Abstract
Purpose
To investigate the relationship between jaw function, patient and treatment variables, and radiation dosimetry of the mandibular muscles and joints in children and young adults receiving radiation for soft tissue and bone sarcomas.
Methods and Materials
Twenty-four pediatric and young adult patients with head and neck sarcomas were treated on an IRB-approved prospective study of focal radiation therapy for local tumor control. Serial jaw depression measurements were related to radiation dosimetry delivered to the medial and lateral pterygoid muscles, masseter muscles, and temporomandibular joints to generate mathematical models of jaw function.
Results
Baseline jaw depression was only influenced by the degree of surgical resection. In the first 12 weeks from initiation of radiation, surgical procedures greater than a biopsy, administration of cyclophosphamide containing chemotherapy regimes, and large gross tumor volumes adversely affected jaw depression. Increasing dose to the pterygoid and masseter muscles above 40 Gy predicted loss of jaw function over the full course of follow-up.
Conclusions
Clinical and treatment factors are related to initial and subsequent jaw dysfunction. Understanding these complex interactions and the affect of specific radiation doses may help reduce the risk for jaw dysfunction in future children and young adults undergoing radiation therapy for the management of soft tissue and bone sarcomas.
Keywords: Jaw Depression, Radiation, Dosimetry, Pediatric Sarcomas, Pterygoid Muscle
Introduction
Children diagnosed with a soft tissue or bone sarcoma, including rhabdomyosarcoma (RMS), other soft tissue sarcomas (STS) and Ewing's sarcoma (ES), often require radiation therapy to achieve a curative outcome. The incidence of these tumors in the head and neck region varies considerably by histology from over 40% in RMS to approximately 6% in ES (1,2). Though tumors of the head and neck in children likely comprise less than 200 cases in the United States annually, approximately 65% of these patients will be long-term survivors. Acute and long-term effects of this aggressive therapy include somatic changes in the musculoskeletal system localized to the region of radiation therapy treatment. Changes are insidious in development, occurring over months to years, and subsequently result in loss of function that affects the patient's daily activities and quality of life. Patients with tumors in the head and neck region may experience symptoms such as trismus or less severe outcomes including reduction in jaw depression or the ability to open the mouth fully (3,4). Despite the readily identifiable long-term complications of radiation therapy, our ability to precisely describe the relationship between dose, volume, a specific musculoskeletal structure and the resulting measurable late treatment effect has been limited (5,6). An approach incorporating treatment parameters with clinical factors has previously resulted in models that more precisely define the causative factors of impairment and its severity (7–9). In this report, we describe a model for the acute and late effects of radiation dose on mandibular muscle (pterygoid and masseter) and temporomandibular joint function based on prospective data from a clinical trial in pediatric, adolescent and young adult patients with sarcomas.
Methods and Materials
Twenty-four pediatric and young adult patients with a diagnosis of primary head and neck bone or soft tissue sarcoma were treated as part of an IRB approved prospective institutional study of focal radiation therapy at St. Jude Children's Research Hospital (SJCRH). Eligible patients included those <25 years of age that required radiation therapy for management of their primary site of disease; patients with metastatic disease were included. The primary objective of this clinical trial was to define the local tumor control rate with a limited margin treatment approach (10,11), and secondary objectives sought to define and relate the early and late toxicity profile of this treatment approach to radiation dosimetry and clinical factors. We present the acute and long-term outcomes relating to jaw function (the ability to open the mouth) for these patients.
