Mutations in the gene DEG-1, which is expressed in the amphid channel neuron ASK, result in defects in low pH avoidance (Wang et al., 2008), raising the possibility that this channel is involved in some aspect of the response. Mutations in another DEG/ENaC channel, acd-1, do not result in acid-avoidance defects; however, acd-1 mutations enhance the avoidance defects of deg-1 mutants. Surprisingly, acd-1 is expressed and functions in amphid sheath glia. Although the precise means by which glial acd-1 modulates acid avoidance is not clear, one possibility is that it regulates the ionic balance in the environment of the neurons. Alternatively, acd-1 may have a more specific role in the detection of low pH; indeed ACD-1 protein exhibits pH-dependent activity in vitro that correlates with in vivo function.
DEG/ENaC channels are expressed in vertebrate glia (Golestaneh et al., 2000; Brockway et al., 2002; Berdiev et al., 2003; Hitomi et al., 2009) and have been implicated in synaptic plasticity (Wemmie et al., 2002; Voglis and Tavernarakis, 2008), further supporting the notion that glia enveloping sensory neuron receptive endings and those supporting neuronal synapses may have overlapping functions.
Glia and sensory neuron plasticity
To survive in a changing environment, animals need to respond appropriately to harsh conditions. Under stress (e.g. lack of food or increased temperature), C. elegans larvae can enter an alternative developmental stage called dauer. Dauer animals are resistant to treatments that kill nondauer animals and can survive up to a year without feeding. Upon establishment of more favorable conditions, animals exit the dauer state and resume development to become fertile adults The integrity of amphid sheath glia is required for dauer entry, as demonstrated by amphid sheath glia ablation studies (Vowels and Thomas, 1994), and by analysis of daf-6 mutants, which were originally isolated as defective in dauer formation (Riddle et al., 1981). More recently, daf-6 and che-14 have been implicated in the dauer stress response in a novel context. Serotonin is involved in the regulation of dauer: animals that produce no serotonin are more likely to enter dauer (Sze and Ruvkun, 2003), and less likely to exit dauer under favorable conditions (Moussaif and Sze, 2009). Mutations in daf-6 or che-14 result in transcriptional upregulation of the serotonin biosynthetic enzyme gene encoding Tryptophan Hydroxylase in the amphid neurons (Moussaif and Sze, 2009), suggesting a possible link between glia and serotonin signaling in neurons, although this link may be indirect. The physiological importance and mechanism underlying the regulation of neuronal serotonin levels by glia remain to be elucidated.
The goal of dauer animals is to escape or outlast harsh environmental conditions and to steer towards better pastures. At least two adaptations to the sensory machinery of dauers may promote such behavior. First, upon dauer entry, the repertoire of odorant receptors expressed by individual sensory neurons in the amphid sensory organ is altered (Peckol et al., 2001), perhaps sensitizing the animal to hints of improvement in the environment. Second, some amphid neurons undergo extensive morphological remodeling upon dauer entry (Albert and Riddle, 1983; Mukhopadhyay et al., 2008), none more striking than the AWC neuron, whose glia-embedded wing-like receptive ending greatly expands in dauer animals. This expansion results in extensive overlap of the receptive endings of the left and right AWC neurons. Although the precise significance of this remodeling is unknown (one hypothesis is that it may increase detection sensitivity towards attractive food cues), it is clear that glia play an important role in reshaping AWC (Fig. 7A and 7B).
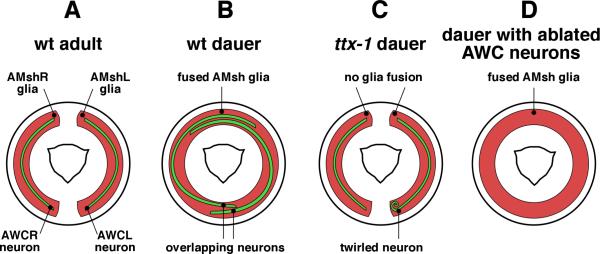
Figure 7.
