Abstract
The prefrontal cortex (PFC) is thought to modulate sensory signals in posterior cortices during top-down attention1,2, yet little is known about the underlying neural circuitry. Experimental and clinical evidence suggest that prefrontal dopamine plays an important role in cognitive functions3, acting predominantly through D1 receptors (D1Rs). Here we show that dopamine D1Rs mediate prefrontal control of signals within visual cortex. We pharmacologically altered D1R-mediated activity within the frontal eye field (FEF) of the PFC and measured its effects on the responses of neurons within visual cortex. This manipulation was sufficient to enhance the response magnitude, orientation selectivity and response reliability of neurons in area V4 to an extent comparable with the known effects of top-down attention. The observed enhancement in V4 signals was restricted to neurons with response fields (RFs) overlapping the part of visual space affected by the D1R manipulation. Altering D1R or D2R-mediated FEF activity increased saccadic target selection, but the D2R manipulation did not enhance V4 signals. Our results identify a role of D1Rs in mediating the control of visual cortical signals by the PFC and demonstrate how processing within sensory areas can be altered in mental disorders involving prefrontal dopamine.
Within the PFC, dopamine D1Rs are expressed by about one fourth of all neurons and are localized primarily in superficial and deep layers4–6. Microiontophoretic application of the selective D1R antagonist, SCH233907, at certain doses can increase the persistent, working memory related, component of single-neuron activity within dorsolateral PFC3,8,9. Given the PFC’s role in visual attention1,2, we hypothesized that D1Rs might also mediate the PFC’s top-down control of visual signals. If true, then changes in D1R-mediated PFC activity might be sufficient to modulate responses within posterior visual cortex, similar to what is observed during selective attention10. The PFC’s influence on visual cortex is achieved in part by the FEF1,11,12, an oculomotor area within posterior PFC. The FEF has a well-established role in saccadic target selection13, but recent evidence also implicates this area in the control of spatial attention2,14,15. To test our hypothesis, we locally infused16 small volumes (0.5–1 µL) of SCH23390 into sites within the FEF of monkeys performing fixation and eye movement tasks (Fig. 1 and Supplementary Fig. 1). We measured the effects of the FEF infusion on target selection using a free-choice, saccade task17. In this task, monkeys were rewarded for choosing between two saccadic targets, one located within the FEFRF and one in the opposite hemifield. In the same experiment, we recorded the visual responses of single neurons within extrastriate area V4 during fixation. In particular, we recorded neurons with RFs that overlapped the FEFRF. Thus, we tested the effects of the D1R manipulation both on visual cortical signals and on saccadic target selection.
Figure 1.
Local manipulation of D1R-mediated activity within the FEF during single neuron electrophysiology in area V4. a, Lateral view of the macaque brain depicts the location of a recording microsyringe within the FEF and of recording sites within area V4. Bottom diagram shows saccades evoked via electrical microstimulation at the infusion site (red traces) and the RF (green ellipse) of a recorded V4 neuron in an example experiment. b, Double-target, saccade task used to measure the monkey’s tendency to make saccades to a target within the FEFRF vs. one at an opposite location across varying temporal onset asynchronies. Positive asynchrony values denote earlier onset of FEFRF targets. Bottom plot shows the leftward shift in the PES, indicating more FEFRF choices, following infusion of SCH23390 into an FEF site. c, Visual responses of a V4 neuron with a RF that overlapped the FEFRF measured during passive fixation. The plot shows mean±S.E.M. visual responses to a bar stimulus presented at orthogonal orientations before (gray) and after (red) the infusion of SCH23390 at the FEF site.
We found that altering D1R-mediated activity at FEF sites increased the tendency of monkeys to choose targets appearing within the FEFRF (Fig. 1B). In the free choice task, the temporal onset of the two targets was systematically varied such that the FEFRF stimulus could appear earlier or later than the opposite stimulus. The monkey’s tendency to select the FEFRF target could then be measured as the temporal onset asynchrony required for equal probability of selecting either stimulus; we termed this the point of equal selection (PES). In the example experiment shown, the monkey chose the FEFRF target as often as the opposite target when the former appeared 76 ms earlier (PES=76). However, infusion of SCH23390 (0.85 µL) into the FEF reduced the PES by 23 ms (binary logistic regression, p=0.007), thereby increasing the proportion of FEFRF target choices.
