Abstract
Background
Late age at first full-term birth (AFB) and nulliparity are known to increase breast cancer risk. The frequency of these risk factors has increased in recent decades.
Methods
We conducted a population-based case-control study to examine associations between parity, AFB, and specific histological subtypes of breast cancer. Women with breast cancer (N=21,266) were identified from cancer registries in Wisconsin, Massachusetts, and New Hampshire. Control women (N=26,677) were randomly selected from population lists. Interviews collected information on reproductive histories and other risk factors. Logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) of ductal, lobular, and mixed ductal-lobular breast cancer diagnosis in association with AFB and nulliparity.
Results
AFB ≥ 30 years of age was associated with a 2.4-fold increase in risk of lobular breast cancer compared to AFB < 20 years (OR 2.4; 95%CI 1.9–2.9). The association was less pronounced for ductal breast cancer (OR 1.3; 95% CI, 1.2–1.4). Nulliparity was associated with increased risk for all breast cancer subtypes, compared to women with AFB <20 years, but the association was stronger for lobular (OR 1.72, 95% CI 1.34–2.20) than for ductal (OR 1.19, 95% CI 1.08–1.31) subtypes (P=0.004). The adverse effects of later AFB was stronger with obesity (P=0.03) in lobular, but not ductal, breast cancer.
Conclusion
Stronger associations observed for late AFB and nulliparity suggests that these preferentially stimulate growth of lobular breast carcinomas. Recent temporal changes in reproductive patterns and rates of obesity may impact the histological presentation of breast cancer.
Keywords: Epidemiology, reproduction, lobular breast cancer, ductal breast cancer, risk factors
INTRODUCTION
Breast cancer incidence and mortality rates in the U.S. increased in successive birth cohorts from 1940 through 1970. 1–3 This increase may be due, in part, to a trend toward an older age at first birth (AFB) and nulliparity in these birth cohorts. 4–7 Concurrent with these trends in AFB, the histological presentation of invasive breast cancer has changed. Ductal carcinoma remains the most commonly diagnosed breast cancer in the U.S., but the occurrence of lobular and mixed ductal-lobular breast cancer increased rapidly during the 1990’s, now accounting for approximately 20% of all cases. 8–10 The increases in lobular and mixed ductal-lobular incidence may be a consequence of recent diagnostic practices, but may also reflect population changes in the distribution of breast cancer subtype specific risk factors, illustrating important biological differences among histologic subtypes. 9
Evidence that later AFB is more strongly associated with lobular than ductal breast cancer has been observed in several studies, 2, 3, 11–13 but is not consistently reported. 14–17 The most convincing data that breast cancer varies by histological type is from studies of postmenopausal hormone use where a greater risk was observed for invasive lobular cancer compared to invasive ductal cancer among users of combined estrogen and progestin postmenopausal hormones. 18–23 A similar association has been reported according to oral contraceptive use as well. 18, 24 A recent case-control study found women with heavier body mass index (BMI) had an increased risk of ductal invasive breast cancer, whereas a null association was found with lobular breast cancer. 14 Most previous studies on histologic subtypes were relatively small in size and lacked power to consider relationships with AFB and other hormonal factors including BMI, use of oral contraceptives, and postmenopausal hormone therapy.
We examined the association between AFB, other hormonal exposures, and specific breast cancer histologies in a large case-control study including nearly 50,000 women born from 1912–1986, a period encompassing major temporal changes in reproductive patterns, hormonal factors, and histological presentation of breast cancer.
METHODS
The Collaborative Breast Cancer Study (CBCS) is a population-based case-control study of risk factors for breast cancer. This study consisted of five consecutive phases conducted in Wisconsin, Massachusetts (excluding metropolitan Boston), and New Hampshire. Details of the case-control studies are provided elsewhere. 25–29 Telephone interviews were completed between 1988 and 2007, and utilized similar procedures. The age eligibility varied over the course of the study and included women ages 20 to 74 years in phase 1 (1988–1991), ages 50–79 years in phase 2 (1992–1996) and ages 20 to 69 years during phases 3 through 5 (1997–2007).
Selection of cases
Cases were women diagnosed with a first invasive breast cancer reported by statutory mandated cancer registries of each state. An eligible case had a published telephone number, a reported date of diagnosis and driver’s license verified by self-report (aged 64 and younger). Of the 29,325 eligible cases, 1,168 were deceased, 653 could not be located and 4,122 refused to participate. Additionally, 984 women were excluded at their doctor’s request. Overall 23,382 cases participated in the study (80% of eligible cases).
Selection of controls
Community controls were chosen randomly within 5 year strata to match the age distribution of the cases from each state. Lists of licensed drivers were used to select women < 65 years, and Medicare beneficiary files were used to identify women ≥ 65. Inclusion criteria for controls required a publicly available telephone number and no personal history of breast cancer. Of the 35,141 eligible controls, 415 were deceased, 1,248 could not be located and 6,571 refused to participate, leaving 26,677 controls eligible for the current analysis (77% of eligible controls).
Data collection
Study participants were sent letters briefly describing the study before they were contacted via telephone by trained interviewers. Women completed a structured 45-minute telephone interviews on average 1–2 years after a reference date; interview questions evaluated exposures prior to this reference date. For cases, the reference date was defined as the date of breast cancer diagnosis. For controls, the reference date was calculated using information from dates of diagnosis and interview for similarly-aged cases. The interview covered questions on exposures occurring prior to the reference date and elicited complete reproductive history, menstrual experiences, medical history, medication use, and lifestyle factors including smoking status, alcohol intake, physical activity, and adult height and weight. Detailed breast cancer screening history and demographics were also collected.
Information about the histology and stage of breast cancer was obtained from each state’s cancer registry. Cases were grouped by histology using International Classification of Diseases-Oncology codes (ICD-10), ductal (code 8010, 8012, 8021, 8140, 8310, 8323, 8410, 8500, 8502, 8530, 8560, 8571), lobular (code 8520) and mixed ductal-lobular (code 8521, 8522, 8523). 30 All other individual tumor types, which each comprised less than 2% of the total sample, were excluded from the present analysis. Extent of disease was also obtained from state cancer registries. In Wisconsin only, information was available on the first course of treatment (surgery, chemotherapy, radiation, and hormonal treatment). Information on personal and family history of cancer was collected at the end of the questionnaire to maintain interviewer blinding; interviewers reported being unaware of the woman’s case-control status until the end of the interview in 85% of cases and 93% of controls.
