Abstract
A community needs assessment focused on colorectal cancer (CRC) screening knowledge, behaviors, and barriers was completed in one Ohio Appalachia county. A CRC screening media campaign was developed based on the findings from the needs assessment and feedback was obtained about the media campaign. The survey was completed by 170 self-reported average-risk adults. In a multivariate model, the CRC screening rate was higher for participants who had received a doctor’s recommendation (OR=6.09), had adequate CRC knowledge (OR=2.88), and was lower among participants employed full-time (OR=0.23). Having health insurance (OR=4.20) and being married (OR=2.58) was associated with having received a doctor’s recommendation for screening. Campaign feedback using a second survey completed by self-reported average-risk adults (n=61) revealed that 69% recognized the campaign image and message, with a billboard being the most cited source. This study highlights the importance of involving community members in the development of CRC screening programs to reduce cancer disparities in Appalachia.
Keywords: Colon cancer screening, Appalachia, health disparities
INTRODUCTION
Colorectal cancer (CRC) is the 3rd leading type of diagnosed cancer and the 3rd leading cause of cancer death among men and women living in the United States (U.S.) (American Cancer Society, 2008). National policy-making expert organizations recommend CRC screening among average-risk adults age 50+ years based on evidence that screening reduces CRC incidence and mortality (Pignone, Rich, Teutsch, Berg, & Lohr, 2002; Winawer et al., 2003; Smith, Cokkinides, & Eyre, 2007). Significant disparities in CRC incidence, mortality, and survival rates exist among underserved populations and CRC screening tests are less likely to be used regularly among underserved populations (American Cancer Society, 2008; Smith, Cokkinides, & Eyre, 2007).
In Ohio, CRC age-adjusted incidence rates were 5% higher and the age-adjusted CRC mortality rates were 9% higher than the U.S. for 1975–2002 (American Cancer Society, 2005). One underserved population that specifically has an excess CRC burden is residents of Appalachia (Appalachia Community Cancer Network, 2009). In Ohio Appalachia, the age-adjusted CRC incidence rate in 2005 was 63.6 per 100,000 compared to 55.7 per 100,000 in non-Appalachia Ohio, a difference of 14.2% (Fisher et al., 2008). The age-adjusted CRC mortality rate in Ohio Appalachia was 25.7 per 100,000 compared to 22.3 per 100,000 in non-Appalachia Ohio, a difference of 15.2% (Fisher et al., 2008). Specifically in Meigs County, Ohio, the residents have a 6.5% higher CRC incidence rate and 7.0% higher CRC mortality rate compared to residents in the state of Ohio, and more than half of the CRC cases were diagnosed at late stage (1998–2002) (American Cancer Society, 2005).
Several cultural factors may be responsible for the CRC disparities in Ohio Appalachia. Residents may have limited access to healthcare including cancer screening, and may have behavioral lifestyle factors (poor nutrition, decreased physical activity levels, and increased tobacco use) that increase their risk of developing CRC (Abramson & Haskell, 2006).
The purpose of this project was to focus on the increased CRC rates in one Ohio Appalachia county. A community needs assessment informed the developed intervention that was evaluated in this pilot study. Since there are increased CRC incidence and mortality rates and decreased CRC screening rates throughout the Appalachian region of the U.S., this pilot study was conducted in expectation that a culturally sensitive CRC screening media campaign could be disseminated throughout Appalachia to address the burden of this disease.
METHODS
A community-based participatory research (CBPR) approach was used in this study by first developing a partnership between an Ohio Appalachia community cancer coalition and academic researchers. The Meigs County Cancer Initiative, Inc. (MCCI) was formed in 2000 and includes community members, cancer survivors, and healthcare professionals. MCCI members identified the increased CRC burden in their county compared to the non-Appalachia Ohio CRC incidence and mortality rates as an issue to address with a collaborative effort. In partnership, a community needs assessment was conducted to gain insight into current CRC knowledge and screening behaviors of the county residents and the findings from the needs assessment were used to develop a culturally sensitive CRC screening media campaign. Campaign feedback was obtained by using a second self-administered survey of county residents. MCCI members were involved in the decision-making, planning, development, and feedback of the CRC screening campaign. The study was approved by the Institutional Review Board of The Ohio State University.
Setting
Meigs County is located in southeast Ohio, is one of six Ohio counties classified as “distressed” by the Appalachian Regional Commission (Appalachia Regional Commission, 2009), and the median household income for Meigs County ranks 87th out of the 88 Ohio counties. Counties are determined to be distressed because they are the most economically depressed and rank in the worst 10% of the nation’s counties (Appalachia Regional Commission, 2009). The residents of Meigs County have a higher percentage of adults without a high school diploma, more residents living below the federal poverty level, higher unemployment rates, and more individuals without health insurance compared to statewide Ohio rates (Ohio Department of Health, 2000; United States Census Bureau, 2000; United States Department of Agriculture, 2006). In addition, there are only six primary care physicians located in Meigs County, for a ratio of population to primary care physician of 3,852:1 compared to a 852:1 ratio statewide for Ohio (Ohio State Medical Board, 2002).
