Abstract
Lower socioeconomic status (SES) has been linked to higher incidence of head and neck cancer (HNC) and lower survival. However, there is little known about the effect of SES on HNC survival in Asians and Pacific Islanders (APIs). This study’s purpose is to examine the effect of SES on disease-specific survival (DSS) and overall survival (OS) in APIs with HNC using population-based data.
Materials and Methods
53,544 HNC patients (4,711 = APIs) were identified from the California Cancer Registry from 1988 – 2007. Neighborhood (block-group-level) SES, based on composite Census 1990 and 2000 data, was calculated for each patient based on address at diagnosis and categorized into statewide quintiles and collapsed into 2 groups for comparison (low SES = quintiles 1-3; high SES = quintiles 4-5). DSS and OS were computed by Kaplan-Meier method. Adjusted hazard ratios (HR) were estimated using Cox proportional hazards regression models.
Results
Among APIs, lower neighborhood SES was significantly associated with poorer DSS (HR range for oral cavity, oropharynx, or larynx/hypopharynx cancer: 1.07–1.34) and OS (HR range: 1.13–1.37) after adjusting for patient and tumor characteristics. Lower SES was significantly associated with poorer survival in API with all HNC sites combined: DSS HR: 1.26 (95% CI: 1.08 – 1.48); OS HR: 1.30 (95% CI: 1.16 – 1.45).
Conclusions
Neighborhood SES is associated with longer DSS and OS in API with HNC. The effect of SES on HNC survival should be considered in future studies, and particular attention should be paid to clinical care of lower-SES HNC patients.
Keywords: socioeconomic status, head and neck cancer, Asians, Pacific Islanders
Introduction
In 2009, there were approximately 48,000 cases of head and neck cancer (HNC) diagnosed in the US, making up about 5% of all cancers [1]. Despite its relatively low incidence, the impact of HNC diagnosis is grave due to its associated morbidity and mortality. Functional morbidity from aggressive multimodality treatment, including dysphasia, dry mouth, hoarseness, and facial disfigurement [2–4], and can lead to subsequent mental distress and impede patients from returning to work [5–6]. The societal burden of HNC treatment ranges from increased use of inpatient and outpatient services [7–8] to increased Medicare expenditure in the range of $25,000 greater than in patients without HNC [9]. Hence, understanding the factors that influence treatment outcomes in this debilitating disease will help us not only in tailoring our treatment to individual patients but also in distributing society resources to the population in need.
Several clinical and pathologic factors have been consistently shown to influence survival in HNC patients; these include age, gender, clinical stage, tumor site, treatment parameters, tobacco use, and human papillomavirus infection. The only social parameter that has been linked to survival outcomes of HNC is socioeconomic status (SES), but this has been evaluated only in a few studies, half of which were performed outside the US [10–18]. Therefore, a better understanding of the relationship between SES and HNC survival can help improve the overall clinical care by heightening provider awareness of existing disparities, as well as by focusing future research to identify and ameliorate the underlying causes of SES disparities.
The prior studies of SES and HNC survival in the US have included non-Hispanic Whites and African-Americans [19–21], but not other racial/ethnic groups. California has the fastest growing Asian and Pacific Islander (API) population in the US [22], yet APIs have been overlooked in most prior studies of HNC survival. In addition, several Asian ethnic groups, such as the Chinese, Vietnamese and Filipinos, are prone to developing a certain HNC, specifically nasopharyngeal carcinoma. To our knowledge, no studies have evaluated the association of SES and survival in APIs diagnosed with HNC in the US. This topic therefore merits investigation, particularly as the effects of SES disparity on cancer survival across racial/ethnic groups can vary [23]. We previously found that lower neighborhood-level SES, based on an index that combines census block-group averages of education, income, occupation, and cost of living [24], was associated with higher HNC incidence among APIs in California [25]. We hypothesize here that lower neighborhood-level SES is also associated with lower disease-specific survival (DSS) among APIs diagnosed with HNC. To address this hypothesis, we evaluate the relationship of neighborhood-level SES with DSS and overall survival (OS) in all HNC and specifically in API patients using population-based cancer registry data from California, the state with the largest population of APIs in the United States.
Materials and Methods
Study Population
Information on all invasive HNC patients diagnosed between January 1st, 1988 and December 31st, 2007 was obtained from the population-based California Cancer Registry (CCR), which operates through state-mandated reporting since 1988, and comprises three of the US National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registries (Greater Bay Area, Los Angeles, and Greater California). Only primary tumors originating in the nasopharynx (NPC, International Classification of Diseases for Oncology, 3rd edition, site codes C110-C119), oral cavity (OC, codes C019-069, C140-148), oropharynx (OP, codes 090-109), larynx (LX, codes C320-329) or hypopharynx (HP, codes C129-139) were included. Tumor histology was determined using ICD-O-3 histology codes to exclude any non-squamous cell carcinoma histologies, including mesotheliomas (codes 9050-9055), Kaposi sarcomas (code 9140), and lymphomas (codes 9590-9989). Patients with unknown race/ethnicity, diagnosed on death certificate or at autopsy, or with unknown survival time were excluded, leaving a sample of 53,544 patients, of whom 4,711 were API. For comparison, our analysis also included 38,892 non-Hispanic Whites.