Statistical analysis
Participants were classified as nulliparous or parous, and among the latter group, the age at first full term birth (AFB) was defined as the age at the first pregnancy of at least 6 months’ duration. The variable AFB was modelled both continuously (in parous women) by age at delivery (years), and was also categorized as < 20 years old, 20–24 years, 25–29 years, and ≥ 30 years at first birth. The relationship between risk of each histological sub-type and AFB was evaluated by the odds ratios (OR) and 95% confidence intervals (CI) obtained from multivariable polytomous logistic regression models. 31
We initially estimated the odds ratios according to categories of AFB, adjusted for age, state of residence and study period. Furthermore, we performed a multivariable analysis that adjusted for additional confounders defined a priori. The final models included age, state of residence, study phase, first degree family history of breast cancer, age of menarche, parity, menopausal status, age at menopause, oral contraceptive use, postmenopausal hormone use, recent alcohol consumption, body mass index (BMI,,kg/m2), history of mammographic screening, and education. The definitions and categories for these variables are shown in Table 1.
Table 1.
Characteristics of women with breast cancer and population controls
| Characteristic | Cases N (%) (N=21,266) | Controls N (%) (N=26,677) |
|---|---|---|
| Reference age | ||
| < 40 | 1,166 (5.5) | 1,671 (6.3) |
| 40–49 | 3,839 (18.1) | 4,977 (18.7) |
| 50–54 | 3,064 (14.4) | 3,892 (14.6) |
| 55–59 | 3,168 (14.9) | 4,171 (15.6) |
| 60–64 | 3,537 (16.6) | 4,472 (16.8) |
| 65–69 | 3,704 (17.4) | 4,740 (17.8) |
| 70–79 | 2,788 (13.1) | 2,754 (10.3) |
| State of residence | ||
| Wisconsin | 14,635 (68.8) | 16,683 (62.5) |
| Massachusetts | 5,054 (23.8) | 7,553 (28.3) |
| New Hampshire | 1,577 (7.4) | 2,441 (9.2) |
| Family history of breast cancer | ||
| Absent | 16,493 (77.6) | 22,740 (85.2) |
| Present | 4,324 (20.3) | 3,411 (12.8) |
| Unknown | 449 (2.1) | 526 (2.0) |
| Age at menarche (years) | ||
| < 12 | 4,179 (19.6) | 4,878 (18.3) |
| 12 | 5,213 (24.5) | 6,227 (23.3) |
| 13 | 5,806 (27.3) | 7,264 (27.2) |
| ≥14 | 5,660 (26.6) | 7,812 (29.3) |
| Parity | ||
| 0 | 2,906 (13.7) | 3,172 (11.9) |
| 1 | 2,382 (11.2) | 2,627 (9.8) |
| 2 | 6,268 (29.5) | 7,302 (27.3) |
| 3 | 4,621 (21.7) | 6,058 (22.7) |
| 4+ | 5,070 (23.8) | 7,471 (28.0) |
| Age at first birth (years) | ||
| <20 | 2,871 (13.5) | 4,323 (16.2) |
| 20–24 | 8,418 (39.6) | 11,476 (43.0) |
| 25–29 | 4,846 (22.8) | 5,534 (20.7) |
| 30+ | 2,225 (10.5) | 2,172 (8.1) |
| Nulliparous | 2,906 (13.7) | 3,172 (11.9) |
| Menopausal status | ||
| Postmenopausal | 14,631 (68.8) | 18,150 (68.0) |
| Premenopausal | 5,508 (25.9) | 7,103 (26.6) |
| Unknown | 1,127 (5.3) | 1,424 (5.3) |
| Age at menopause (years)a | ||
| <45 | 2,721 (12.8) | 4,317 (16.2) |
| 45–49 | 3,124 (14.7) | 3,959 (14.8) |
| 50–54 | 5,020 (23.6) | 5,613 (21.0) |
| 55+ | 1,832 (8.6) | 2,048 (7.7) |
| Oral contraceptive use | ||
| Never | 11,586 (54.5) | 14,609 (54.8) |
| Ever | 9,544 (44.9) | 11,887 (44.6) |
| Type of postmenopausal hormone therapya | ||
| Never | 7,922 (37.3) | 9,909 (37.1) |
| Estrogen only | 2,505 (11.8) | 3,312 (12.4) |
| Estrogen and progestin only | 1,804 (8.5) | 1,740 (6.5) |
| Other combination | 459 (2.2) | 513 (1.9) |
| Recent alcohol consumption (drinks/week) | ||
| None | 4,055 (19.1) | 5,343 (20.0) |
| 1–6 | 13,909 (65.4) | 17,693 (66.3) |
| 7+ | 3,129 (14.7) | 3,382 (12.7) |
| Body Mass Index (kg/m2)a, b | ||
| <25.0 | 6,483 (30.4) | 8,764 (32.8) |
| 25.0–29.9 | 4,748 (22.3) | 5,771 (21.6) |
| ≥30.0 | 3,093 (14.5) | 3,243 (12.2) |
| Unknown | 307 (1.4) | 372 (1.4) |
| History of mammographic screening | ||
| Never | 4,128 (19.4) | 4,069 (15.2) |
| Ever | 14,153 (66.6) | 17,838 (66.9) |
| Unknown | 2,985 (14.0) | 4,770 (17.9) |
| Education | ||
| < High school | 2,355 (11.1) | 3,106 (11.6) |
| High school | 8,928 (42.0) | 11,199 (42.0) |
| Some college | 5,086 (23.9) | 6,713 (25.2) |
| College degree | 4,775 (22.4) | 5,503 (20.6) |
Postmenopausal women only.
