Abstract
Objective
DEK is a nuclear phosphoprotein and autoantigen in a subset of children with juvenile idiopathic arthritis (JIA). Autoantibodies to DEK are also found in a broad spectrum of disorders associated with abnormal immune activation. We previously demonstrated that DEK is secreted by macrophages, released by apoptotic T cells, and attracts leukocytes. As we identified DEK in synovial fluids in JIA patients, we now investigate how DEK protein and/or autoantibodies may contribute to the pathogenesis of JIA.
Methods
DEK autoantibodies, immune complexes, and synovial macrophages were purified from synovial fluids of JIA patients. DEK autoantibodies and immune complexes were purified by affinity column chromatography and analyzed by 2-D gel electrophoresis, immunoblotting and ELISA. DEK in supernates and exosomes was purified by serial centrifugation and magnetic beads, and DEK’s posttranslational modifications were identified by Nano-LC-MS/MS.
Results
DEK autoantibodies and protein are found in synovial fluids from JIA patients. DEK is secreted by synovial macrophages in a free form and via exosomes. DEK autoantibodies (IgG2) may activate the complement cascade, primarily recognize the C-terminal portion of DEK protein and exhibit higher affinity for acetylated DEK. Consistent with these observations, DEK undergoes acetylation on an unprecedented number of lysine residues as demonstrated by Nano-LC-MS/MS.
Conclusion
These results indicate that DEK can contribute directly to joint inflammation in JIA by generating immune complexes through high affinity interaction between DEK and DEK autoantibodies, a process enhanced by acetylation of DEK in the inflamed joint.
Introduction
Juvenile idiopathic arthritis (JIA), a polymorphic chronic inflammatory disease of unknown etiology, is the commonest cause of disability in children (1). Although DEK auto-antibodies are associated with JIA (2), they are also present in patients with other rheumatic diseases, including systemic lupus erythematosus and linear scleroderma (3). The contribution of DEK protein and DEK antibodies to the pathogenesis of JIA and other autoimmune diseases is not yet known.
DEK is a mammalian nuclear phosphoprotein that was initially identified as an oncoprotein resulting from a t(6;9) translocation in a rare subtype of acute myelogenous leukemia (AML) (4). DEK is overexpressed in many malignancies, including hepatocellular carcinoma, glioblastoma, melanoma, bladder cancer, T cell large granular lymphocytic leukemia, and cervical carcinoma; it is also overexpressed in AML, independent of the t6:9 translocation (4–9). Inhibition of apoptosis and senescence by DEK has been shown in recent studies, and DEK has been demonstrated to be a “bona fide” oncogene (10, 11).
DEK bears little resemblance to other known proteins, but it is well conserved among higher eukaryotes. All DEK proteins share a unique conserved region, the “SAP-box” (SAP = Saf/Actinus/PARP), a motif that is found in proteins that are involved in DNA binding, chromatin remodeling, and/or RNA processing (12, 13). We have demonstrated that DEK is capable of binding to the TG-rich pets site in the human immunodeficiency virus type 2 (HIV-2) promoter where it acts as a transcriptional repressor (14, 15). There is sequence similarity between the pets site and the Y box in some class II MHC promoters, in particular, HLA-DQA1*0501; DEK appears to bind in an allele-specific manner at this locus (16), which may be a risk factor for development of oligoarticular onset JIA in northern European populations (17). In addition to its DNA binding properties, DEK has been found in association with mRNA splicing and export factors, as well as with spliced transcripts, where it has been shown to influence 3’ splice fidelity (18–20). DEK also appears to play an active role in maintaining higher-order chromatin architecture (21). Intense post-translational modification of DEK by phosphorylation (22), acetylation (23), and poly(ADP-ribosyl)ation (24) points to the potential importance of these post-translational modifications for DEK’s multiple functions (22, 25). Although DEK’s monomeric molecular size is ∼50 kDa on SDS-PAGE, it can multimerize in a phosphorylation-dependent manner; a 35 kD form of DEK lacking part of the N-terminal domain has also been described (26).