Glia are required for sensory receptive ending remodeling. A: Diagram of a cross-section at the tip of the head. The wing-like processes of the bilateral AWC neurons are ensheathed by the bilateral amphid sheath glia (L and R for left and right respectively). B: In dauer animals the glia expand and fuse to accommodate the expansion of the neuronal wing processes. C: If glia lack ttx-1 they fail to expand and fuse, resulting in lack of neuronal remodeling. D: Conversely, glia remodeling takes place independently of neurons.
Accompanying the remodeling of the AWC neuron is a concomitant remodeling of the amphid sheath glia, which accommodates the large expansion in AWC neuron receptive ending surface area (Fig. 7B). Glial remodeling consists of expansion of the glial processes around the AWC neurons, as well as fusion of the two glia to create a continuous glial torus in the animal's nose. Mutations in the Otx/Otd transcription factor gene ttx-1 prevent glia expansion and fusion, and rescue studies suggest that ttx-1 normally functions within amphid sheath glia (C. Procko, Y. Lu, and S.S., submitted). Remarkably, remodeling of AWC neurons in animals carrying a ttx-1 mutation is defective, and AWC receptive endings fail to overlap in the head of the animal as they would normally, suggesting that glia guide AWC remodeling (Fig. 7C). In support of this hypothesis, the glial processes will expand and fuse in animals in which AWC neurons have been ablated (C. Procko, Y. Lu, and S.S., submitted) (Fig. 7D).
Amphid sheath glia fusion during dauer remodeling requires the fusogen AFF-1 as well as the TTX-1 target gene ver-1, which is related to VEGF receptors. Intriguingly, ver-1 expression is induced in amphid sheath glia not only in dauers, but also in non-dauer animals in response to high ambient temperature, a known stimulus for dauer entry (Golden and Riddle, 1984). Temperature-dependent induction of ver-1 does not require sensory neuron function, suggesting that amphid sheath glia are able to detect temperature directly. The mechanism of temperature sensation in these glial cells is not known.
The ability of amphid sensory organ glia to sense an environmental cue and to promote morphological changes in sensory neurons is strikingly reminiscent of the ability of astrocytes to detect presynaptically-released neurotransmitters and to modulate dendritic spine architecture (Jahromi et al., 1992; Reist and Smith, 1992; Murai et al., 2003), and is consistent with the seemingly conserved aspects of FIG-1 and ACD-1 functions.
Glial control of synaptic function
Most synapses in the C. elegans nerve ring are not ensheathed by the CEPsh glia. However, a very small number of synapses are tightly and reproducibly associated with glial processes, suggesting that glia may play important modulatory roles at these sites. Postembryonic ablation studies of CEPsh glia demonstrate that even after establishment of nerve ring architecture, glia are required for maintenance of aspects of normal neuronal morphology (M. Katz and S.S., unpublished). Furthermore, animals in which CEPsh glia are ablated after nerve ring formation exhibit a set of stereotypical locomotory defects. These defects can be strongly suppressed by ablation of the ALA neuron (M. Katz and S.S., unpublished). Strikingly, the synapse between ALA and its postsynaptic neuron AVE is one of the few synapses tightly associated with a CEPsh glial process (White et al., 1986) (Fig. 2D). Further analysis of this synapse promises to be exciting for two reasons. First, it will allow exploration of roles for glia in synaptic function in an organism with facile genetics and excellent infrastructure for gene discovery. Second, it will allow the study of activity at a single synaptic site using animal behavior as a direct readout. Correlation of single synapse physiology and animal behavior remains essentially impossible in vertebrates or Drosophila, thus C. elegans provides a powerful setting for testing a variety of hypotheses regarding the role of synaptic modulation on animal behavior and learning.