Patient and tumor characteristics that are collected through the CCR and included in this study are age at diagnosis, sex, race/ethnicity, marital status, tumor stage (localized, regional, or remote), tumor histologic grade, treatment modality within the first 4 months of diagnosis, hospital of earliest admission, and vital status. If the patient is deceased, the cause and date of death are also recorded. Registry data on patient race/ethnicity are typically abstracted from hospital medical records, which are obtained primarily through self-report, by assumption of hospital personnel, or on inference from other information including race/ethnicity of parents, maiden name, surname, and birthplace, and from death records [24]. Data on nativity (US-born vs. foreign-born status) are available for Hispanics as well as Chinese, Filipinos, Japanese, Koreans, South Asians, and Vietnamese, but not for other API subgroups that represent approximately 10% of the API cohort. Regardless, we examined nativity in our preliminary models and found that it was not an independent factor in API or Hispanic survival from HNC. Therefore, nativity was excluded from our final analyses. Information on specific API ethnicity is available in the registry, but, for this analysis, APIs were analyzed as one group because of small sample sizes [26].
Neighborhood Socioeconomic Status
Individual patient-level SES information is not collected by cancer registries. Therefore, we determined neighborhood-level SES based on each patient’s residence at diagnosis. The SES index combines census block-group averages of education, income, occupation, and cost of living, as described previously [24]. Neighborhood-level SES is classified in quintiles based on state average distributions; we combined these categories into two groups, with lower SES represented by quintiles 1 – 3 and higher SES represented by quintiles 4 – 5.
Statistical Analysis
Follow-up was measured in months from each patient’s date of diagnosis to the date of death from any cause for overall survival (OS), the date of death from HNC for disease-specific survival (DSS, in which patients who died from other causes were censored on the date of death), the date of last known contact, or the end of the study period on December 31, 2007, whichever occurred earliest. Kaplan-Meier curves stratified by neighborhood SES were generated by race/ethnicity. HP and LX cancers were combined into a single group for analysis due to relatively small case numbers and their intimate proximity in anatomic location, making it difficult on occasion to distinguish between these two primary tumor sites.
Multivariate Cox models proportional hazards models were used to estimate hazard ratios (HR) with 95% confidence intervals (CI) for overall or disease-specific mortality for APIs and non-Hispanic Whites combined and for APIs only. The proportional hazards assumption was assessed by visual inspection of the survival curves (log (-log) of the survival distribution function by log months) and test for time-dependency. There was no evidence of violation of the proportionality assumption. For all patients combined, separate multivariate models were constructed for each tumor site. For API patients, due to limited case numbers, a single multivariate model was constructed for all mucosal HNC sites combined, excluding NPC due to its distinctive natural history, risk factors, and outcomes. Variables included in the multivariate analysis were selected based on univariate associations with the outcome (at p<0.15) and a priori knowledge of potential confounders. These included age at diagnosis, sex, race/ethnicity, marital status, tumor stage, tumor histologic grade, initial treatment modality, and reporting by a university teaching hospital. All statistical tests were two-sided, and p-values <0.05 were considered as statistically significant. Analyses were performed using SAS version 9.1.3.
Results
Patient Characteristics
Table 1 shows patient demographic and disease-related characteristics for non-Hispanic White and API HNC patients included in this study. As expected, HNCs among API patients were more likely than those among non-Hispanic White patients to have NPC, with APIs representing 55.4% of all NPC patients. APIs otherwise represented 6.6% of OC patients, 4.0% of OP patients, and 4.6% of HP/LX patients. The median duration of follow-up was 22.3 months in deceased patients and 79.7 months in living patients.
Table 1.
Demographic and Disease Characteristics in Head and Neck Cancers Diagnosed in California between 1988–2007
| Characteristic | Race, n (%) |
|
|---|---|---|
| Non-Hispanic White (n = 38892) | Asian (n = 4711) | |
| Age at Diagnosis (years) | ||
| <50 | 4919 (13) | 1505 (32) |
| 50–59 | 9540 (25) | 1064 (23) |
| 60–69 | 11792 (30) | 1054 (22) |
| 70+ | 12641 (33) | 1088 (23) |
| Year of Diagnosis | ||
| 1988–1991 | 8588 (22) | 701 (15) |
| 1992–1995 | 7959 (20) | 842 (18) |
| 1996–1999 | 7646 (20) | 985 (21) |
| 2000–2003 | 7317 (19) | 1069 (23) |
| 2004–2007 | 7382 (19) | 1114 (24) |
| Sex | ||
| Male | 27823 (72) | 3284 (70) |
| Female | 11069 (28) | 1427 (30) |
| Marital Status | ||
| Never Married | 5617 (14) | 509 (11) |
| Married | 21141 (54) | 3385 (72) |
| Separated/widowed/divorced | 10858 (28) | 664 (14) |
| Unknown | 1276 (3) | 153 (3) |
| Neighborhood Socioeconomic Status (SES) | ||
| Lower (Statewide Quintiles 1-3) | 21284 (55) | 2529 (54) |
| Higher (Statewide Quintiles 4-5) | 17608 (45) | 2182 (46) |
| University Teaching Hospital? | ||
| No | 34296 (88) | 3993 (85) |
| Yes | 4596 (12) | 718 (15) |
| Cancer Site | ||
| Oral | 11864 (31) | 1020 (22) |
| Oropharynx | 11310 (29) | 573 (12) |
| Hypopharynx/Larynx | 14500 (37) | 905 (19) |
| Nasopharynx | 1218 (3) | 2213 (47) |
| Summary Stage at Diagnosis | ||
| Localized | 15302 (39) | 1341 (28) |
| Regional | 16626 (43) | 2157 (46) |
| Remote | 4598 (12) | 853 (18) |
| Unknown | 2366 (6) | 360 (8) |
| Tumor Histologic Grade | ||
| Well/Moderately Differentiated | 21945 (56) | 1540 (33) |
| Poor/Undifferentiated | 10648 (27) | 2239 (48) |
| Unknown | 6299 (16) | 932 (20) |
| Surgery | ||
| No/Unknown | 18786 (48) | 3106 (66) |
| Yes | 20106 (52) | 1605 (34) |
| Radiotherapy | ||
| No/Unknown | 12314 (32) | 1103 (23) |
| Yes | 26578 (68) | 3608 (77) |
| Chemotherapy | ||
| No | 29758 (77) | 2767 (59) |
| Yes | 8681 (22) | 1899 (40) |
| Unknown | 453 (1) | 45 (1) |
Although not shown in table 1, there were 4,234 AA and 5,707 Hispanic patients included in the survival analyses of all HNC patients. Similar to the literature, both AA and Hispanics were more likely to be in the lower SES quintiles, 85% and 78% respectively. They were both less likely to be married compared to APIs.