Although DEK is a nuclear protein that is primarily associated with chromatin throughout the cell cycle (27), we have recently identified two independent pathways that result in DEK’s presence in the extracellular space. The first of these pathways results in non-classical secretion of DEK by activated human monocyte-derived macrophages (MDM) in both a free form and in exosomes (28). In the second pathway, passive release of poly(ADP-ribosyl)ated, hyperphosphorylated DEK by apoptotic T-lymphocytes may occur as a result of Fas-ligand- or stress-mediated apoptosis (24). In demonstrating these pathways, we have shown that IL-8- induced DEK secretion acts as a chemoattractant of peripheral blood leukocytes (28); identification of DEK in synovial fluids (SF) of patients with JIA suggests that DEK-induced leukocyte accumulation in the extracellular compartment may well result in subsequent joint inflammation (28).
In this paper, we demonstrate secretion of DEK by synovial macrophages purified from the SF of JIA patients. We also report purification of IgG2 antibodies from SF of 10 different JIA patients; these antibodies can interact with complement, and preferentially recognize the C-terminal half of the DEK protein. DEK’s presence in immune complexes purified from synovial fluids of patients with active synovitis also suggests that the interaction of DEK with DEK autoantibodies contributes to the inflammation in the joint. Following mapping of acetylation and phosphorylation patterns on individual amino acids of DEK in extracts derived from primary human MDM and from HeLa cells by Nano-LC-MS/MS mass spectroscopy, we tested the effect of DEK phosphorylation and acetylation on its antigenicity and found that SF auto-antibodies preferentially recognize acetylated DEK species. Taken together, our studies demonstrate that DEK is secreted by synovial macrophages, and that it forms immune complexes in the presence of DEK-specific auto-antibodies that preferentially recognize acetylated DEK. These findings support an active role for the DEK protein and DEK auto-antibodies in chronic joint inflammation seen in patients with JIA.
Materials and Methods
Antibodies and expression of recombinant DEK
DEK-specific polyclonal antibodies, expression and purification of His-tagged full-length DEK as well as the His-tagged DEK-fragments 1–187, 187–375, 310–375, 270–350 and 1–350 have been previously described (12, 22). Monoclonal and goat polyclonal CD81-specific antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Synovial fluids and cells
SF were obtained from patients seen in the Pediatric and Adult Rheumatology Clinics at the University of Michigan and from the Pediatric Rheumatology Tissue Repository at Cincinnati Children’s Hospital Medical Center under a protocol approved by the Institutional Review Board. SF were diluted (1:1) in phosphate buffered saline, centrifuged at 200 × g for 30 min to separate cells and frozen at −70° C. DEK-specific auto-antibodies from SF were purified by affinity column chromatography as previously described (22). Synovial macrophages were purified from SF by adherence (Supplemental Material Figure 1) and were grown as described below.
Isolation of Exosomes
To purify exosomes from synovial macrophages, cells were grown in 10% human serum in RPMI 1640 for 12 days, washed, and incubated in serum-free medium for 48 h. At the end of the culture period, cell viability was still maintained at 80–90% as determined by 7- Amino-Actinomycin D (7-AAD) staining (BD Biosciences, San Diego, CA) and MTT assay (28). Cell culture supernatant from 2.5 × 107 cells was collected and exosomes were isolated as described previously (28, 29). All pellet fractions and supernatants were solubilized using standard SDS-PAGE loading buffer and were further analyzed by immunoblotting.
Purification of exosomes using anti-CD81 antibody-labeled magnetic beads
Dynal beads M-500 (Dynal, Lake Success, NY) coated with anti-CD81 antibody were used to isolate exosomes as described (30). Briefly, Dynal beads were incubated overnight with un-conjugated mouse IgG1 anti-goat linker antibody (10 µg antibody/ 2 × 107 beads) (Sigma, Saint Louis, Missouri), washed thoroughly with PBS, 0.1% BSA, pH 7.4, followed by a 24 h incubation in 0.2 M Tris, 0.1% BSA, pH 8.5 at 4° C. Linker-coated Dynal beads were then incubated overnight at 4° C with 10 µg goat anti–CD81 antibody / 1 × 107 beads (Santa Cruz, CA) followed by washing with PBS/BSA. Purified exosomes from the 70,000 × g fraction were added to the coated beads (100 µg protein per 1 × 107 coated beads) in Dynal buffer A (PBS, pH 7.4, 2 mM EDTA, 5% BSA) and incubated overnight at 4° C. After washing in PBS, beads were isolated, and purified proteins were resolved by SDS-PAGE for subsequent immunoblot analysis.
ELISA for IgG1 and IgG2 antibodies
For details see Supplemental Material and Methods section.
