Abstract
Background
Methamphetamine (METH) is an increasing popular and highly addictive stimulant associated with autonomic nervous system (ANS) dysfunction, cardiovascular pathology, and neurotoxicity. Heart rate variability (HRV) has been used to assess autonomic function and predict mortality in cardiac disorders and drug intoxication, but has not been characterized in METH use. We recorded HRV in a sample of currently abstinent individuals with a history of METH dependence compared to age- and gender-matched drug-free comparison subjects.
Method
HRV was assessed using time domain, frequency domain, and nonlinear entropic analyses in 17 previously METH-dependent and 21 drug-free comparison individuals during a 5 minute rest period.
Results
The METH-dependent group demonstrated significant reduction in HRV, reduced parasympathetic activity, and diminished heartbeat complexity relative to comparison participants. More recent METH use was associated with increased sympathetic tone.
Conclusion
Chronic METH exposure may be associated with decreased HRV, impaired vagal function, and reduction in heart rate complexity as assessed by multiple methods of analysis. We discuss and review evidence that impaired HRV may be related to the cardiotoxic or neurotoxic effects of prolonged METH use.
Keywords: autonomic nervous system, entropy, heart rate variability, methamphetamine
INTRODUCTION
Methamphetamine (METH), a potent and addictive synthetic derivative of amphetamine, is currently one of the most widely abused illegal stimulants in the United States and worldwide (Romanelli and Smith, 2006; Yeo et al., 2007). METH exposure has been associated with myriad adverse effects, including neurotoxicity, neuropsychological deficits, and cardiotoxicity (Citron et al., 1970; Hamamoto and Rhodus, 2009; Scott et al., 2007; Shrem and Halkitis, 2008). The drug exerts profound effects on neurological and cardiac function by mediating the release of monoamine neurotransmitters, including dopamine, norepinephrine, and serotonin (Makisumi et al., 1998; Scott et al., 2007; Yu et al., 2003). While acute METH intoxication stimulates the sympathetic nervous system, resulting in increased heart rate and hypertension (Meredith et al., 2005), chronic METH use is also reported to induce autonomic nervous system (ANS) dysfunction linked to cardiovascular pathology (Kaye et al., 2007), including myocardial infarction, coronary artery disease and cardiomyopathy (Citron et al., 1970; Kalant and Kalant, 1975; Karch et al., 1999; Smith et al., 1976; Swalwell and Davis, 1999).
Heart rate variability (HRV), the quantitative assessment of variation in heartbeat intervals, is increasingly used to detect alterations in ANS function and assess risk for morbidity and mortality associated with cardiovascular pathology (Berntson et al., 1997; Bilchick and Berger, 2006). Cardiac rhythm is regulated by a number of factors, most prominently the sinoatrial node (SA) pacemaker modulated by both the parasympathetic and sympathetic branches of the ANS (Malik and Camm, 1995). Acetycholine released from vagal parasympathetic terminals binds to SA muscarinic cholinergic receptors, thus decreasing SA depolarization and discharge; in contrast, sympathetic terminals on the SA node release norepinephrine and activate beta-adrenergic receptors, consequentially speeding the SA node rhythm (Berntson et al., 1997).
HRV is typically assessed in both time and frequency domains. Variation in heartbeat intervals over a linear time period is quantified by several measures derived from the standard deviation of the beat-to-beat period. Frequency domain analyses separate the heart rate signal into distinct frequency bands that assess the relative contribution, or power, of sympathetic and parasympathetic input to the heart (Cowan, 1995). However, the observation that heart rate can fluctuate in a highly irregular and nonlinear manner has motivated an increasing emphasis on applying nonlinear methods of analysis to characterize the complexity of cardiac function (Peng et al., 1995). The complexity, or predictability, of the heart beat pattern has been recently quantified by various measures of entropy, including sample entropy (SampEn) and dynamical entropy h (Lake et al., 2002). In similar fashion to the traditional time domain HRV measures, higher values of entropy (both SampEn and entropy h) indicate more variability and complexity in the data, while lower values suggest greater regularity in the cardiac rhythm (Richman and Moorman, 2000).
Healthy individuals exhibit a high degree of HRV, reflecting the ability of the ANS to adapt quickly to physical or psychological challenges in the environment (Thayer and Lane, 2000). Normal organisms are characterized by a complex and variable heart rhythm indicative of effective vagal control of the cardiovascular system. In contrast, decreased HRV has been associated with psychiatric disorders as well as explicit cardiac disease (Malik and Camm, 1995; Thayer and Lane, 2009). Reduced HRV and parasympathetic suppression has been reported in schizophrenia and bipolar disorder (Agelink et al., 2002; Henry et al., 2010; Malaspina et al., 1997), and linked to impaired social functioning and cognitive deficits (Berntson et al., 1997; Carney et al., 2005; Egizio et al., 2008; Kim et al., 2006a). Lower HRV and impaired vagal tone (a reduction in SDNN, RMSSD and HF power) have also been associated with dilated and hypertrophic cardiomyopathy (Evrengul et al., 2006; Fauchier et al., 1997; Karcz et al., 2003; Piccirillo et al., 2002), as well as greater mortality risk following myocardial infarction (Kleiger et al., 2005). Similarly, nonlinear heartbeat complexity as assessed by measures such as SampEn is decreased in individuals with cardiomyopathy (Batchinsky et al., 2007; Batchinsky et al., 2009; Claria et al., 2008; Lake et al., 2002).
Although previous work has demonstrated that exposure to drugs such as cocaine and alcohol reduce HRV (John et al., 2007; Vaschillo et al., 2008), the effect of METH on HRV has not been well characterized. The objective of this study was to assess HRV in a sample of abstinent individuals with a history of METH dependence compared to a drug-free comparison group. HRV was quantified using time and frequency domain measures, while nonlinear complexity was assessed with SampEn and dynamic entropy h. Given the reported effects of METH on ANS and cardiovascular function, we hypothesized that METH-dependent subjects would exhibit lower HRV and impaired vagal tone relative to the drug-free comparison sample.
METHODS
Participants
To determine the appropriate number of participants needed for this study, we assumed a large effect size based upon our published data describing HRV differences between individuals with bipolar disorder and healthy comparison subjects (Cohen’s d = 0.95) (Henry et al., 2010). Using the G*POWER software (Erdfelder et al., 1996), a large effect size of d = 0.95 with an alpha level of .05 requires a total sample size of 30 (15 subjects per group) to achieve a power of .80 for a main effect of group on HRV.