Effect of Socioeconomic Status and Other Characteristics on Disease-Specific Survival in Head and Neck Cancers
The median DSS in months for non-Hispanic Whites and APIs combined, stratified by tumor site, sex and SES, is shown in Table 2, and the Kaplan-Meier DSS curves for the two SES groups are shown in Figure 1 for each tumor site. DSS was significantly shorter for patients in the lower SES group than those in the higher SES group, regardless of tumor type or sex.
Table 2.
Median Survival (in Months, with 95% Confidence Intervals) of Head and Neck Cancer Patients in the California Cancer Registry between 1988 – 2007 Based on Tumor Site and Neighborhood Socioeconomic Status
| Neighborhood SES | Oral Cavity | Oropharynx | Hypopharnyx/Larynx | Nasopharynx | ||||
|---|---|---|---|---|---|---|---|---|
| Low (1-3) | High (4-5) | Low (1-3) | High (4-5) | Low (1-3) | High (4-5) | Low (1-3) | High (4-5) | |
| (Statewide Quintiles) | N=8961 | N=6468 | N=8142 | N=6242 | N=12456 | N=7277 | N=2286 | N=1712 |
| Overall | 38.1 (35.5–40.1) | 62.4 (59.1–66.7) | 36.7 (34.4–38.8) | 85.4 (79.2–91.3) | 49.3 (47.3–51.5) | 74.6 (71.2–77.9) | 61.3 (55.9–70.2) | 100.9 (88.7–115-7) |
| Male | 36.1 (33.6–39.2) | 60.8 (56.3–65.6) | 38.9 (36.7–42.0) | 97.2 (90.4–104.4) | 49.6 (47.3–52.0) | 76.9 (72.9–81.8) | 56.3 (50.5–64.8) | 109.7 (88.1–134.6) |
| Female | 40.2 (36.7–44.9) | 65.5 (59.7–72.3) | 28.0 (24.6–33.1) | 53.4 (45.7–64.5) | 48.6 (44.2–53.3) | 61.8 (53.1–72.2) | 76.1 (61.1–91.4) | 94.8 (75.5–108.3) |
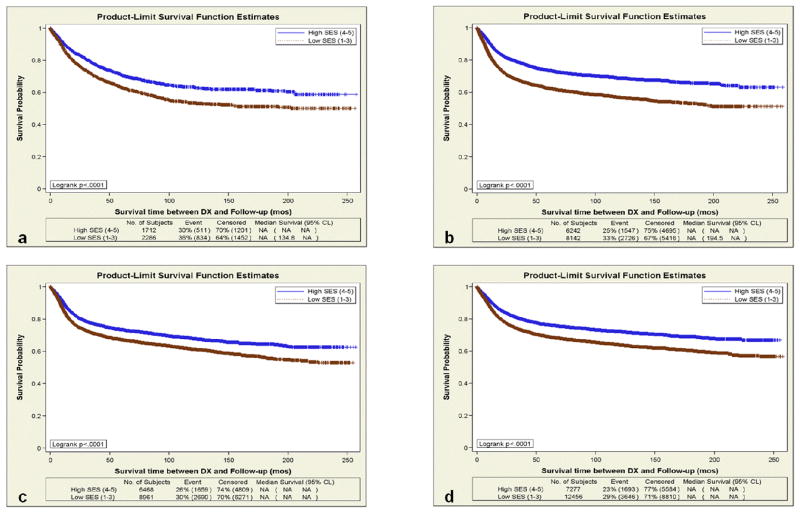
Figure 1.
Disease-Specific Survival of All Patients Diagnosed with Head and Neck Cancer By High (Quintiles 4 – 5) and Low (Quintiles 1 – 3) Neighborhood Socioeconomic Status in California between 1988–2007. (a) Nasopharynx; (b) Oropharynx; (c) Oral cavity; (d) Larynx/Hypopharynx
Univariate analysis revealed that certain variables were statistically significantly associated with shorter DSS for all tumor subsites: non-married status, later stage, higher histologic grade, and lack of treatment with radiotherapy or chemotherapy (data not shown). In multivariate analysis, older age, earlier year of diagnosis, lower SES, and more advanced stage at diagnosis were significantly associated with elevated risk of disease-specific mortality (and therefore, shorter DSS) for each tumor site (Table 3). Compared with women, men had a higher risk of death from HP/LX, but not other tumor sites. Being never married or separated, widowed, or divorced was associated with significantly higher risk of death from OC, OP, and HP/LX. In addition, having been reported to the cancer registry (and, in most cases, diagnosed) by a university teaching hospital was associated with better DSS for OC, HR 1.11 (95% CI 1.02–1.21) and OP, HR 1.10 (95% CI 1.00–1.22). Characteristics not statistically significantly associated with the outcome are not presented in Table 3.