Purification of immune complexes
Immune complexes were purified from patients’ SF by protein G columns (Pierce Chemical Co, Rockford, IL) as described previously (31) and were analyzed by immunoblotting using DEK or C1q–specific antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and secondary HRP conjugated goat anti-rabbit or anti-mouse antibodies (Jackson ImmunoResearch Laboratories, Inc, West Grove, PA).
Nano-LC-MS/MS and MS3 Analysis of the DEK Tryptic Digest and database search
For details see Supplemental Material and Methods section.
In vitro modification of recombinant His-DEK
Preparation of cell extracts from HeLa S3 cells or MDM, and the dephosphorylation procedure for His-DEK has been described previously (22). Recombinant His-DEK (1 µg/reaction) was modified in reactions containing 300 µg total protein derived from HeLa cell or MDM cell lysate, one µM ATP (Roche, Indianapolis, IN), 500 µM of acetyl-CoA (Sigma, St. Louis, MO), and protease inhibitors (EDTA-free) (Roche, Indianapolis, IN) in the presence of either phosphatase inhibitors (5 mM NaF and 0.5 mM sodium-vanadate) or histone deacetylase (HDAC) inhibitors (20 mM sodium-butyrate and 50 ng/ml TSA) for 10 min at 37° C. Reactions were stopped on ice with either 10 mM Tris-C, 800 mM NaCl, 0.5% NP-40, 5 mM Imidazol (Sigma, St. Louis, MO), 20 mM NaF, 1 mM sodium-vanadate for phosphorylation reactions, or 10 mM Tris-Cl, 800 mM NaCl, 0.5% NP-40, 5 mM Imidazol, 20 mM sodium-butyrate, 50 ng/ml TSA for acetylation reactions, or a mix of both.. The modified His-DEK was purified by incubation with Ni2+-NTA agarose (Quiagen, Valencia, CA) for 1 hr at 4° C, followed by six wash steps with ice-cold PBS containing either 150 mM NaCl or 300 mM NaCl on mini columns (Bio-Rad, Hercules, CA). Elutions were carried out with 200 µl of 2% SDS at 37° C for 15 min and eluted proteins were concentrated as described (32). The modified DEK-species (250 ng/ lane) were further analyzed by immunoblotting with anti-His antibodies or antibody from the SF of JIA patients.
Results
DEK is secreted by synovial macrophages
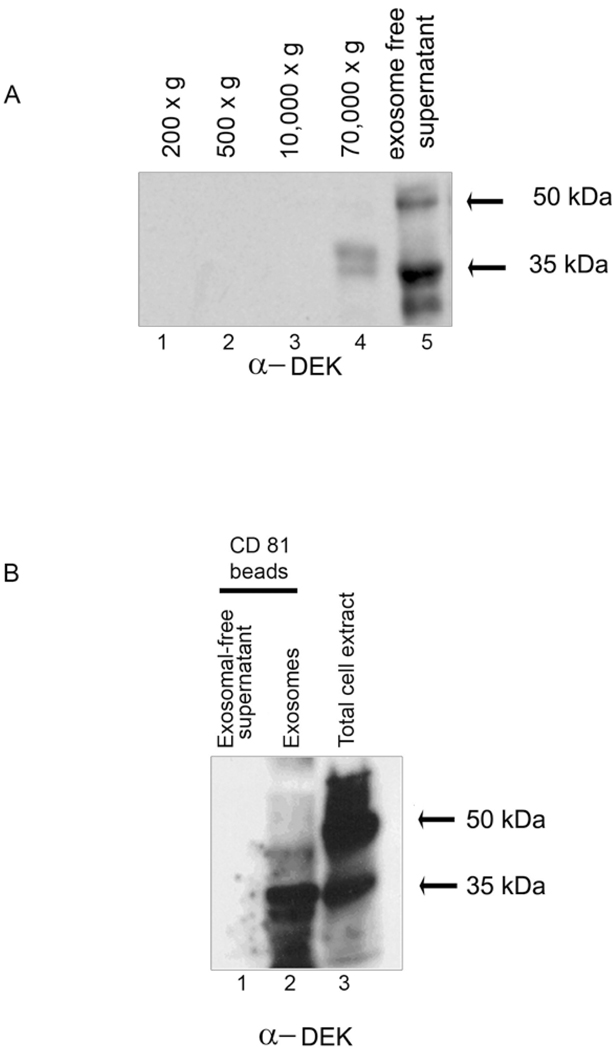
Based on our previous study showing that DEK is a secreted chemotactic factor (28), we hypothesized that accumulation of DEK in the joints of JIA patients is likely to be due to active secretion of DEK by synovial monocytic cells, a predominant cell type in inflammatory arthritis. On this assumption, we purified macrophages from the SF of patients with active JIA and a positive ANA status, and cultured the macrophages for 12 days in the presence of 10% human serum, as previously described for MDM; 88% of these cells were positive for the macrophage marker CD11b (Figure 1, Supplemental Material). Supernatants from the cells were collected and exosomes were isolated by consecutive centrifugation steps (28). DEK in the enriched exosome fraction was detected as proteins of ∼45 kDa and ∼35 kDa (Figure 1 A, 70,000 × g, lane 4). DEK in the concentrated exosome-free supernatant was detected as 50 kDa and 35 kDa proteins (Figure 1A, lane 5) consistent with our previous observations (28). To confirm the presence of DEK in exosomes from synovial macrophages, the 70,000 × g enriched exosome fraction shown in Figure 1A (lane 4), was also subjected to immunoprecipitation by magnetic beads coated with antibodies specific for the exosomal marker CD81 (33) (Figure 1B). Bands corresponding to DEK representing proteins of ∼35 kDa and ∼45 kDa were found to be specifically associated with the CD81 positive enriched exosomal fraction. No DEK was detected in the exosome-free fraction subjected to purification with CD81 beads (Figure 1 B, lane 1), supporting the specificity of the DEK-exosome association. Taken together, our data demonstrate that synovial macrophages derived from a patient with JIA secrete DEK both in a free form (Figure 1A, lane 5) and in exosomes (Figure 1A and 1B).
Figure 1. DEK is secreted by synovial macrophages in a free form and via exosomes.
Macrophages purified from the synovial fluid of a patient with pauciarticular JIA were grown in 10% human serum. (A). Twelve-day macrophages were placed in serum-free medium overnight, and supernatants were subsequentially centrifuged at 200 × g, 500 × g, 10,000 × g and 70,000 × g. The resulting pellets were analyzed by immunoblotting using monoclonal DEK-specific antibodies. Exosomes are present in the 70,000 × g fraction; lane 5 contains an exosome-free fraction. (B). Magnetic beads coated with antibodies to CD81 were used to further purify exosomes from the exosomal enriched fraction (see A, lane 4). The recovered fraction was analyzed by immunoblotting using polyclonal DEK antiserum. Lane 1: negative control, CD81-coated magnetic beads were incubated with concentrated protein from exosome-free supernatant (see A, lane 5). Lane 2: protein recovered from the CD81-coated magnetic beads incubated with 45 µg of protein from the enriched exosomal supernatant fraction obtained by 70,000 × g centrifugation. (Note: Lane 2 contains approximately 10-fold less protein than loaded in Lane 1). Lane 3: intracellular DEK. The 35 kDa form of DEK may represent a breakdown product (3, 15, 19, 26).
DEK-specific antibodies and DEK protein are present in synovial fluids from JIA patients
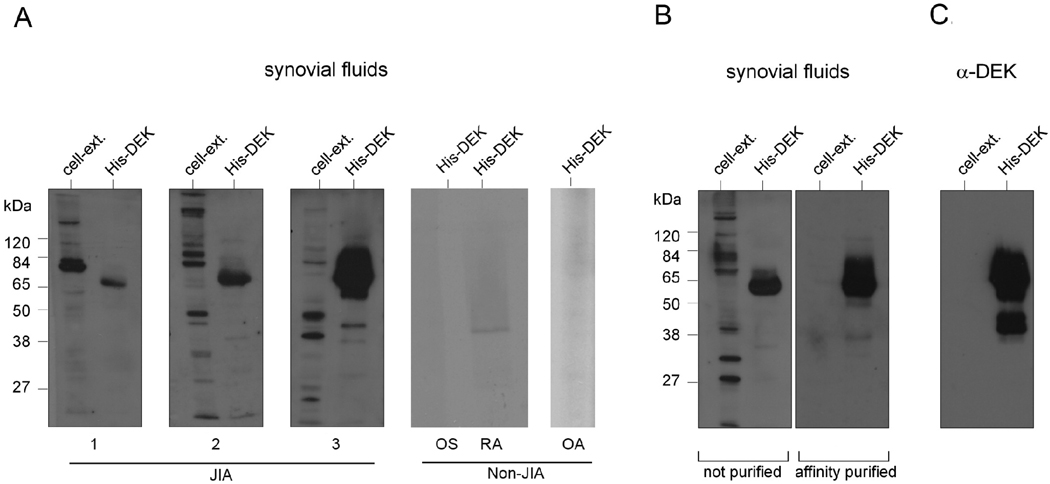