Treatment guidelines
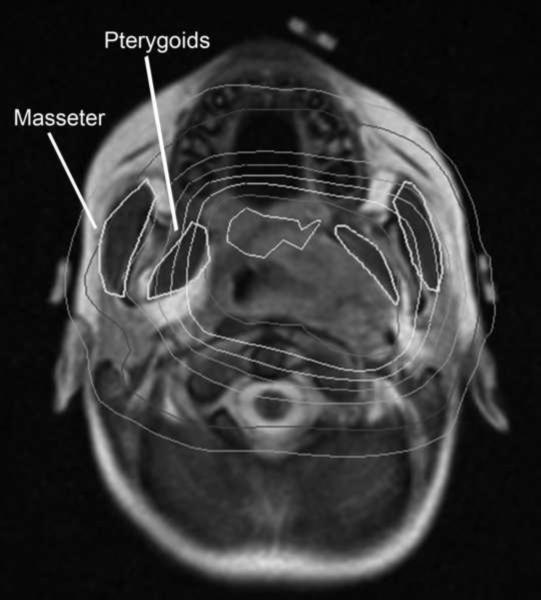
Radiotherapy treatment consisted of ICRU 50 target volumes generated based on CT and MR treatment planning datasets with MR data sets (typically obtained in the treatment position) co-registered to the CT to fully define target volumes (12). The gross tumor volume (GTV) defined by the treating physician was the post-operative tumor bed or gross tumor constrained by barriers to tumor spread (bone, fascial planes). A 1.0, 1.5 or 2.0 cm clinical target volume (CTV) margin in both involved bone and soft tissue was added to the GTV, also anatomically constrained, depending on the patient's tumor histology (ES, RMS or STS, respectively). A patient specific planning target volume (PTV) margin was added that ranged from 0.4 cm to 0.5 cm. Selection of the margin was based on the use of immobilization with a radiocamera localized bite block (Varian, Palo Alto, CA) in conjunction with an aquaplast (WFR/Aquaplast Corp., Avondale, PA) face mask or a face mask alone. Normal tissues delineated for avoidance routinely included the mandible, temporomandibular joints, the masseter and pterygoid muscles, the parotids, as well as the eyes and thyroid. If the pterygoid, masseter and temporomandibular joints were not delineated for routine treatment planning, they were contoured prior to this analysis using a combination of treatment planning MR and CT to generate consistent organ volumes (example shown in figure 1). All contours were reviewed by one physician (M.K.) prior to analysis of the jaw depression data; dosimetry for the muscles and joints was then output as DVH data for analysis. External beam radiation was delivered with 6 or 15 MV photons or megavoltage electrons, the specifics of which have previously been reported (10). Protocol specified total doses of radiation therapy ranged from 41.4–70.2 Gy delivered in fraction sizes of 1.8–2.1 Gy depending on whether a patient received a sequential or integrated boost as part of an intensity modulated treatment plan; this selection was based on improvements in normal tissue dosimetry. Systemic therapy was at the discretion of the treating pediatric oncologist delivered either as a part of a national clinical trial or according to standard therapy guidelines.
Figure 1.
Example of masseter and pterygoid contours overlaid with radiation dosimetry on a coregistered MR.
Muscle and joint dosimetric analysis
One physician (M.K.) reviewed the contours of the right and left pterygoid muscles (medial and lateral combined) and masseter muscles on CT and co-registered MR (when available) and edited for consistency. These muscle groups were merged into the right and left mandibular muscle groups for analysis based on the combined action of these muscles on the closure of the mandible. The temporomandibular joint (TMJ) contours were also verified prior to generation of composite dose distributions from our radiotherapy treatment planning system (PLanUNC, Chapel Hill, NC). Differential dose volume histogram data were exported for the right and left mandibular muscles as well as the right and left TMJ. The volume of GTV was also calculated from the treatment plan for analysis.
Follow-up and rehabilitation evaluations
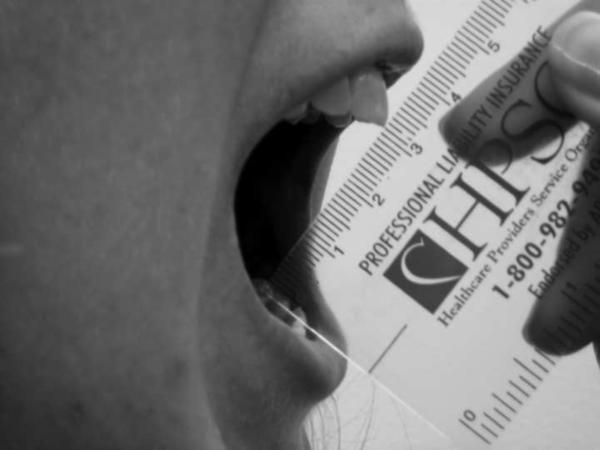
Protocol directed follow-up for patients included a history, physical examination of the primary site of disease, and MR imaging of the primary site every 3 months for 1 year and then every 6 months until 5 years from protocol enrollment. The physical therapy department at SJCRH obtained serial jaw depression measurements in centimeters for all patients using a goniometer (13). These evaluations were obtained by asking the patient to open their mouth to the fullest without assistance by the therapist; measurements were obtained vertically between the incisor teeth (figure 2). Measurements were obtained pretreatment (after any surgical procedure), at 1, 2, 4, and 6 weeks during RT, and at 3, 6, 9, 12, 18, 24, 30, 36, 48, and 60 months post RT. Each measurement, performed once at each evaluation, was a maximal absolute dimensional assessment of the opening of the mouth. If reduction in jaw depression was identified, stretching exercises were prescribed with tongue blades or the TheraBite device (Atos Medical, Hörby, Sweden). Assessments were discontinued at the time of tumor progression or if the patient was removed from study. Missing data occurred due to patient non-compliance and conflicts between assessment appointments and the delivery of chemotherapy.