Table 3.
Multivariate Hazard Ratios (HR) with 95% Confidence Intervals (CI) for Associations with Disease-Specific Survival After Head and Neck Cancer Diagnosis in California, 1988 to 2007.
| Characteristic | Oral | Hazard Ratio* (95% Confidence Interval) | ||
|---|---|---|---|---|
| Oropharynx | Hypopharynx/Larynx | Nasopharynx | ||
| Age (years) | ||||
| <50 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 50–59 | 0.97 (0.87–1.08) | 1.28 (1.15–1.42) | 1.10 (0.99–1.22) | 1.44 (1.25–1.66) |
| 60–69 | 1.02 (0.92–1.13) | 1.59 (1.44–1.76) | 1.33 (1.20–1.48) | 1.76 (1.52–2.05) |
| 70+ | 1.45 (1.32–1.60) | 2.26 (2.09–2.51) | 1.79 (1.61–1.99) | 2.71 (2.31–3.19) |
| Sex | ||||
| Female | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Male | 1.02 (0.96–1.09) | 0.91 (0.85–0.98) | 1.13 (1.05–1.21) | 1.11 (0.98–1.25) |
| Year of Diagnosis | ||||
| 1988–1991 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1992–1995 | 0.91 (0.84–1.00) | 0.91 (0.83–0.99) | 0.97 (0.90–1.05) | 0.88 (0.75–1.03) |
| 1996–1999 | 0.90 (0.82–0.98) | 0.74 (0.68–0.81) | 0.90 (0.83–0.98) | 0.74 (0.63–0.88) |
| 2000–2003 | 0.92 (0.84–1.01) | 0.65 (0.59–0.71) | 0.91 (0.84–1.00) | 0.75 (0.63–0.88) |
| 2004–2007 | 0.84 (0.76–0.94) | 0.52 (0.47–0.58) | 0.78 (0.71–0.86) | 0.55 (0.45–0.66) |
| Race | ||||
| Non-Hispanic White | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Asian/Pacific Islander | 1.09 (0.96–1.23) | 0.97 (0.82–1.14) | 0.90 (0.79–1.03) | 0.90 (0.79–1.02) |
| Marital Status | ||||
| Married | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | – |
| Never Married | 1.22 (1.12–1.34) | 1.37 (1.26–1.50) | 1.50 (1.39–1.62) | – |
| Separated/Widowed/Divorced | 1.23 (1.15–1.32) | 1.37 (1.27–1.47) | 1.45 (1.36–1.55) | – |
| Unknown | 0.91 (0.78–1.06) | 1.05 (0.89–1.24) | 1.26 (1.08–1.48) | – |
| Neighborhood Socioeconomic Status (SES) | ||||
| High SES (Statewide Quintiles 4-5) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Low SES (Statewide Quintiles 1-3) | 1.07 (1.01–1.14) | 1.34 (1.25–1.43) | 1.22 (1.15–1.29) | 1.31 (1.18–1.47) |
| University Teaching Hospital | ||||
| Yes | 1.00 (reference) | 1.00 (reference) | – | – |
| No | 1.11 (1.02–1.21) | 1.10 (1.00–1.22) | – | – |
| Summary Stage at Diagnosis | ||||
| Localized | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Regional | 2.19 (2.04–2.36) | 1.80 (1.61–2.01) | 4.03 (3.75–4.32) | 2.05 (1.64–2.56) |
| Remote | 3.29 (2.97–3.65) | 4.46 (3.94–5.06) | 5.79 (5.34–6.28) | 3.79 (3.01–4.76) |
| Unknown | 2.23 (1.98–2.52) | 1.95 (1.68–2.26) | 1.00 (0.93–1.09) | 1.94 (1.49–2.53) |
| Tumor Histologic Grade | ||||
| Well/Moderately Differentiated | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Poor/Undifferentiated | 1.23 (1.14–1.32) | 0.83 (0.78–0.89) | 1.18 (1.11–1.26) | 0.66 (0.55–0.79) |
| Unknown | 0.84 (0.76–0.92) | 0.93 (1.74–2.01) | 1.00 (0.93–1.09) | 0.67 (0.54–0.81) |
| Surgery | ||||
| Yes | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | – |
| No/Unknown | 2.39 (2.23–2.56) | 1.87 (1.74–2.01) | 1.82 (1.71–1.93) | – |
| Radiotherapy | ||||
| Yes | – | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| No/Unknown | – | 1.86 (1.72–2.00) | 1.61 (1.51–1.71) | 2.05 (1.76–2.38) |
| Chemotherapy | ||||
| Yes | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | – |
| No | 0.84 (0.77–0.92) | 1.15 (1.07–1.23) | 0.96 (0.89–1.03) | – |
| Unknown | 1.64 (1.30–2.06) | 1.33 (1.06–1.67) | 1.28 (1.02–1.61) | – |
Estimates are mutually adjusted for all characteristics shown in the table for each tumor site
The SES disparity in DSS comparing the lower to the higher neighborhood SES group was largest for patients with OP (HR 1.34, 95% CI 1.25–1.43) and NPC (HR 1.31, 95% CI 1.18–1.47). In multivariate models for OS, neighborhood SES was also an important prognostic factor: lower SES was associated with an HR of 1.13 (95% CI 1.09–1.18) for OC; 1.37 (95% CI 1.31–1.43) for OP; HR 1.19 (95% CI 1.15–1.23) for HP/LX; and 1.28 (95% CI 1.17–1.40) for NPC.
