Abstract
Objective
Tetrahydrobiopterin (BH4) is a critical cofactor for Nitric Oxide (NO) synthesis by NO synthase (NOS). Recently, we demonstrated that disturbed flow produced by partial carotid ligation decreases BH4 levels in vivo. We therefore aimed to determine whether atherosclerosis induced by disturbed flow is due to BH4 deficiency and NOS uncoupling and whether increasing BH4 would prevent endothelial dysfunction, plaque inflammation and atherosclerosis.
Methods and Results
We produced a region of disturbed flow in ApoE−/− mice using partial carotid ligation and fed these animals a high-fat diet. This caused eNOS uncoupling as characterized by increased vascular superoxide production, altered vascular reactivity and a change in eNOS migration on low-temperature gel. These perturbations were accompanied by severe atherosclerosis, infiltration of T cells and macrophages, and an increase in cytokine production. Treatment with BH4 recoupled NOS, decreased superoxide production, imporoved endothelium-dependent vasodilatation and virtually eliminated atherosclerosis. BH4 treatment also markedly reduced vascular inflammation and improved the cytokine milieu induced by disturbed flow.
Conclusions
Our results highlight a key role of BH4 deficiency and NOS uncoupling in atherosclerosis induced by disturbed flow, and provide insight into the effect of modulating vascular BH4 levels on atherosclerosis and inflammation at these sites of the circulation.
Keywords: tetrahydrobiopterin, NOS uncoupling, atherosclerosis, disturbed flow, inflammation
Introduction
Disturbances of blood flow, such as low and oscillatory shear stress, often exist at branch points and curved regions of the circulation such as the aortic arch1, 2. Human and experimental atherosclerosis occurs preferentially at these sites of flow alteration. In contrast, laminar shear stress, which exists in straight portions of arteries, inhibits atherosclerotic lesion formation3. One mechanism involved in shear regulation of atherosclerosis is the endothelial production of nitric oxide (NO). Laminar shear stress acutely stimulates endothelial production of NO and over the long term enhances endothelial NO synthase (NOS) gene expression4. NO inhibits atherosclerosis by suppressing inflammation, inhibiting platelet aggregation and leukocyte adhesion, and preventing smooth muscle cell proliferation5.
Tetrahydrobiopterin (BH4) is a critical cofactor for all three isoforms of NOS, and is involved in the reduction of the heme iron of the enzyme to ultimately form an iron-oxy species that hydroxylates L-arginine to produce NO. In the absence of BH4, the NOS enzymes produce superoxide rather than NO, a situation referred to as NOS uncoupling6 BH4 deficiency and NOS uncoupling have been shown to be present in experimental vascular disease models of hypercholesterolemia and atherosclerosis7, and in humans with hypercholesterolemia8.
The rate-limiting enzyme for de novo synthesis of BH4 is GTP cyclohydrolase I (GTPCH-1). The GTP cyclohydrolase feedback regulatory protein (GFRP) is an important modulator of GTPCH-1 enzyme activity and BH4 promotes inhibition of GTPCH-1 by GFRP in a negative feedback fashion9. We have demonstrated that in human endothelial cells, laminar shear stress stimulates GTPCH-1 phosphorylation and this dramatically increases endothelial cell BH4 levels by reducing the binding of GTPCH-1 and GFRP10. In contrast, oscillatory shear only modestly affects GTPCH-1 activity and BH4 levels. In accordance to this finding in cultured endothelial cells, we found that disturbed flow in vivo reduces GTPCH-1 phosphorylation and BH4 production11. In addition, disturbed flow also increases BH4 oxidation, likely attributable to increased reactive oxygen species (ROS) production caused by oscillatory shear stress. The precise role of NOS uncoupling in the development of atherosclerotic lesions at sites of disturbed flow remains undefined.
The purpose of this study was therefore to determine whether atherosclerosis induced by disturbed flow is due to low levels of BH4 and NOS uncoupling, and to determine if increasing BH4 prevents endothelial dysfunction, plaque inflammation and atherosclerosis. To perform these studies, we used an in vivo model of disturbed flow by inducing partial carotid ligation in hyperlipidemic mice12. This model is associated with rapid atherosclerotic lesion formation and facilitates the examination of therapeutic interventions.
Methods
Reagents and materials
BH4 and 5,6,7,8-tetrahydro-D-neopterin (NH4) were from Schircks Laboratories (Switzerland). All other biochemicals were purchased in the highest available grade from Sigma-Aldrich (St Louis, MO). Live/Dead Fixable Near-IR stain and PE-TR rat anti-mouse CD45 antibody were from Invitrogen (Carlsbad, CA). PE Cy7 rat anti-mouse F4/80 antibody was from eBiosciences (San Diego, CA). All other antibodies for flow cytometry were from BD Biosciences (San Jose, CA). The eNOS antibody used for Western blot was from BD Biosciences (San Jose, CA).
Animals studied
The Institutional Animal Care and Use Committee at Emory University approved all experimental protocols. C57Bl/6 and ApoE−/− mice were obtained from Jackson Laboratories. Partial ligation of left common carotid artery (LCA) was carried out at 8 weeks of age as previously described13 to create low and oscillatory shear stress in the LCA. The right common carotid artery (RCA) was not ligated and used as a control.
To augment vascular levels of BH4, this pterin was added to the drinking water to provide an approximate dose of 10 mg/kg per day. To minimize oxidation of BH4 in the drinking water, 0.04% vitamin C was also added. In preliminary experiments, we found that this prevented BH4 oxidation for up to 24 hours. As a vehicle control, mice not receiving BH4 received only vitamin C in the drinking water. In some experiments, mice were also treated with 10 mg/kg NH4 per day. Treatment with BH4, NH4 or vehicle was started three days before the carotid ligation. Mice were fed a high fat diet containing 45% kcal% fat (OpenSource Diets, New Brunswick, NJ) immediately following ligation until sacrificed.
Plasma lipid analysis
Blood was aspirated directly into syringes containing heparin via cardiac puncture. Plasma was obtained through centrifugation of blood for 15 minutes at 5500 g and 4°C, and then stored at −20°C until each assay was performed. Lipid analysis was performed commercially (Cardiovascular Specialty Laboratories, Atlanta, GA). All lipid determinations were performed using a Beckman CX7 chemistry analyzer and reagents from Beckman Diagnostics (Fullerton, CA) for total cholesterol and triglycerides.
