Abstract
Background
A substantial body of evidence amassed from epidemiologic, correlative and experimental studies strongly associates atherosclerotic vascular disease (AVD) with Alzheimer's disease (AD). Depending on the precise interrelationship between AVD and AD, systematic application of interventions to maintain vascular health and function as a component of standard AD therapy offers the prospect of mitigating what is presently the inexorable course of dementia. To assess this hypothesis it is vital to rigorously establish the measures of AVD that are most strongly associated with an AD diagnosis.
Methods
A precise neuropathological diagnosis was established for all subjects using a battery of genetic, clinical, and histological methods. The severity of atherosclerosis in the circle of Willis (CW) was quantified by direct digitized measurement of arterial occlusion in postmortem specimens and compared between AD and non-demented control (NDC) groups by calculating a corresponding index of occlusion.
Results
Atherosclerotic occlusion of the CW arteries was more extensive in the AD group than the NDC group. Statistically significant differences were also observed between control and AD groups with regard to Braak stage, total plaque score, total NFT score, total white matter rarefaction score, brain weight, MMSE scores and apolipoprotein E allelic frequencies.
Conclusions
Our results, combined with a consideration of the multifaceted impacts of impaired cerebral circulation, suggest an immediate need for prospective clinical trials to assess the efficacy of AD prevention using anti-atherosclerotic agents.
Keywords: Alzheimer's disease, vascular dementia, intracranial atherosclerosis, circle of Willis, brain hypoperfusion
1. Introduction
Genetic investigations suggest that the amyloid-beta (Aβ) peptide has a central role in Alzheimer's disease (AD). However, genetically determined familial AD is rare, while sporadic AD is the most common form of this dementia. Nearly 25 years after the Aβ molecule was identified as a potential therapeutic target, the exact cause(s) of AD dementia remains undefined. For the foreseeable future the standard pharmacologic treatments are virtually palliative, offering only steadily diminishing functional maintenance without hope of cure.
Alzheimer's disease may result from the combined and chronic, cascading effects of multiple systemic diseases affecting the elderly, among them cardiovascular, immune/inflammatory, endocrine and ultimately a dysfunctional brain energy metabolism. In recent years, a broad body of evidence derived from epidemiologic, correlative and experimental studies has strongly linked atherosclerotic vascular disease (AVD) with AD (reviewed in reference [1]). Postmortem studies have recently shown that individuals with AD have significantly more atherosclerotic narrowing of the intracranial arteries [1-5].
Despite the epidemiological and neuropathological evidence, the question of whether intracranial AVD has a significant causal role in AD pathogenesis still remains unanswered, although there are data consistent with causation. As atherosclerosis generally begins much earlier in life than AD, the temporal relationship suggests that AVD may cause or accelerate AD, rather than the reverse. Results from several longitudinal life-history studies have shown that elevated AVD risk factors in midlife are associated with increased AD risk in old age [6-9]. Individuals with higher midlife cholesterol levels have a higher risk of developing AD, and patients with clinically or neuropathologically diagnosed AD have higher cholesterol levels compared to non-demented control (NDC) individuals [8;10-12]. There is also considerable evidence from experimental studies suggestive of a causative effect for increased blood cholesterol. The production of APP and the Aβ peptide, the main biochemical AD marker, in cell culture and animal models is regulated by cholesterol and decreased by cholesterol-lowering drugs such as statins, and some molecular mechanisms have been proposed for these interactions (reviewed in reference [13]). The independent association of AD with multiple AVD risk factors suggests, however, that cholesterol is not the sole culprit in dementia.
That hypercholesterolemia, hypertension, diabetes, hyperhomocysteinemia, tobacco smoking and other AVD risk factors would produce pathology through completely separate molecular mechanisms seems improbable. A common mechanism may be hypoperfusion. The circulatory system is preeminent in the development of the brain and the maintenance of its vital functions. Thus, any pathology that impedes circulation, including the normal age-related decline in cardiovascular function and its increasing inability to adapt and repair is deleterious. Consequently, the importance of recognizing the interrelationship between cardiovascular disease and brain perfusion in AD cannot be overstated.
Ischemic brain disease is the generic designation for a group of closely related syndromes resulting from a disparity between the supply (perfusion) and the demand imposed by the brain for oxygenated blood. In addition, it involves reduced availability of nutrient substrates and ineffective removal of CO2 and noxious metabolites. It has been established that hypoperfusion or chronic oligemia could induce cortical atrophy through slow starvation of brain parenchyma [14]. Intracranial atherosclerosis is a major cause of brain hypoperfusion and stroke. Furthermore, infarcts are present in approximately 40% of subjects with AD and the presence of infarcts has been shown to significantly increase the likelihood of dementia in subjects harboring both infarcts and AD histopathology [15-18].
The reports by ourselves and others [1-5] of increased intracranial AVD in AD indicate that stenosis of the arteries supplying the brain may be at least partially responsible for reduced cerebral perfusion in AD. Possible molecular mechanisms linking AD pathology and hypoperfusion include ischemia-induced alterations in Aβ precursor protein (APP) expression and APP cleavage [19], both of which increase Aβ production. In addition, brain ischemia induces the production of hypoxia inducible factor that increases the production of β-secretase and increases Aβ levels [20]. An even simpler hypothesis is that decreased cortical perfusion may reduce Aβ clearance from brain to the blood, analogous to the declining clearance of blood urea to urine with decreased renal perfusion seen, for example, in congestive heart failure.