Role of SES in APIs
In API, the median follow-up time was 22.9 months for deceased patients and 79.7 months for living patients. Table 4 shows the median DSS for each primary HNC tumor site among APIs. Similar to the results noted for the non-Hispanic Whites and APIs combined, lower neighborhood SES was associated with shorter DSS for male APIs with each type of HNC. There was also a trend toward longer DSS among higher-SES compared with lower-SES female API patients with OC or OP cancer, but the number of females with HNC was too small to make any definitive conclusions.
Table 4.
Median Survival (in Months, with 95% Confidence Intervals) in Asians and Pacific Islanders Diagnosed with Head and Neck Cancer in the California Cancer Registry between 1988 – 2007, Based on Tumor Site and Neighborhood Socioeconomic Status
| Neighborhood SES | Oral Cavity | Oropharynx | Hypopharnyx/Larynx | Nasopharynx | ||||
|---|---|---|---|---|---|---|---|---|
| Low (1-3) | High (4-5) | Low (1-3) | High (4-5) | Low (1-3) | High (4-5) | Low (1-3) | High (4-5) | |
| (Statewide Quintiles) | N=506 | N=514 | N=312 | N=261 | N=531 | N=374 | N=1180 | N=1033 |
| Overall | 40.9 (29.4–59.2) | 91.0 (75.2–119.0) | 38.8 (24.6–59.2) | 101.7 (60.2–153.7) | 59.3 (47.2–80.8) | 119.1 (99.1–152.7) | 83.1 (74.2–94.9) | 148.4 (114.7–184.2) |
| Male | 32.5 (24.2–49.3) | 77.2 (56.9–107.1) | 31.5 (22.0–56.2) | 89.4 (52.4–153.7) | 58.3 (44.4–79.3) | 118.7 (98.9–150.8) | 70.6 (57.6–82.5) | 148.6 (110.9–184.9) |
| Female | 59.4 (31.5–140.7) | 116.4 (81.6–171.9) | 75.8 (24.6–**) | 126.3 (58.3–**) | 89.1 (30.5–129.2) | ** | 145.7 (100.7–**) | 126.8 (98.6–**) |
- not estimable
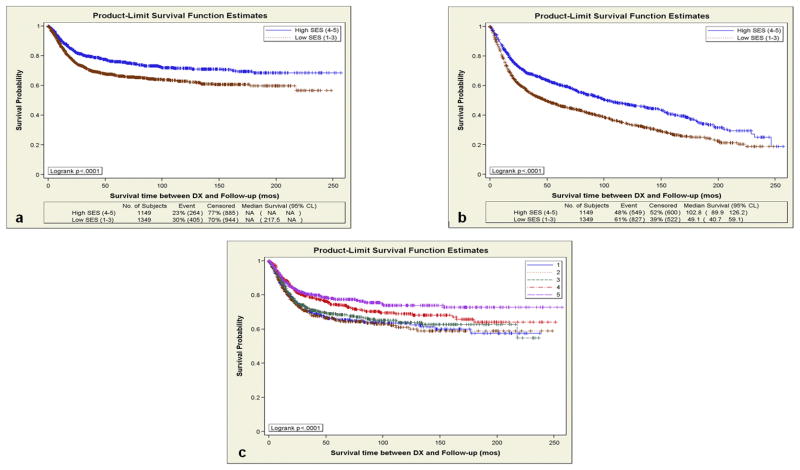
Figures 2a and 2b show the Kaplan-Meier curves for DSS and OS by neighborhood SES among API patients with OC, OP and HP/LX tumors combined, with significantly shorter survival among patients with lower SES. To ensure that our results were not due only to the grouping of SES quintiles, we also generated the Kaplan-Meier curves for DSS among API patients with OC, OP and HP/LX tumors combined, stratified by each quintile of neighborhood SES. As illustrated in Figure 2c, DSS increased gradually with each additional SES quintile, except for the transition from quintile 1 to 2.
Figure 2.
(a) Disease Specific Survival (DSS) in Asian-Pacific Islanders (API) Diagnosed with OC, OPC, and LX/HPC By High (Quintiles 4 – 5) and Low (Quintiles 1 – 3) Neighborhood Socioeconomic Status in California between 1988 – 2007. (b) Combined Overall Survival (OS) in API Diagnosed with OC, OPC, and LX/HPC based on Socioeconomic Quintiles in California between 1988 – 2007. (c) Disease Specific Survival in Asian Pacific Islanders Diagnosed with OC, OPC, or LX/HPC based on each Socioeconomic Status Quintile in California, 1988 – 2007.
Table 5 shows the results of the multivariate analysis for DSS in API patients with HNC. Lower neighborhood SES continued to be significantly associated with shorter DSS (HR 1.26, 95% CI 1.08–1.48) after adjusting for age, sex, year of diagnosis, marital status, stage, histologic tumor grade, and treatment. Other factors associated with significantly shorter DSS on multivariate analysis were older age at diagnosis, more advanced disease stage, and lack of surgical intervention (Table 5). Lower neighborhood SES was also associated with shorter OS after HNC among APIs, with a multivariate adjusted HR of 1.30 (95% CI 1.16–1.45).
Table 5.