BH4 measurements by HPLC
Tissue biopterins (BH4 and more oxidized species) were measured by HPLC as previously described10. Carotids were homogenized with lysis buffer (50mM Tris-HCl, 1mM DTT, 1mM EDTA) and oxidized by exposure to 1% I2 and 2% KI at room temperature for 1 hour under dark conditions. Ascorbic acid was added to stop the reaction, and the mixture was then centrifuged for 10 minutes at 12,000 g. Biopterins in the supernatant were quantified by HPLC on a C18 column with fluorescence detection.
Measurement of vascular superoxide production
Carotid superoxide production was quantified by measuring formation of 2-hydroxyethidium from dihydroethidium (DHE) by HPLC. This product specifically reflects the reaction of superoxide with DHE as previously validated14 To localize superoxide within the vascular wall, we employed DHE staining. As previously described frozen sections (30 µm) obtained from LCA and RCA of partially ligated mice were stained in 2 µmol/L DHE for 30 min at 37°C13. Slides were mounted with DAKO mounting media and immediately imaged with a Zeiss LSM 510 META confocal microscope.
Western blot analysis for eNOS dimers/monomers
Low-temperature Western blot was performed to detect eNOS dimers and monomers as described previously15.
Assessment of atherosclerosis by Oil red O staining
Mice were sacrificed and perfused at physiological pressure with saline containing heparin. The left and right carotid arteries were removed en bloc with the trachea and esophagus. For frozen sections, tissue was embedded in Tissue-Tek optimum cutting temperature medium, frozen in liquid nitrogen, and stored at −80°C until stained. Oil red O staining was carried out using frozen sections as previously described13 and nuclei were stained with hematoxylin. Images were captured with a Zeiss epifluorescence microscope. Images were analyzed with Image J software to quantify lesion size as previously described13. For en face oil red O staining to examine intercostal artery lesions, the aortas were removed, cleaned, and opened longitudinally with the luminal surface facing up. Aortas were rinsed with 60% isopropanol for 1 min. Staining was then performed with filtered Oil red O solution (Cayman Chemical, Ann Arbor, Michigan) for 30 min. Vessels were then rinsed with 60% isopropanol and pinned to black wax to reveal the entire luminal surface area. Images were obtained using a camera connected to a Zeiss dissecting microscope.
Vascular isometric tension studies
Endothelium-dependent and independent vasodilatation was examined using carotid rings obtained from LCA and RCA of partially ligated ApoE−/− mice as previously described13. Isometric tension was measured using Multi Wire Myograph System Model 610M (DMT). After equilibrating for at least 30 minutes, the rings were preconstricted using phenylephrine. After a stable contraction was achieved, the rings were exposed to acetylcholine to assess endothelium-dependent vasodilation. Endothelium-independent relaxation to sodium nitroprusside (SNP) was also examined.
Flow cytometry for measurement of vascular inflammatory cells
Mouse carotids were minced with fine scissors and digested using collagenase type IX (125 U/ml), collagenase type I (450 U/ml), and hyaluronidase I (60 U/ml) dissolved in PBS containing calcium and magnesium for 1 hr at 37°C, with agitation every 20 minutes. Digested samples were then homogenized using an 18 gauge needle yielding single cell suspensions. Cells were then centrifuged at 1,200 rpm and resuspended in FACS buffer. Cells were stained for 30 min at 4°C with antibodies and then washed and resuspended in FACS buffer. Antibodies used for staining were FITC rat anti-mouse CD4, PE-TR rat anti-mouse CD45, PerCP-Cy5.5 rat anti-mouse CD3, V450 rat anti-mouse CD8, APC rat anti-mouse CD44, APC Cy7 rat anti-mouse CD62L, FITC rat anti-mouse CD45, PerCP-Cy5.5 rat anti-mouse cd11c, APC rat anti-mouse cd11b, PE rat anti-mouse CD86, PE Cy7 rat anti-mouse F4/80, and Live/Dead Fixable Near-IR stain. Absolute cell counting was performed by adding a known quantity of calibration beads to a known sample volume. After staining, cells were analyzed immediately on a LSR-II flow cytometer with DIVA software (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, Inc.).
Cytokine measurements
Mouse carotids were placed into each well of a 96-well plate containing 60 µL DMEM with 10% fetal calf serum, 100 U/ml penicillin, and 100 µg/mL streptomycin and maintained at 37°C under 5% CO2 for 16 hours. During this time, a combination of phorbol myristate acetate (PMA, 10 µmol/L) and ionomycin (2 µmol/L) was added to stimulate vascular cytokine production. Following this, levels of cytokines TNF-α, IFN-γ, and IL-2 in the media were measured using cytokine bead array kit (BD Biosciences).
Statistical Analysis
Data are expressed as mean ± standard error of the mean. When two groups were compared, unpaired t tests were employed. ANOVA was employed for multiple comparisons and when significance was indicated, a two-tailed Bonferroni post hoc test was used to make selected comparisons. When one group was served as a control, the Dunnett post hoc test was used. A value of p<0.05 was considered significant.