Participants
The community needs assessment and campaign feedback were completed by different convenience samples of self-reported average-risk (no personal history of CRC or colorectal polyps, no family CRC history, no history of inflammatory bowel disease) adult (≥50 years) residents of Meigs County. An individual may have participated in both surveys. MCCI members working on the project identified popular locations (grocery store, county festival, restaurant, and community center) to collect surveys. The coalition members’ presence created an element of trust and made it easier to recruit community members from different locations throughout the county to complete both surveys. Participants completed the survey after signing a consent form about the study and informed them that they would be given an American Cancer Society (ACS) CRC brochure entitled “Colorectal Cancer: Reduce Your Risk” and a $10 Meigs County Chamber of Commerce gift card for appreciation of their time.
Pre-CRC Screening Campaign Survey (Community Needs Assessment)
Members of MCCI and the academic researchers developed and administered a survey that included demographics, self health rating (poor to excellent), date of last medical visit (within 1 year, 1–2 years, >2 years ago), CRC personal and family history, CRC knowledge, CRC screening behaviors and barriers to CRC screening. Each CRC screening test was described to reduce measurement error (Vernon, et al., 2004). An example of a screening test description used on the survey is: “A fecal occult blood test (FOBT) is a colon cancer screening test done at home. You place your stool from three different bowel movements on three cards and return the cards to the doctor to be tested for blood.” Participants who had not completed CRC screening were able to choose from a list of barriers (lack of recommendation, no symptoms, embarrassment, fear of cancer, cost, lack of time or transportation, and fear of procedure) for each screening test or write in the reason for not completing each test.
CRC Media Campaign
MCCI and the academic researchers shared the results of the community needs assessment with the general public at an open forum held in Meigs County, Ohio. Two findings from the community needs assessment influenced the MCCI members’ decision to conduct a media campaign and the campaign’s message. First, since many average-risk adults who completed the needs assessment were not within recommended CRC screening guidelines, MCCI members thought that a media campaign may be important to increase CRC screening awareness to all county residents. Second, since many adults reported that they would complete a CRC screening test if it was recommended by their doctor, the MCCI members thought that a cue to talk to their doctor about CRC screening should be the campaign message.
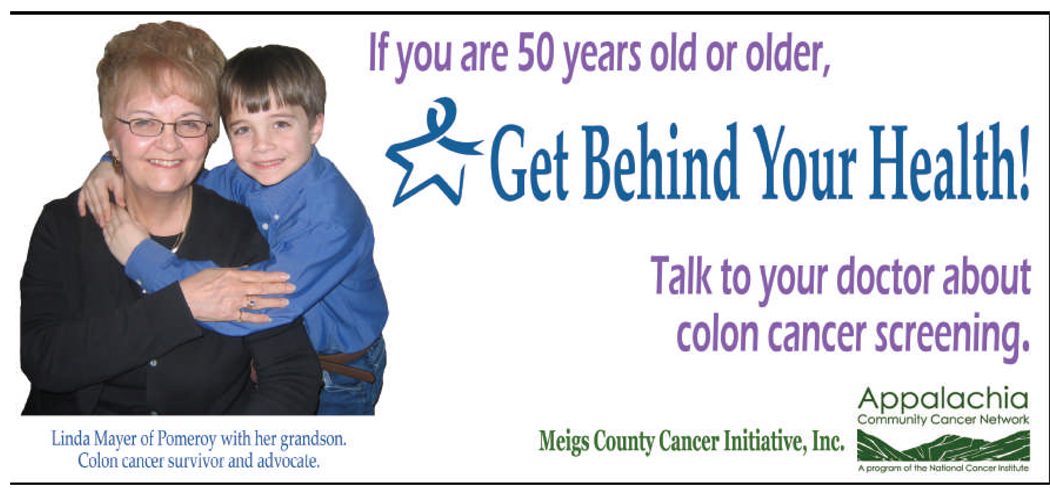
MCCI members in partnership with academic researchers developed a media campaign entitled, “Get Behind Your Health! Talk to your doctor about colon cancer screening.” The campaign image and message was used in all campaign materials including the billboard, posters, brochures, and newspaper ads. Although the information on the billboard was limited, the posters, brochures, radio, TV, and newspaper articles included information about CRC, CRC risk factors and symptoms, CRC screening, and that CRC screening saves lives.
The Social Cognitive Theory (SCT) (Bandura, 1986) provided a structure for creating the CRC screening media campaign. SCT constructs addressed by the campaign were: outcome expectations (outcomes that result from the behavior; e.g. “Get Behind Your Health!,” information in newspaper articles, and using a local community member in the campaign), observational learning (learning a behavior by exposure to media display; e.g. “Talk to your doctor about colon cancer screening,” and radio and television spots), and the promotion of CRC awareness and social norms focused on the importance of CRC screening (e.g. community members talk about media campaign).