Multivariate Hazard Ratios (HR) with 95% Confidence Intervals (CI) for Associations with Disease-Specific Survival after Head and Neck Cancer Diagnosis among Asians and Pacific Islanders in California, 1988 to 2007.
| Characteristics | Hazard Ratio* (95% Confidence Interval) |
|---|---|
| Age (years) | |
| <50 | 1.00 (reference) |
| 50–59 | 0.99 (0.74–1.34) |
| 60–69 | 1.27 (1.45–2.39) |
| 70+ | 1.86 (1.45–2.39) |
| Sex | |
| Female | 1.00 (reference) |
| Male | 0.99 (0.83–1.19) |
| Year of Diagnosis | |
| 1988–1991 | 1.00 (reference) |
| 1992–1995 | 0.84 (0.62–1.08) |
| 1996–1999 | 0.89 (0.70–1.14) |
| 2000–2003 | 0.83 (0.65–1.06) |
| 2004–2007 | 0.92 (0.71–1.20) |
| Marital Status | |
| Married | 1.00 (reference) |
| Never Married | 1.24 (0.96–1.61) |
| Separated/Widowed/Divorced | 1.09 (0.89–1.34) |
| Unknown | 0.83 (0.49–1.40) |
| Neighborhood socioeconomic status (SES) | |
| High SES (Statewide Quintiles 4-5) | 1.00 (reference) |
| Low SES (Statewide Quintiles 1-3) | 1.26 (1.08–1.48) |
| Summary Stage at Diagnosis | |
| Localized | 1.00 (reference) |
| Regional | 2.94 (2.39–3.60) |
| Remote | 4.82 (3.78–6.15) |
| Unknown | 3.13 (2.25–4.34) |
| Tumor Histologic Grade | |
| Well/Moderately Differentiated | 1.00 (reference) |
| Poor/Undifferentiated | 1.02 (0.86–1.22) |
| Unknown | 0.81 (0.64–1.02) |
| Surgery | |
| Yes | 1.00 (reference) |
| No/Unknown | 1.63 (1.37–1.93) |
| Chemotherapy | |
| Yes | 1.00 (reference) |
| No | 1.12 (0.92–1.37) |
| Unknown | 1.76 (0.89–3.45) |
Estimates are mutually adjusted for all characteristics shown in the table.
Discussion
Our results from a large, population-based, state cancer registry show that lower neighborhood-level SES is an independent negative prognostic factor among API patients diagnosed with HNC. Among APIs, living in a lower-SES neighborhood was associated with a 26% increased risk of HNC-specific death and a 30% increased risk of overall death, independent of age, year of diagnosis, tumor stage, treatment, and other factors. This difference in survival is much larger than that observed for the survival benefit of chemotherapy when added to radiation therapy for these cancers. More importantly, SES remained a significant prognostic factor for HNC after adjusting for other patient and disease-related characteristics. These results suggest that a research strategy that aims to identify and ameliorate the underlying causes of SES disparities will effectively improve the overall survival results in API patients with HNC.
While SES has been shown to significantly impact survival in more common cancers such as breast, prostate, and lung [27–31], its role in HNC-specific survival is less well studied, particularly within specific racial/ethnic groups. Most of the prior reports analyzed the impact of SES among all racial/ethnic groups, and not specifically in individual racial/ethnic groups. The few reports that have focused on SES and HNC survival focused primarily on African-Americans [19–21]. Molina et al. found in a large, population-based dataset in Florida that lower patient-level SES and African-American race are both significantly associated with worse survival for patients with HNC, independently of other demographics, comorbidities, clinical characteristics, and treatment modality [19]. Ours is the first study, to our knowledge, to document the association with SES in the large and rapidly growing API population.
A Danish population-based study, which used various factors including education, social class, housing district and world market affiliation to determine SES, demonstrated that survival from mouth, pharynx, and larynx cancers was shorter with lower SES, especially in men [14]. Similarly, a population-based study of larynx cancer in Wales showed a large survival disparity between affluent and poor males, with a 17% absolute difference in 5-year survival between the groups [32]. In the US, both poorer health care insurance status (Medicaid dependent or uninsured patients) and living in an area with 15% of residents below the poverty line conferred a poorer outcome with HNC [11, 19]. Such findings in predominantly non-Hispanic White patient populations indicate that the impact of SES is an independent predictor of HNC survival that cannot be ignored.
Despite the fact that API overall have some of the lowest mortality rates for HNC in comparison to other ethnic groups [33], our data still show that SES is a significant prognostic factor for HNC-specific survival within the API group and the role of SES in this group cannot be ignored. In our own previous work among California APIs [25] and in other studies, SES has also been associated with HNC incidence. A study based in the Thames Cancer Registry noted a significantly higher incidence of OC and pharynx cancers in English South Asians who lived in lower-SES compared to higher-SES neighborhoods [34]. A recent meta-analysis of 41 studies, which included 15,344 cases and 33,852 controls, found that the odds ratio for developing OC cancer was 1.85 for those with lower education levels, 1.84 for those with lower occupational/social class and 2.41 for those with lower income, when compared to those in the highest SES strata [35]. This association was universal in both high- and low-income countries, and remained significant after adjusting for other potential confounders. These results and our current findings suggest that the relationship between SES and HNC development and prognosis is independent of ethnicity and should be explicitly recognized in future effort for prevention, early detection and treatment of HNC among all patients, including APIs.
Although our data are consistent with previous reports regarding the association between SES and survival in HNC, our results should be interpreted with some limitations in mind. While we had data on patients’ neighborhood-level SES based on residential address at diagnosis, we did not have information on individual-level measures of SES, such as education and income. While neighborhood-level and individual-level SES are correlated, the two groups of measures capture different types of exposures that may be independently associated with health outcomes [36–37]. For example, neighborhood-level measures of SES capture both aspects of individuals within the neighborhood as well as characteristics of the neighborhood itself. Interestingly, both individual-level and neighborhood-level SES measures have been shown to impact the development of HNC, as Conway et al. noted that the risk of developing HNC was significantly higher in patients who were unemployed (OR 2.27, 95% CI 1.21 – 4.26) and lived in deprived areas (OR 4.66, 95% CI 1.79 – 12.18), although these associations were largely explained by smoking and alcohol consumption [12].