Results
Disturbed flow induced by partial carotid ligation causes NOS uncoupling
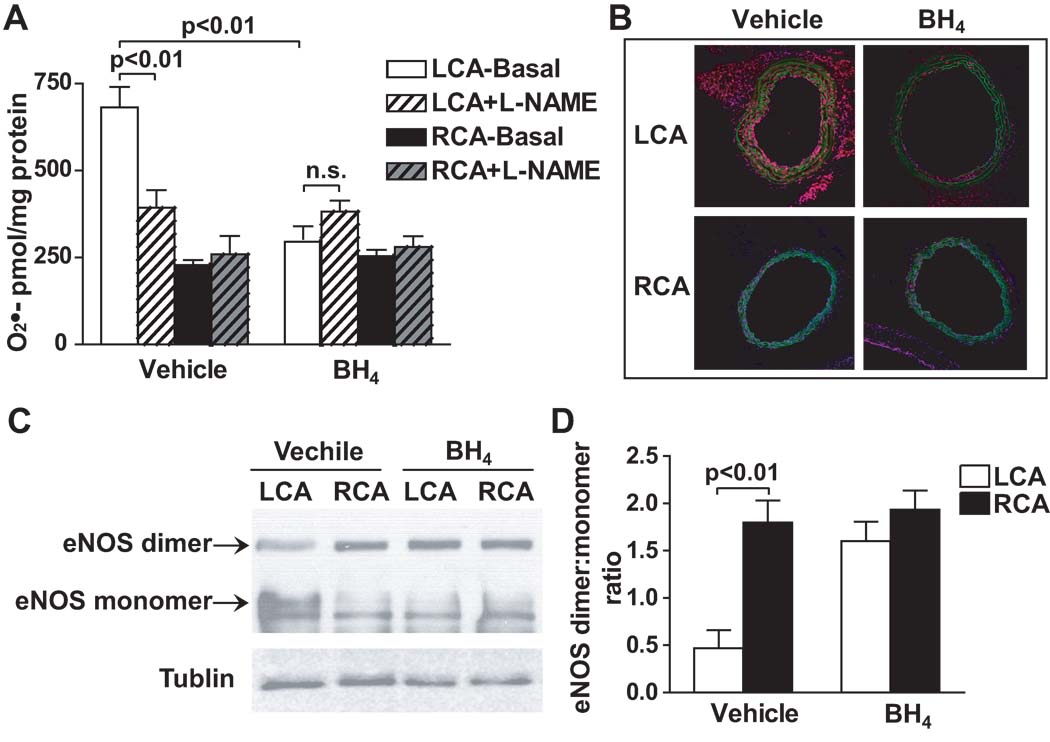
We have previously shown that partial carotid ligation reduces total biopterin production and increases BH4 oxidation to BH2 in the LCA of the C57bl/6 mice11. In the present study, we confirmed that this also occurred in ApoE−/− mice fed a high fat diet. In these animals, total biopterin and BH4 were markedly reduced in the LCA after 1 week of ligation (Supplemental Figure IA through IC). Notably, the BH4/BH2 ratio in the LCA was lower than the RCA, indicating the higher oxidative stress in the LCA induced by disturbed flow (Supplemental Figure ID). We hypothesized that these defects in BH4 synthesis and stability could cause NOS uncoupling. In keeping with this, in ApoE−/− mice that underwent carotid ligation and were treated with vehicle alone for one week, vascular superoxide production by the LCA was increased two-fold compared to the RCA. The NOS inhibitor NG-L-nitro-arginine methyl ester (L-NAME) inhibited superoxide production in the LCA, suggesting NOS was uncoupled and was a source of superoxide (Figure 1A). To localize superoxide production within the vessel wall, we also employed DHE staining. DHE staining was significantly increased not only in the endothelium, but also in the medium and adventitia of the LCA in vehicle treated animals (Figure 1B). Consistent with these results, low-temperature Western blot for eNOS revealed that in vehicle treated animals, carotid ligation decreased the formation of intact eNOS dimers and increased monomer formation, indicating uncoupling of eNOS (Figure 1C and 1D).
Figure 1.
Identification of NOS uncoupling at areas of disturbed flow. ApoE−/− mice underwent partial carotid ligation and were fed a high fat diet for one week. Mice were further randomized to receive either oral BH4 or vehicle in the drinking water. A. During incubation with DHE ex vivo, vessels were co-incubated with and without L-NAME. and formation of 2-hydroxyethidium was subsequently measured by using HPLC (n=6). Values were compared using ANOVA and selected comparisons were made using a Bonferroni post hoc test. B. Representative images of DHE fluorescence (red) in frozen sections of carotid arteries. Autofluorescence of the elastic laminas is green. C. Representative western blot for eNOS dimer/monomer in the carotids using a low-temperature gel. D. Densitometry ratios for eNOS dimers and monomers (n=3). Values were compared using ANOVA and selected comparisons were made using a Bonferroni post hoc test.
Effect of BH4 treatment on NOS uncoupling and vascular superoxide production at areas of disturbed flow
We treated mice with BH4 in the drinking water to examine if increasing BH4 levels can reverse NOS uncoupling and decrease superoxide levels at areas of disturbed flow. BH4 treatment for 1 week normalized the total biopterin and BH4 levels in the LCA of the ApoE−/− mice (Supplemental Figures IA and IC). This confirmed that oral BH4 supplementation increased vascular BH4 levels. BH4 treatment also reduced superoxide production in the LCA while not affecting RCA superoxide levels. Moreover, in BH4 treated mice, L-NAME increased superoxide production, suggesting that BH4 treatment was able to recouple NOS in the setting of disturbed flow (Figure 1A). Using DHE staining, we found that BH4 treatment markedly reduced superoxide production in all three layers of the vessel wall (Figure 1B). In keeping with this, BH4 treatment also normalized the ratio of eNOS dimers to monomers observed on low-temperature Western blots (Figure 1C and 1D). Taken together, these results indicate that NOS uncoupling enhances vascular superoxide production at areas of disturbed flow and that this can be ameliorated by BH4 treatment.
Effect of NOS uncoupling on atherosclerosis at areas of disturbed flow