To make the campaign culturally relevant, MCCI members invited a local CRC survivor who was featured in the campaign (Figure 1) and MCCI members invited a local physician to participate in the public service announcements (PSAs). The county-wide campaign was implemented from April to June 2007. The campaign included local radio PSAs (n=100; 60 second spots) and television PSAs (n=5 times per day), local newspaper stories (n=2) and newspaper ads (n=2), a billboard, posters (n=20) and brochures (n=1000) displayed in stores, offices, and agencies throughout the county. Locations for campaign materials and local radio and newspapers were identified by MCCI members.
Figure 1.
Image and message used in the “Get Behind Your Health! Talk to your doctor about colon cancer screening” media campaign
Post-CRC Screening Campaign Survey (Media Campaign Feedback)
A self-administered survey was developed focusing on the CRC screening campaign, “Get Behind Your Health! Talk to your doctor about colon cancer screening.” The survey contained questions about participants’ media habits during the past 3 months, CRC screening message recall, and following the picture of the “Get Behind Your Health! Talk to your doctor about colon cancer screening” campaign image and message there were questions about if the message was seen or heard, where the message was seen or heard (television, radio, newspaper, billboard, brochures, posters), frequency of seeing it (1, 2–5, 6–10, >10), importance of the message (not important to very important), and if they discussed or planned to discuss the message with anyone (doctor, nurse, family members, friends, co-workers, others). Additionally, a picture of a CRC screening campaign from another state (sham campaign) and similar questions addressing the sham campaign were included in the survey to serve as a control (Broadwater, Heins, Hoelscher, Mangone, & Rozanas, 2004). Participants also provided demographic information, CRC personal and family history, and CRC screening behaviors.
Data Analysis
The community needs assessment survey data were used to analyze the differences between adults who were within or not within CRC screening guidelines and who received or did not receive a doctor’s recommendation for CRC screening. A doctor’s recommendation for CRC screening was determined by self-report and screening within guidelines was determined by the self-report of a CRC screening test completed within ACS recommended guidelines. The ACS CRC screening guidelines recommend a Fecal Occult Blood Test (FOBT) annually, a flexible sigmoidoscopy (FS) every 5 years, or a colonoscopy every 10 years (American Cancer Society, 2008). Participants within and not within CRC screening guidelines were compared on demographic factors, CRC and CRC screening knowledge, and CRC screening barriers.
Demographic information included: gender (female/male), age (50–59/60–69/70+ years), marital status (separated, divorced, widowed, never married/married, living together), education (<high school graduate/high School graduate+), household income (<county median/≥county median), work status (unemployed, retired, disabled, part-time/full-time), health insurance (no/yes), doctor recommendation for FOBT, FS, or colonoscopy (no/yes), and CRC knowledge (inadequate/ adequate). Individuals were determined to be at average-risk or high-risk (any of the following: personal history of polyps or CRC, or family history of CRC) for CRC.
Knowledge of CRC was assessed using ten true-false items based on the Centers for Disease Control Screen For Life Campaign and the strategic plan of the National Colorectal Cancer Roundtable (Centers for Disease Control, 2008; Levin et al., 2002). A response was considered incorrect if answered incorrectly, left blank, or marked “Do not know.” If the participants answered at least 7 of the 10 items correctly, they were categorized as having adequate CRC knowledge. Participants had inadequate CRC knowledge if they answered less than seven items correctly.
Logistic regression modeling using forward selection was then used to determine the set of demographic and other factors most predictive of: 1) completing CRC screening within guidelines for average-risk adults, and 2) receiving a doctor recommendation for screening for average-risk adults. At each stage in the model building process, all potential predictors were added to the model one at a time and the variable with the smallest p-value was selected into the model, provided the p-value was less than p=0.05. This process was repeated in a forward stepwise fashion until no variables were statistically significant at the p=0.05 level. The presence of any significant two-way interactions was then assessed. Odds ratios, 95% confidence intervals, and p-values were calculated to describe the odds of being within CRC screening guidelines versus not being within screening guidelines and for receiving a doctor recommendation for CRC screening versus not receiving a doctor recommendation for CRC screening. The overall fit of the models were evaluated using the Hosmer-Lemeshow Goodness of Fit.
Frequencies for the media campaign feedback were calculated for average-risk (for CRC) participants. Questionnaires are available by request. All analyses were performed using SAS Version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Pre-CRC Screening Campaign Survey (Community Needs Assessment)
A convenience sample (n=300) of adult (>50 years) residents of Meigs County completed the self-administered questionnaire at 6 different community locations (a wings & ribs festival, an indoor flea market, local grocery stores, a senior center, and a fast food restaurant) on 7 different days in June 2006. It was determined that one participant was less than 50 years old, so a total of 299 participants were included in the study. Only a few individuals approached to participate in the study declined and all who declined stated it was because of the lack of time. The number of surveys completed by residents of each township within Meigs County reflected the population density of the county.