Figure 2.
Example of a jaw depression measurement being obtained (measured between the incisors).
Statistical analysis
Trends for jaw depression, treatment variables (Table 1) and the dosimetric parameters were included in the statistical analysis. Jaw depression was analyzed at baseline (pre-RT), during the first 12 weeks from the start of radiation (for acute effects), and finally over the years following RT (for late effects) in order to assess the effects of multiple covariates as this treatment effect evolved over time. A mixed effects model with random coefficients for intercept (the baseline) and slope (the rate of change in time) were used to estimate the longitudinal trends of jaw depression. A step-wise model selection was used to select the best fit. The influence of potential covariates was investigated for each model including degree of surgical resection (marginal / intralesional resection vs. biopsy), specific chemotherapeutic agents administered (cyclophosphamide, doxorubicin, actinomycin, based on interactions with radiation or the direct effect of mucositis), tumor size at the time of RT (the GTV), age (in years), mean RT dose to the mandibular muscles and TMJs, and the integral of the percent volume of the mandibular muscles above 40 Gy (5 Gy bin size). Selection of 40 Gy as the point above which to integrate dose to the mandibular muscles was based on several factors, including the need for a dose level that is frequently encountered in this population, our prior analysis of MR T2 signal number change noting 40 Gy to be the point where significant increases in T2 are seen (14), as well as adult literature suggesting that doses near 40 Gy affect pterygoid muscle function (15,16).
Table 1.
Treatment variables for all patients (n=24)
| Characteristics | No. | % |
|---|---|---|
| Surgery | ||
| Core needle biopsy | 16 | 66.7% |
| Intralesional excision | 3 | 12.5% |
| Marginal excision | 5 | 20.8% |
| Chemotherapy | ||
| No | 3 | 12.5% |
| Yes | 21 | 87.5% |
| Chemotherapy agents | ||
| Cyclophosphamide | 16 | 66.7% |
| Doxorubicin | 7 | 29.2% |
| Actinomycin | 15 | 62.5% |
Results
Patient characteristics
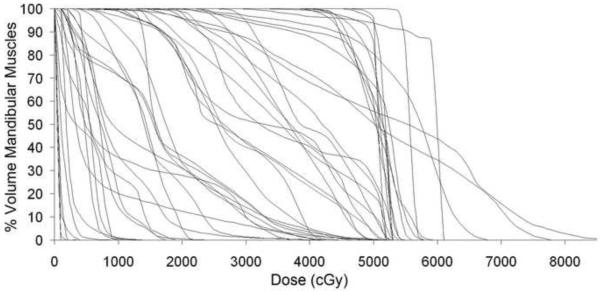
24 patients with musculoskeletal tumors of the head and neck were treated on protocol between February 2003 and May 2007. Tumor types included RMS (16 pts), STS (3 pts), ES (3 pts), and one each of osteosarcoma and chordoma. The median age at the time of enrollment and delivery of RT was 10.2 years (range, 1.4–23.8). All patients included in this study received external-beam radiotherapy with a median cumulative delivered dose of 50.4 Gy (range, 41.4–70.2 Gy). The median and mean effective uniform dose (average dose) for all of the mandibular muscle groups was 27.2 Gy and 27.0 Gy (range 0.5–57.9 Gy). The median and mean volume treated above 40 Gy (V40Gy) was 4.5% and 35.2% (range 0–100%) and the cumulative DVH data for all patients' mandibular muscles is shown in figure 3. The temporomandibular joint received a median and mean dose of 22.9 and 23.7 Gy (range 0–59.8 Gy). Other treatments included chemotherapy (21 pts) and surgery at diagnosis (8 pts) and are detailed in table 1. A total of 209 jaw depression measurements were made (mean 8.7/pt) over the period of time analyzed. Jaw depressions of 2 cm or less and 3 cm or less were seen in 19% and 24% of patients at initial evaluation, respectively, and no patient started protocol therapy with a measurement over 5 cm. The median follow-up for patients remaining on study at the time of analysis was 33 months (range, 3.1–61.5 months).