BMI levels as defined by WHO.32
We examined modification of the relationship between AFB and risk of specific histological breast cancer types by postmenopausal BMI, oral contraceptive history, and postmenopausal hormone therapy. Adult BMI was categorized in three groups as average weight (<25 kg/m2), overweight (25–29.9 kg/m2) and obese (≥30 kg/m2). 32 Analyses of oral contraceptives were restricted to women <65 years of age who had greatest opportunity for regular use of oral contraceptives, which were not widely marketed until the late 1960s. 33 Participants were considered postmenopausal hormone users if they reported past or current use for three months or more. Analyses were stratified by exclusive users of estrogen alone (E only) and by users of formulations which combined both estrogen and progestin (E + P only). We restricted analyses to specific hormone therapy type since previous studies have reported varying strength of associations with breast cancer by histologic subtype. 2, 3, 11–13 Postmenopausal hormone and oral contraceptive (OC) use were modeled both as never/ever categorizations and by years of duration of use. To determine the significance of the interactions, log-likelihood values were compared between models with and without the cross-product interaction term for AFB (in parous women only) multiplied by the term for BMI, oral contraceptive use, or postmenopausal hormone use. All P-values were two-sided and statistical significance was defined as a P value of less than 0.05. All statistical analyses were performed using SAS System, version 9.1. Questionnaire data for 38 breast cancer cases and 37 controls were deemed unreliable by the interviewers because of inconsistent participant responses, and an additional 201 cases and 193 controls did not report sufficient reproductive history for calculation of age at first birth. Therefore, this analysis is based upon 21,266 cases and 26,677 controls.
RESULTS
In general, control participants were more likely to have given birth, experienced menopause at an earlier age, had a lower adult BMI (if postmenopausal), and consumed lower levels of alcohol than breast cancer cases (Table 1). Additionally, control women were less likely to have a family history of breast cancer and to have used combined estrogen and progestin postmenopausal hormone therapy. The majority of women with breast cancer in this analysis were diagnosed with ductal carcinoma (79%, N = 18,329). Lobular and mixed ductal-lobular carcinoma followed in frequency of diagnosis with approximately 10% (N = 2,030) and 4% (N = 907), respectively. The diagnosis of lobular and mixed ductal-lobular tumor types increased in occurrence in our study population over calendar time. During the first phase of the study (1988–1991), mixed ductal-lobular carcinoma accounted for 1.7% of the case population and increased to 7.5% by the enrollment of the final phase (2004–2007).
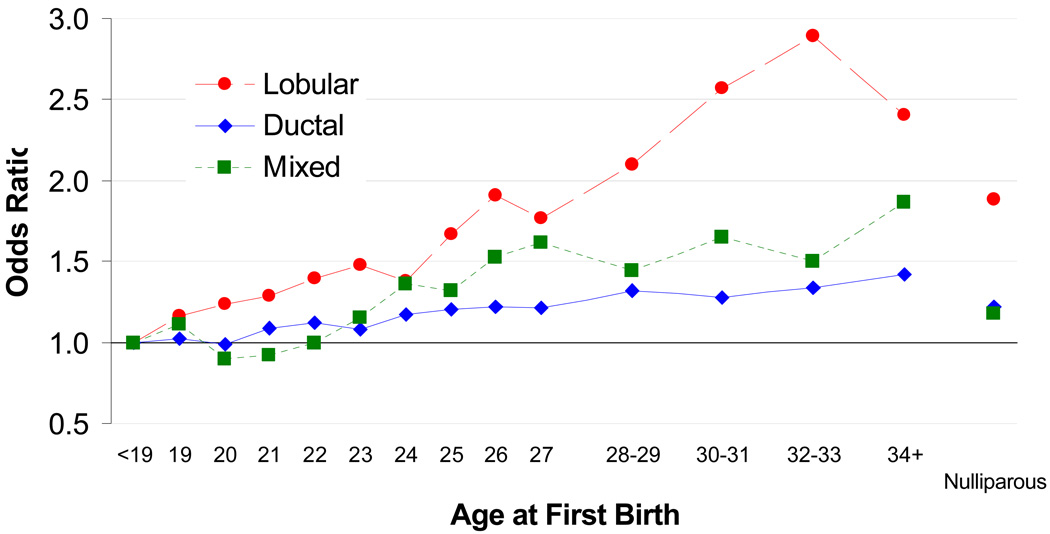
Later AFB was associated with an increasing risk of all histological subtypes of breast cancer, including ductal, lobular, and mixed ductal-lobular carcinoma (Figure). The increase in risk was greater for lobular than for other histologies. The ORs for the comparison of AFB ≥30 to AFB <20 were 2.38 (95% CI, 1.94–2.93) for lobular breast cancer, 1.31 (95% CI 1.20–1.43) for ductal breast cancer, and 1.55 (95% CI 1.15–2.07) for mixed ductal-lobular breast cancer (Table 2). The difference between these ORs was statistically significant for ductal vs. lobular (P < 0.001) and mixed vs. lobular (P = 0.01).
Figure.
The relationship between age at first birth and incidence of breast cancer sub-types
Table 2.
Odds ratios for specifica histologic types of breast cancer by age at first full-term birth
| Controls (N=26,677) |
Ductal breast cancer (N=18,329) |
Lobular breast cancer (N=2,030) |
P-value ductal vs. lobular |
Mixed ductal-lobular breast cancer (N=907) |
P-value Mixed vs. lobular |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age at first birth | N | N | OR (95% CI) | N | OR (95% CI) | N | OR (95% CI) | |||
| Nulliparous | 3,172 | 2,548 | 1.19 (1.08–1.31) | 247 | 1.72 (1.34–2.20) | 0.004 | 111 | 1.09 (0.77–1.54) | 0.03 | |
| <20 | 4,323 | 2,516 | 1 | 234 | 1.00 | 121 | 1 | |||
| 20–24 | 11,476 | 7,298 | 1.07 (1.01–1.13) | 790 | 1.25 (1.07–1.46) | 0.05 | 330 | 0.99 (0.79–1.23) | 0.08 | |
| 25–29 | 5,534 | 4,118 | 1.22 (1.14–1.31) | 796 | 1.71 (1.44–2.04) | <0.001 | 232 | 1.36 (1.06–1.73) | 0.12 | |
| 30+ | 2,172 | 1,849 | 1.31(1.20–1.43) | 263 | 2.38 (1.94–2.93) | <0.001 | 113 | 1.55 (1.15–2.07) | 0.01 | |
| P-trend (per 1 year)c | <0.001 | <0.001 | <0.001 | |||||||
| 5-Year increment | 1.11 (1.08–1.14) | 1.32 (1.24–1.40) | 1.23 (1.12–1.34) | |||||||
Includes ductal, lobular, and mixed ductal-lobular only (other histologic subtypes were excluded from analysis (N=1577)). Odds ratios are adjusted for age, state of residence, study period, parity, age of menarche, menopausal status, age at menopause, postmenopausal hormone use, oral contraceptive use, family history of breast cancer, recent alcohol consumption, body mass index, history of mammographic screening, and education.
P-trend calculated among parous women only.