It is evident that cardiovascular disease and AD are likely to have a synergistic effect on dementia [21]. This statement is made with a strong caveat that although the statistical linkage between AD and intracranial AVD is significant, it is clear that AVD is not a precondition for the development of AD. The existence of cases of AD with very little AVD, and of very old NDC individuals with severe AVD, demonstrates that the association is not invariant.
Atherosclerotic vascular disease arises from the complex interactions of genetic and environmental factors [22;23]. However, some of the complications of AVD are largely preventable by lifestyle modification and pharmacological manipulation [24], suggesting therefore, AD may be at least partially preventable by similar methods. Furthermore, even if AD and AVD are only coincidentally related, about one-half of AD cases have significant contributory AVD that impairs cognition through ischemia/hypoxia and infarction in an additive fashion [15-17]. On this basis alone, the systematic application of AVD prevention as a component of standard AD therapy should reduce functional impairment and decline in AD. If AVD is a synergistic or convergent disease with AD [21], by accelerating disease onset and cognitive decline, then AVD therapy will have a correspondingly greater clinical impact in AD patients.
The clinical utility of a causal link between AD and AVD can only be definitively established by prospective clinical prevention trials using anti-atherosclerotic agents. Postmortem evaluation of the circle of Willis (CW) and major cerebral arteries seeks to establish the groundwork for such trials by revealing those AVD measures most strongly associated with the diagnosis of AD. In the present study, we compare the degree of CW atherosclerosis between AD and NDC individuals by rigorously measuring the index of occlusion in postmortem specimens. In addition, the functional repercussions of arterial stenosis on brain hemodynamics and hydrodynamics are discussed.
2. Methods
2.1 Human specimens
Circle of Willis specimens were collected at Banner Sun Health Research Institute, a private, non-profit organization located in Sun City, Arizona. Volunteers for the Brain Donation Program receive annual neurological and psychological assessments, as well as apolipoprotein (ApoE) genotyping [25]. All CW arteries were removed in the immediate postmortem, rinsed with phosphate buffer, fixed in 10% paraformaldehyde for 7 days and stored at 4° C in phosphate buffer with 0.01% sodium azide until the time of analysis. For this study, we measured the CW arteries from 36 NDC and 61 AD cases.
2.2 Neuropathological diagnosis
For neurodegenerative diseases, the neuropathologic diagnosis was made as outlined in a published algorithm [26]. Cases with dementia were rated for AD changes according to NIA/Reagan Institute [27] and CERAD criteria [28], and by Braak stage [29]. The diagnosis of AD was made when an NIA/Reagan Institute rating of “high” or “intermediate” was present in a subject clinically diagnosed with dementia.
The average values for age, gender, Braak stage, total amyloid plaque score, total neurofibrillary tangle (NFT) score, total white matter rarefaction (WMR) score, brain weight and the last mini-mental state examination (MMSE) score as well as ApoE allelic frequency are summarized in Table 1. The assessment of Braak stage, total amyloid plaque score, total NFT score, total WMR score and MMSE procedure have been described elsewhere [2].
Table 1.
Characteristics of NDC and AD Subjects
| Characteristic | NDC (n=36) | AD (n=61) |
|---|---|---|
| Percentage | ||
| Female | 50 | 57 |
| ApoE*1 | ||
| 2/2 | 2.8 | 0.0 |
| 2/3 | 16.7 | 5.0 |
| 3/3 | 55.6 | 58.3 |
| 3/4 | 25.0 | 30.0 |
| 4/4 | 0.0 | 6.7 |
| Braak stage* | ||
| I | 8.3 | 0.0 |
| II | 25.0 | 4.9 |
| III | 50.0 | 11.5 |
| IV | 16.7 | 14.8 |
| V | 0.0 | 34.4 |
| VI | 0.0 | 34.4 |
| Mean (SD) | ||
| Age (years) | 84.9 (6.1) | 85.1 (7.3) |
| Total plaque score* | 5.5 (4.3) | 12.6 (1.9) |
| Total NFT score* | 4.1(2.3) | 11.0 (4.3) |
| Total WMR score*2 | 2.3 (3.1) | 5.1 (4.0) |
| Brain weight*, g | 1175 (108.0) | 1061 (145.9) |
| Last MMSE score*3 | 28 (1.8) | 10 (7.6) |
| CW index of occlusion* | 51.6 (7.5) | 58.9 (12.5) |
p<.001; all other differences between NDC and AD subjects are not significant (p>.05)
Sample size is 96 (n=36 NDC, 60 AD)
Sample size is 94 (n=34 NDC, 60 AD)
Sample size is 66 (n=26 NDC, 40 AD)
Abbreviations: NDC, non-demented control; AD, Alzheimer's disease; ApoE, apolipoprotein E; NFT, neurofibrillary tangle; WMR, white matter rarefaction; MMSE, Mini-mental state examination; CW, circle of Willis. Total plaque, total NFT and total WMR score are based on 0-15. Each of the 4 cerebral lobes plus the hippocampus is scored from 0-3, where 0 = none, 1 = mild, 2 = moderate and 3 = severe. Braak stage ranges from 0-VI, according to the increasing regional distribution of the NFT in the brain. The MMSE (range 0 to 30) assesses cognitive function, with decreasing scores reflecting lower cognitive performance.