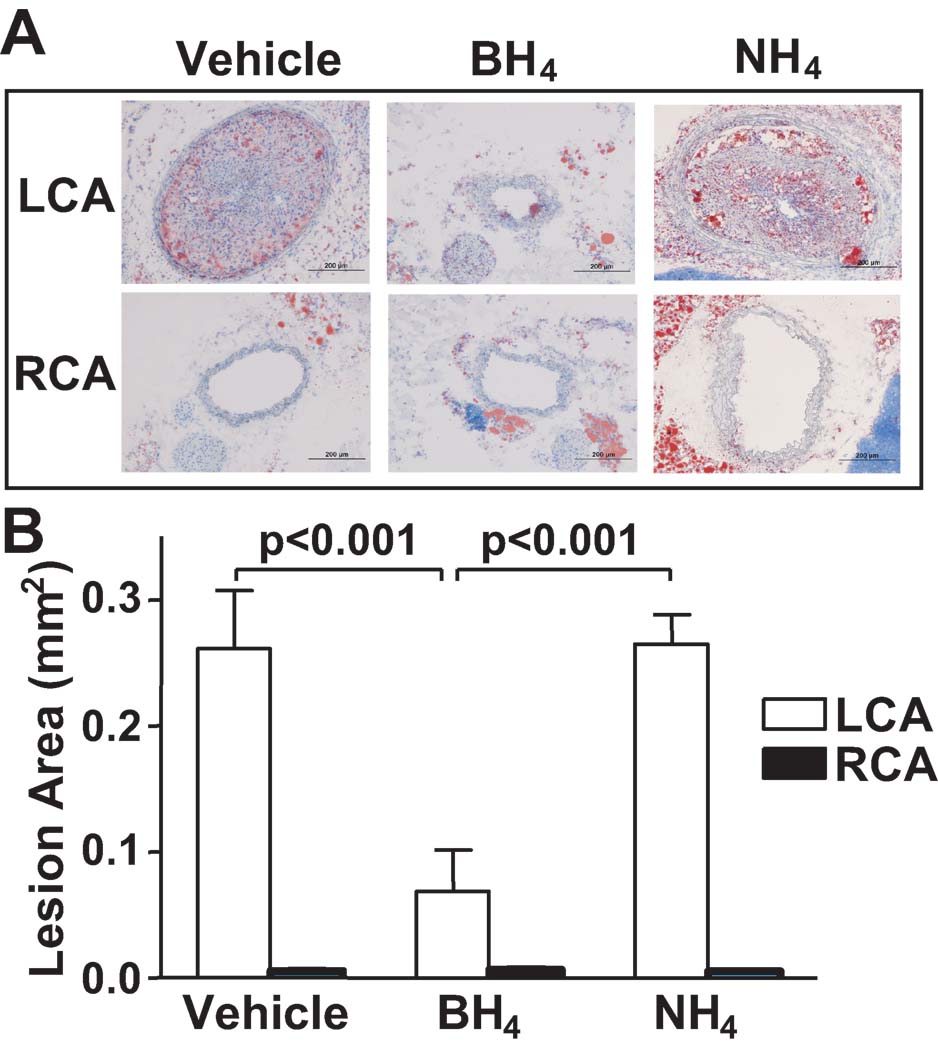
We next sought to determine the role of NOS uncoupling on the severity of atherosclerosis at sites of disturbed flow. Nam at al. has previously shown that partial carotid ligation in ApoE−/− mice fed a high fat diet causes accelerated atherosclerosis within 3 weeks.13 In keeping with this, Oil red O staining showed that the left carotid artery (LCA) of vehicle treated mice developed severe atherosclerosis. In striking contrast, BH4 treatment for 3 weeks virtually eliminated atherosclerotic lesion formation in the LCA (Figure 2A and 2B). The unligated right carotid artery (RCA) did not develop atherosclerotic lesions in this time frame. BH4 treatment for 3 weeks did not affect plasma cholesterol levels of the ApoE−/− mice fed a high fat diet for 3 weeks (Supplemental Figure II). To exclude the possibility that the benefit of BH4 was due to a non-specific anti-oxidant effect, we also treated mice with 5,6,7,8-tetrahydro-D-neopterin (NH4). NH4 has a similar redox potential to BH4, but does not sustain NOS catalysis. In contrast to BH4 treatment, oral treatment with NH4 did not affect atherosclerosis development (Figure 2A and 2B). Taken together, these findings indicate that NOS uncoupling plays a major role in promoting atherosclerosis at sites of disturbed flow and that this is markedly reduced by oral administration of BH4. The benefit of BH4 is likely due to improved NOS catalysis rather than direct anti-oxidant properties.
Figure 2.
Role of NOS uncoupling on atherosclerosis induced by partial carotid ligation. ApoE−/− mice underwent partial carotid ligation, fed a high fat diet and were treated with BH4, 5,6,7,8-tetrahydro-D-neopterin (NH4) or vehicle for 3 weeks. A. Representative images of Hematoxylin and Oil red O staining of the carotid frozen sections. B. Quantification of the intimal lesion areas using Image J (n=6). Values were compared using ANOVA and selected comparisons were made using a Bonferroni post hoc test.
Prior studies of ApoE−/− mice have shown that atherosclerosis preferentially develops at the origin of the brachiocephalic and intercostal arteries, areas were shear stresses are likely reduced3,16 In keeping with these prior observations, we found that high fat feeding induced small lesions in the brachiocephalic artery and in some of the intercostal artery branches of the aorta. These were reduced by BH4 treatment (Supplemental Figure III).
Effect of NOS uncoupling on endothelial dysfunction induced by partial ligation
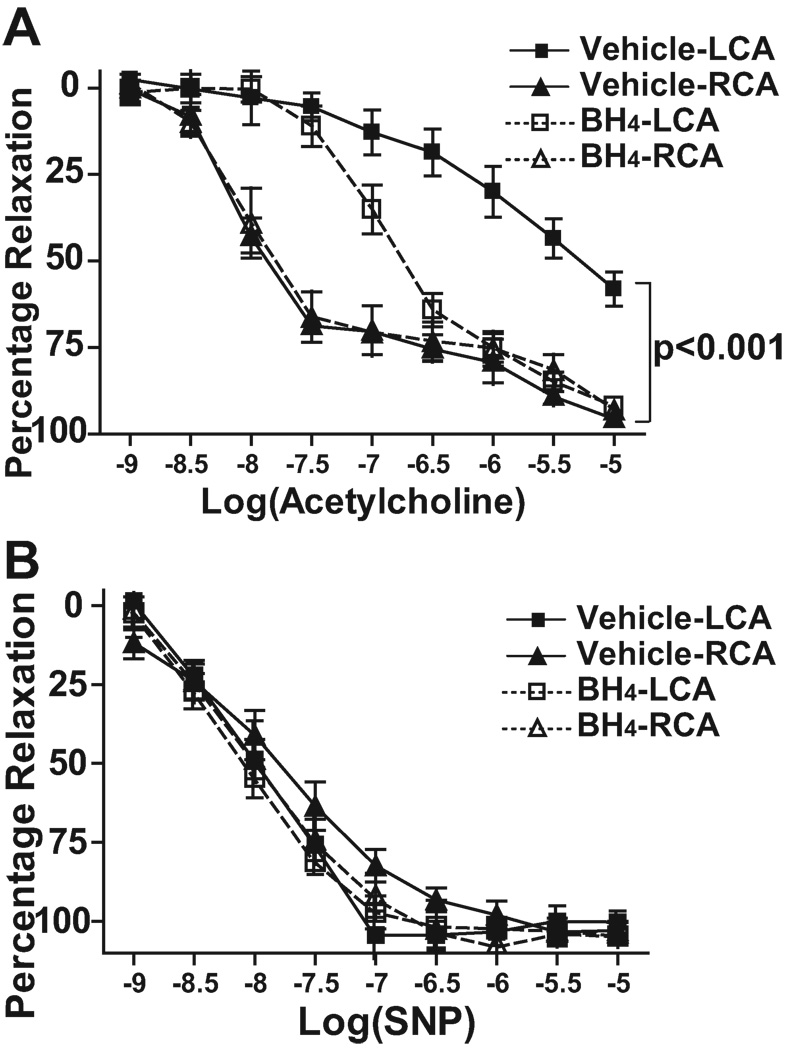
Endothelial dysfunction is an early marker for atherosclerosis17. A defect in BH4 results in NOS uncoupling and reduces production of NO, leading to impaired endothelium-dependent vasodilation. We therefore sought to determine if the endothelial dysfunction observed at sites of disturbed flow was due to NOS uncoupling. Carotid rings were obtained from LCA and RCA of partially ligated ApoE−/− mice fed a high fat diet for 1 week. Carotid rings were pre-constricted with phenylephrine and the relaxation evoked by increasing concentrations of acetylcholine was examined. In the unligated RCA, acetylcholine elicited potent relaxations reaching 90% of the pre-constricted tension at EC50 of 10−8 mol/L (Table 1). LCA segments from vehicle treated mice exhibited significantly blunted relaxations to acetylcholine both in terms of peak relaxation and the EC50. BH4 treatment normalized the peak relaxation to acetylcholine and improved but did not normalize EC50 (Figure 3A and Table 1). Endothelium-independent relaxation in response to the NO donor sodium nitroprusside (SNP) was identical among all the groups (Figure 3B). Thus, the alteration of endothelium-dependent vasodilatation observed at areas of disturbed flow is largely related to BH4 deficiency and NOS uncoupling.