In the current study, 17 participants with a history of METH dependence were recruited through the HIV Neurobehavioral Research Center (HNRC) in San Diego, an institute that collaborates with a network of drug treatment facilities in the local community. Participants met SCID (Structured Clinical Interview for DSM-IV) criteria (First, 1994) for lifetime METH dependence, as well as DSM-IV criteria for METH abuse or dependence within the past two years. Subjects were also required to be abstinent from the drug for at least 7 days before testing. Drug use history was obtained through a substance use questionnaire (Table 1). 21 drug-free comparison subjects who had never met SCID criteria for any substance dependence were recruited from advertisements placed in the San Diego community. Comparison and METH groups were matched for age, gender, education, body mass index (BMI), and smoking status. Female participants were also matched for menstrual cycle. All participants provided written informed consent to the current protocol approved by the UCSD institutional review board.
Table 1.
Demographic factors and drug use history for drug-free comparison subjects (n = 21) and participants with a history of METH dependence (n = 17). Data are represented as means ± S.E.M.
| Parameter | Comparison | METH-Dependent |
|---|---|---|
| Age (years) | 33.3 ± 2.0 | 37.2 ± 2.1 |
| Gender | 19 M, 2 F | 15 M, 2 F |
| Education (years) | 13.8 ± 0.3 | 13.5 ± 0.5 |
| Body Mass Index (BMI) | 26.3 ± 1.2 | 27.6 ± 1.0 |
| Smokers / Non-smokers | 8 / 13 | 9 / 8 |
| Menstrual cycle (days) | 13.0 ± 7.0 | 14.0 ± 7.0 |
| Duration of continuous METH use (years) | ---------- | 10.8 ± 1.8 |
| Average frequency of METH use (times per month during periods of abuse) |
---------- | 21.5 ± 2.6 |
| Total amount of METH used (in grams) | ---------- | 4548.5 ± 733.1 |
| Average number of days of METH use in past year | ---------- | 55.1 ± 21.9 |
| Average duration of METH Abstinence (days) | ---------- | 299.1 ± 55.7 |
Participants were excluded if: 1) they met SCID criteria for schizophrenia, bipolar disorder, or current major depression; 2) they were taking any medication that could impair HRV (such as antidepressants or other psychotropic medication); 3) a history of stroke, heart attack, or cardiac disease; 4) diabetes; 5) any neurological conditions or head trauma, 6) infection with HIV or hepatitis C; 7) treatment with electroconvulsive therapy; 8) a positive result for cocaine, amphetamine, PCP, opiates, or cannabis on a urine toxicology Rapid Drug screen (Pharmatic Inc., San Diego, CA) administered during the test session; 9) substance dependence on illegal drugs (other than METH in the METH group) during the past 5 years; 10) alcohol abuse or dependence within the past 12 months; 11) a remote (i.e., more than 5 years prior to study enrollment) but significant history of alcohol or other substance dependence, as described in previous studies (Rippeth et al., 2004; Woods et al., 2005). Three participants included in the drug-free comparison group reported infrequent and remote stimulant exposure. One participant used cocaine twice in high school, while two individuals reported using METH between one and two dozen times from 1991 to 1995, but did not meet criteria for drug dependence and did not indicate any stimulant use in the past 15 years.
HRV Assessment
Subjects were fitted with the LifeShirt (LS), a continuous monitoring system that records the physiological activity of the myocardium via 3 electrocardiogram (ECG) leads placed on the skin of the upper chest and lateral abdomen (Vivometrics, 2002). The sensor array of the LS is embedded in a sleeveless Lycra undergarment attached to a PDA that continuously encrypts and stores participant data on a compact flash memory card. Cardiac data are sampled at 200 Hz and analyzed with VivoLogic™, proprietary PC-based software. This program decrypts the data, images the continuous stream of cardiac autonomic output over time, and exports processed data in ASCII format.
Digitized ECG data were analyzed to detect the R-wave peaks of the QRS complex. RR interval artifacts ± 15% of reference RR duration were manually removed using linear interpolation. Ectopic beats were identified and removed using the Vivologic automated ectopic beat identification procedure. Only normal RR intervals were included in the analysis. The power spectrum of the HRV signal was assessed using nonparametric Fast Fourier Transform (FFT) and the Welch periodogram method as per standard procedure (Vivometrics, 2002).
LS assessment of cardiac function has been shown to be equivalent to more widely used HRV laboratory measures such as Biopac (Heilman and Porges, 2007). Heilman and colleagues (2007) quantified heart rate and HRV in subjects during short intervals of rest and exercise using both the LS (sample rate 200 Hz) and Biopac (sample rate 1000 Hz). They concluded that the LS algorithm for R-wave detection and timing precision were sufficiently robust to provide precise and accurate cardiac data.
Data Collection
ECG recordings were obtained from participants during a 5 minute interval in a seated position at complete rest between 9 a.m. and 5 p.m. The 5 minute test period was selected as a standard length of HRV assessment (Malik and Camm, 1995). Participants were instructed to relax and sit quietly during the test period and were required to refrain from smoking for at least 30 minutes prior to the test session. Heart rate (HR) was monitored during the session and all subjects exhibited a baseline HR below 100 beats-per-minute (bpm).
HRV data were analyzed in both time and frequency domains. Variation in the heart rhythm over time was quantified by assessing the standard deviation of sinus RR intervals (SDNN), or the differences in the length of time between R peaks that signal ventricular depolarization (Cowan, 1995). Differences between adjacent RR intervals were measured by variables that include the RMSSD (the root mean square of successive RR differences) and pNN50 (the percentage of adjacent RR intervals that differ by more than 50 milliseconds).
In the frequency domain, the low frequency (LF) signal (0.04 –0.15 Hz) is mediated by both sympathetic and parasympathetic activity, while high frequency signal (HF) (0.15-0.4 Hz) is mediated primarily by parasympathetic input to the heart (Cowan, 1995). LF and HF power were quantified for each subject, and the relative intensity of cardiac sympathetic activity was assessed by quantifying the LF/HF ratio, representing the comparative balance of the two branches of the ANS. To account for individual variability in total power (Pagani et al., 1986), LF and HF were normalized to total power (TP) and analyzed as normalized low frequency power: (LFn, LF/(TP - Very Low Frequency)) and normalized high frequency power (HFn, LF/(TP - Very Low Frequency)).