2.3 Measurement of the index of occlusion
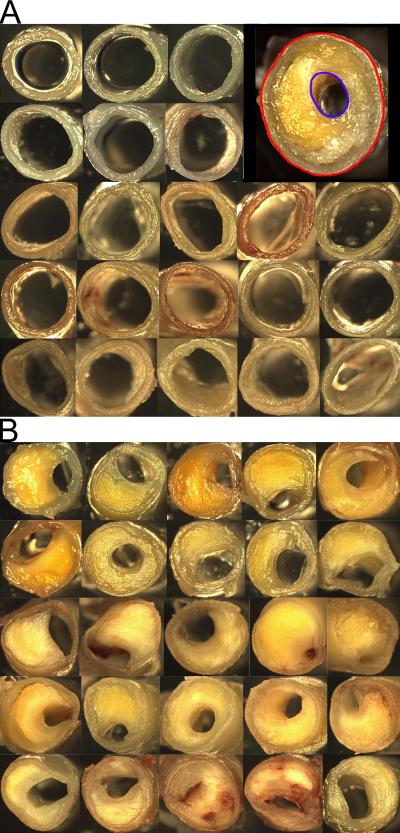
Digital photographs of the intact vessels were taken prior to dissection. The arteries included in this study were right and left vertebral arteries (VA), basilar artery (BA), right and left posterior cerebral arteries (PCA), right and left middle cerebral arteries (MCA), right and left internal carotid arteries (ICA) and right and left anterior cerebral arteries (ACA). All arteries were cut into ~5 mm cross-sections and examined under a Leica S8APO dissecting microscope, and the point of minimum cross-sectional lumenal area in each arterial segment was selected for morphometric assessment. A total of 2,108 cross-sections were measured. The segments were photographed with an Optronics Magnafire SP camera and software program (Optronics, Goleta, CA). Measurements of the cross-sectional external and lumenal areas were taken from the digital photographs with the calibrated ImagePro Express, v. 4.0 software (Media Cybernetics, Silver Spring, MD). By definition, the arterial wall structure included the intima, media and adventitia layers while the arterial external and lumenal areas were obtained by subtracting the area bounded by the intima from that calculated for the complete circumference of the outer limit of the adventitia as shown in the Figure 1A insert. The measurements of these areas, reported in mm2, were exported to an Excel database spreadsheet (Microsoft, Redmond, WA). Since there is a wide variation in arterial size, an index of occlusion (stenosis) was calculated for each cross-section by subtracting the lumenal area from the external area, dividing the difference by the external area and multiplying the quotient by 100.
Figure 1.
Representative cross-sections of the circle of Willis arteries. The top panel (A) illustrates a series of arterial sections in which the lumenal area is minimally reduced. The inset indicates the external area (red) and luminal area (blue) that were manually encircled. The area was calculated automatically with ImagePro Express, v 4.0 software and an index of occlusion is derived from these numbers. The bottom panel (B) shows arteries with severe atherosclerosis. In some cases the arteries are almost occluded by the atheroma plaque.
2.4 Medical history assessments
Four years of private medical records were generally available for each subject with two- year histories obtained at the time of program initiation and two more years requested at the time of death. We reviewed the medical records of the subjects and recorded the presence or absence of clinically related cardiovascular ailments or interventions, risk factors for AVD, respiratory diseases and other relevant co-morbidities (see Table 2).
Table 2.
Co-morbidities of NDC and AD Subjects
| Co-morbidity (%) | NDC (n = 36) | AD (n = 61) |
|---|---|---|
| Coronary artery disease | 36 | 36 |
| Angina | 11 | 3 |
| Stent, Angioplasty or CABG | 14 | 11 |
| Carotid artery disease | 11 | 3 |
| Valvular diseases | 11 | 7 |
| Myocardial infarction | 8 | 15 |
| Congestive heart failure | 33 | 16 |
| Left ventricular hypertrophy | 14 | 8 |
| Dysfunctions of rhythm and conduction* | 61 | 23 |
| Peripheral vascular disease | 8 | 7 |
| Hyperlipidemia | 31 | 18 |
| Hypertension | 64 | 48 |
| Transient ischemic attack | 17 | 13 |
| Syncope | 8 | 8 |
| Stroke, hemorrhage or embolism | 19 | 28 |
| Diabetes mellitus | 19 | 16 |
| Peripheral neuropathy | 17 | 5 |
| Renal disease* | 31 | 8 |
| COPD | 33 | 18 |
| Emphysema | 8 | 2 |
| Asthma | 8 | 5 |
| Pulmonary embolism | 6 | 2 |
| Severe head trauma | 3 | 7 |
| Cancer* | 56 | 30 |
| Osteoporosis* | 28 | 10 |
| Hypothyroidism | 25 | 30 |
difference between NDC and AD subjects significant at p<.05
Abbreviations: NDC, non-demented control; AD, Alzheimer's disease; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease.
2.5 Statistical Analysis
The association of cognitive status group with subject characteristics, co-morbidities and index of occlusion was analyzed using Fisher's exact chi-square tests and unpaired, 2-tailed t-tests with Satterthwaite's unequal variance assumption. Multiple logistic regression models were used to examine stenosis as a predictor of AD, and were adjusted for the covariates age, gender and ApoE-ε4 allele status. Standard lacks of fit and regression diagnostics (residual and collinearity tests) were assessed. Analyses were conducted with SAS software, version 9.1 (SAS Institute Inc., Cary, North Carolina).