A total of 170 (57%) of the 299 completed questionnaires were completed by self-reported average-risk (for CRC) adults and 129 (43%) surveys by high-risk adults. The increased number of high-risk adults in this convenience sample may reflect their interest in helping with a project focused on CRC or may be due to the increased CRC rates in the county. Only data from the average-risk adults are presented because surveillance of high-risk adults varies with physician recommendation (Table 1).
Table 1.
Characteristics of the Meigs County, Ohio average-risk participants within and not within colorectal cancer (CRC) screening guidelines (n=170)
| CRC Screening Within Guidelines |
|||||
|---|---|---|---|---|---|
| No n (%) |
Yes n (%) |
Total¶ n (%) |
Odds Ratio** (95% CI ) |
||
| Gender | Female | 78 (74) | 28 (26) | 106 (62) | 1.00 |
| Male | 42 (66) | 22 (34) | 64 (38) | 1.46 (0.75,2.86) | |
| Age (years) | 50–59 | 39 (81) | 9 (19) | 48 (28) | 1.00 |
| 60–69 | 30 (60) | 20 (40) | 50 (29) | 2.89 (1.15,7.25) | |
| 70+ | 51 (71) | 21 (29) | 72 (42) | 1.78 (0.74, 4.32) | |
| Marital status | Divorced/Widowed/ Separated/Single |
47 (81) | 11 (19) | 58 (35) | 1.00 |
| Married/Living with Partner |
71 (65) | 39 (36) | 110 (66) | 2.35 (1.09,5.04)* | |
| Education | Less than High School | 20 (80) | 5 (20) | 25 (15) | 1.00 |
| High School + | 98 (69) | 45 (32) | 143 (85) | 1.84 (0.65,5.21) | |
| Household Income | <County median | 54 (75) | 18 (25) | 72 (56) | 1.00 |
| ≥County median | 37 (65) | 20 (35) | 57 (44) | 1.62 (0.76,3.47) | |
| Employment Status | Employed part-time/ Unemployed / Disabled / Retired |
95 (67) | 46 (33) | 141 (86) | 1.00 |
| Employed Full-time | 20 (87) | 3 (13) | 23 (14) | 0.31 (0.09,1.10) | |
| Health Insurance | No | 19 (95) | 1 (5) | 20 (12) | 1.00 |
| Yes | 100 (67) | 49 (33) | 149 (88) | 9.31 (1.21,71.53)* | |
| Doctor recommendation |
No | 86 (86) | 14 (14) | 100 (59) | 1.00 |
| Yes | 34 (49) | 35 (51) | 69 (41) | 6.32 (3.03,13.20)* | |
| CRC knowledge | Inadequate | 56 (82) | 12 (18) | 68 (40) | 1.00 |
| Adequate | 64 (63) | 38 (37) | 102 (60) | 2.77 (1.32,5.82)* | |
p<0.05
crude odds ratio
missing data, totals do not equal 170
CI = Confidence Interval
Approximately two-thirds (62%) of the average-risk participants (n=170) were females, and 57% were 50–69 years of age. Race and ethnicity of the participants were characteristic of Ohio Appalachia with 98% reporting being White and 94% reporting being Non-Hispanic. Sixty-six percent of the participants were currently married or living with a partner and 15% had not graduated from high school. Over half (56%) of the participants reported a total household income that was below the county median (<$30,000), only 14% worked full-time, and 12% reported having no form of health insurance.
Almost one-fourth (23%) of the participants self-reported their health as poor or fair. Most participants (91%) had a regular healthcare provider and 82% reported having a regular check up in the past year. Many (84%) reported knowing that there was a test to check for colon cancer. Among the participants who knew there was a CRC screening test (n=143), the test identified most often from a list was colonoscopy (92%). Participants could choose multiple answers, and the other tests chosen included: rectal exam (52%), FOBT (47%), flexible sigmoidoscopy (37%), X-ray (11%), Pap smear (4%), and mammography (1%).
The rate of CRC screening within recommended ACS guidelines for average-risk adults was 29% (50/170). Having adequate CRC knowledge was documented in 60% (102/170) of the participants and 41% (69/169) reported a previous doctor recommendation for a CRC screening test. Among individuals not within recommended CRC screening guidelines, 78% (94/120) reported that they would complete a CRC screening test if their doctor recommended a test. The two most frequent CRC screening barriers reported by participants not within screening guidelines were lack of physician recommendation and having no symptoms. Screening barriers were similar for fecal occult blood test, flexible sigmoidoscopy, and colonoscopy. All other screening barriers (embarrassment, fear of cancer, cost, lack of time or transportation, fear of procedure) were each reported less than 5% for each of the screening tests.