Figure 3.
Cumulative dose-volume histograms for all patients' mandibular muscles.
Baseline jaw depression
In the 17 patients receiving chemotherapy with available baseline values, jaw depression was only affected by the degree of surgical resection (marginal / intralesional resection – median 2.3 cm vs. biopsy – median 3.6 cm, p=0.05, std dev 1.47 and 0.96, respectively).
Trends in the first 12 weeks
Using a mixed effects model, we analyzed the effects of the degree of surgical resection, chemotherapeutic agents administered, tumor size (GTV), and patient age on trends for jaw depression over the first 12 weeks from initiation of RT on all 24 patients. Age (< or ≥ 11 years) and tumor size (GTV < or ≥ 37.8cc) were re-categorized based on their median values into low and high categorical variables. Jaw depression was adversely affected by having a surgical procedure that was more than a biopsy, receiving cyclophosphamide and by the effect of large gross tumor volume. Age was not found to be a significant factor for this analysis. The effect on jaw depression can be expressed by the following function:
The coefficients and p-values for this model are shown in table 2. Analysis of only the first 6 weeks of jaw depression data, corresponding to the period of time during RT delivery yielded the same results and significance.
Table 2.
Coefficients and p-values for mixed model for short-term jaw depression
| Variable | Estimate | Std Error | p-value |
|---|---|---|---|
| Intercept | 4.26 | 0.41 | < 0.0001 |
| Degree of surgery | −0.66 | 0.22 | 0.0051 |
| Cyclophosphamide | −1.01 | 0.23 | < 0.0001 |
| Time | −0.01 | 0.03 | 0.77 |
| Time × GTVvolume group | −0.08 | 0.04 | 0.05 |
Long-term trends for jaw depression
Analysis of mean dose received by the MMGs and TMJs was initially analyzed for its effect on jaw depression over the entire period of follow-up. Only mean dose to the MMGs was found to be a significant dosimetric predictor in this exploratory analysis of normal tissue doses so further analyses focused only on these structures. The integral of the percent volume of the left and right MMG receiving a dose above 40 Gy was a significant predictor of reduced long term jaw depression and is shown below as a function and with p-values in table 3.
Table 3.
Coefficients and p-values for mixed model for long-term jaw depression
| Variable | Estimate | Std Error | p-value |
|---|---|---|---|
| Intercept | 3.87 | 0.11 | < 0.0001 |
| Integral_lt_MMG>40Gy | −0.037 | 0.0039 | < 0.0001 |
| Integral_rt_MMG>40Gy | −0.02 | 0.0034 | < 0.0001 |
| Int_lt_MMG>40Gy× Int_rt_MMG>40Gy | 0.0008 | 0.0002 | < 0.0001 |
| Time | 0.005 | 0.002 | 0.0156 |
Based on this model, for each 10% volumetric increment of the mandibular muscles treated above 40 Gy, a patient's jaw depression is reduced by approximately 2mm. We have calculated the predicted loss in jaw depression in table 4, demonstrating the potential incremental effect of a uniform threshold dose delivered to the masseter and pterygoid muscles (mandibular muscles). This table may be used as a reference to determine the significance of a patient's mandibular muscle dosimetry relative to other risks and benefits of a specific treatment plan.
Table 4.