Comparing glia in C. elegans with glia in other animals
Until recently, C. elegans researchers tended to use terms such as “support” or “glia-like” cells when referring to C. elegans glia, a sign of caution over whether these intriguing cells indeed resemble vertebrate glia. The studies summarized in this review have uncovered similarities between glia in C. elegans and those of other animals on several levels: morphology, development, anatomy, and function, establishing C. elegans as an exciting system in which to study neuron-glia interactions. Nonetheless, differences between glia in C. elegans and other organisms are also apparent. These differences point towards C. elegans as a reductionist setting for studying glial function, and reveal where the power of the system is best exploited. Two main differences are apparent:
Myelin
Axonal myelination by glia has arisen independently several times during animal evolution, in response to the need for either higher speeds or longer distances of electrical conduction (Hartline and Colman, 2007). C. elegans does not exhibit axonal myelination, probably because neuronal process length is short (less than 1 mm) and current loss, therefore, negligible.
Trophic support
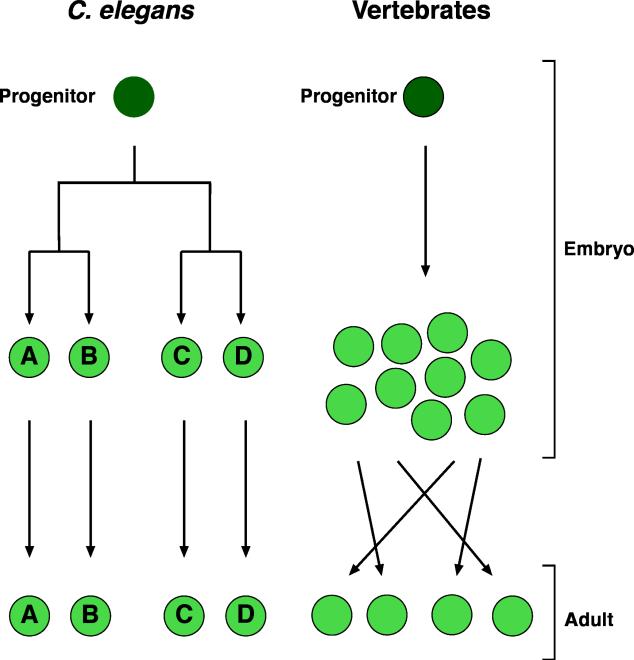
In contrast to neurons in other model systems, C. elegans neurons can survive without trophic support from glia. This difference likely stems from differences in modes of development between nematodes and other metazoans. In Drosophila and vertebrates, neurons are born in excess, and the final neuronal complement of the organism is determined by competition and pruning. Culling of cells in such a setting requires an extracellular signal that communicates to individual cells whether they are in excess, and glia provide components of this signal (Fig. 8). By contrast, the number of C. elegans neurons is predetermined by lineage, is identical between all C. elegans individuals, and is not governed by cell interactions. Therefore, with no competition for growth signals, it is not surprising that glia have no influence on the survival or demise of neuronal cells (Fig. 8). Indeed, to date, none of the programmed cell death that takes place in the soma of C. elegans has been shown to occur in response to an extracellular cue. The lack of trophic support of neurons by glia provides an obvious and powerful technical advantage: the opportunity to dissociate such support functions from regulatory functions, a problem that has plagued analysis of glia-neuron interactions in other model systems. Many of the studies described here have taken advantage of this unique in vivo arena to study glial influences on neuronal development and function.
Figure 8.
Neuronal development in vertebrates and C. elegans. In vertebrates, neurons are born in excess and then undergo a selection process that is hypothesized to include competition for glia-derived survival factors. In C. elegans, neurons, like all cells of the soma, are born in a predetermined fashion, and in predetermined numbers. Their survival does not depend on signals from other cells, including glia. Therefore, in contrast to vertebrates, C. elegans glia can be ablated without neuronal demise, and the role of glia in neuronal function can be probed in vivo.
Concluding remarks
Glia are one of the great mysteries in neuroscience today. Although the possibility that glia actively participate in the nervous system was acknowledged over 100 years ago (Cajal, 1911), progress in exploring possible roles played by these cells has been slow. A number of reasons are likely to have contributed to this state of affairs. Unlike glia, the output of neuronal function can be readily assayed by electrophysiology, thus attracting the interest of neuroscientists long before the revolution ushered in by molecular biology. Furthermore, the dependence of neurons on glial trophic factors has hindered progress in understanding how glia influence neuronal activity because manipulation of glia often leads to the demise of their associated neurons.