Nonlinear complexity was assessed using SampEn. This measure quantifies the conditional probability that two sequences similar for m points remain similar at the next data point, where sequence self-matches are not included in calculating the probability (Lake et al., 2002). SampEn can be accurately estimated from a group of 100 to 5000 data points if the sequence length to be compared (m) is set at 1 or 2 and the tolerance level (r) for determining sequence matches is set between 0.1 and 0.25 of the data set standard deviation (Groome et al., 1999; Pincus and Huang, 1992), although some studies have used higher values of m (Lake et al., 2002). Higher values of r increase the probability of sequence matches and decrease the observed complexity of the data, driving the value of SampEn towards zero. Preliminary analyses with the HRV data obtained from METH and comparison subjects indicated that SampEn values approached or effectively reached zero when the value of r was set at or above 0.2; thus the r value for the current dataset was set at 0.1 and SampEn was subsequently calculated for m values of 1, 2, and 3 as previously reported. SampEn was computed using Matlab software with tools available on the PhysioNet website (Goldberger et al., 2000).
Our lab has used the dynamical entropy h measure to assess the complexity of human behavior and physiology in several domains, including quantifying decision-making behavior in schizophrenia (Paulus et al., 2001), motor activity in bipolar disorder (Perry et al., 2009), and HRV in both psychiatric disorders (Henry et al., 2010). In brief, entropy h is calculated by determining the minimal length of unique subsequences contained in a data set and computed via log (number of data points)/[unique subsequence length] (Perry et al., 2009). In the current study, we applied the dynamic entropy h measure to the RR interval data generated by the LS as previously described (Henry et al., 2010).
Statistical Analyses
Statistical analyses were performed using SPSS and data were examined for normality of distribution and homogeneity of variance. Square root transformations were applied to RMSSD, pNN50, HFn, the LF/HF ratio, SampEn, and data for the length and quantity of METH use to maximize normality and minimize skew and kurtosis.
Mean values for HR, SDNN, RMSSD, pNN50, HFn, LFn, the LF/HF ratio, RR entropy h, and SampEn were calculated for the 5 minute rest period. Group differences for HRV measures were assessed using a multivariate analysis of covariance (MANCOVA), followed up by univariate ANOVAs for each parameter. Gender, smoking status, and BMI were included as covariates to account for the potential effect of these factors on HRV. Post-hoc differences were assessed using Bonferroni-adjusted multiple t-test comparisons.
Bivariate Pearson r correlations were performed to compare relationships between HRV measures and characteristics of METH use, including length of use and duration of drug abstinence. To further examine the effect of METH use on HRV, METH participants were divided into 2 groups to compare: 1) the effect of lifetime METH exposure (above or below the median group amount of 4000 grams); 2) length of abstinence (longer or shorter than 1 year); 3) the amount of METH use in the last 12 months (greater or fewer than 20 days of reported use). Independent samples t-tests were performed to compare the effect of these variables on SDNN, the LF/HF ratio, and entropy h. Finally, additional correlations were conducted to assess the association between the traditional (time and frequency domain) and nonlinear HRV measures. To reduce the probability of a Type 1 error associated with a large number of statistical analyses, the level of significance for Pearson r comparisons was set at p < 0.025, rather than p < 0.05.
RESULTS
Time and Frequency Domain analyses
The MANOVA performed for HRV data during the 5 minute rest period indicated a significant effect of group [F(7,27) = 2.57, p < 0.05], but no significant effects of gender [F(7,27) = 1.49, ns], smoking [F(7,27) = 1.32, ns], or BMI [F(7,27) = 1.72, ns]. Subsequent univariate ANOVAs revealed a main effect of group on the LF/HF ratio [F(1, 33) = 10.95, p < 0.01], HFn [F(1, 33) = 13.98, p < 0.01], LFn [F(1, 33) = 6.97, p < 0.05], RMSSD [F(1, 33) = 5.67, p < 0.05] and pNN50 [F(1,33) = 6.32, p < 0.05]. There was a non-significant trend towards increased heart rate in METH participants [F(1, 33) = 3.20, p = 0.08]; but no group difference was observed for SDNN. Bonferroni post-hoc tests indicated that participants with a history of METH dependence exhibited a significant increase in the LF/HF ratio, LFn, and a decrease in HFn, RMSSD, and pNN50 compared to drug-free comparison subjects (Table 2). Group differences in HRV were characterized by moderate to large effect sizes (r values) (Table 2).
Table 2.
Autonomic parameters for drug-free comparison subjects (n = 21) and participants with a history of METH dependence (n = 17) during the 5 minute rest period. SampEn tolerance level r for determining RR sequence matches was set at 10% (0.1) of the standard deviation of the data set. Data are represented as means ± S.E.M. Asterisks indicate significant group differences
| Parameter | Comparison | METH-Dependent | Effect Size (r) |
|---|---|---|---|
| Time Domain | |||
|
|
|||
| HR (beats/min) | 64.0 ± 2.1 | 70.8 ± 2.4† | 0.32 |
| SDNN (ms) | 69.5 ± 6.9 | 56.0 ± 5.7 | 0.23 |
| RMSSD (ms) | 54.6 ± 8.3 | 31.0 ± 4.3* | 0.37 |
| pNN50 (%) | 0.28 ± 0.05 | 0.09 ± 0.03* | 0.39 |
| Frequency Domain | |||
|
|
|||
| HFn | 0.40 ± 0.04 | 0.21 ± 0.03** | 0.51 |
| LFn | 0.53 ± 0.04 | 0.65 ± 0.03* | 0.36 |
| LF/HF ratio | 2.0 ± 0.36 | 4.10 ± 0.64** | 0.46 |
| Nonlinear Analysis | |||
|
|
|||
| entropy h | 0.35 ± 0.01 | 0.30 ± 0.01** | 0.46 |
| SampEn (m = 1) | 0.21 ± 0.05 | 0.09 ± 0.03† | 0.30 |
| SampEn (m = 2) | 0.19 ± 0.04 | 0.09 ± 0.02* | 0.31 |
| SampEn (m = 3) | 0.16 ± 0.04 | 0.08 ± 0.02† | 0.31 |
p < 0.05
p < 0.01.
indicates a trend towards a group effect (p < 0.1).