In the same experiment, we also measured the responses of V4 neurons to oriented bars during fixation in a separate task (Fig. 1C, Supplementary methods). We found that the increase in target selection following the SCH23390 infusion was accompanied by an enhanced V4 neuronal response to oriented bars appearing within the overlapping V4RF and FEFRF. The example neuron shown was selective for orientation; it responded more to the 45° than to the 135° bar stimulus (p<10−3). Following the infusion of SCH23390, there was not only a significant increase in the overall visual response of this neuron, but also a significant increase in the differential response to the two orientations (Two-way ANOVA, SCH23390 effect, p<10−3; SCH23390-orientation interaction, p<10−3). Thus, the local perturbation of D1R-mediated FEF activity not only caused the monkey to select FEFRF stimuli as saccade targets more frequently, but it also led to enhanced and more selective visual responses of a V4 neuron representing the same part of space.
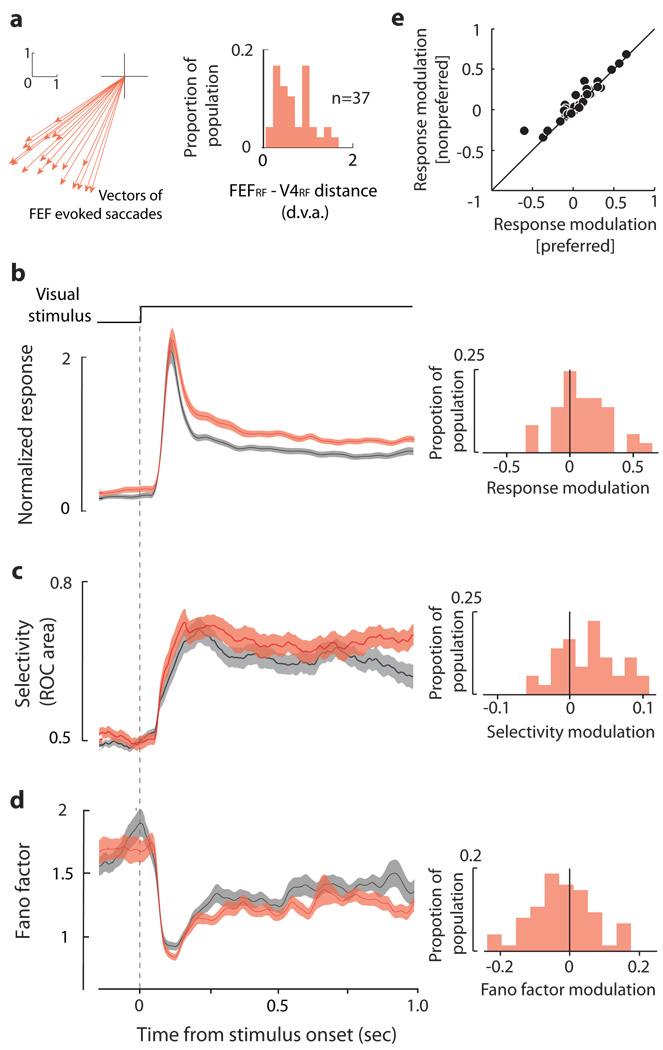
We studied the visual responses of 37 V4 neurons with RFs that overlapped the RFs of FEF infusion sites. The average (mean±SEM) distance between V4RF and FEFRF centers was 0.71±0.07 degrees of visual angle (Fig. 2A). As with the example neuron, we measured the responses of all neurons to oriented bars appearing in their RF during a 1-second fixation period (Fig. 2B). Prior to onset of the visual stimulus, there was a significant elevation in baseline activity following the D1R manipulation (Δbaseline=0.077±0.186, p=0.030). In addition to the baseline increase, the visually driven response of V4 neurons was enhanced by 17% above the control response (Δresponse=0.121±0.054, p=0.018). We confirmed that the enhancement in the visual response was not due to systematic changes in eye position during stimulus presentation (Supplementary Fig. 2). The enhancement of the visual response was independently significant for both preferred (Δpreferred=0.264±0.087; p=0.004) and for non-preferred stimuli (Δnon-preferred=0.132±0.062; p=0.032). In addition, there was an increase in the response difference between the preferred and non-preferred orientations (Δresponse difference=0.132±0.041; p=0.004) (Supplementary Fig. 3), suggesting an increase in orientation selectivity. To measure selectivity more quantitatively, we used a receiver-operating characteristic (ROC) analysis to quantify the degree to which each neuron’s responses could be used to judge stimulus orientation (Fig. 2C). This analysis confirmed that V4 neurons were more orientation selective following changes in D1R-mediated FEF activity (ΔROC area=0.035±0.009, p<10−3). The enhancement in V4 response magnitude and selectivity was accompanied by a decrease in the trial-to-trial variability of visual responses. We measured the variability of V4 responses across trials by computing the Fano factor (FF), which is the variance in the spike count divided by its mean. We found that the FF of V4 responses was reduced after the D1R manipulation (ΔFF=−0.105±0.045; p<10−3)(Fig. 2D)(Supplementary Fig. 4). All three V4 effects were comparable in magnitude to the known effects of top-down attention, and consistent with a multiplicative increase in the gain of visual signals18,19 (Fig. 2E).