Only recently has it become apparent that the popular invertebrate model systems, Drosophila and C. elegans, have glia that are developmentally, morphologically and functionally homologous or analogous to the glia of vertebrate systems. C. elegans offers a unique arena in which to explore glia since the trophic dependence of neurons on glia is absent in this animal. The powerful tools of genetics and genomics, combined with the complete anatomical reconstruction of the nervous system (Ward et al., 1975; White et al., 1986; Hall and Russell, 1991) and with the ability to perform studies in vivo at single cell and single synapse resolution all suggest that exploration of glia in this organism is likely to provide important insight into the roles and functions of these enigmatic cells.
Acknowledgements
We would like to thank members of the Shaham lab for sharing unpublished data and for discussions. We apologize to those whose work we did not cite due to oversight on our part or space limitations. This work is supported by NIH grants R01NS064273, R01HD052677, and R01NS073121 to S.S.
References
- Albert PS, Brown SJ, Riddle DL. Sensory control of dauer larva formation in Caenorhabditis elegans. J Comp Neurol. 1981;198:435–451. doi: 10.1002/cne.901980305. [DOI] [PubMed] [Google Scholar]
- Albert PS, Riddle DL. Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J Comp Neurol. 1983;219:461–481. doi: 10.1002/cne.902190407. [DOI] [PubMed] [Google Scholar]
- Aldskogius H, Kozlova EN. Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. doi: 10.1016/s0301-0082(97)00093-2. [DOI] [PubMed] [Google Scholar]
- Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, Filho WJ, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Bacaj T, Tevlin M, Lu Y, Shaham S. Glia are essential for sensory organ function in C. elegans. Science. 2008;322:744–747. doi: 10.1126/science.1163074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI. Chemosensation in C. elegans. WormBook: the online review of C elegans biology. 2006:1–29. doi: 10.1895/wormbook.1.123.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem. 2003;278:15023–15034. doi: 10.1074/jbc.M300991200. [DOI] [PubMed] [Google Scholar]
- Brockway LM, Zhou ZH, Bubien JK, Jovov B, Benos DJ, Keyser KT. Rabbit retinal neurons and glia express a variety of ENaC/DEG subunits. Am J Physiol, Cell Physiol. 2002;283:C126–34. doi: 10.1152/ajpcell.00457.2001. [DOI] [PubMed] [Google Scholar]
- Bundgaard M, Abbott NJ. All vertebrates started out with a glial blood-brain barrier 4–500 million years ago. Glia. 2008;56:699–708. doi: 10.1002/glia.20642. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal RS. Histology of the Nervous System. Oxford University Press; New York, NY: 1911. p. 202. [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Colón-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson EL, Sternberg PW, Horvitz HR. A genetic pathway for the specification of the vulval cell lineages of Caenorhabditis elegans. Nature. 1987;326:259–267. doi: 10.1038/326259a0. [DOI] [PubMed] [Google Scholar]
- Fuentes-Medel Y, Logan MA, Ashley J, Ataman B, Budnik V, Freeman MR. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 2009;7:e1000184. doi: 10.1371/journal.pbio.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliot B, de Vargas C, Miller D. Evolution of homeobox genes: Q50 Paired-like genes founded the Paired class. Dev Genes Evol. 1999;209:186–197. doi: 10.1007/s004270050243. [DOI] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- Golestaneh N, Nicolas C, Picaud S, Ferrari P, Mirshahi M. The epithelial sodium channel (ENaC) in rodent retina, ontogeny and molecular identity. Curr Eye Res. 2000;21:703–709. [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, Russell RL. The posterior nervous system of the nematode Caenorhabditis elegans: serial reconstruction of identified neurons and complete pattern of synaptic interactions. J Neurosci. 1991;11:1–22. doi: 10.1523/JNEUROSCI.11-01-00001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Current biology: CB. 2007;17:R29–35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Heiman MG, Shaham S. Ancestral roles of glia suggested by the nervous system of Caenorhabditis elegans. Neuron Glia Biol. 2007;3:55–61. doi: 10.1017/S1740925X07000609. [DOI] [PubMed] [Google Scholar]