3. Results
No significant differences existed between the NDC and AD groups with respect to age or gender (Table 1). However, statistically significant differences were found between NDC and AD groups on average CW index of occlusion as well as ApoE genotype, Braak stage, total plaque score, total NFT score, total WMR score, brain weight and MMSE score. Since we consider AD as a multifactorial disease closely related to the natural decay of multiple systems associated with aging, we reviewed the prevalence of several relevant cardiovascular, respiratory and other co-morbidities in the NDC and AD groups (Table 2). Of the extensive list of co-morbidities, only renal disease, cancer, osteoporosis and dysfunctions of rhythm and conduction differed significantly between the two groups, with these co-morbidities more common in NDC subjects. Previous studies have demonstrated that, for reasons not well understood, AD individuals seem protected against oncologic diseases [30]. The above data emphasize the large number of maladies and their complex interactions related to vital functions that alter blood and oxygen supply to the brain or disrupt its metabolism. Ultimately, the impact of these diseases on the prevention, pathogenesis or course of AD would reflect their age of onset, pathology intensity, combination of morbidities and their timely and adequate pharmacological or surgical management.
Disease duration did not correlate with the average index of occlusion, percentage of sections ≥ 60% occlusion, percentage of sections ≥ 70% occlusion or percentage of sections ≥ 80% occlusion (data not shown, R2 = 0.17, 0.17, 0.17, and 0.19, respectively).
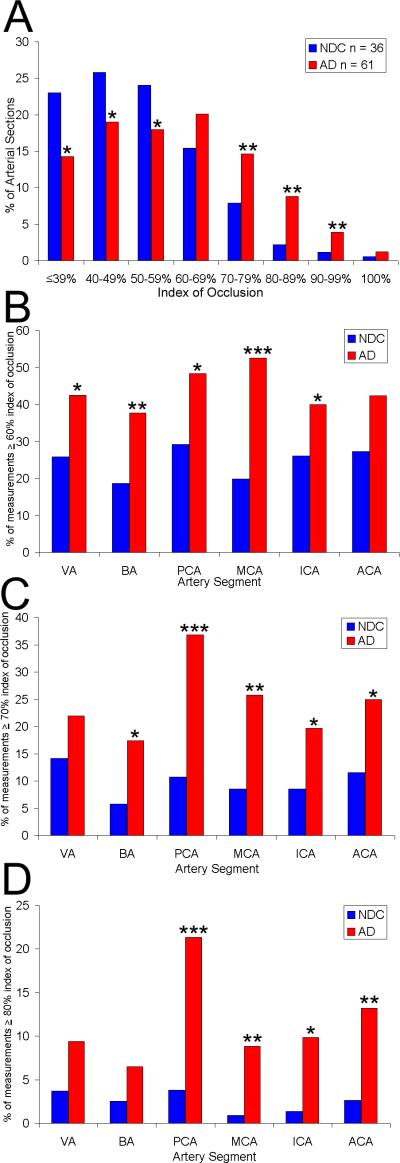
Arteries of the CW were more severely occluded by atherosclerotic lesions in the AD group than in the NDC group. A graphic representative example of the magnitude of CW atherosclerosis in AD subjects is illustrated in Figure 1. Figure 2A shows the percentage of all arterial measurements taken along the y-axis and the index of occlusion, in deciles, along the x-axis. For example, the proportion of cross-sectional measurements with the lowest index of occlusion (less than or equal to 39%) was 23% in the NDC compared with 14% in the AD group. The histogram illustrates the gross differences in the degree of atherosclerosis between the control and affected cohorts. Significant differences exist at all occlusion levels except 60-69%. Below 60-69%, AD subjects are significantly less likely to be found in these lower occlusion level categories, while above 60-69%, AD subjects are significantly more likely to be found in these higher occlusion level categories. The 60-69% occlusion level appears to be a transition point between these patterns and thus shows no significant differences.
Figure 2.
A) The y-axis represents of the percentage of all arterial measurements taken. The index of occlusion, separated in deciles, is along the x-axis. The index of occlusion was also higher in the individual arteries of AD subjects compared to NDC subjects when separating by the percentage of measurements ≥ 60% (B), 70% (C) and 80% (D) index of occlusion. NDC, non-demented control; AD, Alzheimer's disease; VA, vertebral arteries; BA, basilar artery; PCA, posterior cerebral arteries; MCA, middle cerebral arteries; ICA, intracranial arteries; ACA, anterior cerebral arteries; * p<.05; **p<.01; ***p<.001.
In the AD group, an average of 48% of arterial sections were 60% or more occluded, 29% were 70% or more occluded and 14% were 80% or more occluded. By contrast, in the NDC group for the identical index of occlusion, the corresponding percentages were: 27%, 12% and 4%. Comparisons at all three occlusion extent percentiles (≥60%, ≥70%, ≥80%) between AD and NDC groups revealed statistically significant differences at p<.0001. The index of occlusion of individual major intracranial arteries (VA, BA, PCA, MCA, ICA and ACA) differed significantly between the NDC and AD groups, with greater stenosis in AD subjects (Figures 2B, C and D). Multivariate logistic regression models also support an association between stenosis in the CW arteries and likelihood of AD, even after adjusting for the effects of age, gender and ApoE-ε4 (Table 3). Higher levels of stenosis were more likely in AD subjects compared with NDC subjects (for all arteries combined, the odds ratio=1.06; i.e., 6% change per 1-unit increase in index of occlusion). When considering the entire population under study, the mean index of occlusion ranged from 33% (lowest) to 84% (highest); there would thus be a 51-unit or 306% (three-fold) increase in likelihood of AD from the lowest to the highest levels of stenosis. This number is similar to the odds ratios from carrying one ApoE ε4 allele and agrees with our previous observations using semi-quantitative atherosclerotic scoring on a much larger sample [1]. This association was also significant for specific CW arteries: BA, ICA, MCA, and PCA. When the percentage of arterial sections that were 60% or more occluded was assessed, there were significant associations again with both individual arteries and all arteries combined.