The final model examining predictors of being within CRC screening guidelines (Table 2A) for an average-risk adult revealed that the adjusted odds ratio of being within CRC screening guidelines was significantly higher for participants with a doctor’s recommendation (Odds Ratio (OR)=6.09; 95% CI=2.80,13.21; p<0.0001), with adequate CRC knowledge (OR)=2.88; 95% CI=1.25,6.65;p=0.013), and significantly lower if participants were employed full-time (OR=0.23; 95%CI=0.06,0.89; p=0.034). The final model examining predicators of receiving a doctor’s recommendation for CRC screening (Table 2B) for an average-risk adult revealed that the odds of receiving a doctor’s recommendation was significantly higher for participants with health insurance (OR=4.20; 95% CI=1.16,15.24; p=0.029), and for those who were married (OR)=2.58; 95% CI=1.27,5.25; p=0.009). The Hosmer-Lemeshow tests did not indicate a lack of fit for the screening within guidelines model (p=0.4011) or the receiving a doctor’s recommendation for colon cancer screening model (p=0.7282).
Table 2A.
Final model for variables significantly associated with an average-risk adult completing CRC screening within guidelines
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Doctor recommendation. (yes vs. no) | 6.09 | 2.80–13.21 | <0.0001 |
| CRC Knowledge (adequate vs. not adequate) | 2.88 | 1.25–6.65 | 0.013 |
| Employed (employed full time vs. other) | 0.23 | 0.06–0.89 | 0.034 |
| Table 2B. Final model for variables significantly associated with an average-risk adult receiving a doctor’s recommendation for CRC screening | |||
|---|---|---|---|
| Variable | OR | 95% CI | p-value |
| Health Insurance (yes vs. no) | 4.20 | 1.16–15.24 | 0.029 |
| Marital Status (married vs. other) | 2.58 | 1.27–5.25 | 0.009 |
Note: Order of variables entered into the model were: doctor recommendation, CRC knowledge, and employment.
Note: Order of variables entered into the model were: marital status and health insurance
Post-CRC Screening Campaign Survey (Media Campaign Feedback)
A second convenience sample of residents (≥50 years) of Meigs County completed the media campaign questionnaire at two different community locations (local grocery store and a fast food restaurant) on two different days in July 2007. Once again, a few individuals approached refused to participate and all who declined stated it was because of the lack of time. Although 100 residents completed the survey, it was later determined that only 97 were 50 years and older. Of the Meigs County residents (50 years and older) completing the survey, 61 (63%) were average-risk and 36 (37%) were high-risk adults for CRC. To be consistent, only data from the average-risk adults participating in the CRC screening media campaign feedback are presented in Table 3.
Table 3.
“Get Behind Your Health!” Media Campaign Feedback
| Average-Risk n (%) |
||
|---|---|---|
| Reported seeing the “Get Behind Your Health” message in the past 3 months (n=61) |
Yes | 42/61 (69) |
| No | 19/61 (31) | |
| Frequency of seeing the message in the past 3 months by those who reported seeing the campaign (n=42) |
1 | 5/42 (12) |
| 2 – 5 | 19/42 (45) | |
| 6 – 10 | 9/42 (21) | |
| > 10 | 9/42 (21) | |
| Source of message by those who reported seeing the campaign (n=42) |
Billboard | 33/42 (79) |
| Newspaper | 16/42 (38) | |
| Poster | 8/42 (19) | |
| Television | 6/42 (14) | |
| Importance of message by those who reported seeing the campaign (n=42)* |
Very important | 16/42 (38) |
| Important | 18/42 (43) | |
| Moderately important | 5/42 (12) | |
| Of little importance | 2/42 (5) | |
| Unimportant | 0 (0) | |
| They had talked to someone about message by those who reported seeing the campaign (n=42)* |
Yes | 14/42 (33) |
| No | 27/42 (64) | |
| Of those who reported that they had already talked to someone about the media campaign (n=14), they reported they had talked to (could report more than one) |
Family | 11/14 (79) |
| Friend | 5/14 (36) | |
| Doctor | 2/14 (14) | |
| Nurse | 2/14 (14) | |
| Of those who reported they saw the campaign and were planning to talk to someone in the future about the message (n=27)* |
Yes | 8/27 (30) |
| No | 16/27 (59) | |
| Of those who planned to talk to someone about the campaign (n=8), they reported they planned to talk to |
Family | 3/8 (38) |
| Friend | 1/8 (13) | |
| Doctor | 4/8 (50) | |
| Reported seeing the sham CRC screening message in the past 3 months (n=61) |
Yes | 4/61 (7) |
| Yes | 57/61 (93) | |
missing data
Colon cancer screening message recall was reported about CRC screening in general (n=10; 16%) and specifically about the media campaign (n=8; 13%). Following questions about the campaign message recall, the campaign image and message was presented and 69% (42/61) of the average-risk for CRC participants reporting seeing a “Get Behind Your Health! Talk to your doctor about colon cancer screening” message from at least one source during the 3 months prior to the survey. Of the 69% who reported seeing an ad for the CRC screening campaign, a few (12%) reported seeing it once, most (45%) reported seeing it 2–5 times, 21% saw it 6–10 times, and 21% saw it over 10 times.