Although several clinical studies have revealed DEK autoreactivity in sera of patients with JIA and other autoimmune diseases (2, 3, 26), the presence of DEK antibodies in inflamed joints has not yet been described. In this study, we detected DEK-specific antibodies in crude SF by probing recombinant DEK protein (expressed in the baculovirus system) or a control mock protein preparation (High5 insect cell extracts) with SF from patients with active JIA (Figure 2A, left panel). The presence of DEK-specific antibodies was seen in 10 of 13 SF from JIA patients with active disease, including ANA-negative patients. Very-low to no reactivity to DEK was detected in SF from non-JIA patients, such as those with osteoarthritis (OA), rheumatoid arthritis (RA), and orthopedic surgery patients with non-inflammatory conditions (Figure 2A, right panel). Purification of crude SF by DEK-affinity column chromatography resulted in increased antibody sensitivity and specificity (Figure 2B, right panel). Column-purified antibody isolated from the SF is comparable in sensitivity and specificity to a monoclonal DEK-specific antibody (Figure 2C). Since the physiological function of any antibody is dependent on its isotype, we analyzed the IgG subclass of DEK-specific antibodies in SF from JIA patients. DEK-specific antibodies from SF of 9 JIA patients were purified by affinity column chromatography prior to being subjected to ELISA and immunoblot analysis. We found that all nine SF samples contained IgG2 DEK antibodies, while five of the nine samples contained a mixed population of IgG1 and IgG2 DEK antibodies. Some IgG4 reactivity was detected in 2 of the 9 distinct SF, but there was no IgG3 or IgA anti-DEK detected in patient SF (Table 1, Supplemental Material section).
Figure 2. DEK auto-antibodies are detected in synovial fluids from patients with JIA.
(A). Proteins from mock-infected insect cell extracts (negative control) or recombinant histidine-tagged DEK expressed and purified from High5 insect cells (1 µg per lane) were resolved by SDS-PAGE and subjected to immunoblotting with a 1:200 dilution of crude synovial fluids. Shown are Western blots using synovial fluids from 3 different JIA patients that tested positive for DEK-specific auto-antibodies and are representative of 10 out of 13 synovial fluids from JIA patients that were screened. Also shown are results from three non-JIA patients’ synovial fluids that had no-to-very low levels of DEK-specific auto-antibodies and are representative of 6 orthopedic surgery (OS), 1 rheumatoid arthritis (RA), and 2 osteoarthritis (OA) patients’ synovial fluids. (B). Samples were also probed with synovial fluid that had been affinity purified using SulfoLink sepharose columns covalently conjugated with recombinant His-DEK. (C). Insect cell extract and recombinant His-DEK were probed with a monoclonal antibody to DEK as a positive control.