Other limitations of our study, as in any other registry-based studies, are the lack of detailed information on tumor stage, treatment, pattern of relapse, and other risk factors that may impact HNC survival, such as comorbidities, tobacco and alcohol consumption, and tumor human papillomavirus or Epstein-Barr virus status. In particular, there is literature supporting that a substantial cause of SES disparity is from tobacco use. In one Australian study, low education (below or up to primary school) negatively impacted survival from all causes (HR 1.36, 95% CI 1.12 – 1.65). But once smoking was taken into account, the effect of low education decreased to 1.22 (95% CI 1.01 – 1.49) [38]. Another study from the UK looking at cancer mortality specifically demonstrates that once smoking was accounted for in the model, the effect of low SES on cancer specific mortality was no longer significant [39]. Similar to these studies, it is possible that the negative impact on DSS in API based on low SES is at least partly related to tobacco consumption. However, our registry sample size was incomparably large for our population and highly enriched for API, making the CCR one of the unique databases available to specifically address the role of SES in HNC survival among API in the US.
In summary, we found that neighborhood-level SES is significantly associated with DSS and OS among the large and growing API population living in California. This association may reflect a lack of preventative care in the lower-SES populations, as well as barriers to healthcare that may delay treatment after diagnosis. We believe that future studies on HNC management should control for the disparity in SES. More importantly, future education efforts on HNC risk and management should target lower-SES patients in to improve the overall outcome of this debilitating cancer.
Acknowledgments
This research was also supported by a Stanford Cancer Center Developmental Cancer Research Award in Population Sciences. We would like to thank Kari Fish for her assistance in some of the data analysis.
The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Cancer Prevention Institute of California (formerly the Northern California Cancer Center), and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Health Services, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Footnotes
Conflict of Interest: none
References
- 1.American Cancer Society. Cancer Facts & Figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 2.Givens DJ, Karnell LH, Gupta AK, et al. Adverse events associated with concurrent chemoradiation therapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1209–17. doi: 10.1001/archoto.2009.174. [DOI] [PubMed] [Google Scholar]
- 3.Terrell JE, Fisher SG, Wolf GT. Long-term quality of life after treatment of laryngeal cancer. The Veterans Affairs Laryngeal Cancer Study Group. Arch Otolaryngol Head Neck Surg. 1998;124:964–71. doi: 10.1001/archotol.124.9.964. [DOI] [PubMed] [Google Scholar]
- 4.Mowry SE, Tang C, Sadeghi A, Wang MB. Standard chemoradiation versus intensity-modulated chemoradiation: a quality of life assessment in oropharyngeal cancer patients. Eur Arch Otorhinolaryngol. 2010;267(7):1111–6. doi: 10.1007/s00405-009-1183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckwalter AE, Karnell LH, Smith RB, Christensen AJ, Funk GF. Patient-reported factors associated with discontinuing employment following head and neck cancer treatment. Arch Otolaryngol Head Neck Surg. 2007;133:464–70. doi: 10.1001/archotol.133.5.464. [DOI] [PubMed] [Google Scholar]
- 6.Verdonck-de Leeuw IM, van Bleek WJ, Leemans CR, de Bree R. Employment and return to work in head and neck cancer survivors. Oral Oncol. 2010 Jan;46:56–60. doi: 10.1016/j.oraloncology.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;15(68):1110–20. doi: 10.1016/j.ijrobp.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 8.Lang K, Sussman M, Friedman M, et al. Incidence and costs of treatment-related complications among patients with advanced squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2009;135:582–8. doi: 10.1001/archoto.2009.46. [DOI] [PubMed] [Google Scholar]
- 9.Lang K, Menzin J, Earle CC, Jacobson J, Hsu MA. The economic cost of squamous cell cancer of the head and neck: findings from linked SEER-Medicare data. Arch Otolaryngol Head Neck Surg. 2004;130:1269–75. doi: 10.1001/archotol.130.11.1269. [DOI] [PubMed] [Google Scholar]
- 10.Groome PA, Schulze KM, Keller S, et al. Explaining socioeconomic status effects in laryngeal cancer. Clin Oncol (R Coll Radiol) 2006;18:283–92. doi: 10.1016/j.clon.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Kwok J, Langevin SM, Argiris A, Grandis JR, Gooding WE, Taioli E. The impact of health insurance status on the survival of patients with head and neck cancer. Cancer. 2010;116:476–85. doi: 10.1002/cncr.24774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway DI, McMahon AD, Smith K, et al. Components of socioeconomic risk associated with head and neck cancer: a population-based case-control study in Scotland. Br J Oral Maxillofac Surg. 2010;48:11–7. doi: 10.1016/j.bjoms.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Johnson S, McDonald JT, Corsten MJ. Socioeconomic factors in head and neck cancer. J Otolaryngol Head Neck Surg. 2008;37:597–601. [PubMed] [Google Scholar]