Of the participants who reported seeing the campaign, the most frequent source was the billboard (79%), the newspaper (38%) and posters (19%). The majority of average-risk (81%) participants who reported seeing the message stated that the message said something very important or important. Additionally, among the average-risk participants who reported seeing the message in the prior 3 months, two reported having already spoken to their doctor about the message and 4 participants reported that they were planning to talk to their doctor about the message. Only 4 (7%) of the average-risk participants reported seeing the sham campaign.
DISCUSSION
Colorectal cancer remains a significant public health problem, especially among minority and underserved populations, such as poor and rural populations (Friedell, Linville, & Hullet, 1998; Holmes-Rovner et al., 2002). Interventions to increase CRC screening rates are important because CRC screening tests are effective in reducing both the incidence and mortality from CRC (Pignone, Rich, Teutsch, Berg, & Lohr, 2002; Winawer et al., 2003). This study was designed and implemented by a partnership between the community members and the academic researchers to assess current CRC knowledge and CRC screening behaviors among residents living in a poor, medically underserved county in Ohio Appalachia to gain insight into the barriers to CRC screening. In addition, this information guided the development of the CRC screening awareness campaign to address the increased CRC burden in that county.
In the community needs assessment, only 29% of the average-risk participants had completed a CRC screening test within recommended guidelines. This is similar to previous reports documenting that Ohio Appalachia residents (≥50 years) complete CRC screening at a lower rate compared to residents in non-Appalachia counties (Fisher et al., 2008; Appalachia Community Cancer Network, 2009). In this study, for an average-risk adult, having health insurance and being married increased the odds of receiving a doctor’s recommendation for CRC screening, and receiving a doctor’s recommendation for CRC screening, having adequate CRC knowledge, and not being employed full-time and thus likely having the time to complete screening increased the odds of being within recommended CRC screening guidelines. The results in this study are similar to previous studies conducted among different racial, SES, and cultural groups (Coughlin & Thompson, 2004; Katz et al., 2004; Klabunde et al., 2005).
Physician recommendation for CRC screening remains a constant motivating factor for individuals to complete CRC screening within recommended guidelines (Vernon, 1997), including studies conducted in rural populations (Coughlin & Thompson, 2004). In a report comparing the barriers to CRC screening from two separate national surveys, several interesting differences emerged between perspectives of primary care physicians (PCP) compared to average-risk adults (Klabunde et al., 2005). In this report, for example, 56% of the PCPs identified that patient embarrassment or anxiety about undergoing a CRC screening test was a major barrier while less than 10% of patients indicated this as a barrier, and 46% of PCPs identified cost or the lack of health insurance as a major barrier compared to <1% of the patients. Although we did not survey healthcare providers in Meigs County, the barriers to the various CRC screening tests reported by the adults in this study were similar to those in the national survey suggesting that perhaps the differences in the perceptions about the barriers to CRC screening may be associated with the ongoing low rates of CRC screening. Since perceptions of providers and patients may differ, considerable effort to increase CRC screening must continue at the patient, provider, and system levels.
Although patient-doctor communication about CRC screening is important to increase CRC screening rates, the discussion itself may not always lead to the completion of the screening test (Brawarsky, Brooks, Mucci, & Wood, 2004; Lafata, Divine, Moon, & Williams, 2006). Other factors that may play a role in test completion following a doctor’s recommendation for CRC screening are the patient’s beliefs and attitudes toward CRC and the various screening tests, receiving enough information regarding the test, and receiving assistance in making the screening test appointment (Brawarsky, Brooks, Mucci, & Wood., 2004; Lafata, Divine, Moon & Williams, 2006).
The media campaign developed for this study focused on activating the patient to initiate a discussion about CRC screening with their doctor. In only 3 months, 69% of the average-risk participants reported seeing the campaign message at least once; with most participants reporting that they had seen the message between 2 and 5 times. The most common source of the message was the one billboard in the county, followed by newspaper stories and posters displayed throughout the county. Among the average-risk adults, 2 participants had already talked to their doctor about the message and 4 participants reported that they were planning to discuss the message with their provider. In addition, the media campaign developed also addressed changing social norms by using multiple channels to start the discussion about CRC screening among residents and between individuals and their healthcare providers.
The documented success of mass media health campaigns has varied because of the difficulty in evaluating their effectiveness (Hornik, 2002). Public media campaigns to promote CRC screening have been developed and implemented during the past decade (Broadwater et al., 2004; Centers for Disease Control, 2008; Schroy et al., 2008), although often their effectiveness has not always been thoroughly evaluated. In one study, a state-wide (Utah) CRC screening campaign was conducted in 2003 using local media talent (Broadwater, Heins, Hoelscher, Mangone & Rozanas, 2004). A random-digit dialing protocol was used following the media campaign to conduct a telephone survey. Seventy-nine percent of the 403 participants reported seeing or hearing CRC screening messages in the past three months, and 85% of those participants could recall one of the main messages from the campaign. In that study, the source of the message most often reported was television (86%), followed by newspapers or magazines (18%), and radio (15%).