Table 1.
EC50 and peak relaxation values of LCA and RCA of ApoE−/− mice treated with vehicle or BH4 in response to acetylcholine.
| Experimental groups | EC50 Log M[Ach] | Peak relaxation (%) |
|---|---|---|
| Vehicle-LCA | −5.82 ± 0.64* | 58.1 ± 14.0† |
| Vehicle-RCA | −8.33 ± 0.64 | 95.3 ± 3.1 |
| BH4-LCA | −6.80 ± 0.34¶ | 92.0 ± 3.3 |
| BH4-RCA | −8.09 ± 0.34 | 93.1 ± 4.8 |
p<0.001 vs. RCA,
p<0.01 vs. RCA,
p<0.05 vs. Vehicle-LCA (n=6–8).
Figure 3.
Role of NOS uncoupling on endothelial dysfunction induced by partial carotid ligation. ApoE−/− mice underwent partial carotid ligation and were fed a high fat diet for 1 week. The animals were randomized to receive oral BH4 or vehicle following carotid ligation. Arterial rings were obtained from LCA and RCA of these mice for vascular ring activity measurements. A. Rings were pre-constricted with phenylephrine and responses evoked by increasing concentrations of acetylcholine for endothelium-dependent relaxation (n=6–8). B. Responses to increasing concentrations of sodium nitroprusside (SNP) were studied to assess endothelium-independent relaxation (n=6–8). Values were compared using repeated measures ANOVA and selected comparisons were made using a Bonferroni post hoc test.
Role of NOS uncoupling on infiltration of activated immune cells at sites of disturbed flow
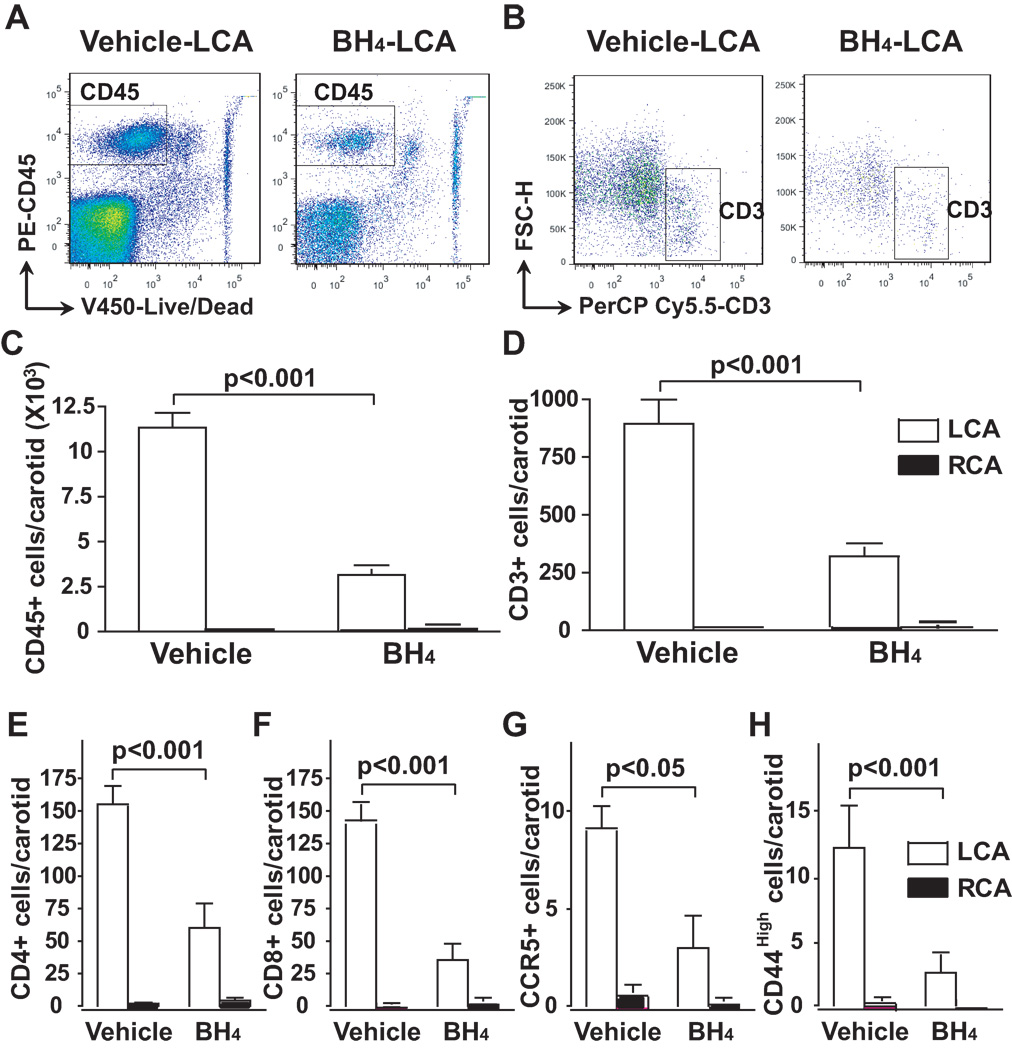
Inflammatory cells, including T cells, antigen-presenting dendritic cells and macrophages are present in atherosclerotic lesions and are thought to promote the progression of atherosclerosis and plaque instability by releasing inflammatory cytokines.18 We therefore examined the role of NOS uncoupling in this inflammatory response in regions of disturbed flow. Using flow cytometry, we found that partial ligation of the LCA for 1 week caused marked infiltration of total leukocytes and T cells, characterized by the surface markers, CD45+ and CD3+, respectively. BH4 treatment reduced the number of leukocytes and T cells in the LCA (Figure 4A to 4D). Within the T cell population, we found that BH4 treatment reduced both CD4+ and CD8+ cells in the LCA (Figure 4E and 4F). In addition to reducing the total number of T cells in the LCA, BH4 also decreased the number of activated T cells, as indicated by a reduced number of CCR5+ and CD44High cells (Figure 4G and 4H). Immune cells in the RCA were virtually undetectable. Of note, BH4 treatment had no effect on levels of total leukocytes, T cells, CD4+ cells, CD8+ cells or activated T cells in the peripheral blood or spleen (Supplemental Figure IV).