Nonlinear HRV analyses
Univariate ANOVA performed for entropy measures calculated from RR interval sequences indicated a main effect of group on dynamical entropy h [F(1,33) = 9.28, p < 0.01] and SampEn for m = 2 [F(1,33) = 4.47, p < 0.05], with a strong trend towards a group effect on SampEn for m = 1 [F(1,33) = 4.11, p = 0.05] and m =3 [F(1,33) = 4.04, p = 0.05] (Table 2). The Bonferroni post-hoc tests for entropy h and SampEn indicated that METH participants exhibited a significant decrease in entropy relative to comparison subjects, signifying less variability in the RR interval pattern (Table 2). While there were no significant effects of smoking or BMI on entropy, female participants exhibited greater entropy compared to male subjects for all values of SampEn [m = 1, p < 0.01; m = 2, p < 0.01; m = 3, p < 0.01].
Subsequent correlations between nonlinear, time and frequency domain HRV measures revealed that both entropy h and SampEn were positively correlated with HFn power, SDNN, RMSSD, and pNN50, but negatively correlated with the LF/HF ratio and LFn power (Table 3). The two measures of entropy were also correlated with each other (Table 3).
Table 3.
Pearson r correlations between time and frequency domain HRV measures and nonlinear measures of entropy h and SampEn (n = 38 ). SampEn was calculated with sequence match tolerance r set to 0.1 and m represents sequence length. Asterisks indicate significant correlations
| entropy h | SampEn (m = 1) | SampEn (m = 2) | SampEn (m = 3) | |
|---|---|---|---|---|
| SDNN (ms) | 0.54*** | 0.89*** | 0.88*** | 0.87*** |
| RMSSD (ms) | 0.72*** | 0.92*** | 0.91*** | 0.90*** |
| pNN50 (%) | 0.72*** | 0.85*** | 0.86*** | 0.84*** |
| HFn | 0.70*** | 0.59*** | 0.59*** | 0.56*** |
| LFn | −0.63*** | −0.59*** | −0.59*** | −0.55*** |
| LF/HF ratio | −0.63*** | −0.52** | −0.53** | −0.50** |
| entropy h | 0.70*** | 0.70*** | 0.68*** |
p < 0.01
p < 0.001.
Characteristics of METH use
The amount of METH use in the past year (number of days) was positively correlated with LFn power (r = 0.50, p = 0.04) and HR (r = 0.45, p = 0.07), although these relationships did not reach quite reach significance. In addition, we observed a trend towards a negative correlation between HR and the length of abstinence (r = −0.42, p = 0.09). Individuals with greater lifetime METH use (over 4000 grams) showed slightly lower SDNN and a marginally higher LF/HF ratio compared to those with less exposure to the drug, but these differences were not significant. However, METH participants with less than 1 year of abstinence exhibited a trend towards a higher LF/HF ratio (p = 0.07) compared to subjects with more distant METH use (e.g., more than 1 year of abstinence). In addition, participants with greater METH use in the past year (more than 20 days), exhibited a significantly greater LF/HF ratio (p < 0.05) compared to drug users with less METH exposure in the past 12 months (Table 4).
Table 4.
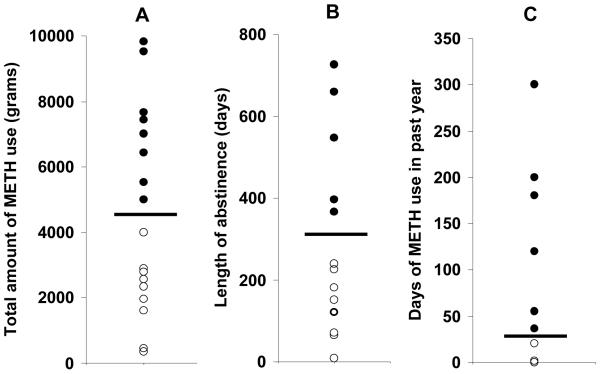
HRV parameters for subgroups of METH participants differentiated by the lifetime quantity of METH use (A), length of abstinence (B), and number of days of METH use in the past year (C). The distribution of these variables is illustrated in Figure 1. Subjects with more recent or greater drug use in the past year exhibited higher sympathetic activity relative to individuals with more remote exposure to METH. Asterisks indicate significant group differences
| A. Total METH use | ||
|
| ||
| n = 9 < 4000 grams ○ |
n = 8 > 4000 grams ● |
|
|
|
||
| SDNN (ms) | 60.0 ± 11.5 | 51.5 ± 4.4 |
| LF/HF ratio | 3.7 ± 0.9 | 4.6 ± 1.0 |
| entropy h | 0.32 ± 0.04 | 0.29 ± 0.02 |
| B. Length of abstinence | ||
|
| ||
| n = 10 > 1 year ● |
n = 7 < 1 year ○ |
|
|
|
||
| SDNN (ms) | 63.5 ± 11.2 | 50.7 ± 5.7 |
| LF/HF ratio | 2.7 ± 0.5 | 5.1 ± 0.9† |
| entropy h | 0.31 ± 0.02 | 0.29 ± 0.01 |
| C. Days of METH use in the past year | ||
|
| ||
| n = 11 < 20 days ○ |
n = 6 > 20 days ● |
|
|
|
||
| SDNN (ms) | 62.8 ± 7.5 | 44.4 ± 6.9 |
| LF/HF ratio | 2.9 ± 0.5 | 6.2 ± 1.3* |
| entropy h | 0.31 ± 0.01 | 0.30 ± 0.02 |
|
| ||
p < 0.05.
indicates a trend towards a group effect (p < 0.1).
While three participants in the drug-free comparison group reported infrequent and remote stimulant exposure more than 15 years ago (e.g., using cocaine or METH a few times) they did not display any evidence of impaired HRV. These individuals exhibited an equivalent mean LF/HF ratio (1.8) relative to the completely stimulant-naïve comparison subjects (2.0). They also showed a trend towards higher entropy h (mean = 0.36) and elevated SDNN (mean = 100.8) compared to the drug-free participants with no past stimulant exposure (mean entropy h = 0.34; mean SDNN = 64.3). While the sample size is extremely small, these findings support our observations in the METH-dependent group, which suggest that HRV measures begin to normalize following more than 1 year of abstinence from the drug.