Table 3.
Association of Occlusion with AD
| Measure of Occlusion | n (NDC/AD) | Adjusted1 Odds Ratio | p |
|---|---|---|---|
| Mean index of occlusion | |||
| ACA | 36/60 | 1.03 | .065 |
| BA | 35/60 | 1.05 | .015 |
| ICA | 36/60 | 1.05 | .014 |
| MCA | 36/60 | 1.06 | .003 |
| PCA | 35/60 | 1.04 | .0099 |
| VA | 31/49 | 1.04 | .052 |
| All arteries | 36/60 | 1.06 | .006 |
| Percentage of sections ≥60% occluded | |||
| ACA | 36/60 | 1.01 | .101 |
| BA | 35/60 | 1.02 | .018 |
| ICA | 36/60 | 1.01 | .084 |
| MCA | 36/60 | 1.03 | .0003 |
| PCA | 35/60 | 1.01 | .027 |
| VA | 31/49 | 1.02 | .022 |
| All arteries | 36/60 | 1.03 | .002 |
Models were adjusted for age at death, gender and presence of an apolipoprotein E *E4 allele. The maximum possible sample size in this multivariate analyses for the AD group is 60, due to the absence of apolipoprotein genotyping of one individual.
Abbreviations: NDC, non-demented control; AD, Alzheimer's disease; ACA, anterior cerebral arteries; BA, basilar artery; ICA, internal carotid arteries; MCA, middle cerebral arteries; PCA, posterior cerebral arteries; VA, vertebral arteries.
The index of occlusion for carriers of an ApoE ε4 allele was compared to non-carriers for each diagnosis group. There was no statistically significant difference between carriers and non-carriers within the AD or NDC group (data not shown; p > 0.05, unpaired, 2-tailed t-test). These results are similar to those found in other studies [1;5;31].
4. Discussion
Atherosclerosis of the CW and major cerebral arteries is almost universal after 80 years of age. It is more severe in males than in females by age 60, with the male predominance decreasing with age and finally disappearing by age 80 [32]. When compared to NDC individuals, our multivariate regression models clearly indicate a link between the degree of atherosclerosis of the CW and AD (adjusted odds ratio: 1.06 (95% CI = 1.02-1.11, p = 0.006).Overall, an index of occlusion above 60% is more frequent in AD cases than in NDC, while a larger number of arterial segments with a lower index of occlusion (<60%) is more abundant in the NDC group. Significant differences were also found between AD and NDC in many individual arteries, with the frequency and degree of occlusion more severe in AD. These pathological differences between the two groups were reflected hemodynamically in an altered pulsatility index and mean flow velocity values (see previous article). Measurements of cerebral blood flow in terms of velocity and resistivity are assessments of great value in AD, since apart from suggesting diffuse microvascular disease, they also reveal the hemodynamic conditions of the individual and the adaptive responsiveness of the CW and major cerebral arteries through the development of collateral circulation.
The presence of severe atherosclerotic lesions in major cerebral arteries can obstruct the ostia or invade the lumina of important secondary branches supplying vital brain regions [4;33;34], such as the hippocampal arteries arising from the PCA [35], the most affected arteries in our study (Figure 2C and D). Likewise, occlusion of the small anterior perforating branches originating from the ACA that supply the cholinergic nucleus basalis of Meynert [36] would have dire consequences for both cognition and cholinergic/nitric oxide-mediated vasodilatation of the cerebral arteries. With a chronic, progressive regional decrease of blood flow, metabolic and electrophysiological functions decline and eventually fail [37]. Moreover, lacunar infarcts result from perforating artery obstruction triggered by cardiac disease, atherosclerosis, and hypertension [38] and play a role in modifying the clinical expression of AD, as shown by the Nun Study [17]. In addition, hypoxic/ischemic conditions can result in a gross disruption of the blood brain barrier (BBB) integrity and bleeding [39;40]. It has been suggested that microhemorrhages may induce a localized, beneficial cerebrovascular amyloidosis in an attempt to patch ruptured capillary and arteriolar walls [41;42]. However, the global, untargeted deposition of amyloid in the walls of capillaries, arterioles and small arteries in the cerebral cortex and leptomeningeal vessels characteristic of AD may represent the ultimate pathological consequence of brain ischemia and hypoxia.
The normal arterial tree is morphologically and functionally designed as both a conduit and cushion. Failure to cushion systolic pulsation due to arterial wall stiffening, results in larger amplitude of intracranial blood beating and microvasculature damage [43;44], and consequent endothelial cell shedding, myocyte damage, BBB failure and vessel rupture [45]. In general, accumulating diffuse microangiopathic disease promotes increased vascular resistance [46-48] escalating hypertension and impaired venous outflow [43;49].