Figure 4.
Role of NOS uncoupling on infiltation of T cells and T cell activation induced by partial carotid ligation. ApoE−/− mice were fed a high fat diet and underwent partial carotid ligation for 1 week. Animals also received BH4 or vehicle in the drinking water. Two carotids from the same treatment group were pooled to obtain adequate cell numbers for flow cytometry. Flow cytometry was performed to detect CD45 (Panel A) and CD3 (Panel B) in the carotids. Absolute numbers of vascular CD45+ (Panel C) and CD3+ cells (Panel D) were quantified using using counting beads and FlowJo software (n=6–8). Numbers of CD4+ (Panel E), CD8+ (Panel F), CD4+CCR5+ (Panel G), and CD4+CD44High (Panel F) cells are shown (n=6–8). Values were compared using ANOVA and selected comparisons were made using a Bonferroni post hoc test.
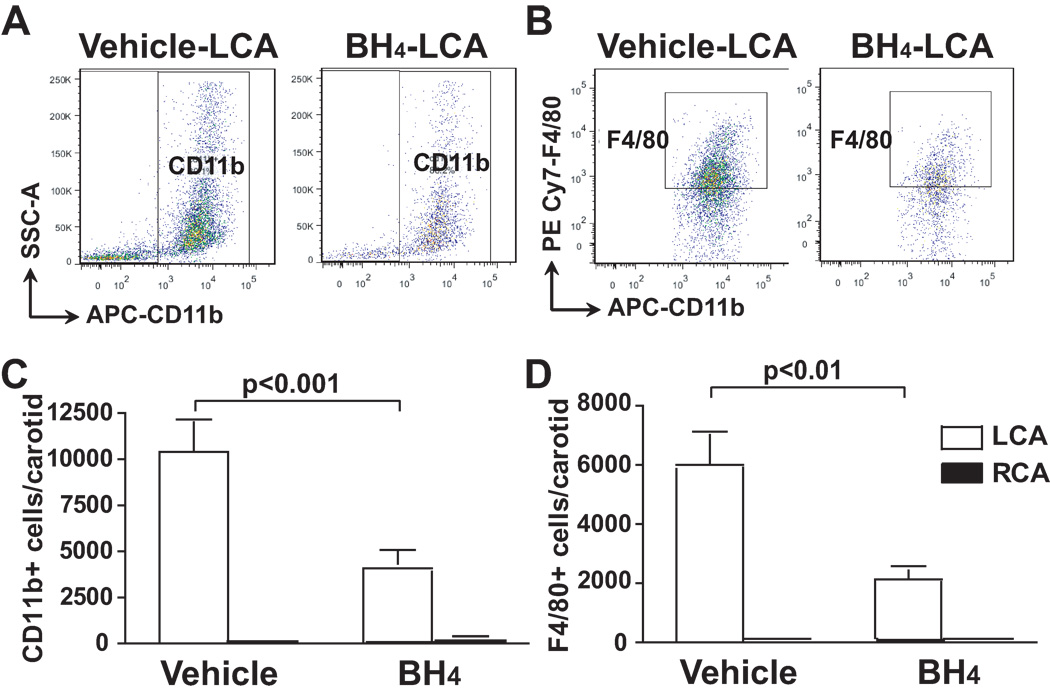
Vascular monocyte infiltration and differentiation into macrophages is one of the hallmarks of atherosclerosis. We therefore examined vascular levels of monocytes and macrophages, characterized by surface markers, CD11b+ and F4/80+, respectively, at areas of disturbed flow. In the LCA of vehicle treated animals, both CD11b+ and F4/80+ cells were markedly increased compared to the unligated RCA. BH4 treatment reduced infiltration of these cells by approximately one half (Figure 5A to 5D). Taken together, these data suggest that vascular leukocyte and monocyte infiltration at sites of disturbed flow is in part due to NOS uncoupling and that this inflammatory response can be reduced by BH4 treatment.
Figure 5.
Role of NOS uncoupling on infiltration and activation of monocytes and macrophages induced by partial carotid ligation. ApoE−/− mice were fed a high fat diet and underwent partial carotid ligation for 1 week. Animals also received BH4 or vehicle in the drinking water. Flow cytometry was performed to detect CD11b (Panel A) and F4/80 (Panel B) in the carotids. Absolute numbers of CD11b+ (Panel C) and F4/80+ cells (Panel D) in the carotids were shown (n=6–8). Values were compared using ANOVA and selected comparisons were made using a Bonferroni post hoc test.
Role of NOS uncoupling on ex vivo cytokine production of the carotid exposed to disturbed flow
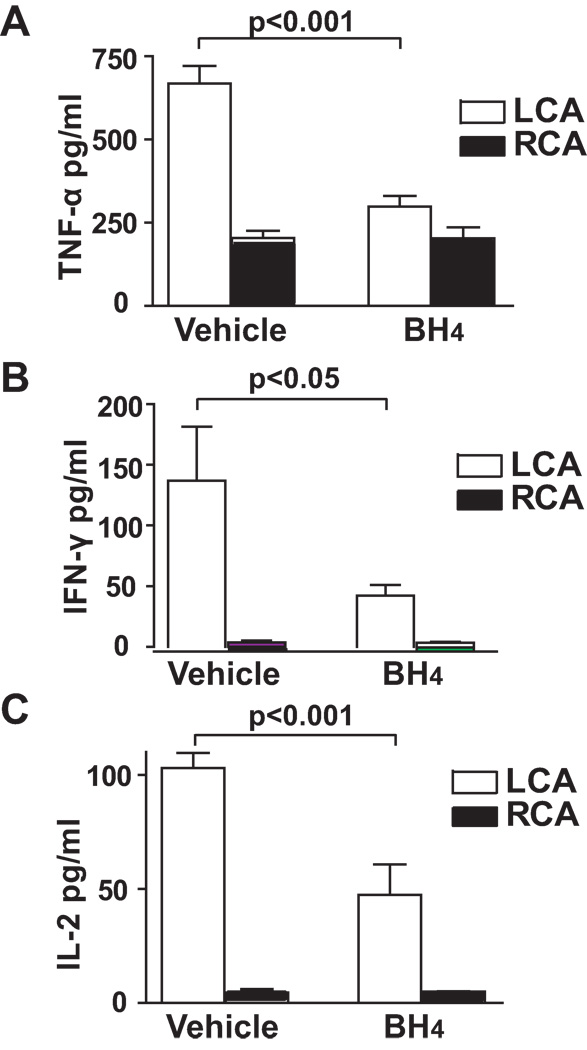
It is known that activated immune cells in the plaque produce cytokines (TNF-α, INF-γ, etc.) that promote further inflammation, induce plaque progression, and stimulate matrix proteases and ROS production. To examine the role of NOS uncoupling on cytokine levels in the LCA, we measured carotid cytokine production by incubating isolated mouse carotid ex vivo and stimulating it with a combination of phorbol myristate acetate (PMA) and ionomycin. The cytokines released into the media were then measured. We found that BH4 treatment dramatically decreased levels of cytokines, including TNF-α, INF-γ, and IL-2 in the LCA of ApoE−/− mice that were partially ligated for 1 week (Figure 6A to 6C). These data further demonstrate NOS uncoupling promotes the inflammatory cascade that contributes to atherosclerosis at areas of disturbed flow and that this is suppressed by BH4 treatment.