Nulliparity was associated with increased risks for ductal and lobular breast cancer when compared to women with a first birth < 20 years of age, and the association was stronger for lobular (OR, 1.72; 95% CI, 1.34–2.20) than for ductal (OR, 1.19; 95% CI, 1.08–1.31) histology (Table 2). No excess risk was demonstrated for the smaller group of mixed ductal-lobular breast cancer (OR, 1.09; 95% CI, 0.77–1.54). The difference between these ORs was statistically significant for ductal vs. lobular (P = 0.004) and mixed vs. lobular (P = 0.03).
We examined the association between later AFB and risk of breast cancer histological types according to BMI and exogenous hormone use. Among postmenopausal women, ductal breast cancers increased with higher BMI for each AFB comparison (Table 3). The association between AFB and ductal breast cancer risk were only minimally elevated among women in the non-obese categories of BMI, and the interaction between BMI (highest vs. lowest category) and AFB (continuous) was not statistically significant (Pinteraction = 0.81). The trend was more pronounced in the lobular breast cancer group. Women in the higher categories of BMI had a progressively increasing risk of lobular breast cancer (Pinteraction = 0.03). The odds ratio for lobular breast cancer in women who were ≥ 30 years and obese was 3.17 (95% CI, 1.92–5.24). There were no significant associations between AFB and risk of mixed ductal-lobular breast cancer within any BMI strata (Pinteraction = 0.86). Similarly, in nulliparous women, high BMI (≥25 kg/m2) was associated with a statistically significantly elevated lobular breast cancer risk, but not for ductal or mixed ductal-lobular breast cancer.
Table 3.
Odds ratiosa for histologic types of postmenopausal breast cancer according to age at first birth and body mass index (BMI)b
| Ductal breast cancer (N=12,301) |
Lobular breast cancer (N=1,460) |
Mixed ductal-lobular breast cancer (N=563) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BMI | BMI | BMI | |||||||
| <25.0 kg/m2 | 25.0–29.9 kg/m2 | ≥30 kg/m2 | <25.0 kg/m2 | 25.0–29.9 kg/m2 | ≥30 kg/m2 | <25.0 kg/m2 | 25.0–29.9 kg/m2 | ≥30 kg/m2 | |
| Age at first birth |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
| Nulliparous | 1.15 (0.96–1.37) |
1.29 (1.04–1.61) |
1.11 (0.84–1.47) |
1.21 (0.77–1.89) |
2.94 (1.07–5.06) |
1.52 (1.82–2.82) |
0.91 (0.46–1.80) |
0.47 (0.20–1.12) |
0.71 (0.29–1.72) |
| <20 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 20–24 | 0.99 (0.88–1.10) |
1.14 (1.01–1.29) |
1.24 (1.07–1.44) |
1.01 (0.77–1.34) |
1.61 (1.14–2.27) |
1.32 (0.93–1.88) |
0.82 (0.54–1.25) |
1.01 (0.63–1.61) |
1.13 (0.68–1.89) |
| 25–29 | 1.10 (0.97–1.25) |
1.28 (1.11–1.49) |
1.37 (1.14–1.65) |
1.23 (0.90–1.67) |
2.39 (1.63–3.49) |
1.96 (1.29–3.00) |
1.00 (0.62–1.60) |
1.34 (0.79–2.29) |
1.27 (0.69–2.36) |
| 30+ | 1.19 (1.01–1.40) |
1.39 (1.14–1.69) |
1.50 (1.16–1.94) |
1.56 (1.07–2.28) |
4.16 (2.68–6.46) |
3.17 (1.92–5.24) |
1.58 (0.90–2.78) |
1.53 (0.78–2.99) |
1.27 (0.57–2.80) |
| Pinteractionc | 0.81 | 0.03 | 0.86 | ||||||
Odds ratios are adjusted for age, state of residence, study period, parity, age of menarche, menopausal status, age at menopause, postmenopausal hormone use, oral contraceptive use, family history of breast cancer, recent alcohol consumption, body mass index, history of mammographic screening, and education. Analysis includes 17,778 controls.
BMI levels as defined by WHO32
Pinteraction calculated among lowest and highest levels of BMI and continuous age at first birth, parous women only.
In all three breast cancer histologic subgroups, later AFB was a more prominent risk factor among OC never-users than ever-users (Table 4). The magnitude of effect of AFB was greatest in the lobular subgroup and least strong in the ductal subgroup: the highest odds ratio was observed for lobular breast cancer comparing extreme categories of AFB in women who had never used OCs (OR, 4.03; 95% CI 2.65–6.11). However, the interaction between OC duration of use and AFB was not statistically significant for lobular (Pinteraction = 0.15) histology, but was statistically significant for the ductal and mixed (Pinteraction = 0.003 and 0.01, respectively) subtypes. This pattern was also suggested for nulliparous women in all subtype groups.
Table 4.