Figure 2.
Manipulation of D1R-mediated activity enhances V4 visual signals. a, Average vectors of saccades evoked at all FEF sites that overlapped V4 RFs (left). Distribution of distances between the endpoint of evoked saccades and the centers of overlapping V4RFs for 37 V4 neurons. The mean normalized response magnitude (b), orientation selectivity (c) and response variability (FF) (d) of V4 neurons before (gray) and after (red) microinfusion of SCH23390 into the FEF. Shown are means ± S.E.M. within a 100-ms moving window measured during the 1-second RF stimulus presentation (top event plot). Histograms to the right of each response profile show the distributions of modulation indices for response magnitude (b), selectivity (c) and variability (d) across the population of neurons. e, Comparison of V4 response modulation following the SCH23390 infusion for preferred and non-preferred RF stimuli.
The effect of the D1R manipulation on saccadic target selection was highly consistent across the two monkeys tested. Of 21 double-target experiments, the PES was reduced in every case (Fig. 3A). The mean PES shifted in favor of the FEFRF stimulus by an average of 27 ms (ΔPES=−26.934±3.086, p<10−3), significantly increasing the overall proportion of FEFRF choices (Chi-square=80.60, p<10−3), thus indicating that the D1R manipulation increased the monkeys’ tendency to target FEFRF stimuli. The increase in target selection was apparent across a range of drug dosages (Supplementary Fig. 5). In addition to the D1R manipulation, we tested the effects of the D2R-agonist quinpirole. Previous studies using this drug found that it does not affect persistent activity, but rather increases saccade-related activity within dorsolateral PFC20. We found that, like the D1R effect, local manipulation of D2R-mediated FEF activity increased the selection of FEFRF targets (Fig. 3A). The PES shifted an average of 22 ms (ΔPES=−21.993±6.758, p=0.010), increasing the proportion of FEFRF choices (Chi-square= 13.86, p<10−3). Thus, the D1R and D2R-mediated manipulations of FEF activity resulted in equivalent increases in saccadic target selection.
Figure 3.
Changes in saccadic target selection and V4 visual responses. a, Scatter plot shows the consistent increase in FEFRF target choices (decrease in PES) after manipulation of both D1R (circles) and D2R-mediated (triangles) FEF activity. For both drug effects, the increase in FEFRF target selection was constant across a range of control PES values; the slope in the linear fit did not differ significantly from unity in either case (D1R: slope=0.96, p=0.552; D2R: slope=0.97, p=0.502). b, Changes in response magnitude, orientation selectivity and response variability (FF) following each drug manipulation. Changes shown are mean differences from pre-infusion values. Error bars denote S.E.M.. Single, double and triple asterisks denote significance at p<0.05, p<0.01, and p<0.001, respectively.
In spite of the increase in target selection, manipulation of D2R-mediated activity in FEF failed to enhance the responses of V4 neurons. We found no significant effect on the visual response magnitude, orientation selectivity or response variability of V4 neurons following the D2R manipulation (Δresponse=0.001±0.048, p=0.999; ΔROC area=−0.007±0.010, p=0.426; ΔFF=0.037±0.052, p=0.338; n=15) (Fig. 3B). Moreover, the changes in these measures were all significantly different from the changes we observed with the D1R manipulation (ΔresponseD2R<ΔresponseD1R, p=0.045; ΔselectivityD2R<Δ selectivityD1R, p=0.011; ΔFFD2R> ΔFFD1R, p=0.019). Thus, the equivalent effects of D1R and D2R on saccadic target selection were accompanied by contrasting effects in V4, in which the enhancement of visual signals was specific to D1R-mediated activity.
We also found that the enhancement of visual signals following the D1R manipulation was confined to V4 neurons with RFs that overlapped the FEFRF. For V4 neurons with RFs that did not overlap the FEFRF (mean distance between V4RF and FEFRF=9.00±0.86 d.v.a.; n=15), we found no significant effect of the D1R manipulation on response magnitude (Δresponse=−0.028±0.087, p=0.9780), orientation selectivity (ΔROCarea=−0.017±0.010, p=0.187) or the FF (ΔFF=0.010±0.043, p=.688). Importantly, the changes in these measures were all significantly different from the changes observed in neurons with overlapping RFs (Δresponsenon-overlap.<Δresponseoverlap, p=0.044; Δselectivitynon-overlap<Δselectivityoverlap, p=0.007; ΔFFnon-overlap>ΔFFoverlap, p=0.034) (Fig. 3B). Thus, the enhancement in visual cortical signaling produced by the manipulation of D1R-mediated FEF activity was spatially specific.