DISCUSSION
The aim of this study was to examine HRV in a group of abstinent individuals with a history of METH dependence during a 5 minute rest period. Compared to drug-free comparison subjects, METH participants exhibited a decrease in HRV, reduced vagal tone, and a reduction in heartbeat complexity as assessed by nonlinear entropy. While the lifetime quantity of METH exposure was not significantly associated with any HRV variable, our data indicate that more recent METH use was linked with autonomic abnormalities. Greater METH use in the previous 12 months was associated with elevated sympathetic tone as evidenced by a higher LF/HF ratio in METH participants with more than 20 days of reported use. In addition, METH exposure in the past year was positively correlated with LF power. In contrast, subjects with more than 1 year of abstinence from the drug exhibited LF/HF and SDNN values that were more similar to drug-free comparison subjects. Finally, our results also suggest that more recent METH use may be associated with elevated HR.
While time domain measures such as SDNN reflect both sympathetic and parasympathetic activity, RMSSD and pNN50 are strongly correlated with the HF signal and are believed to be more specific indicators of parasympathetic activity (Kleiger et al., 1991). In the current report, METH subjects demonstrated significantly reduced RMSSD, pNN50, and lower HF power relative to the drug-free comparison sample. Overall, these data indicate that chronic METH use is associated with suppression of parasympathetic function.
Entopy h and SampEn were positively correlated with RMSSD, pNN50, and HF power and negatively correlated with LF power and the LF/HF ratio, indicating that reduced complexity of the heartbeat pattern is associated with impaired parasympathetic tone. These two measures of entropy were also significantly correlated with each other (r = 0.7), replicating earlier work (Henry et al., 2010) in support of the concept that entropy h may be a reliable measure of HRV complexity. Our results also showed an independent effect of gender on entropy, with female participants demonstrating higher SampEn compared to male subjects. While interpretation of this finding is limited by a small number of total female subjects (n = 4), the results support previous work indicating that women exhibit greater parasympathetic activity compared to men (Sztajzel et al., 2008).
Our data are supported by previous research indicating stimulant exposure is associated with impaired HRV and ECG abnormalities (Haning and Goebert, 2007; Vongpatanasin et al., 2004). Newborn infants exposed to cocaine in utero are reported to exhibit reduced HRV and diminished parasympathetic activity (John et al., 2007; Mehta et al., 2001). Acute cocaine administration in healthy adults also reduces HF power, indicating decreased vagal tone (Vongpatanasin et al., 2004). One recent study reported abnormal ECG in 36% of a METH-dependent cohort (Haning and Goebert, 2007). The most prominent abnormality was a prolonged QTc interval, a risk factor for arrthymia and potential marker for cardiomyopathy. In addition, chronic alcohol exposure is characterized by impaired ANS function and reduced HRV (Agelink et al., 1998), a phenomenon thought to be mediated by autonomic neuropathy, including degeneration of the vagus nerve (Guo et al., 1987).
METH administration has been associated with numerous adverse effects, including cardiotoxic and neurotoxic consequences that may have a direct impact on HRV (Scott et al., 2007; Yu et al., 2003). Chronic METH exposure is linked to a wide variety of cardiovascular disorders associated with impaired HRV and vagal abnormalities, including cardiomyopathy, coronary artery disease, arrthymia, and myocardial infarction (Kaye et al., 2007). Cardiovascular pathology mediated by METH use may be induced by distinct mechanisms, but is generally attributed to the effect of the stimulant on catecholamine levels (Yu et al., 2003). Elevated catecholamine activity decreases oxygen supply to the myocardium by inducing vasoconstriction and vasospasm, while also boosting oxygen demand by causing tachycardia and hypertension (Karch, 2002; Yu et al., 2003). The resulting oxygen deficit can thus cause cardiac tissue necrosis and the formation of fibrotic tissue that can impair cardiovascular function. Preclinical studies have demonstrated that METH exposure can also damage cardiac tissue by increasing intracellular calcium concentration, thus inhibiting myosin synthesis and damaging cardiomyocytes (Guo et al., 1986; Salomon, 1978). In addition, several weeks of METH administration in rat (1 mg/kg per day) induced cardiac myocte lesions similar to those observed in human cardiomyopathy (He et al., 1996). While individuals with a diagnosis of cardiac disease were excluded from the current study, subclinical METH-induced cardiac pathology, including silent left ventricular systolic dysfunction, may have mediated alterations in HRV.
Alternatively, it is conceivable that HRV differences may be related to the neurotoxic effects of the drug. An extensive literature has documented neuropathological effects of chronic METH use on human frontostriatal circuitry (Barr et al., 2006). Relative to comparison subjects, METH-dependent individuals have exhibited multiple abnormalities in frontal cortex, including reduced metabolite levels (indicative of reduced neuronal density) (Ernst et al., 2000), decreased cerebral blood flow (Chang et al., 2002), impaired glucose metabolism (Kim et al., 2005), lower fractional anisotropy (Chung et al., 2007), and reduced grey matter density (Kim et al., 2006b). Many of these changes have been observed after extended abstinence from the drug (Kim et al., 2006b; Kim et al., 2005), indicating long-term neurotoxicity.
Recent reports have suggested that regions such as the prefrontal cortex (PFC) could play a critical role in ANS regulation (Hansen et al., 2004; Thayer and Lane, 2007, 2009); for example, inactivation and reduced blood flow in the PFC has been associated with decreased HRV (Ahern et al., 2001; Lane et al., 2009). The PFC is proposed to regulate ANS function by inhibiting the amygdala, an area that suppresses parasympathetic activity by inhibiting vagal pathways originating in the nucleus ambiguus and dorsal motor nucleus (Thayer and Lane, 2009). It is thus feasible that METH-induced ANS dysregulation could be affected by the failure of a damaged frontal cortex to appropriately regulate subcortical structures, resulting in impaired parasympathetic function.