Although recognition rates of the CRC media campaign conducted in this study were similar to the study conducted in Utah, the source reported most frequently in this study was the billboard and newspaper articles. This difference could be due to the smaller scale and budget of the campaign used in this study or because the community members suggested that campaign materials be placed in locations they knew would be seen by the residents of that county. Since CRC has a complex set of determinants, like other public health problems, we believe the use of CBPR strategies in this study enhanced the media campaign. Additionally, we believe that the use of a local CRC survivor and physician in the media campaign were important campaign components to resonate with this rural population. Using a local community member in a media campaign enhances the credibility of the message and potentially increases an individual’s self-identification with the message which may influence the desired behavioral change (Bowen, Hickman & Powers, 1997). This affective identification has been documented in educational materials focused on improving cancer screening rates and other public health issues (Rudd & Coming, 1994; Yancey, Tanjasiri, Klein & Tunder, 1995) and may have implications for cancer primary and secondary prevention media campaigns in the Appalachia region and other regions in the United States. Although it has been documented that media campaigns can increase cancer screening awareness and screening rates, the current challenge is to create messages that reach diverse populations that are theoretically-based, culturally relevant, delivered through the appropriate channels, and undergo rigorous evaluation (Broadwater et al., 2004; Hannon et al., 2009; Viswanath, 2005; Southwell et al., 2202; Evans et al., 2009).
The limitations of this study are that the community needs assessment and the media campaign evaluation were based on convenience samples and may not represent all adults living in Ohio Appalachia. In addition, completing a CRC screening test within a specific time frame was based on self-report which may vary in accuracy compared to medical records (Baier et al., 2000).
This study is important because we partnered with community members, focused on the poor county in Ohio Appalachia that has increased CRC incidence and mortality rates. The strengths of this study are that the needs assessment was conducted on 7 different days and locations in an Ohio Appalachia county and included individuals from 10 of the 12 county townships. In addition, in partnership with community members, we used the findings from the community needs assessment to develop the campaign that featured a local CRC survivor and physician, and only a few average-risk participants reported seeing the sham campaign.
In conclusion, this report describes a community-based approach to the development and feedback about a CRC screening campaign in Ohio Appalachia. This campaign to increase CRC screening awareness addressed a cue to action (talk to your doctor about CRC screening) and may influence social norms. The results of this study suggests that a culturally sensitive media campaign focused on increasing CRC screening in rural Appalachia may be an effective strategy to increase CRC screening.
Acknowledgements
Supported by: Ohio Department of Health/Federal Government, Bureau of Health Promotion and Risk Reduction, Comprehensive Cancer Program, A Community Investment Subcontract from the American Cancer Society, Ohio Division; NCI Grant #: CA114622 (Appalachia Community Cancer Network); NCI Grant # CA107079 (MLK); NCI Grant # CA016058 (The Behavioral Measurement Shared Resource at The Ohio State University Comprehensive Cancer Center).
References
- Abramson R, Haskell J, editors. Encyclopedia of Appalachia, Knoxville. Tennessee: The University of Tennessee Press; 2006. [Google Scholar]
- American Cancer Society, Ohio Division. Ohio Cancer Facts and Figures. Columbus: American Cancer Society; 2005. [Google Scholar]
- American Cancer Society. Colorectal Cancer Facts and Figures. Atlanta: American Cancer Society; 2008. [Google Scholar]
- Appalachia Community Cancer Network. The Cancer Burden in Appalachia. 2009 http://www.accnweb.com/
- Washington, DC: Appalachia Regional Commission; 2009. http://www.arc.gov/index.jsp. [Google Scholar]
- Baier M, Calonge N, Cutter G, McClatchey M, Schoentgen S, Hines S, et al. Validity of self-reported colorectal cancer screening behavior. Cancer Epidemiol Biomarkers Prev. 2000;9(2):229–232. [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, New Jersey: Prentice Hall; 1986. [Google Scholar]
- Bowen D, Hickman KM, Powers D. Importance of psychological variables in understanding risk perceptions and breast cancer screening of African American women. Womens Health. 1997;3(3–4):227–242. [PubMed] [Google Scholar]
- Brawarsky P, Brooks DR, Mucci LA, Wood PA. Effect of physician recommendation and patient adherence on rates of colorectal cancer testing. Cancer Detect Prev. 2004;28(4):260–268. doi: 10.1016/j.cdp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Broadwater C, Heins J, Hoelscher C, Mangone A, Rozanas C. Skin and colon cancer media campaigns in Utah. Prev Chronic Dis. 2004;1(4):A18. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. [accessed January 7, 2008];Screen for Life Campaign. http://www.cdc.gov/cancer/colorectal/sfl/