We also tested the effects of complete inactivation of FEF sites on the responses of V4 neurons with overlapping RFs. Previous studies have shown that local inactivation of the FEF disrupts saccadic target selection and impairs attention17,21. We therefore wondered if inactivation could reduce the components of V4 responses that were enhanced by the D1R manipulation. We locally inactivated FEF sites with the GABAA agonist muscimol. Unlike the sparsely localized D1Rs, GABAA receptors are widely expressed by all neurons in all cortical layers22. As in previous studies, local inactivation of FEF sites with muscimol decreased the targeting of FEFRF stimuli. FEF inactivation also significantly reduced V4 orientation selectivity (ΔROC area=−0.030±0.011, p=0.003; n=33). However, the inactivation did not change the response magnitude or variability of V4 neurons (Δresponse= 0.016±0.061, p=0.809; ΔFF=−0.002±0.023, p=0.921) (Fig. 3B). Thus, in contrast to the D1R manipulation, which altered all 3 components of V4 activity, complete inactivation altered only one. All 3 inactivation effects were significantly different from the D1R effects (Δresponsemuscimol<ΔresponseD1R, p=0.024; Δselectivitymuscimol<ΔselectivityD1R, p<10−3; ΔFFmuscimol>ΔFFD1R, p=0.007). Although the reduction in orientation selectivity is consistent with previous electrical microstimulation studies12, and with the effects of inactivation on orientation discrimination21, the lack of a reduction in response magnitude may seem less congruent. However, we suggest that this difference is due to variation between experimental paradigms (Supplemental discussion). Finally, we tested for any effects of vehicle (saline) infusions into the FEF. The infusion of saline failed to change the response magnitude, selectivity or variability of V4 neurons (Δresponse= 0.018±0.048, p=0.380; ΔROC area=−0.010±0.013, p=0.569; ΔFF=−0.035±0.061, p=0.179; n=12) (Fig. 3B). All 3 measures were significantly different from the D1R effects (Δresponsesaline<ΔresponseD1R, p=0.045; Δselectivitysaline<ΔselectivityD1R, p=0.013; ΔFFsaline>ΔFFD1R, p=0.009).
Our results identify prefrontal D1Rs as a component of the neural circuitry controlling signals within visual cortex. Manipulation of D1R-mediated FEF activity was sufficient to enhance the magnitude, reliability and visual selectivity of neuronal responses within area V4, three known effects of visual attention. The observed enhancement might account for the benefits in visually guided behavior that accompany attentional deployment (Supplementary Fig. 6), though a causal link between attentional modulation of visual cortical signals and visual perception remains to be established. Our results demonstrate how visual representations within posterior areas can be altered merely by changes in dopamine tone within the PFC. Given the complex effects of dopamine through D1Rs, one might predict that at “optimum” dopamine levels9, optimal effects of top-down control of visual cortical signals, would be achieved.
The circuitry underlying top-down control of visual cortex likely involves a number of different neuromodulators e.g. 23 and an array of different brain structures e.g. 24. Our results show that this circuitry involves prefrontal dopamine acting via D1Rs. Within dorsolateral PFC, dopamine D1Rs are thought to modulate recurrent glutamatergic connections, thereby influencing working memory related activity in this area25,26. The present results suggest that D1Rs contribute to the FEF’s control of visual signals by an analogous mechanism, namely by modulating (long-range) recurrent connections between the FEF and visual cortex (Supplementary Fig. 7). Since superficial layer FEF neurons are reciprocally connected with neurons in V42,27, dopaminergic modulation of these connections via superficial layer D1Rs would be expected to mediate the FEF’s control of V4 signals. The specificity of V4 effects to D1Rs, rather than D2Rs, might be explained by the relative absence of D2Rs in superficial layers of prefrontal cortex4-6. The equivalent target selection effects of D1R and D2R manipulations might be explained by the presence of both receptor subtypes within infragranular layers of cortex4-6, where layer V FEF neurons project to the superior colliculus27. Importantly, impairments in saccadic control are prominent among the set of impairments exhibited in attention deficit/hyperactivity disorder (ADHD)28. The observed influence of prefrontal D1Rs on saccadic target selection and visual cortical signals, combined with their known influence on persistent activity, may explain the behavioral links between saccadic control, attention and working memory29, and the coincidence of their corresponding impairments in ADHD30.