Odds ratiosa for histologic types of breast cancer according to age at first full-term birth stratified by oral contraceptive and postmenopausal hormone use
| Oral contraceptive useb | |||||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |||||
| Ductal breast cancer | Lobular breast cancer | Mixed ductal-lobular breast cancer | |||||
| Age at first birth | Never | Ever Use | Never | Ever Use | Never | Ever Use | |
| Nulliparous | 1.33 (1.11–1.60) |
1.19 (1.02–1.39) |
1.92 (1.18–3.12) |
1.64 (1.10–2.44) |
1.16 (0.61–2.22) |
1.28 (0.78–2.11) |
|
| <20 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 20–24 | 1.21 (1.08–1.36) |
0.98 (0.89–1.07) |
1.58 (1.15–2.16) |
1.15 (0.91–1.46) |
1.32 (0.86–2.03) |
0.84 (0.61–1.14) |
|
| 25–29 | 1.32 (1.16–1.51) |
1.20 (1.08–1.34) |
2.07 (1.45–2.95) |
1.69 (1.29–2.22) |
1.73 (1.07–2.79) |
1.26 (0.91–1.81) |
|
| 30+ | 1.64 (1.37–1.96) |
1.28 (1.12–1.46) |
4.03 (2.65–6.11) |
2.02 (1.46–2.80) |
3.02 (1.73–5.27) |
1.08 (0.66–1.58) |
|
| Pinteractionc | 0.003 | 0.15 | 0.01 | ||||
| Postmenopausal hormone used | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||||||
| Ductal breast cancer | Lobular breast cancer | Mixed ductal–lobular breast cancer | ||||||||
| Age at first birth | Never | E only | E+P Only | Never | E only | E+P Only | Never | E only | E+P Only | |
| Nulliparous | 1.15 (0.97–1.36) |
1.23 (0.92–1.64) |
0.94 (0.64–1.38) |
1.64 (1.09–2.47) |
2.42 (1.18–4.96) |
1.21 (0.56–2.65) |
0.57 (0.30–1.08) |
2.56 (0.78–8.39) |
0.32 (0.12–0.89) |
|
| <20 | 1 | 1 | 1 | 1 | 1 | 1 | 1.00 | 1 | 1.00 | |
| 20–24 | 1.11 (1.01–1.22) |
1.04 (0.89–1.22) |
0.97 (0.77–1.21) |
1.19 (0.93–1.53) |
1.48 (0.98–2.23) |
0.89 (0.57–1.38) |
1.01 (0.70–1.45) |
1.14 (0.57–2.30) |
0.79 (0.43–1.45) |
|
| 25–29 | 1.21 (1.08–1.36) |
1.17 (0.96–1.42) |
0.97 (0.74–1.28) |
1.61 (1.22–2.12) |
1.69 (1.04–2.76) |
1.23 (0.73–2.06) |
1.13 (0.75–1.73) |
2.18 (1.01–4.73) |
0.60 (0.29–1.27) |
|
| 30+ | 1.26 (1.08–1.46) |
1.44 (1.07–1.94) |
1.57 (1.10–2.24) |
2.35 (1.69–3.25) |
2.86 (1.51–5.44) |
2.02 (1.05–3.89) |
1.48 (0.90–2.44) |
4.01 (1.55–10.40) |
0.63 (0.23–1.67) |
|
| Pinteractione | 0.13 | 0.47 | 0.84 | 0.61 | 0.03 | 0.20 | ||||
Odds ratios are adjusted for age, state of residence, study period, parity, age of menarche, menopausal status, age at menopause, family history of breast cancer, recent alcohol consumption, body mass index, history of mammographic screening, education, postmenopausal hormone use, or oral contraceptive use.
OR calculated only among women less than age 65. Analysis included 19,056 controls, 12,652 ductal cases, 1,321 lobular cases, and 699 mixed ductal-lobular cases.
Pinteraction calculated using duration of use and lowest and highest categories of age of first birth.
OR calculated only among postmenopausal women. E only postmenopausal hormone use is defined as using any postmenopausal hormone supplements containing estrogen alone for 3 month or more. E+P only use refers to combined postmenopausal hormone use and is defined as using postmenopausal hormone supplements containing estrogen and progestin for 3 month or more. Analysis included 14,961 controls, 10,449 ductal cases, 1,284 lobular cases and 498 mixed ductal-lobular cases.
Pinteraction calculated using never versus ever hormone use and lowest and highest categories of age of first birth.
In ductal and lobular histologies there were no statistically significant differences in the associations between AFB and breast cancer by women exclusively using postmenopausal hormones johnformulations containing estrogen-only, or combined estrogen and progestin, the type most strongly associated with lobular breast cancer (Table 4). Later AFB was associated with an increased risk of ductal and lobular cancer in non-users and users of both types of postmenopausal hormones. Opposing associations were found in women diagnosed with mixed ductal-lobular by postmenopausal hormone type. Estrogen-only participants with an AFB after age 20 were at an increased risk of mixed-ductal lobular disease; women with an AFB at or after age 30 experienced the highest risk (OR, 4.01; 95% CI 1.55–10.40). Whereas, the association between AFB and mixed ductal-lobular breast cancer was null among never users of hormone therapy (Pinteraction = 0.03). In combined hormone users an inverse association was seen for mixed ductal-lobular histology women with an AFB after age 20 were at a slight decreased risk for breast cancer. Nulliparous users of combined postmenopausal hormones were at the lowest risk of mixed ductal-lobular breast cancer (OR, 0.32; 95% CI 0.12–0.89).
CONCLUSION
In this large case-control study, women with earlier childbirth were less likely to develop ductal, lobular, and mixed ductal-lobular breast cancer than women who had children at later ages or remained nulliparous. However, of these histological types, lobular breast cancer was more strongly associated with AFB than was ductal or mixed ductal-lobular subtypes. A 2.4-fold increase in risk of lobular breast cancer was observed for women with AFB of at least 30 years of age, when compared to women with first birth before the age of 20 years. There was also evidence that AFB associations were modified by postmenopausal BMI and history of oral contraceptive use.
Several earlier studies also indicated a stronger association of AFB with lobular than ductal breast cancer. 2, 3, 11–13, 34, 35 Other studies, however, have found no difference in associations between AFB and ductal or lobular cancers. 15–17, 36 In a meta-analysis combining data from nine studies, lobular cancer was found to be more strongly associated with later AFB than ductal carcinoma.11 The summary odds ratios for a five year delay in AFB was 1.23 (1.17–1.29) for lobular breast cancer and 1.10 (1.07–1.12) for ductal breast cancer; differences that were less marked than found in the current investigation (Table 2 and Figure). Past studies did not examine possible AFB interactions by endogenous and exogenous hormonal exposures, but we observed that the elevated risk of lobular breast cancer associated with late AFB was modified by aspects of the hormonal milieu specifically by oral contraceptive use and adult obesity.
In our study, we found that women who were overweight with later AFB had a three-fold increased risk of developing lobular breast cancer when compared to women with AFB less than 20; for average weight women, there was a 56% increased risk of developing lobular breast cancer in women with later AFB. In contrast, for ductal and mixed ductal-lobular breast cancer, there was no evidence for effect modification by BMI. Postmenopausal obesity is a consistent risk factor for breast cancer. 37 Obesity in postmenopausal women is associated with alterations in sex hormones (e.g. estrogen, progesterone, and androgens) 38–42 and other growth factors such as insulin and IGFs 43 that have important promoting elements. Increased levels of endogenous hormones may act to enhance the promotion of cellular aberrations in the breast. 44 Overweight and obese postmenopausal women frequently also have high BMIs in their premenopausal years as well. 45, 46 This finding is of particular public health significance as obesity levels continue to rise in U.S women. 45, 47 Societal changes relating to both increasing age at first birth and BMI may be reflected in the increase lobular breast cancer occurrence during portions of our study period. From an individual perspective these findings may provide additional motivation for women to lose or maintain body weight.