- Coughlin SS, Thompson TD. Colorectal cancer screening practices among men and women in rural and nonrural areas of the United States, 1999. J Rural Health. 2004;20(2):118–124. doi: 10.1111/j.1748-0361.2004.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Evans WD, Uhrig J, Davis K, McCormack L. Efficacy methods to evaluate health communication and marketing campaigns. Journal of Health Communication. 2009;14:315–330. doi: 10.1080/10810730902872234. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Engelhardt HL, Stephens JA, Smith BR, Haydu GG, Indian RW, et al. Cancer-related disparities among residents of Appalachia Ohio. J Health Disparities Research and Practice. 2008;2(2):61–74. [Google Scholar]
- Friedell G, Linville LH, Hullet S. Cancer control in rural Appalachia. Cancer. 1998;83:1868–1871. [Google Scholar]
- Hannon P, Lloyd GP, Viswanath K, Smith T, Basen-Engquist K, Vernon SW, et al. Mass media and marketing communication promoting primary and secondary cancer prevention. Journal of Health Communication. 2009;14:30–37. doi: 10.1080/10810730902806802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes-Rovner M, Williams GA, Hoppough S, Quillan L, Butler R, Given CW. Colorectal cancer screening barriers in persons with low income. Cancer Pract. 2002;10(5):240–247. doi: 10.1046/j.1523-5394.2002.105003.x. [DOI] [PubMed] [Google Scholar]
- Hornik R. Public health communication: making sense of contradictory evidence. In Public Health Communication: Evidence for Behavior Change. Mahwah, New Jersey: Lawrence Erlbaum Associates; 2002. pp. 1–22. [Google Scholar]
- Katz ML, James AS, Pignone MP, Hudson MA, Jackson E, Oates V, et al. Colorectal cancer screening among African American church members: a qualitative and quantitative study of patient-provider communication. BMC Public Health. 2004;4:62. doi: 10.1186/1471-2458-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43(9):939–944. doi: 10.1097/01.mlr.0000173599.67470.ba. [DOI] [PubMed] [Google Scholar]
- Lafata JE, Divine G, Moon C, Williams LK. Patient-physician colorectal cancer screening discussions and screening use. Am J Prev Med. 2006;31(3):202–209. doi: 10.1016/j.amepre.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B, Smith RA, Feldman GE, Colditz GA, Fletcher RH, Nadel M, Rothenberger DA, Schroy PS, Vernon SW, Wender R. Promoting early detection tests for colorectal carcinoma and adenomatous polyps: a framework for action: the strategic plan of the National Colorectal Cancer Roundtable. Cancer. 2002;95(8):1618–1628. doi: 10.1002/cncr.10890. [DOI] [PubMed] [Google Scholar]
- MMWR. Cancer death rates-Appalachia, 1994–1998. MMWR Weekly. 2002;51:527–529. [PubMed] [Google Scholar]
- Ohio Department of Health, Center for Public Health Data and Statistics. 1998 Ohio Family Health Survey and Ohio Small Area Estimation Survey. 2000
- Ohio State Medical Board. Registered Physicians. 2002 [Google Scholar]
- Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average-risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(2):132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- Rudd RE, Comings JP. Learner developed materials: An empowering product. Health Ed Q. 1994;21:313–327. doi: 10.1177/109019819402100304. [DOI] [PubMed] [Google Scholar]
- Schroy PC, 3rd, Glick JT, Robinson PA, Lydotes MA, Evans SR, Emmons KM. Has the surge in media attention increased public awareness about colorectal cancer and screening? J Community Health. 2008;33(1):1–9. doi: 10.1007/s10900-007-9065-5. [DOI] [PubMed] [Google Scholar]
- Smith RA, Cokkinides V, Eyre HJ. Cancer screening in the United States, 2007: a review of current guidelines, practices, and prospects. CA Cancer J Clin. 2007;57(2):90–104. doi: 10.3322/canjclin.57.2.90. [DOI] [PubMed] [Google Scholar]
- Southwell BG, Barmada CH, Hornik RC, Maklan DM. Can we measure encoded exposure? Validation evidence from a national campaign. Journal of Health Communication. 2002;7:445–453. doi: 10.1080/10810730290001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. Ohio Department of Health, Primary Care Section. 2000 [Google Scholar]
- United States Department of Agriculture. Economic Research Service. Ohio Data Set, Bureau of the Census. 2006 [Google Scholar]
- Viswanath K. Science and Society. The communications revolution and cancer control. Nature Reviews: Cancer. 2005;5:828–835. doi: 10.1038/nrc1718. [DOI] [PubMed] [Google Scholar]
- Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89(19):1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- Vernon SW, Meissner H, Klabunde C, Rimer BK, Ahnen DJ, Bastani R, et al. Measures for ascertaining use of colorectal cancer screening in behavioral, health services, and epidemiologic research. Cancer Epidemiol Biomarkers Prev. 2004;13(6):898–905. [PubMed] [Google Scholar]
- Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124(2):544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- Yancey AK, Tanjasiri SP, Klein M, Tunder J. Increased cancer screening behavior in women of color by culturally sensitive video exposure. Prev Med. 1995;24:142–148. doi: 10.1006/pmed.1995.1027. [DOI] [PubMed] [Google Scholar]