- 14.Andersen ZJ, Lassen CF, Clemmensen IH. Social inequality and incidence of and survival from cancers of the mouth, pharynx and larynx in a population-based study in Denmark, 1994–2003. Eur J Cancer. 2008;44:1950–61. doi: 10.1016/j.ejca.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Demiral AN, Sen M, Demiral Y, Kinay M. The effect of socioeconomic factors on quality of life after treatment in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:23–7. doi: 10.1016/j.ijrobp.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 16.Wong YK, Tsai WC, Lin JC, et al. Socio-demographic factors in the prognosis of oral cancer patients. Oral Oncol. 2006;42:893–906. doi: 10.1016/j.oraloncology.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Konski A, Berkey BA, Kian Ang K, Fu KK. Effect of education level on outcome of patients treated on Radiation Therapy Oncology Group Protocol 90-03. Cancer. 2003;98:1497–503. doi: 10.1002/cncr.11661. [DOI] [PubMed] [Google Scholar]
- 18.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. New Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 19.Molina MA, Cheung MC, Perez EA, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer. 2008;113:2797–806. doi: 10.1002/cncr.23889. [DOI] [PubMed] [Google Scholar]
- 20.Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116:1093–106. doi: 10.1097/01.mlg.0000224939.61503.83. [DOI] [PubMed] [Google Scholar]
- 21.Arbes SJ, Jr, Olshan AF, Caplan DJ, Schoenbach VJ, Slade GD, Symons MJ. Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States) Cancer Causes Control. 1999;10:513–23. doi: 10.1023/a:1008911300100. [DOI] [PubMed] [Google Scholar]
- 22.www.census.gov
- 23.Yin D, Morris C, Allen M, Cress R, Bates J, Liu L. Does socioeconomic disparity in cancer incidence vary across racial/ethnic groups? Cancer Causes Control. 2010 Jun 22; doi: 10.1007/s10552-010-9601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different races/ethnic groups. Cancer Causes and Control. 2001;12:703–11. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 25.Filion EJ, McClure LA, Huang D, et al. Higher incidence of head and neck cancers among Vietnamese American men in California. Head Neck. 2010 Jan 20; doi: 10.1002/hed.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez SL, Le GM, West DW, Satariano WA, O’Connor L. Hospital policy and practice regarding the collection of data on race, ethnicity, and birthplace. Am J Public Health. 2003;93:1685–8. doi: 10.2105/ajph.93.10.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keegan TH, John EM, Fish KM, Alfaro-Velcamp T, Clarke CA, Gomez SL. Breast cancer incidence patterns among California Hispanic women: differences by nativity and residence in an enclave. Cancer Epidemiol Biomarkers Prev. 2010;19:1208–18. doi: 10.1158/1055-9965.EPI-10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Hooijdonk C, Droomers M, Deerenberg IM, Mackenbach JP, Kunst AE. The diversity in associations between community social capital and health per health outcome, population group and location studied. Int J Epidemiol. 2008;37:1384–92. doi: 10.1093/ije/dyn181. [DOI] [PubMed] [Google Scholar]
- 29.van der Aa MA, Siesling S, Louwman MW, Visser O, Pukkala E, Coebergh JW. Geographical relationships between sociodemographic factors and incidence of cervical cancer in the Netherlands 1989–2003. Eur J Cancer Prev. 2008;17:453–9. doi: 10.1097/CEJ.0b013e3282f75ed0. [DOI] [PubMed] [Google Scholar]
- 30.Berglund A, Holmberg L, Tishelman C, Wagenius G, Eaker S, Lambe M. Social inequalities in non-small cell lung cancer management and survival: a population-based study in central Sweden. Thorax. 2010;65:327–33. doi: 10.1136/thx.2009.125914. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz K, Powell IJ, Underwood W, 3rd, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology. 2009;74:1296–302. doi: 10.1016/j.urology.2009.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rachet B, Quinn MJ, Cooper N, Coleman MP. Survival from cancer of the larynx in England and Wales up to 2001. Br J Cancer. 2008;99:S35–S37. doi: 10.1038/sj.bjc.6604581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2006 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2010. [Google Scholar]
- 34.Moles DR, Fedele S, Speight PM, Porter SR, dos Santos Silva I. Oral and pharyngeal cancer in South Asians and non-South Asians in relation to socioeconomic deprivation in South East England. Br J Cancer. 2008;98:633–5. doi: 10.1038/sj.bjc.6604191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conway DI, Petticrew M, Marlborough H, Berthiller J, Hashibe M, Macpherson LM. Socioeconomic inequalities and oral cancer risk: a systematic review and meta-analysis of case-control studies. Int J Cancer. 2008;122:2811–9. doi: 10.1002/ijc.23430. [DOI] [PubMed] [Google Scholar]
- 36.van Jaarsveld CH, Miles A, Wardle J. Pathways from deprivation to health differed between individual and neighborhood-based indices. J Clin Epidemiol. 2007;60:712–9. doi: 10.1016/j.jclinepi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Winkleby M, Cubbin C, Ahn D. Effect of cross-level interaction between individual and neighborhood socioeconomic status on adult mortality rates. Am J Public Health. 2006;96:2145–53. doi: 10.2105/AJPH.2004.060970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siahpush M, English D, Powles J. The contribution of smoking to socioeconomic differentials in mortality: results from the Melbourne Collaborative Cohort Study, Australia. J Epidemiol Community Health. 2006 Dec;60:1077–9. doi: 10.1136/jech.2005.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McFadden E, Luben R, Wareham N, Bingham S, Khaw KT. Occupational social class, educational level, smoking and body mass index, and cause-specific mortality in men and women: a prospective study in the European Prospective Investigation of Cancer and Nutrition in Norfolk (EPIC-Norfolk) cohort. Eur J Epidemiol. 2008;23:511–22. doi: 10.1007/s10654-008-9267-x. [DOI] [PubMed] [Google Scholar]