- Heiman MG, Shaham S. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell. 2009;137:344–355. doi: 10.1016/j.cell.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman RK. Mosaic analysis of two genes that affect nervous system structure in Caenorhabditis elegans. Genetics. 1987;116:377–388. doi: 10.1093/genetics/116.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi Y, Suzuki A, Kawano Y, Nozawa-Inoue K, Inoue M, Maeda T. Immunohistochemical detection of ENaCbeta in the terminal Schwann cells associated with the periodontal Ruffini endings of the rat incisor. Biomed Res. 2009;30:113–119. doi: 10.2220/biomedres.30.113. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Takizawa K, Nitta K, Hotta Y. glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell. 1995;82:1025–1036. doi: 10.1016/0092-8674(95)90281-3. [DOI] [PubMed] [Google Scholar]
- Jahromi BS, Robitaille R, Charlton MP. Transmitter release increases intracellular calcium in perisynaptic Schwann cells in situ. Neuron. 1992;8:1069–1077. doi: 10.1016/0896-6273(92)90128-z. [DOI] [PubMed] [Google Scholar]
- Jones BW, Fetter RD, Tear G, Goodman CS. glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell. 1995;82:1013–1023. doi: 10.1016/0092-8674(95)90280-5. [DOI] [PubMed] [Google Scholar]
- Krause M, Harrison SW, Xu SQ, Chen L, Fire A. Elements regulating cell- and stage-specific expression of the C. elegans MyoD family homolog hlh-1. Dev Biol. 1994;166:133–148. doi: 10.1006/dbio.1994.1302. [DOI] [PubMed] [Google Scholar]
- Labouesse M, Hartwieg E, Horvitz HR. The Caenorhabditis elegans LIN-26 protein is required to specify and/or maintain all non-neuronal ectodermal cell fates. Development. 1996;122:2579–2588. doi: 10.1242/dev.122.9.2579. [DOI] [PubMed] [Google Scholar]
- Legan PK, Rau A, Keen JN, Richardson GP. The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J Biol Chem. 1997;272:8791–8801. doi: 10.1074/jbc.272.13.8791. [DOI] [PubMed] [Google Scholar]
- Logan MA, Freeman MR. The scoop on the fly brain: glial engulfment functions in Drosophila. Neuron Glia Biol. 2007;3:63–74. doi: 10.1017/S1740925X07000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMiller TL, Johnson CM. Molecular characterization of HLH-17, a C. elegans bHLH protein required for normal larval development. Gene. 2005;356:1–10. doi: 10.1016/j.gene.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaux G, Gansmuller A, Hindelang C, Labouesse M. CHE-14, a protein with a sterol-sensing domain, is required for apical sorting in C. elegans ectodermal epithelial cells. Curr Biol. 2000;10:1098–1107. doi: 10.1016/s0960-9822(00)00695-3. [DOI] [PubMed] [Google Scholar]
- Moussaif M, Sze JY. Intraflagellar transport/Hedgehog-related signaling components couple sensory cilium morphology and serotonin biosynthesis in Caenorhabditis elegans. J Neurosci. 2009;29:4065–4075. doi: 10.1523/JNEUROSCI.0044-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Lu Y, Shaham S, Sengupta P. Sensory signaling-dependent remodeling of olfactory cilia architecture in C. elegans. Dev Cell. 2008;14:762–774. doi: 10.1016/j.devcel.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience. 2002;113:221–233. doi: 10.1016/s0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Peckol EL, Troemel ER, Bargmann CI. Sensory experience and sensory activity regulate chemosensory receptor gene expression in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2001;98:11032–11038. doi: 10.1073/pnas.191352498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perens EA, Shaham S. C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev Cell. 2005;8:893–906. doi: 10.1016/j.devcel.