We also observed significant differences in the associations between lobular breast cancer risk and AFB based on another hormone-related potential risk factor, history of oral contraceptive use. 44 The four-fold increase in risk of lobular breast cancer associated with late AFB among women who never used oral contraceptives may be due to the true impact of late AFB. Oral contraceptives generally provide low levels of the hormones estrogen and progestin, 33 mimicking the hormonal effects of pregnancy. Due to their hormonal effects users of oral contraceptives may be less influenced by the effects of late AFB as a risk factor of lobular breast cancer. We did not find evidence for effect modification by postmenopausal hormone use either for E only or E + P, in relation to ductal or lobular histologies. Differences in risks were observed in mixed ductal-lobular participants by use of estrogen-only hormonal supplements. This finding may point to the subtle biological differences in histologic subtype.
Regardless of histological subtype, a higher incidence of breast cancer with later age at first birth or nulliparity is a consistent finding in epidemiologic studies, though the mechanism is unclear. 44, 48 Russo et al. proposed that mammary glands become fully differentiated at pregnancy, and that less differentiated ducts are more susceptible to carcinogens. 49, 50 As carcinogen exposures accumulate with increasing age, later AFB then places the breast at greater risk. 51, 52 Both progesterone and estrogen are mitogenic for epithelial breast cells, and may stimulate breast cell proliferation. 53–55 Increased susceptibility to breast cancer might occur because of the greater number of menstrual cycles with hormones acting on the undifferentiated breast. During pregnancy, progesterone induces lobular–aveolar development in preparation for lactation, 56 and its effect on the lobules could well depend upon AFB. In addition to the unique clinical and molecular characteristics of histological types of breast cancer, 57–62 epidemiologic evidence is also consistent that lobular tumors are etiologically distinct and more hormonally dependent than other subtypes, based upon associations with exogenous hormones containing progestin. 2, 3, 11, 14, 18, 22, 24 While epidemiologic results have not been completely supportive of a role for circulating progesterone levels and breast cancer risk, 55, 63–66 none of these studies specifically considered lobular histology.
An interesting finding from this study is the proportion of women diagnosed with lobular breast cancer increased over the study period. Although one possible explanation for this increase is a change in the definitions of histology over time, it is most likely attributable to the common use of combined estrogen plus progestin postmenopausal hormone therapy through the 1990’s which have been shown to increase the risk of lobular breast cancer to a greater degree than ductal breast cancer. 18–23, 67 U.S. lobular breast cancer rates have declined recently, along with the concurrent decline in use of hormone replacement formulations involving estrogen plus progestin after the Women’s Health Initiative found users experienced increased incidence in breast cancer.68, 69 This decline provides support for the hypothesis that lobular breast cancers are more strongly associated with hormonal factors than are other histological subtypes.
The large size of this study permitted examination of AFB in groups defined by BMI and oral contraceptive use, and high participation rates reduced concerns about selection bias. However, several limitations should be considered in interpreting our results. The possibility of misclassification of histology is a potential concern as pathology reports for our study came from community labs across three states, and there was no centralized review. However, one study showed high levels of agreement among 23 pathologists in data from 12 European registries when assigning breast cancer histology. 70 Moreover, in the current study, the distribution of breast cancer histologies was similar by state, and the overall results were also similar by study center, suggesting that information reported by cancer registries was reasonably accurate. We did not have information on hormone receptor-status for this analysis. It has been hypothesized that receptor status may confound the relationship between reproductive factors and breast cancer histological subtypes, 12 because invasive lobular carcinomas more often express estrogen or progesterone receptors than do invasive ductal carcinomas, 59, 67 and positive-receptor status is also associated with later age at first birth. 12, 71, 72 However, these findings suggest, as do our own, a greater influence of reproductive factors on hormonally sensitive tumors. 72
In this study, the associations of late AFB and nulliparity were stronger in lobular than ductal or mixed ductal-lobular breast cancer sub-types. These associations appeared to be modified by obesity and use of oral contraceptives. Temporal changes in childbearing practices and lifestyle factors that influence obesity may lead to an increasing prominence of lobular breast cancer in the coming years.
Acknowledgments
This study was supported by National Institutes of Health Cancer Institute grants CA47147, CA47305, CA69664, CA82004, and the Avon Foundation.
Footnotes
There are no financial disclosures from any authors.
REFERENCES
- 1.Tarone RE, Chu KC, Gaudette LA. Birth cohort and calendar period trends in breast cancer mortality in the United States and Canada. J Natl Cancer Inst. 1997;89(3):251–256. doi: 10.1093/jnci/89.3.251. [DOI] [PubMed] [Google Scholar]
- 2.Li CI, Daling JR, Malone KE, et al. Relationship between established breast cancer risk factors and risk of seven different histologic types of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(5):946–954. doi: 10.1158/1055-9965.EPI-05-0881. [DOI] [PubMed] [Google Scholar]
- 3.Wohlfahrt J, Mouridsen H, Andersen PK, Melbye M. Reproductive risk factors for breast cancer by receptor status, histology, laterality and location. Int J Cancer. 1999;81(1):49–55. doi: 10.1002/(sici)1097-0215(19990331)81:1<49::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.White E. Projected changes in breast cancer incidence due to the trend toward delayed childbearing. Am J Public Health. 1987;77(4):495–497. doi: 10.2105/ajph.77.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn RA, Moolgavkar SH. Nulliparity, decade of first birth, and breast cancer in Connecticut cohorts, 1855 to 1945: an ecological study. Am J Public Health. 1989;79(11):1503–1507. doi: 10.2105/ajph.79.11.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li CI, Moe RE, Daling JR. Risk of mortality by histologic type of breast cancer among women aged 50 to 79 years. Archives of Internal Medicine. 2003;163(18):2149–2153. doi: 10.1001/archinte.163.18.2149. [DOI] [PubMed] [Google Scholar]
- 7.Statistics NCfH. Table 1–30. Central Birth Rates by Live-Birth Order, Current Age of Mother, According to Race of Child, for Women in Selected Groups of Cohorts From 1896–1900 to 1980–1984. [Accessed July 11, 2006]; http://www.cdc.gov/nchs/data/statab/tab1_30.pdf.