In addition to declining brain perfusion, the loss of arterial elasticity from atherosclerosis, arteriosclerosis and calcification has ominous hemodynamic and cerebrospinal fluid (CSF) hydrodynamic consequences for the maintenance of brain homeostasis. Magnetic resonance phase imaging has demonstrated that when an arterial pulse wave enters the rigid cranium, the force propagates perpendicular to the skull surface, resulting in an immediate increase in CSF pressure which will be offset by the rigidity of the calvarium, propelling CSF through the tentorial notch and foramen magnum into the spinal canal. The pulsatile movements result in arterial expansion and concomitant brain volume enlargement assisted by systolic capillary dilation and venous and subarachnoid space engorgement. Recoil of CSF from the spinal canal into the brain's subarachnoid space occurs during diastole [50;51]. Dilation of the brain ventricles in AD correlates with the degree of cerebral blood flow impingement created by advanced atherosclerotic and arteriosclerotic lesions. Increased resistance in the CSF and cerebral vessels may also impair venous drainage and further contribute to decrease in cerebral blood flow [52]. Furthermore, the microvascular pulsations also impel the drainage of the brain's interstitial fluid (IF) along the perivascular spaces that drain into the deep lymphatics of head and cervical venous circulation [53;54]. Clogging of the perivascular spaces in the cerebral cortex by amyloid deposition results in the stagnation of the IF in the white matter with dilation of the periarterial spaces (état criblé) and overflow into the ventricular space [55]. In this loop of detrimental events, decreased cerebral blood flow velocity in the arteries of the brain may ultimately be due to reduced brain metabolic demands [56].
In summary, mechanical obstruction and reduction in cerebral arterial inflow due to atherosclerotic lesions damages the microvasculature, eventually leading to severe brain hypoxia, leukoariosis, lacunar infarcts, brain atrophy, ventricular dilation, retention of IF and accumulation of noxious substrates [57;58]. The cumulative effects of these interdependent hemodynamic and hydrodynamic dysfunctions play a pivotal role in accelerating and augmenting the pathogenesis and evolution of AD [59].
Preventative therapies for both AVD and AD would be most efficacious if implemented prior to the development of clinically evident AVD. For this reason, systematic, preemptive diagnostic studies involving lipid screenings, hemodynamic imaging and ultrasound studies should be a routine aspect of patient assessment to enable early recognition of impending AD. It has been recently found that timely treatment of vascular risk factors slows the decline of AD [60]. For example, several clinical trials suggest that aggressive intervention with statins leads to a reduction of low-density lipoprotein cholesterol and regression of coronary atherosclerosis measured by intravascular ultrasound [61;62]. In contrast, evidence exists from retrospective clinical trials that statins given late in life offer no dementia prevention or amelioration benefit (reviewed in reference [63]). However, this disappointing result must be balanced with a caveat that these trials were performed on elderly patients exhibiting clinically evident mild to moderate AD. At this stage, the virtually universal presence of advanced atherosclerotic/calcification damage and permanent brain injury may have precluded any hope for clinically meaningful beneficial effects. Therefore, it is imperative that statin therapies commence prior to the establishment of advanced AVD and any appearance of brain lesions associated with AD. In addition, statins may have other important contributory factors to the reduction of atherosclerosis through their anti-thrombotic, vasodilatory-antihypertensive and antimitotic effects (reviewed in reference [64]). The predictive strength of a family history and preclinical evidence of AVD must also be considered as critical rationalizations for the recommendation of intense pharmacological intervention, lifestyle and dietary modifications as well as the adoptation of disciplined exercise regimens to preempt dementia development. Systematic implementation of educational campaigns promoting radical changes in cultural and societal values will be important factors in the broad patient adoption of proactive AD-defeating strategies. In addition, such actions may provide potentially huge dividends by preventing both cardiovascular disease and dementia.
Acknowledgements
This work was in part supported by Science Foundation Arizona, the Arizona Department of Health Services (Contract 211002 Arizona Alzheimer Disease Research Consortium), the National Institute on Aging (R01-AG19795 and R01-NS38674), the Arizona Alzheimer's Disease Core Center (P30 AG-19610) and the Prescott Family Initiative of the Michael J. Fox Foundation for Parkinson's Research. We are indebted to Dr. Douglas Walker for ApoE genotyping and to Drs. Walter M. Kalback, Dean C. Luehrs and R. Lyle Patton for helpful discussions.
Abbreviations
- Aβ
amyloid-beta
- ACA
anterior cerebral arteries
- AD
Alzheimer's disease
- ApoE
apolipoprotein E
- APP
amyloid-beta precursor protein
- AVD
atherosclerotic vascular disease
- BA
basilar artery
- BBB
blood brain barrier
- CABG
coronary artery bypass graft
- COPD
chronic obstructive pulmonary disease
- CSF
cerebrospinal fluid
- CW
circle of Willis
- ICA
internal carotid arteries
- IF
interstitial fluid
- MCA
middle cerebral arteries
- MCI
mild cognitive impairment
- MMSE
mini-mental state examination
- NDC
non-demented control
- NFT
neurofibrillary tangle
- PCA
posterior cerebral arteries
- TCD
transcranial Doppler
- VA
vertebral arteries
- WMR
white matter rarefaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Marwan N. Sabbagh received grant/clinical trial support from Baxter, Lilly, Wyeth, Avid, BMS, Medivation and Elan. Dr. Sabbagh is a consultant for Lilly, Wyeth, Glaxo Smith Kline, and Amerisciences. The rest of the Authors involved in this project declare no competing interests.