Predicted loss of jaw depression at 3 years based on dose received by the mandibular muscles groups (MMGs) and the volume of the MMGs irradiated
| % volume both MMGs irradiated | Volume of the mandibular muscles above: |
||
|---|---|---|---|
| 40 Gy | 50 Gy | 60 Gy | |
| 10% | 2 mm | 3 mm | 3 mm |
| 20% | 4 mm | 5 mm | 6 mm |
| 30% | 6 mm | 7 mm | 8 mm |
| 40% | 7 mm | 8 mm | 9 mm |
| 50% | 8 mm | 9 mm | 9 mm |
When the clinical and treatment parameters of diagnosis, age (in years) as a continuous variable, chemotherapeutic agents and degree of surgery were modeled individually with the dosimetric parameters described above, only the histologic diagnosis of rhabdomyosarcoma and younger age remained significant. These two factors were not found to be statistically correlated. In all of these analyses, dose to the mandibular muscle groups above 40 Gy remained highly significant.
The function, incorporating age (a), is as follows:
Discussion
Late effects of radiation therapy in the musculoskeletal system, resulting in muscle fibrosis, dysfunction and loss of bone growth, are frequently cited to argue for radiation avoidance. The general incidence of these complications has been described in the pediatric literature, but the severity, and more importantly, the relation between the effects and dose, volume and other clinical factors are poorly quantified (3,15). Adult studies have previously reported that radiation exposure to the pterygoid muscles results in jaw dysfunction (15–17). They have also noted, as we have in this report, the lack of a relationship between radiation to the temporal-mandibular joint and reduced jaw depression. The lack of dose-volume analysis accounting for patient and treatment factors leaves Radiation Oncologists treating children with no guidance as to what tissues to avoid or what doses are safe or appropriate. In this analysis, we have related jaw depression to dosimetric and clinical factors in the population of head and neck sarcoma patients treated on a prospective institutional clinical trial of conformal or intensity modulated radiation therapy. These models predict incremental loss of function with pre-radiation treatment factors and increasing dose and volume to the pterygoid and masseter muscles above 40 Gy. Based on normative data for children 10–17 years of age, the average jaw depression is 5.0 cm (18). Patients in our study often had reductions in jaw depression to levels significantly less than these normative values. Though our models are not predictive for an individual patient, understanding their components can guide Radiation Oncologists and Medical Dosimetrists during the treatment planning process. The resultant effect of treatment to the pterygoid and masseter muscles is incremental loss of function. Also of importance is the lack of a dose-volume relationship with the temporal-mandibular joint, suggesting muscle injury and fibrosis may be the etiology of radiation induced jaw dysfunction. This report now provides the first quantitative data in children and young adults predicting the degree of jaw dysfunction based on dosimetry and clinical factors.
Limitations of this study relate to the population of patients and inherent boundaries of dosimetric models. The study population, comprised of children and young adults with rhabdomyosarcoma, Ewing's sarcoma, soft tissue sarcoma and other bone sarcomas in the head and neck region, may reduce the widespread applicability of this data to the more typical adult population with head and neck cancer. In addition, the physical and occupational therapists and speech language pathologists involved in this trial routinely followed and managed patients to improve reduced jaw depression; this may have resulted in less severe dysfunction than if patients were left unmanaged. Our models, based on patient data from this trial, incorporate radiation dose to left and right muscle groups individually. The coefficients for the left and right sides are thus specific to this population. Though this may be seen as a limitation, it provides insight that radiation to either side, whether alone or in combination with the contralateral muscle group, may yield an adverse effect on jaw depression.
Despite inherent difficulties when modeling the effects of radiation on biologic systems, RT has a measurable, incremental effect on jaw depression. Treating physicians should anticipate early reductions in jaw depression both before the start of RT and shortly after, due to prior surgery, large tumor volumes, and the administration of cyclophosphamide containing chemotherapy regimes. These early reductions are not necessarily permanent. Care must be exercised in RT planning to minimize the volume of the left and right pterygoid and masseter muscles receiving doses above 40 Gy. This provides the greatest opportunity for maintenance or recovery of jaw function over the long term in a population of children and young adults that are often long-term cancer survivors.
Radiation therapy will continue to be an important local modality in the management of pediatric patients with head and neck sarcomas. With the availability of increasingly conformal delivery methods, such as proton beam therapy, the ability to deliver focal conformal radiation has become easier and the availability of guidelines based on patient data to direct acceptable doses to normal tissue have become increasingly important. The approach presented here for studying this specific normal tissue effect can be replicated throughout the musculoskeletal system making the delivery of radiation therapy safer and the risk and benefit ratio more clear.