- 8.SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 9.Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289(11):1421–1424. doi: 10.1001/jama.289.11.1421. [DOI] [PubMed] [Google Scholar]
- 10.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005. [Accessed April 20, 2008]; http://seer.cancer.gov/csr/1975_2005/ [Google Scholar]
- 11.Reeves GK, Pirie K, Green J, Bull D, Beral V. Reproductive factors and specific histological types of breast cancer: prospective study and meta-analysis. Br J Cancer. 2009;100(3):538–544. doi: 10.1038/sj.bjc.6604853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ursin G, Bernstein L, Lord SJ, et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br J Cancer. 2005;93(3):364–371. doi: 10.1038/sj.bjc.6602712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phipps AI, Li CI, Kerlikowske K, Barlow WE, Buist DS. Risk factors for ductal, lobular, and mixed ductal-lobular breast cancer in a screening population. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1643–1654. doi: 10.1158/1055-9965.EPI-10-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li CI, Malone KE, Porter PL, Weiss NS, Tang MT, Daling JR. Reproductive and anthropometric factors in relation to the risk of lobular and ductal breast carcinoma among women 65–79 years of age. Int J Cancer. 2003;107(4):647–651. doi: 10.1002/ijc.11465. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg LU, Magnusson C, Lindstrom E, Wedren S, Hall P, Dickman PW. Menopausal hormone therapy and other breast cancer risk factors in relation to the risk of different histological subtypes of breast cancer: a case-control study. Breast Cancer Res. 2006;8(1):R11. doi: 10.1186/bcr1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaber EF, Holt VL, Malone KE, Porter PL, Daling JR, Li CI. Reproductive factors, age at maximum height, and risk of three histologic types of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3427–3434. doi: 10.1158/1055-9965.EPI-08-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Closas M, Brinton LA, Lissowska J, et al. Established breast cancer risk factors by clinically important tumour characteristics. Br J Cancer. 2006;95(1):123–129. doi: 10.1038/sj.bjc.6603207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li CI, Malone KE, Porter PL, et al. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. JAMA. 2003;289(24):3254–3263. doi: 10.1001/jama.289.24.3254. [DOI] [PubMed] [Google Scholar]
- 19.Li CI, Weiss NS, Stanford JL, Daling JR. Hormone replacement therapy in relation to risk of lobular and ductal breast carcinoma in middle-aged women. Cancer. 2000;88(11):2570–2577. doi: 10.1002/1097-0142(20000601)88:11<2570::aid-cncr20>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Newcomb PA, Titus-Ernstoff L, Egan KM, et al. Postmenopausal estrogen and progestin use in relation to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11(7):593–600. [PubMed] [Google Scholar]
- 21.Daling JR, Malone KE, Doody DR, et al. Relation of regimens of combined hormone replacement therapy to lobular, ductal, and other histologic types of breast carcinoma. Cancer. 2002;95(12):2455–2464. doi: 10.1002/cncr.10984. [DOI] [PubMed] [Google Scholar]
- 22.Newcomer LM, Newcomb PA, Potter JD, et al. Postmenopausal hormone therapy and risk of breast cancer by histologic type (United States) Cancer Causes Control. 2003;14(3):225–233. doi: 10.1023/a:1023634907723. [DOI] [PubMed] [Google Scholar]
- 23.Chen CL, Weiss NS, Newcomb P, Barlow W, White E. Hormone replacement therapy in relation to breast cancer. JAMA. 2002;287(6):734–741. doi: 10.1001/jama.287.6.734. [DOI] [PubMed] [Google Scholar]
- 24.Newcomer LM, Newcomb PA, Trentham-Dietz A, Longnecker MP, Greenberg ER. Oral contraceptive use and risk of breast cancer by histologic type. Int J Cancer. 2003;106(6):961–964. doi: 10.1002/ijc.11307. [DOI] [PubMed] [Google Scholar]
- 25.Newcomb PA, Storer BE, Longnecker MP, et al. Lactation and a reduced risk of premenopausal breast cancer. N Engl J Med. 1994;330(2):81–87. doi: 10.1056/NEJM199401133300201. [DOI] [PubMed] [Google Scholar]
- 26.Titus-Ernstoff L, Egan KM, Newcomb PA, et al. Exposure to breast milk in infancy and adult breast cancer risk. J Natl Cancer Inst. 1998;90(12):921–924. doi: 10.1093/jnci/90.12.921. [DOI] [PubMed] [Google Scholar]
- 27.Nichols HB, Trentham-Dietz A, Sprague BL, Hampton JM, Titus-Ernstoff L, Newcomb PA. Effects of birth order and maternal age on breast cancer risk: modification by whether women had been breast-fed. Epidemiology. 2008;19(3):417–423. doi: 10.1097/EDE.0b013e31816a1cff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprague BL, Trentham-Dietz A, Newcomb PA, Titus-Ernstoff L, Hampton JM, Egan KM. Lifetime recreational and occupational physical activity and risk of in situ and invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(2):236–243. doi: 10.1158/1055-9965.EPI-06-0713. [DOI] [PubMed] [Google Scholar]
- 29.Newcomb PA, Nichols HB, Beasley JM, et al. No difference between red wine or white wine consumption and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(3):1007–1010. doi: 10.1158/1055-9965.EPI-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. International Classification of Diseases (ICD-10) Geneva: WHO; 1994
- 31.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd edition. Wiley and Sons: 2000. [Google Scholar]
- 32.WHO. Geneva: World Health Organization; Physical status: The Use and Interpretation of Anthropometry. 1995
- 33.IARC. Combined Estrogen-Progestogen Contraceptives and Combined Estrogen-Progestogen Menopausal Therapy. Lyon, France: WHO/IARC; 2007. [PMC free article] [PubMed] [Google Scholar]
- 34.Granstrom C, Sundquist J, Hemminki K. Population attributable risks for breast cancer in Swedish women by morphological type. Breast Cancer Res Treat. 2008;111(3):559–568. doi: 10.1007/s10549-007-9814-2. [DOI] [PubMed] [Google Scholar]