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Reist NE, Smith SJ. Neurally evoked calcium transients in terminal Schwann cells at the neuromuscular junction. Proc Natl Acad Sci USA. 1992;89:7625–7629. doi: 10.1073/pnas.89.16.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DL, Swanson MM, Albert PS. Interacting genes in nematode dauer larva formation. Nature. 1981;290:668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- Schneitz K, Spielmann P, Noll M. Molecular genetics of Aristaless, a prd-type homeo box gene involved in the morphogenesis of proximal and distal pattern elements in a subset of appendages in Drosophila. Genes Dev. 1993;7:911. doi: 10.1101/gad.7.5.911. [DOI] [PubMed] [Google Scholar]
- Shaham S. Chemosensory organs as models of neuronal synapses. Nature Reviews Neuroscience. 2010;11:212–217. doi: 10.1038/nrn2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003;112:619–630. doi: 10.1016/s0092-8674(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- Spacek J. Three-dimensional analysis of dendritic spines. III. Glial sheath. Anat Embryol. 1985;171:245–252. doi: 10.1007/BF00341419. [DOI] [PubMed] [Google Scholar]
- Strømme P, Mangelsdorf ME, Scheffer IE, Gécz J. Infantile spasms, dystonia, and other X-linked phenotypes caused by mutations in Aristaless related homeobox gene, ARX. Brain Dev. 2002;24:266–268. doi: 10.1016/s0387-7604(02)00079-7. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol. 1980;78:542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Sze JY, Ruvkun G. Activity of the Caenorhabditis elegans UNC-86 POU transcription factor modulates olfactory sensitivity. Proc Natl Acad Sci USA. 2003;100:9560–9565. doi: 10.1073/pnas.1530752100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M, Sieber M, Morphew M, Han M. The Caenorhabditis elegans aristaless orthologue, alr-1, is required for maintaining the functional and structural integrity of the amphid sensory organs. Mol Biol Cell. 2005;16:4695–4704. doi: 10.1091/mbc.E05-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S, Vonesch JL, Giangrande A. Glide directs glial fate commitment and cell fate switch between neurones and glia. Development. 1996;122:131–139. doi: 10.1242/dev.122.1.131. [DOI] [PubMed] [Google Scholar]
- Voglis G, Tavernarakis N. A synaptic DEG/ENaC ion channel mediates learning in C. elegans by facilitating dopamine signalling. EMBO J. 2008;27:3288–3299. doi: 10.1038/emboj.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowels JJ, Thomas JH. Multiple chemosensory defects in daf-11 and daf-21 mutants of Caenorhabditis elegans. Genetics. 1994;138:303–316. doi: 10.1093/genetics/138.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. doi: 10.1016/s0896-6273(00)80021-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Apicella A, Lee SK, Ezcurra M, Slone RD, Goldmit M, Schafer WR, Shaham S, Driscoll M, Bianchi L. A glial DEG/ENaC channel functions with neuronal channel DEG-1 to mediate specific sensory functions in C. elegans. EMBO J. 2008;27:2388–2399. doi: 10.1038/emboj.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- Ware RW, Clark D, Crossland K, Russell RL. The nerve ring of the nematode Caenorhabditis elegans: sensory input and motor output. J Comp Neurol. 1975;162:71–110. [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode C. elegans. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Murray JI, Lu Y, Waterston RH, Shaham S. mls-2 and vab-3 Control glia development, hlh-17/Olig expression and glia-dependent neurite extension in C. elegans. Development. 2008;135:2263–2275. doi: 10.1242/dev.019547. [DOI] [PubMed] [Google Scholar]
- Yu RY, Nguyen CQ, Hall DH, Chow KL. Expression of ram-5 in the structural cell is required for sensory ray morphogenesis in Caenorhabditis elegans male tail. EMBO J. 2000;19:3542–3555. doi: 10.1093/emboj/19.14.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]