While determining the mechanism mediating the effect of METH on HRV is beyond the scope of the current report, future studies could examine this relationship using a variety of methods. Several groups have utilized Positron Emission Tomography (PET) and functional Magnetic Resonance Imaging (fMRI) to assess the relationship between regions of the brain that regulate the ANS (such as the PFC) and HRV measures including HF power (Critchley et al., 2003; Gianaros et al., 2004; Napadow et al., 2008; Thayer and Lane, 2009); these methods could also be employed to determine if METH-induced changes in HRV correspond to altered activity in central ANS structures. In addition, direct effects of METH on the heart, including left ventricular dysfunction, could be assessed by an echocardiogram administered concurrently with HRV measures (Ito et al., 2009). Finally, HRV could also be assessed in rodents exposed to a chronic METH regimen to examine the relationship between this measure and indications of cardiotoxicity such as myocardial lesions (He et al., 1996; Rowan et al., 2007; Yu et al., 2003).
There are a number of limitations in the current study. The level of general physical activity and the degree of exposure to small particulate air pollution, two factors that may impact HRV (Simkhovich et al., 2008), were not quantified in this sample of participants. It is relevant to note that all subjects were recruited from urban areas of San Diego and HRV assessed within 2 to 4 hours of exposure to traffic (e.g., traveling by bus or car to the testing site); thus, we would not predict substantial group differences in living environment (urban vs. rural) or acute exposure to ambient particulate matter, which has been reported to reduce HF power (Zanobetti et al., 2010). However, it is possible that differences in lifestyle (e.g., variation in daily exercise and physical activity) could affect HRV measures. In addition, although participants in the current study were excluded for any history of stroke, heart attack, or cardiac disease, cardiac medical history in family members was not obtained. Finally, the current findings were observed in a relatively small sample. Although the effects of potentially confounding variables, such as gender and smoking behavior, were addressed by treating these factors as covariates in our data analyses, we can not exclude the possibility that HRV differences between our groups may remain influenced by these characteristics. Thus, future studies assessing the effect of acute and chronic METH on HRV in larger cohorts would improve our understanding of how stimulants can impact this measure.
While HRV is frequently assessed during a 5 minute time period, as this interval is deemed sufficient to assess clinical measures of HF and LF variability (Task Force, 1996), longer intervals (such as a 24 hour recording) are also recommended (Vanderlei et al., 2009). Data from shorter recording periods are more vulnerable to distortion by artifacts and do not take into account potential circadian variation, while 24 hour recordings have higher predictive value in heart disease (Majercak, 2002; Malpas and Purdie, 1990). Therefore, additional studies examining the effect of chronic METH use on HRV during longer recording intervals are also warranted.
In conclusion, a sample of abstinent individuals with a history of METH dependence exhibited significant impairment in time domain, frequency domain, and nonlinear complexity measures of HRV compared to drug-free comparison subjects. This finding supports previous work demonstrating the adverse effects of stimulants on HRV and contributes to a considerable literature reporting detrimental health effects associated with chronic METH use.
Figure 1.
These plots illustrate the distribution of the data for lifetime quantity of METH use (A), length of abstinence (B), and number of days of METH use in the past year (C) in participants with a history of METH dependence. To examine the relationship between METH use and HRV, participants were divided into two groups as indicated by the horizontal bar in each plot. Participants with high METH use (greater than 4000 grams), longer abstinence (greater than 1 year), and greater METH use in the past year are designated by solid circles; lower values are indicated by open circles. Group comparison data are shown in Table 4.
Acknowledgements
The manuscript was supported by National Research Service Award F32DA024524-01A1, Program Project DA12065 from the National Institute On Drug Abuse (NIDA), and a grant from the National Institute of Mental Health (NIMH) (R01-MH071916-05). Additional support was provided by the HIV Neurobehavioral Research Center CSPAR Developmental Grant Award (# HNRC-819). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA or the HNRC. The authors gratefully thank Rodney von Jaeger and Terence Hendrix for their contribution in recruiting participants involved in this study.
References
- Agelink MW, Boz C, Ullrich H, Andrich J. Relationship between major depression and heart rate variability. Clinical consequences and implications for antidepressive treatment. Psychiatry research. 2002;113:139–149. doi: 10.1016/s0165-1781(02)00225-1. [DOI] [PubMed] [Google Scholar]
- Agelink MW, Malessa R, Weisser U, Lemmer W, Zeit T, Majewski T, Klieser E. Alcoholism, peripheral neuropathy (PNP) and cardiovascular autonomic neuropathy (CAN) Journal of the neurological sciences. 1998;161:135–142. doi: 10.1016/s0022-510x(98)00266-4. [DOI] [PubMed] [Google Scholar]
- Ahern GL, Sollers JJ, Lane RD, Labiner DM, Herring AM, Weinand ME, Hutzler R, Thayer JF. Heart rate and heart rate variability changes in the intracarotid sodium amobarbital test. Epilepsia. 2001;42:912–921. doi: 10.1046/j.1528-1157.2001.042007912.x. [DOI] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Batchinsky AI, Cancio LC, Salinas J, Kuusela T, Cooke WH, Wang JJ, Boehme M, Convertino VA, Holcomb JB. Prehospital loss of R-to-R interval complexity is associated with mortality in trauma patients. The Journal of trauma. 2007;63:512–518. doi: 10.1097/TA.0b013e318142d2f0. [DOI] [PubMed] [Google Scholar]
- Batchinsky AI, Salinas J, Kuusela T, Necsoiu C, Jones J, Cancio LC. Rapid Prediction of Trauma-Patient Survival by Analysis of Heart-Rate Complexity: Impact of Reducing Dataset Size. Shock (Augusta, Ga. 2009 doi: 10.1097/SHK.0b013e3181a993dc. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr., Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bilchick KC, Berger RD. Heart rate variability. Journal of cardiovascular electrophysiology. 2006;17:691–694. doi: 10.1111/j.1540-8167.2006.00501.x. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosomatic medicine. 2005;67(Suppl 1):S29–33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry research. 2002;114:65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, Sim ME, Song IC, Kim J, Chang KH, Renshaw PF. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2007;10:765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- Citron BP, Halpern M, McCarron M, Lundberg GD, McCormick R, Pincus IJ, Tatter D, Haverback BJ. Necrotizing angiitis associated with drug abuse. The New England journal of medicine. 1970;283:1003–1011. doi: 10.1056/NEJM197011052831901. [DOI] [PubMed] [Google Scholar]