Methods
The effects of pharmacologic perturbations of FEF activity on target selection and the visual responses of V4 neurons was studied in three macaque monkeys performing fixation and eye movement tasks (supplementary information). All experimental procedures were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Society for Neuroscience Guidelines and Policies, and approved by the Stanford University Animal Care and Use Committee. Eye position was monitored with a scleral search coil. In each experiment, we infused small volumes of drug into sites within the FEF through a surgically implanted titanium chamber overlaying the arcuate sulcus using a custom-made recording microinjectrode. We identified FEF sites by eliciting short-latency, fixed-vector saccadic eye movements with trains (50 – 100 ms) of biphasic current pulses (≤50 µA; 250 Hz; 0.25 ms duration). In the same experiment, recordings from V4 neurons were made through a chamber overlaying the prelunate gyrus. Response fields (RF) of V4 neurons were all located in the lower quadrant of the contralateral hemifield (<12° eccentricity). The position of the FEF microinjectrode was adjusted so that the saccade elicited by FEF microstimulation either shifted the monkey’s gaze to within the V4 RF (overlapping) or far outside of it (non-overlapping).
Supplementary Material
Acknowledgements
We thank Doug S. Aldrich for technical assistance, N. Steinmetz for help with the Fano factor analysis, and W.T. Newsome, E.I. Knudsen, K. M. Armstrong, and R.F. Squire for valuable comments on the manuscript. This work was supported by NIH EY014924, NSF IOB-0546891, The McKnight Foundation, and an IBRO Fellowship to B.N.
Footnotes
Contributions:
B.N. designed and performed experiments, analysed data and wrote the paper; T.M. designed and performed experiments and wrote the paper.
Competing financial interests:
The authors declare no competing financial interests.
References
- 1.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 2.Noudoost B, Chang MH, Steinmetz NA, Moore T. Top-down control of visual attention. Curr. Opin. Neurobiol. 2010;20:183–190. doi: 10.1016/j.conb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- 5.Lidow MS, Wang F, Cao Y, Goldman-Rakic PS. Layer V neurons bear the majority of mRNAs encoding the five distinct dopamine receptor subtypes in the primate prefrontal cortex. Synapse. 1998;28:10–20. doi: 10.1002/(SICI)1098-2396(199801)28:1<10::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex. 2009;19:849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- 7.Bourne JA. SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS. Drug Rev. 2001;7:399–414. doi: 10.1111/j.1527-3458.2001.tb00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 9.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu. Rev. Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- 11.Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 13.Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature. 1993;366:467–469. doi: 10.1038/366467a0. [DOI] [PubMed] [Google Scholar]
- 14.Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J. Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong KM, Chang MH, Moore T. Selection and maintenance of spatial information by frontal eye field neurons. J. Neurosci. 2009;29:15621–15629. doi: 10.1523/JNEUROSCI.4465-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noudoost B, Moore T. A reliable microinjectrode system for use in behaving monkeys. J. Neurosci. Methods. 2011;194:218–223. doi: 10.1016/j.jneumeth.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiller PH, Tehovnik EJ. Cortical inhibitory circuits in eye-movement generation. Eur. J. Neurosci. 2003;18:3127–3133. doi: 10.1111/j.1460-9568.2003.03036.x. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 19.McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J. Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 21.Monosov IE, Thompson KG. Frontal eye field activity enhances object identification during covert visual search. J. Neurophysiol. 2009;102:3656–3672. doi: 10.1152/jn.00750.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huntsman MM, Isackson PJ, Jones EG. Lamina-specific expression and activity-dependent regulation of seven GABAA receptor subunit mRNAs in monkey visual cortex. J. Neurosci. 1994;14:2236–2259. doi: 10.1523/JNEUROSCI.14-04-02236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrero JL, et al. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 26.Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nat. Neurosci. 2000;(3 Suppl):1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- 27.Pouget P, et al. Visual and motor connectivity and the distribution of calcium-binding proteins in macaque frontal eye field: implications for saccade target selection. Front Neuroanat. 2009;3:2. doi: 10.3389/neuro.05.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munoz DP, Armstrong IT, Hampton KA, Moore KD. Altered control of visual fixation and saccadic eye movements in attention-deficit hyperactivity disorder. J. Neurophysiol. 2003;90:503–514. doi: 10.1152/jn.00192.2003. [DOI] [PubMed] [Google Scholar]
- 29.Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 2006;10:124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat. Rev. Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.