Reference List
- 1.Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, et al. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113:13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 2.Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, et al. Circle of Willis atherosclerosis is a risk factor for sporadic Alzheimer's disease. Arterioscler Thromb Vasc Biol. 2003;23:2055–62. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- 3.Kalback W, Esh C, Castano EM, Rahman A, Kokjohn T, Luehrs DC, et al. Atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer's disease. Neurol Res. 2004;26:525–39. doi: 10.1179/016164104225017668. [DOI] [PubMed] [Google Scholar]
- 4.Roher AE, Esh C, Rahman A, Kokjohn TA, Beach TG. Atherosclerosis of cerebral arteries in Alzheimer disease. Stroke. 2004;35:2623–27. doi: 10.1161/01.STR.0000143317.70478.b3. [DOI] [PubMed] [Google Scholar]
- 5.Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer's Coordinating Center. Neurology. 2005;64:494–500. doi: 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- 6.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 7.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 8.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–51. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–60. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 10.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–82. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 11.Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, et al. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer's disease. Neuroepidemiology. 1998;17:14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- 12.Kuo YM, Emmerling MR, Bisgaier CL, Essenburg AD, Lampert HC, Drumm D, et al. Elevated low-density lipoprotein in Alzheimer's disease correlates with brain abeta 1-42 levels. Biochem Biophys Res Commun. 1998;252:711–15. doi: 10.1006/bbrc.1998.9652. [DOI] [PubMed] [Google Scholar]
- 13.Wolozin B, Brown J, III, Theisler C, Silberman S. The cellular biochemistry of cholesterol and statins: insights into the pathophysiology and therapy of Alzheimer's disease. CNS Drug Rev. 2004;10:127–46. doi: 10.1111/j.1527-3458.2004.tb00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caroli A, Testa C, Geroldi C, Nobili F, Guerra UP, Bonetti M, et al. Brain perfusion correlates of medial temporal lobe atrophy and white matter hyperintensities in mild cognitive impairment. J Neurol. 2007;254:1000–1008. doi: 10.1007/s00415-006-0498-z. [DOI] [PubMed] [Google Scholar]
- 15.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–55. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 16.Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, Davis D, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- 17.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–17. [PubMed] [Google Scholar]
- 18.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–36. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 19.Bennett SA, Pappas BA, Stevens WD, Davidson CM, Fortin T, Chen J. Cleavage of amyloid precursor protein elicited by chronic cerebral hypoperfusion. Neurobiol Aging. 2000;21:207–14. doi: 10.1016/s0197-4580(00)00131-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao FF, et al. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J Biol Chem. 2007;282:10873–80. doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]
- 21.Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer's disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363:1139–46. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- 22.Ding K, Kullo IJ. Genome-wide association studies for atherosclerotic vascular disease and its risk factors. Circ Cardiovasc Genet. 2009;2:63–72. doi: 10.1161/CIRCGENETICS.108.816751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotta LA. Genome-wide association studies in atherothrombosis. Eur J Intern Med. 2010;21:74–78. doi: 10.1016/j.ejim.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Zaiou M, Benachour H, Marteau JB, Visvikis-Siest S, Siest G. Genomics and the prospects of existing and emerging therapeutics for cardiovascular diseases. Curr Pharm Des. 2009;15:3193–206. doi: 10.2174/138161209789058011. [DOI] [PubMed] [Google Scholar]
- 25.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987-2007. Cell Tissue Bank. 2008;9:229–45. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickson DW. Required techniques and useful molecular markers in the neuropathologic diagnosis of neurodegenerative diseases. Acta Neuropathol. 2005;109:14–24. doi: 10.1007/s00401-004-0950-z. [DOI] [PubMed] [Google Scholar]
- 27.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–97. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 29.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 30.Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC. Alzheimer disease and cancer. Neurology. 2005;64:895–98. doi: 10.1212/01.WNL.0000152889.94785.51. [DOI] [PubMed] [Google Scholar]
- 31.Luoto TM, Haikonen S, Haapasalo H, Goebeler S, Huhtala H, Erkinjuntti T, et al. Large vessel cerebral atherosclerosis is not in direct association with neuropathological lesions of Alzheimer's disease. Eur Neurol. 2009;62:93–98. doi: 10.1159/000222779. [DOI] [PubMed] [Google Scholar]
- 32.Sawabe M, Arai T, Kasahara I, Hamamatsu A, Esaki Y, Nakahara K, et al. Sustained progression and loss of the gender-related difference in atherosclerosis in the very old: a pathological study of 1074 consecutive autopsy cases. Atherosclerosis. 2006;186:374–79. doi: 10.1016/j.atherosclerosis.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Ostrow PT, Miller LL. Pathology of small artery disease. Adv Neurol. 1993;62:93–123. [PubMed] [Google Scholar]
- 34.Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. 1989;39:1246–50. doi: 10.1212/wnl.39.9.1246. [DOI] [PubMed] [Google Scholar]
- 35.Duvernoy HM. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. 2nd ed. Springer; Berlin: [Google Scholar]
- 36.Pullicino PM. The course and territories of cerebral small arteries. Adv Neurol. 1993;62:11–39. [PubMed] [Google Scholar]