Acknowledgments
Support: American Lebanese Syrian Associated Charities (ALSAC), Lance Armstrong Foundation and Cancer Center Support Grant #P30 CA021765-31
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification No conflicts of interest exist.
References
- 1.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19(12):3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 2.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. New Eng J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 3.Bensadoun RJ, Riesenbeck D, Lockhart PB, et al. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer. 2010 doi: 10.1007/s00520-010-0847-4. e-pub. [DOI] [PubMed] [Google Scholar]
- 4.Specht L. Oral complications in the head and neck radiation patient: Introduction and scope of the problem. Support Care Cancer. 2002;10:36–39. doi: 10.1007/s005200100283. [DOI] [PubMed] [Google Scholar]
- 5.Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2002;95:2431–2441. doi: 10.1002/cncr.10978. [DOI] [PubMed] [Google Scholar]
- 6.Paulino AC. Late effects of radiotherapy for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys. 2004;60:265–274. doi: 10.1016/j.ijrobp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Krasin MJ, Hoth KA, Hua C, et al. Incidence and correlates of radiation dermatitis in children and adolescents receiving radiation therapy for the treatment of paediatric sarcomas. Clin Oncol-UK. 2009;21:781–785. doi: 10.1016/j.clon.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Merchant TE, Goloubeva O, Pritchard DL, et al. Radiation dose-volume effects on growth hormone secretion. Int J Radiat Oncol Biol Phys. 2002;52:1264–1270. doi: 10.1016/s0360-3016(01)02788-2. [DOI] [PubMed] [Google Scholar]
- 9.Krasin MJ, Xiong X, Wu S, et al. The effects of external beam irradiation on the growth of flat bones in children: Modeling a dose-volume effect. Int J Radiat Oncol Biol Phys. 2005;62:1458–1462. doi: 10.1016/j.ijrobp.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Hua C, Gray JM, Merchant TE, et al. Treatment planning and delivery of external beam radiotherapy for pediatric sarcoma: The St. Jude Children's Research Hospital experience. Int J Radiat Oncol Biol Phys. 2008;70:1598–1606. doi: 10.1016/j.ijrobp.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Krasin MJ, Davidoff AM, Xiong X, et al. Preliminary results from a prospective study using limited margin radiotherapy in pediatric and young adult patients with high-grade nonrhabdomyosarcoma soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2010;76(3):874–878. doi: 10.1016/j.ijrobp.2009.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Commission of Radiation Units and Measurements . Report No. 50. ICRU; Bethesda, MD: 1993. Prescribing, recording, and reporting photon beam therapy from the International Commission of Radiation Units and Measurements (ICRU) [Google Scholar]
- 13.Gajdosik RL, Bohannon RW. Clinical measurement of range of motion: Review of goniometry emphasizing reliability and validity. Phys Ther. 1987;67(12):1867–1872. doi: 10.1093/ptj/67.12.1867. [DOI] [PubMed] [Google Scholar]
- 14.Krasin MJ, Xiong X, Reddick WE, et al. A model for quantitative changes in the magnetic resonance parameters of muscle in children after therapeutic irradiation. Magn Reson Imaging. 2006;24:1319–1324. doi: 10.1016/j.mri.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein M, Maxymiw WG, Cummings BJ, et al. The effects of antitumor irradiation on mandibular opening and mobility. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:365–373. doi: 10.1016/s1079-2104(99)70044-2. [DOI] [PubMed] [Google Scholar]
- 16.Teguh DN, Levendag PC, Voet P, et al. Trismus in patients with oropharyngeal cancer: Relationship with dose in structures of mastication apparatus. Head Neck. 2008;30:622–630. doi: 10.1002/hed.20760. [DOI] [PubMed] [Google Scholar]
- 17.Hsiung CY, Huang EY, Ting HM, et al. Intensity-modulated radiotherapy for nasopharyngeal carcinoma: The reduction of radiation-induced trismus. Brit J Radiol. 2008;81:809–814. doi: 10.1259/bjr/17942449. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch C, John MT, Lautenschläger C, et al. Mandibular jaw movement capacity in 10–17-yr-old children and adolescents: Normative values and the influence of gender, age, and temporomandibular disorders. Eur J Oral Sci. 2006;114:465–470. doi: 10.1111/j.1600-0722.2006.00402.x. [DOI] [PubMed] [Google Scholar]