DEK is found in immune complexes in synovial fluids of JIA patients
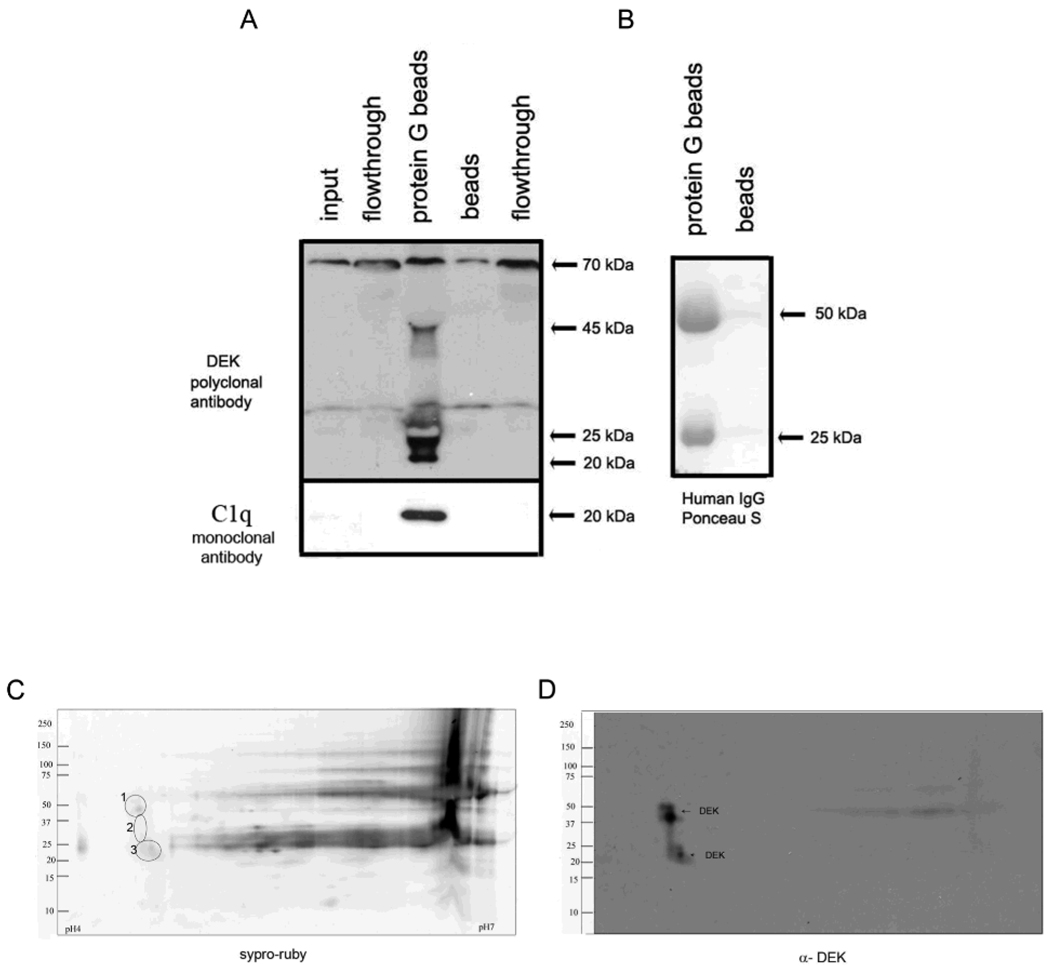
The presence of both DEK protein and abundant IgG1 and IgG2 DEK antibodies in synovial fluid of JIA patients suggested the likelihood of finding DEK/anti-DEK immune complexes (ICs) in the synovial space. Antigen-antibody ICs have previously been found in the blood and SF of JIA patients, although the contribution of immune complexes to the pathogenesis of JIA remains controversial (34, 35). To determine if extracellular DEK and DEK antibodies in the synovial fluid result in ICs, we used protein G coated beads (31) to pull down immunoglobulins from synovial fluids. DEK was detected as ∼50 kDa and ∼20 kDa proteins among the IC proteins using DEK-specific polyclonal antibodies (Figure 3A); the immunoglobulin heavy and light chains were identified by total protein staining (Figure 3B). To confirm that we had pulled down immune complexes, we probed for the C1q protein, a component of active immune complexes. C1q–specific monoclonal antibodies detected a ∼20 kDa protein in the DEK-containing sample (Figure 3A, bottom panel), supporting the association of DEK with immune complexes in the JIA SF. The specificity of the antibodies, and hence these results, was further confirmed as shown in the Supplemental Material, Figure 2.
Figure 3. DEK is detected in synovial fluid immune complexes (ICs).
(A). Protein G beads or beads alone were used to pull down ICs from synovial fluids. IC proteins were analyzed by immunoblotting using DEK-specific polyclonal antibodies and monoclonal antibodies to C1q (left panel and bottom left panel, respectively). DEK antibodies detected: 45 kDa, (identified DEK protein size), 70 kDa (possibly non-specific), and 20 kDa band (most likely a breakdown product (36), still to be characterized). Flow-through lane represents all the proteins that did not bind protein G beads. The specificity of the antibodies is shown in the Supplemental Material section, Figure 3. The bottom panel shows the presence of C1q in the ICs. The right panel (B) shows total protein staining with Ponceau S of the pull-down proteins, with the heavy and light chain of the human Ig pulled down from the synovial fluids by the protein G beads, as opposed to beads alone (right lane). (C). Total protein as in B, stained with Syper ruby after separation on a 2-D gel. (D). Immunoblotting of the 2-D gel by DEK mono-specific polyclonal antibodies (DEK corresponding proteins are marked in circles labeled as 1, 2 and 3 in panel C).