- Claria F, Vallverdu M, Baranowski R, Chojnowska L, Caminal P. Heart rate variability analysis based on time-frequency representation and entropies in hypertrophic cardiomyopathy patients. Physiological measurement. 2008;29:401–416. doi: 10.1088/0967-3334/29/3/010. [DOI] [PubMed] [Google Scholar]
- Cowan MJ. Measurement of heart rate variability. Western journal of nursing research. 1995;17:32–48. doi: 10.1177/019394599501700104. discussion 101-111. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Egizio VB, Jennings JR, Christie IC, Sheu LK, Matthews KA, Gianaros PJ. Cardiac vagal activity during psychological stress varies with social functioning in older women. Psychophysiology. 2008;45:1046–1054. doi: 10.1111/j.1469-8986.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behavior Research Methods, Instruments, and Computers. 1996;28:1–11. [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Evrengul H, Tanriverdi H, Kose S, Amasyali B, Kilic A, Celik T, Turhan H. The relationship between heart rate recovery and heart rate variability in coronary artery disease. Ann Noninvasive Electrocardiol. 2006;11:154–162. doi: 10.1111/j.1542-474X.2006.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchier L, Babuty D, Cosnay P, Autret ML, Fauchier JP. Heart rate variability in idiopathic dilated cardiomyopathy: characteristics and prognostic value. Journal of the American College of Cardiology. 1997;30:1009–1014. doi: 10.1016/s0735-1097(97)00265-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, editors. Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) Psychiatric Press; Washington D.C.: 1994. [Google Scholar]
- Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101:E215–220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- Groome LJ, Mooney DM, Holland SB, Smith LA, Atterbury JL, Loizou PC. Human fetuses have nonlinear cardiac dynamics. J Appl Physiol. 1999;87:530–537. doi: 10.1152/jappl.1999.87.2.530. [DOI] [PubMed] [Google Scholar]
- Guo JX, Jacobson SL, Brown DL. Rearrangement of tubulin, actin, and myosin in cultured ventricular cardiomyocytes of the adult rat. Cell motility and the cytoskeleton. 1986;6:291–304. doi: 10.1002/cm.970060306. [DOI] [PubMed] [Google Scholar]
- Guo YP, McLeod JG, Baverstock J. Pathological changes in the vagus nerve in diabetes and chronic alcoholism. Journal of neurology, neurosurgery, and psychiatry. 1987;50:1449–1453. doi: 10.1136/jnnp.50.11.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto DT, Rhodus NL. Methamphetamine abuse and dentistry. Oral diseases. 2009;15:27–37. doi: 10.1111/j.1601-0825.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- Haning W, Goebert D. Electrocardiographic abnormalities in methamphetamine abusers. Addiction (Abingdon, England) 2007;102(Suppl 1):70–75. doi: 10.1111/j.1360-0443.2006.01776.x. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Sollers JJ, 3rd, Stenvik K, Thayer JF. Heart rate variability and its relation to prefrontal cognitive function: the effects of training and detraining. European journal of applied physiology. 2004;93:263–272. doi: 10.1007/s00421-004-1208-0. [DOI] [PubMed] [Google Scholar]
- He SY, Matoba R, Fujitani N, Sodesaki K, Onishi S. Cardiac muscle lesions associated with chronic administration of methamphetamine in rats. Am J Forensic Med Pathol. 1996;17:155–162. doi: 10.1097/00000433-199606000-00014. [DOI] [PubMed] [Google Scholar]
- Heilman KJ, Porges SW. Accuracy of the LifeShirt (Vivometrics) in the detection of cardiac rhythms. Biological psychology. 2007;75:300–305. doi: 10.1016/j.biopsycho.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Paulus MP, Geyer MA, Perry W. Heart rate variability in bipolar mania and schizophrenia. J Psychiatr Res. 2010;44:168–176. doi: 10.1016/j.jpsychires.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Yeo KK, Wijetunga M, Seto TB, Tay K, Schatz IJ. A comparison of echocardiographic findings in young adults with cardiomyopathy: with and without a history of methamphetamine abuse. Clinical cardiology. 2009;32:E18–22. doi: 10.1002/clc.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John V, Dai H, Talati A, Charnigo RJ, Neuman M, Bada HS. Autonomic alterations in cocaine-exposed neonates following orthostatic stress. Pediatric research. 2007;61:251–256. doi: 10.1203/01.pdr.0000252436.62151.67. [DOI] [PubMed] [Google Scholar]
- Kalant H, Kalant OJ. Death in amphetamine users: causes and rates. Canadian Medical Association journal. 1975;112:299–304. [PMC free article] [PubMed] [Google Scholar]
- Karch SB. Karch’s Pathology of Drug Abuse. CRC Press; Boca Raton: 2002. [Google Scholar]
- Karch SB, Stephens BG, Ho CH. Methamphetamine-related deaths in San Francisco: demographic, pathologic, and toxicologic profiles. Journal of forensic sciences. 1999;44:359–368. [PubMed] [Google Scholar]
- Karcz M, Chojnowska L, Zareba W, Ruzyllo W. Prognostic significance of heart rate variability in dilated cardiomyopathy. International journal of cardiology. 2003;87:75–81. doi: 10.1016/s0167-5273(02)00207-3. [DOI] [PubMed] [Google Scholar]
- Kaye S, McKetin R, Duflou J, Darke S. Methamphetamine and cardiovascular pathology: a review of the evidence. Addiction (Abingdon, England) 2007;102:1204–1211. doi: 10.1111/j.1360-0443.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- Kim DH, Lipsitz LA, Ferrucci L, Varadhan R, Guralnik JM, Carlson MC, Fleisher LA, Fried LP, Chaves PH. Association between reduced heart rate variability and cognitive impairment in older disabled women in the community: Women’s Health and Aging Study I. Journal of the American Geriatrics Society. 2006a;54:1751–1757. doi: 10.1111/j.1532-5415.2006.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, Kwon DH, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2006b;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Sung YH, Lee HY, Lee DS, Jeong DU, Renshaw PF. Frontal glucose hypometabolism in abstinent methamphetamine users. Neuropsychopharmacology. 2005;30:1383–1391. doi: 10.1038/sj.npp.1300699. [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Bigger JT, Bosner MS, Chung MK, Cook JR, Rolnitzky LM, Steinman R, Fleiss JL. Stability over time of variables measuring heart rate variability in normal subjects. The American journal of cardiology. 1991;68:626–630. doi: 10.1016/0002-9149(91)90355-o. [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Stein PK, Bigger JT., Jr. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake DE, Richman JS, Griffin MP, Moorman JR. Sample entropy analysis of neonatal heart rate variability. American journal of physiology. 2002;283:R789–797. doi: 10.1152/ajpregu.00069.2002. [DOI] [PubMed] [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. NeuroImage. 2009;44:213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Majercak I. The use of heart rate variability in cardiology. Bratislavske lekarske listy. 2002;103:368–377. [PubMed] [Google Scholar]