- 37.Hossmann KA. The hypoxic brain. Insights from ischemia research. Adv Exp Med Biol. 1999;474:155–69. [PubMed] [Google Scholar]
- 38.Derouesne C, Poirier J. [Cerebral lacunae: still under debate]. Rev Neurol (Paris) 1999;155:823–31. [PubMed] [Google Scholar]
- 39.Kaur C, Ling EA. Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res. 2008;5:71–81. doi: 10.2174/156720208783565645. [DOI] [PubMed] [Google Scholar]
- 40.Emmrich P, Hahn J, Ogunlade V, Geiger K, Schober R, Mohr FW. [Neuropathological findings after cardiac surgery-retrospective study over 6 years]. Z Kardiol. 2003;92:925–37. doi: 10.1007/s00392-003-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, et al. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:10836–40. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cullen KM, Kocsi Z, Stone J. Microvascular pathology in the aging human brain: evidence that senile plaques are sites of microhaemorrhages. Neurobiol Aging. 2006;27:1786–96. doi: 10.1016/j.neurobiolaging.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Henry-Feugeas MC, Onen F, Claeys ES. Classifying late-onset dementia with MRI: is arteriosclerotic brain degeneration the most common cause of Alzheimer's syndrome? Clin Interv Aging. 2008;3:187–99. doi: 10.2147/cia.s2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirata K, Yaginuma T, O'Rourke MF, Kawakami M. Age-related changes in carotid artery flow and pressure pulses: possible implications for cerebral microvascular disease. Stroke. 2006;37:2552–56. doi: 10.1161/01.STR.0000242289.20381.f4. [DOI] [PubMed] [Google Scholar]
- 45.O'Rourke MF, Hashimoto J. Arterial stiffness: a modifiable cardiovascular risk factor? J Cardiopulm Rehabil Prev. 2008;28:225–37. doi: 10.1097/01.HCR.0000327179.21498.38. [DOI] [PubMed] [Google Scholar]
- 46.Biedert S, Forstl H, Hewer W. The value of transcranial Doppler sonography in the differential diagnosis of Alzheimer disease vs multi-infarct dementia. Mol Chem Neuropathol. 1993;19:15–23. doi: 10.1007/BF03160165. [DOI] [PubMed] [Google Scholar]
- 47.Lee KY, Sohn YH, Baik JS, Kim GW, Kim JS. Arterial pulsatility as an index of cerebral microangiopathy in diabetes. Stroke. 2000;31:1111–15. doi: 10.1161/01.str.31.5.1111. [DOI] [PubMed] [Google Scholar]
- 48.Kidwell CS, el-Saden S, Livshits Z, Martin NA, Glenn TC, Saver JL. Transcranial Doppler pulsatility indices as a measure of diffuse small-vessel disease. J Neuroimaging. 2001;11:229–35. doi: 10.1111/j.1552-6569.2001.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 49.Henry-Feugeas MC. MRI of the ‘Alzheimer syndrome’. J Neuroradiol. 2007;34:220–227. doi: 10.1016/j.neurad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Greitz D, Wirestam R, Franck A, Nordell B, Thomsen C, Stahlberg F. Pulsatile brain movement and associated hydrodynamics studied by magnetic resonance phase imaging. The Monro-Kellie doctrine revisited. Neuroradiology. 1992;34:370–380. doi: 10.1007/BF00596493. [DOI] [PubMed] [Google Scholar]
- 51.Stivaros SM, Jackson A. Changing concepts of cerebrospinal fluid hydrodynamics: role of phase-contrast magnetic resonance imaging and implications for cerebral microvascular disease. Neurotherapeutics. 2007;4:511–22. doi: 10.1016/j.nurt.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greitz T. Effect of brain distension on cerebral circulation. Lancet. 1969;1:863–65. doi: 10.1016/s0140-6736(69)91903-5. [DOI] [PubMed] [Google Scholar]
- 53.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol. 2008;18:253–66. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 55.Roher AE, Kuo YM, Esh C, Knebel C, Weiss N, Kalback W, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer's disease. Mol Med. 2003;9:112–22. [PMC free article] [PubMed] [Google Scholar]
- 56.Franceschi M, Alberoni M, Bressi S, Canal N, Comi G, Fazio F, et al. Correlations between cognitive impairment, middle cerebral artery flow velocity and cortical glucose metabolism in the early phase of Alzheimer's disease. Dementia. 1995;6:32–38. doi: 10.1159/000106919. [DOI] [PubMed] [Google Scholar]
- 57.Henry-Feugeas MC. Alzheimer's disease in late-life dementia: a minor toxic consequence of devastating cerebrovascular dysfunction. Med Hypotheses. 2008;70:866–75. doi: 10.1016/j.mehy.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 58.Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE. Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer's disease. Am J Pathol. 1998;153:725–33. doi: 10.1016/s0002-9440(10)65616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schreiber SJ, Doepp F, Spruth E, Kopp UA, Valdueza JM. Ultrasonographic measurement of cerebral blood flow, cerebral circulation time and cerebral blood volume in vascular and Alzheimer's dementia. J Neurol. 2005;252:1171–77. doi: 10.1007/s00415-005-0826-8. [DOI] [PubMed] [Google Scholar]
- 60.Deschaintre Y, Richard F, Leys D, Pasquier F. Treatment of vascular risk factors is associated with slower decline in Alzheimer disease. Neurology. 2009;73:674–80. doi: 10.1212/WNL.0b013e3181b59bf3. [DOI] [PubMed] [Google Scholar]
- 61.Sipahi I, Nicholls SJ, Tuzcu EM, Nissen SE. Coronary atherosclerosis can regress with very intensive statin therapy. Cleve Clin J Med. 2006;73:937–44. doi: 10.3949/ccjm.73.10.937. [DOI] [PubMed] [Google Scholar]
- 62.Takayama T, Hiro T, Yamagishi M, Daida H, Hirayama A, Saito S, et al. Effect of rosuvastatin on coronary atheroma in stable coronary artery disease: multicenter coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Circ J. 2009;73:2110–2117. doi: 10.1253/circj.cj-09-0358. [DOI] [PubMed] [Google Scholar]
- 63.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2009:CD003160. doi: 10.1002/14651858.CD003160.pub2. [DOI] [PubMed] [Google Scholar]
- 64.Sipahi I, Tuzcu EM. Candidate mechanisms for regression of coronary atherosclerosis with high-dose statins: insight from intravascular ultrasonography trials. Am J Cardiovasc Drugs. 2008;8:365–71. doi: 10.2165/0129784-200808060-00003. [DOI] [PubMed] [Google Scholar]