Figure 6.
Role of NOS uncoupling on ex vivo cytokine production of the carotid induced by partial ligation. ApoE−/− mice that underwent partial carotid ligation were fed a high fat diet and treated with BH4 or vehicle for 1 week. Isolated mouse carotids were incubated in DMEM medium containing 10 µmol/L PMA and 2 µmol/L ionomycin for 16 hours. TNF-α (A), IFN-γ (B), and IL-2 (C) in the culture media were measured by cytokine bead array (n=7–8). Values were compared using ANOVA and selected comparisons were made using a Bonferroni post hoc test.
Discussion
Recently, our laboratory demonstrated that disturbed flow produced by partial carotid ligation reduces GTPCH-1 phosphorylation, decreases total biopterin production, and augments BH4 oxidation in vivo11. In the present study, we show that these perturbations of BH4 homeostasis lead to NOS uncoupling and contribute to the accelerated lesion development at regions of altered shear stress, including areas of disturbed flow induced by partial carotid ligation and naturally formed in the arterial tree. In addition to promoting lesion development, our findings indicate that BH4 deficiency and NOS uncoupling contribute to vascular superoxide production and endothelial dysfunction associated with atherosclerosis at sites of disturbed flow. Finally, our data indicate that BH4 deficiency and NOS uncoupling likely contribute to the vascular inflammation and abnormal cytokine milieu induced by disturbed flow without affecting systemic immune cell numbers.
Evidence from animal models and human studies suggests that atherosclerosis preferentially develops at areas where blood flow is known to be disturbed, including the lesser curvature of the aortic arch, the infrarenal aorta, the carotid bulb and the proximal coronary arteries1. Flow separations occur at these sites, reducing wall shear stress. In addition, flow reversal occurs at some of these sites, leading to oscillatory shear. Low and oscillatory shear stress promote atherosclerotic lesion development through a number of molecular and cellular mechanisms, including attenuation of NO-dependent atheroprotection, promotion of oxidative stress and inflammation, induction of vascular smooth muscle cell (VSMC) migration, and enhancement of low-density lipoprotein cholesterol (LDL) uptake19. Oscillatory shear stress has been shown to activate the NADPH oxidases and cause eNOS uncoupling. Our previous studies have shown that oscillatory shear can reduce BH4 levels and cause eNOS uncoupling by at least two mechanisms. One is that oscillatory shear causes oxidation of BH4; the other being that it reduces the synthesis of BH4 by reducing GTPCH-1 phosphorylation and activation10. Our current findings indicate that the reduction in NO and concomitant increase in superoxide caused by NOS uncoupling likely play a critical role in the accelerated lesion formation caused by disturbed flow in vivo.
In the present study, we employed a model of rapid developing atherosclerosis caused by partial carotid ligation in ApoE−/− mice fed a high fat diet13. This intervention leads to both low and oscillatory shear stress in the carotid proximal to the site of ligation. The resulting lesions share many characteristics of complex human atherosclerotic lesions, including lipid deposition, inflammation and fibrosis. This model allows examination of interventions such as BH4 administration over a short period, but does not provide insight into any long term benefits of BH4 therapy. In keeping with our studies, Schmidt et al. have shown that oral BH4 reduces atherosclerosis in the aortic root over 12 weeks20. Likewise, Hattori et al. have shown a benefit of BH4 treatment on plaque development in ApoE−/− mice over 10 weeks of high fat feeding21. Of note, in the study of Hattori et al., BH4 therapy seemed to have a striking effect on lesion development in the lesser curvature of aorta, where shear stress has been shown to be oscillatory21.
BH4 exhibits modest anti-oxidant effects, and therefore could have prevented atherosclerosis via its ability to scavenge ROS. We and others have previously shown that BH4 reacts with strong oxidants, including peroxynitrite, thiyl and carbonate radicals22, 23. To exclude the possibility that the effect of BH4 was mediated by oxidant scavenging, we performed additional experiments with NH4, an anti-oxidant structurally similar to BH4 but without cofactor activity for NOS. Our data demonstrate that NH4 did not protect against disturbed flow induced atherosclerosis, suggesting that the effect of BH4 was primarily mediated by its ability to sustain NOS catalysis.
We found that BH4 treatment decreased superoxide production not only in the endothelium, but also in the media and adventitia. This might have been due to its ability to sustain catalysis of NOS isoforms in all these cell layers. In additional to eNOS, neuronal NOS (nNOS) is expressed in rat and human vascular smooth muscle cells24, 25. Expression of inducible NOS (iNOS) in the vessel wall can also occur in the setting of oxidative stress and inflammation26. Importantly, Wilcox et al. showed that atherosclerosis is associated with expression of all forms of NOS in the intima and adventitia27. It is also conceivable that ROS produced by uncoupled NOS could activate other sources of superoxide, such as the NADPH oxidase, in a feed forward fashion, and that these could contribute to ROS production throughout the vessel wall.
In keeping with improved NOS catalysis, endothelium-dependent vasodilatation of the ligated carotid arteries was partially enhanced by BH4 treatment. It is of interest that in normal vessels, the dose response curve to acetylcholine seemed biphasic, rather than sigmoidal. This response is similar to that observed by others28, 29. Of interest, BH4 treatment normalized the relaxation to higher doses of acetylcholine and thus the peak response to acetylcholine, while having a partial beneficial effect on the responses to the lower doses and thus partially shifted EC50s. It is conceivable that this might reflect a differential effect of BH4 on different mechanisms of carotid artery relaxation.