- 35.Li CI, Malone KE, Daling JR, et al. Timing of menarche and first full-term birth in relation to breast cancer risk. Am J Epidemiol. 2008;167(2):230–239. doi: 10.1093/aje/kwm271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ewertz M, Duffy SW. Risk of breast cancer in relation to reproductive factors in Denmark. Br J Cancer. 1988;58(1):99–104. doi: 10.1038/bjc.1988.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 38.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 39.Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987;45 1 Suppl:277–282. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- 40.Cleary MP, Maihle NJ. The role of body mass index in the relative risk of developing premenopausal versus postmenopausal breast cancer. Proc Soc Exp Biol Med. 1997;216(1):28–43. doi: 10.3181/00379727-216-44153b. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiol Rev. 1993;15(1):48–65. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- 42.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3(9):565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 43.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–1543. [PubMed] [Google Scholar]
- 44.Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia. 2002;7(1):3–15. doi: 10.1023/a:1015714305420. [DOI] [PubMed] [Google Scholar]
- 45.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 46.Trentham-Dietz A, Newcomb PA, Storer BE, et al. Body size and risk of breast cancer. Am J Epidemiol. 1997;145(11):1011–1019. doi: 10.1093/oxfordjournals.aje.a009057. [DOI] [PubMed] [Google Scholar]
- 47.Vital signs: state-specific obesity prevalence among adults --- United States. MMWR Morb Mortal Wkly Rep. 2009;59(30):951–955. [PubMed] [Google Scholar]
- 48.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15(1):36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 49.Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2(1):5–73. doi: 10.1007/BF01805718. [DOI] [PubMed] [Google Scholar]
- 50.Russo J, Mailo D, Hu YF, Balogh G, Sheriff F, Russo IH. Breast differentiation and its implication in cancer prevention. Clin Cancer Res. 2005;11(2 Pt 2):931s–936s. [PubMed] [Google Scholar]
- 51.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133–140. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 52.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21(3):427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 53.Soderqvist G. Effects of sex steroids on proliferation in normal mammary tissue. Ann Med. 1998;30(6):511–524. [PubMed] [Google Scholar]
- 54.Feng Z, Marti A, Jehn B, Altermatt HJ, Chicaiza G, Jaggi R. Glucocorticoid and progesterone inhibit involution and programmed cell death in the mouse mammary gland. J Cell Biol. 1995;131(4):1095–1103. doi: 10.1083/jcb.131.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Helzlsouer KJ, Alberg AJ, Bush TL, Longcope C, Gordon GB, Comstock GW. A prospective study of endogenous hormones and breast cancer. Cancer Detect Prev. 1994;18(2):79–85. [PubMed] [Google Scholar]
- 56.Lange CA, Richer JK, Horwitz KB. Hypothesis: Progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol Endocrinol. 1999;13(6):829–836. doi: 10.1210/mend.13.6.0290. [DOI] [PubMed] [Google Scholar]
- 57.Sims AH, Howell A, Howell SJ, Clarke RB. Origins of breast cancer subtypes and therapeutic implications. Nat Clin Pract Oncol. 2007;4(9):516–525. doi: 10.1038/ncponc0908. [DOI] [PubMed] [Google Scholar]
- 58.Yoder BJ, Wilkinson EJ, Massoll NA. Molecular and morphologic distinctions between infiltrating ductal and lobular carcinoma of the breast. Breast J. 2007;13(2):172–179. doi: 10.1111/j.1524-4741.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 59.Stierer M, Rosen H, Weber R, Hanak H, Spona J, Tuchler H. Immunohistochemical and biochemical measurement of estrogen and progesterone receptors in primary breast cancer. Correlation of histopathology and prognostic factors. Ann Surg. 1993;218(1):13–21. doi: 10.1097/00000658-199307000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Acs G, Lawton TJ, Rebbeck TR, LiVolsi VA, Zhang PJ. Differential expression of E-cadherin in lobular and ductal neoplasms of the breast and its biologic and diagnostic implications. Am J Clin Pathol. 2001;115(1):85–98. doi: 10.1309/FDHX-L92R-BATQ-2GE0. [DOI] [PubMed] [Google Scholar]
- 61.Dixon JM, Anderson TJ, Page DL, Lee D, Duffy SW. Infiltrating lobular carcinoma of the breast. Histopathology. 1982;6(2):149–161. doi: 10.1111/j.1365-2559.1982.tb02712.x. [DOI] [PubMed] [Google Scholar]
- 62.Lee AH, Dublin EA, Bobrow LG, Poulsom R. Invasive lobular and invasive ductal carcinoma of the breast show distinct patterns of vascular endothelial growth factor expression and angiogenesis. J Pathol. 1998;185(4):394–401. doi: 10.1002/(SICI)1096-9896(199808)185:4<394::AID-PATH117>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 63.Secreto G, Toniolo P, Pisani P, et al. Androgens and breast cancer in premenopausal women. Cancer Res. 1989;49(2):471–476. [PubMed] [Google Scholar]
- 64.Thomas HV, Key TJ, Allen DS, et al. A prospective study of endogenous serum hormone concentrations and breast cancer risk in premenopausal women on the island of Guernsey. Br J Cancer. 1997;75(7):1075–1079. doi: 10.1038/bjc.1997.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wysowski DK, Comstock GW, Helsing KJ, Lau HL. Sex hormone levels in serum in relation to the development of breast cancer. Am J Epidemiol. 1987;125(5):791–799. doi: 10.1093/oxfordjournals.aje.a114596. [DOI] [PubMed] [Google Scholar]
- 66.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96(24):1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 67.Biglia N, Mariani L, Sgro L, Mininanni P, Moggio G, Sismondi P. Increased incidence of lobular breast cancer in women treated with hormone replacement therapy: implications for diagnosis, surgical and medical treatment. Endocr Relat Cancer. 2007;14(3):549–567. doi: 10.1677/ERC-06-0060. [DOI] [PubMed] [Google Scholar]
- 68.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 69.Eheman CR, Shaw KM, Ryerson AB, Miller JW, Ajani UA, White MC. The changing incidence of in situ and invasive ductal and lobular breast carcinomas: United States, 1999–2004. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1763–1769. doi: 10.1158/1055-9965.EPI-08-1082. [DOI] [PubMed] [Google Scholar]
- 70.Sloane JP, Amendoeira I, Apostolikas N. Pathology ECWGoBS. Consistency achieved by 23 European pathologists from 12 countries in diagnosing breast disease and reporting prognostic features of carcinomas. Virchows Archive. 1999;434:3–10. doi: 10.1007/s004280050297. [DOI] [PubMed] [Google Scholar]
- 71.Korhonen T, Huhtala H, Holli K. A comparison of the biological and clinical features of invasive lobular and ductal carcinomas of the breast. Breast Cancer Res Treat. 2004;85(1):23–29. doi: 10.1023/B:BREA.0000021038.97593.8b. [DOI] [PubMed] [Google Scholar]
- 72.Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93(9):1046–1052. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]