- Makisumi T, Yoshida K, Watanabe T, Tan N, Murakami N, Morimoto A. Sympatho-adrenal involvement in methamphetamine-induced hyperthermia through skeletal muscle hypermetabolism. European journal of pharmacology. 1998;363:107–112. doi: 10.1016/s0014-2999(98)00758-4. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Bruder G, Dalack GW, Storer S, Van Kammen M, Amador X, Glassman A, Gorman J. Diminished cardiac vagal tone in schizophrenia: associations to brain laterality and age of onset. Biological psychiatry. 1997;41:612–617. doi: 10.1016/s0006-3223(96)00161-8. [DOI] [PubMed] [Google Scholar]
- Malik M, Camm AJ. Heart Rate Variability. Futura Publishing; New York: 1995. [Google Scholar]
- Malpas SC, Purdie GL. Circadian variation of heart rate variability. Cardiovascular research. 1990;24:210–213. doi: 10.1093/cvr/24.3.210. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Super DM, Salvator A, Singer L, Connuck D, Fradley LG, Harcar-Sevcik RA, Kaufman ES. Heart rate variability in cocaine-exposed newborn infants. American heart journal. 2001;142:828–832. doi: 10.1067/mhj.2001.118112. [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harvard review of psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. NeuroImage. 2008;42:169–177. doi: 10.1016/j.neuroimage.2008.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circulation research. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rapaport MH, Braff DL. Trait contributions of complex dysregulated behavioral organization in schizophrenic patients. Biological psychiatry. 2001;49:71–77. doi: 10.1016/s0006-3223(00)00984-7. [DOI] [PubMed] [Google Scholar]
- Peng CK, Havlin S, Hausdorff JM, Mietus JE, Stanley HE, Goldberger AL. Fractal mechanisms and heart rate dynamics. Long-range correlations and their breakdown with disease. Journal of electrocardiology. 1995;28(Suppl):59–65. doi: 10.1016/s0022-0736(95)80017-4. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo G, Germano G, Quaglione R, Nocco M, Lintas F, Lionetti M, Moise A, Ragazzo M, Marigliano V, Cacciafesta M. QT-interval variability and autonomic control in hypertensive subjects with left ventricular hypertrophy. Clin Sci (Lond) 2002;102:363–371. [PubMed] [Google Scholar]
- Pincus SM, Huang W. Approximate entropy: Statistical properties and applications. Commun Statist-Theory Meth. 1992;21:3061–3077. [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:H2039–2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Romanelli F, Smith KM. Clinical effects and management of methamphetamine abuse. Pharmacotherapy. 2006;26:1148–1156. doi: 10.1592/phco.26.8.1148. [DOI] [PubMed] [Google Scholar]
- Rowan WH, 3rd, Campen MJ, Wichers LB, Watkinson WP. Heart rate variability in rodents: uses and caveats in toxicological studies. Cardiovascular toxicology. 2007;7:28–51. doi: 10.1007/s12012-007-0004-6. [DOI] [PubMed] [Google Scholar]
- Salomon R. The effect of amphetamines on culture myotubes: selective inhibition of protein synthesis. Life sciences. 1978;23:1941–1949. doi: 10.1016/0024-3205(78)90561-1. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychology review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Shrem MT, Halkitis PN. Methamphetamine abuse in the United States: contextual, psychological and sociological considerations. Journal of health psychology. 2008;13:669–679. doi: 10.1177/1359105307082461. [DOI] [PubMed] [Google Scholar]
- Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. Journal of the American College of Cardiology. 2008;52:719–726. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Smith HJ, Roche AH, Jausch MF, Herdson PB. Cardiomyopathy associated with amphetamine administration. American heart journal. 1976;91:792–797. doi: 10.1016/s0002-8703(76)80545-5. [DOI] [PubMed] [Google Scholar]
- Swalwell CI, Davis GG. Methamphetamine as a risk factor for acute aortic dissection. Journal of forensic sciences. 1999;44:23–26. [PubMed] [Google Scholar]
- Sztajzel J, Jung M, Bayes de Luna A. Reproducibility and gender-related differences of heart rate variability during all-day activity in young men and women. Ann Noninvasive Electrocardiol. 2008;13:270–277. doi: 10.1111/j.1542-474X.2008.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of affective disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biological psychology. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and biobehavioral reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Vanderlei LC, Pastre CM, Hoshi RA, Carvalho TD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc. 2009;24:205–217. doi: 10.1590/s0102-76382009000200018. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Bates ME, Vaschillo B, Lehrer P, Udo T, Mun EY, Ray S. Heart rate variability response to alcohol, placebo, and emotional picture cue challenges: effects of 0.1-Hz stimulation. Psychophysiology. 2008;45:847–858. doi: 10.1111/j.1469-8986.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivometrics . The Lifeshirt System ™. Ventura, CA: 2002. [Google Scholar]
- Vongpatanasin W, Taylor JA, Victor RG. Effects of cocaine on heart rate variability in healthy subjects. The American journal of cardiology. 2004;93:385–388. doi: 10.1016/j.amjcard.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, Cherner M, Heaton RK, Grant I. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19:35–43. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]
- Yeo KK, Wijetunga M, Ito H, Efird JT, Tay K, Seto TB, Alimineti K, Kimata C, Schatz IJ. The association of methamphetamine use and cardiomyopathy in young patients. The American journal of medicine. 2007;120:165–171. doi: 10.1016/j.amjmed.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Yu Q, Larson DF, Watson RR. Heart disease, methamphetamine and AIDS. Life sciences. 2003;73:129–140. doi: 10.1016/s0024-3205(03)00260-1. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Gold DR, Stone PH, Suh HH, Schwartz J, Coull BA, Speizer FE. Reduction in heart rate variability with traffic and air pollution in patients with coronary artery disease. Environmental health perspectives. 2010;118:324–330. doi: 10.1289/ehp.0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]