It is well known that inflammation plays a key role in the pathogenesis of atherosclerosis18. Flow disturbances promote endothelial expression of leukocyte adhesion molecules and chemokines, such as VCAM-1 and MCP-1. These could in turn induce monocyte rolling, adhesion and transmigration into the sub-endothelial space, promoting early lesion development. Monocytes differentiate into macrophages and dendritic cells that present antigens and trigger T cell activation within the lesion. These activated T cells produce Th1 cytokines, such as TNF-α and INF-γ, which further lead to inflammation and lesion progression. Our data indicate that NOS uncoupling has a dramatic impact on these inflammatory events. We found that BH4 treatment reduced vascular accumulation of T cells and macrophages. Of note, the T cells present in the lesions caused by disturbed flow seemed to have the phenotype of effector T cells, in that they express CCR5 and are CD44High. In addition to preventing the accumulation of these cells, BH4 treatment also markedly reduced the ability of vascular segments to elaborate Th1 cytokines, such as TNF-α, INF-γ, and IL-2. Taken together, these data indicate that recoupling of NOS has dramatic effects in reducing vascular inflammation in the setting of rapid atherosclerosis development.
In summary, our findings demonstrate that NOS uncoupling is a key mechanism by which disturbed flow induces accelerated atherosclerosis. We find that oral BH4 supplementation prevents NOS uncoupling and improves endothelial function in the carotid exposed to disturbed flow induced by partial carotid ligation. The reduction in oxidative stress elicited by BH4 treatment is associated with diminished monocyte adhesion and T cell activation as well as blunted cytokine production from the vessel wall. Taken together, these indicate that BH4 treatment, by correcting NOS uncoupling and rectifying the oxidative status at areas of disturbed flow, targets the underlying mechanism of atherosclerosis progression by inhibiting inflammation. These results highlight a pivotal role of BH4 deficiency and NOS uncoupling in atherosclerosis progression, particularly under the patterns of low and oscillatory shear flow, and indicate that modulation of vascular BH4 levels could be a therapeutic target for preventing atherosclerosis at branches and curvatures in the arterial tree.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH grants P01HL58000 and R01HL39006 (to D.G.H.) and an American Heart Association Predoctoral Grant (to L.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27:346–351. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- 2.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 3.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 4.Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Forstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Vasquez-Vivar J, Kalyanaraman B, Martasek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res. 2003;37:121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- 7.Channon KM. Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med. 2004;14:323–327. doi: 10.1016/j.tcm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoneyama T, Hatakeyama K. Decameric GTP cyclohydrolase I forms complexes with two pentameric GTP cyclohydrolase I feedback regulatory proteins in the presence of phenylalanine or of a combination of tetrahydrobiopterin and GTP. J Biol Chem. 1998;273:20102–20108. doi: 10.1074/jbc.273.32.20102. [DOI] [PubMed] [Google Scholar]
- 10.Widder JD, Chen W, Li L, Dikalov S, Thony B, Hatakeyama K, Harrison DG. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circ Res. 2007;101:830–838. doi: 10.1161/CIRCRESAHA.107.153809. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Rezvan A, Salerno JC, Husain A, Kwon K, Jo H, Harrison DG, Chen W. GTP cyclohydrolase I phosphorylation and interaction with GTP cyclohydrolase feedback regulatory protein provide novel regulation of endothelial tetrahydrobiopterin and nitric oxide. Circ Res. 2010;106:328–336. doi: 10.1161/CIRCRESAHA.109.210658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison DG, Giddens DP, Jo H. A model of disturbed flow-induced atherosclerosis in mouse carotid artery by partial ligation and a simple method of RNA isolation from carotid endothelium. J Vis Exp. 2010 doi: 10.3791/1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–H1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 15.Klatt P, Schmidt K, Lehner D, Glatter O, Bachinger HP, Mayer B. Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and L-arginine in the formation of an SDS-resistant dimer. EMBO J. 1995;14:3687–3695. doi: 10.1002/j.1460-2075.1995.tb00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng. 2008;36:554–562. doi: 10.1007/s10439-007-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 18.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 19.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt TS, McNeill E, Douglas G, Crabtree MJ, Hale AB, Khoo J, O'Neill CA, Cheng A, Channon KM, Alp NJ. Tetrahydrobiopterin supplementation reduces atherosclerosis and vascular inflammation in apolipoprotein E-knockout mice. Clin Sci (Lond) 2010;119:131–142. doi: 10.1042/CS20090559. [DOI] [PubMed] [Google Scholar]
- 21.Hattori Y, Hattori S, Wang X, Satoh H, Nakanishi N, Kasai K. Oral administration of tetrahydrobiopterin slows the progression of atherosclerosis in apolipoprotein E-knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:865–870. doi: 10.1161/01.ATV.0000258946.55438.0e. [DOI] [PubMed] [Google Scholar]
- 22.Patel KB, Stratford MR, Wardman P, Everett SA. Oxidation of tetrahydrobiopterin by biological radicals and scavenging of the trihydrobiopterin radical by ascorbate. Free Radic Biol Med. 2002;32:203–211. doi: 10.1016/s0891-5849(01)00777-8. [DOI] [PubMed] [Google Scholar]
- 23.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 24.Boulanger CM, Heymes C, Benessiano J, Geske RS, Levy BI, Vanhoutte PM. Neuronal nitric oxide synthase is expressed in rat vascular smooth muscle cells: activation by angiotensin II in hypertension. Circ Res. 1998;83:1271–1278. doi: 10.1161/01.res.83.12.1271. [DOI] [PubMed] [Google Scholar]
- 25.Buchwalow IB, Podzuweit T, Bocker W, Samoilova VE, Thomas S, Wellner M, Baba HA, Robenek H, Schnekenburger J, Lerch MM. Vascular smooth muscle and nitric oxide synthase. FASEB J. 2002;16:500–508. doi: 10.1096/fj.01-0842com. [DOI] [PubMed] [Google Scholar]
- 26.Gunnett CA, Lund DD, McDowell AK, Faraci FM, Heistad DD. Mechanisms of inducible nitric oxide synthase-mediated vascular dysfunction. Arterioscler Thromb Vasc Biol. 2005;25:1617–1622. doi: 10.1161/01.ATV.0000172626.00296.ba. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, Marsden PA. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol. 1997;17:2479–2488. doi: 10.1161/01.atv.17.11.2479. [DOI] [PubMed] [Google Scholar]
- 28.Stec DE, Vera T, McLemore GR, Jr, Kelsen S, Rimoldi JM, Gadepalli RS, Ryan MJ. Heme oxygenase-1 induction does not improve vascular relaxation in angiotensin II hypertensive mice. Am J Hypertens. 2008;21:189–193. doi: 10.1038/ajh.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2576–2581. doi: 10.1161/ATVBAHA.107.153080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.