Detection of the ∼20 kDa DEK protein has previously been described by another group as being associated with the exon junction complex that is involved in mRNA processing (36). To confirm the specificity of these bands, we separated DEK from the other proteins and antibodies in the ICs by 2-D gel electrophoresis. Proteins corresponding to ∼50 kDa , 35 kDa, and ∼20 kDa MW (Figure 3C, marked in circles as 1, 2, and 3 respectively) were detected by monospecific DEK antibodies (Figure 3D).
The C-terminal domain of DEK is immunogenic
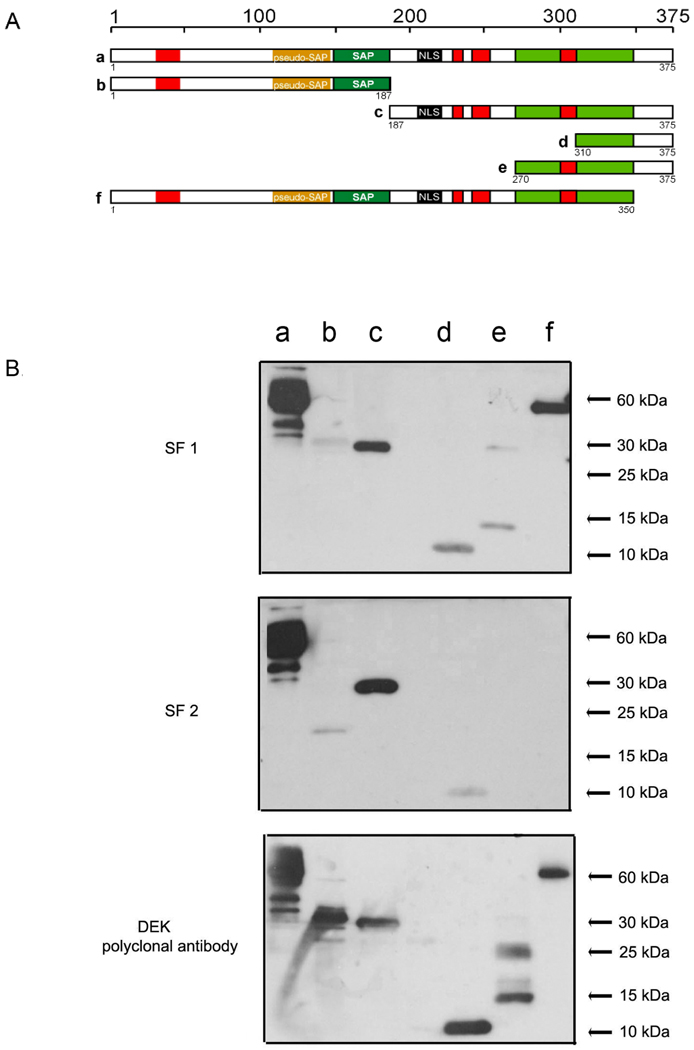
Because little is known about auto-reactive domain(s) of DEK, our identification of DEK antibodies in SF led us to investigate which epitopes of DEK may be immunogenic. Using SF from 8 different JIA patients as a source of DEK antibodies, we probed recombinant full-length DEK and five recombinant DEK-fragments, including: N-terminal half, C-terminal half, C-terminal 65 aa fragment, 105 aa fragment, and full length DEK lacking only the C-terminal 25 amino acids (Figure 4A). All of the synovial fluids reacted with the C-terminal half of DEK and none showed any reactivity to the N-terminal part of DEK (Figure 4 B). Five of 8 SF samples failed to recognize DEK in the absence of the last 25 amino acids (for example, Figure 4 B, SF2, lane f), suggesting that this epitope may represent the most immunogenic region of DEK.
Figure 4. Antibodies from synovial fluids of JIA patients primarily recognize the C-terminal half of the DEK protein.
A. Illustration of recombinant His-DEK fragments utilized in these experiments. Shown are functional domains and predicted functional domains as follows: red boxes: acidic regions, yellow box: pseudo-SAP-box (13), green box: SAP-box, light green box: second DNA binding domain (12, 13); and black box: the putative nuclear localization signal (NLS). B. DEK fragments were expressed and purified from insect cells, separated by SDS-PAGE (30 pmole, lane), and detected by immunoblotting using a 1:200 dilution of crude synovial fluid collected from eight individual JIA patients, or with DEK-specific polyclonal antibodies as control (bottom panel). Shown are two examples representative of experiments done with eight different SFs.
Analysis of the posttranslational modification pattern of DEK
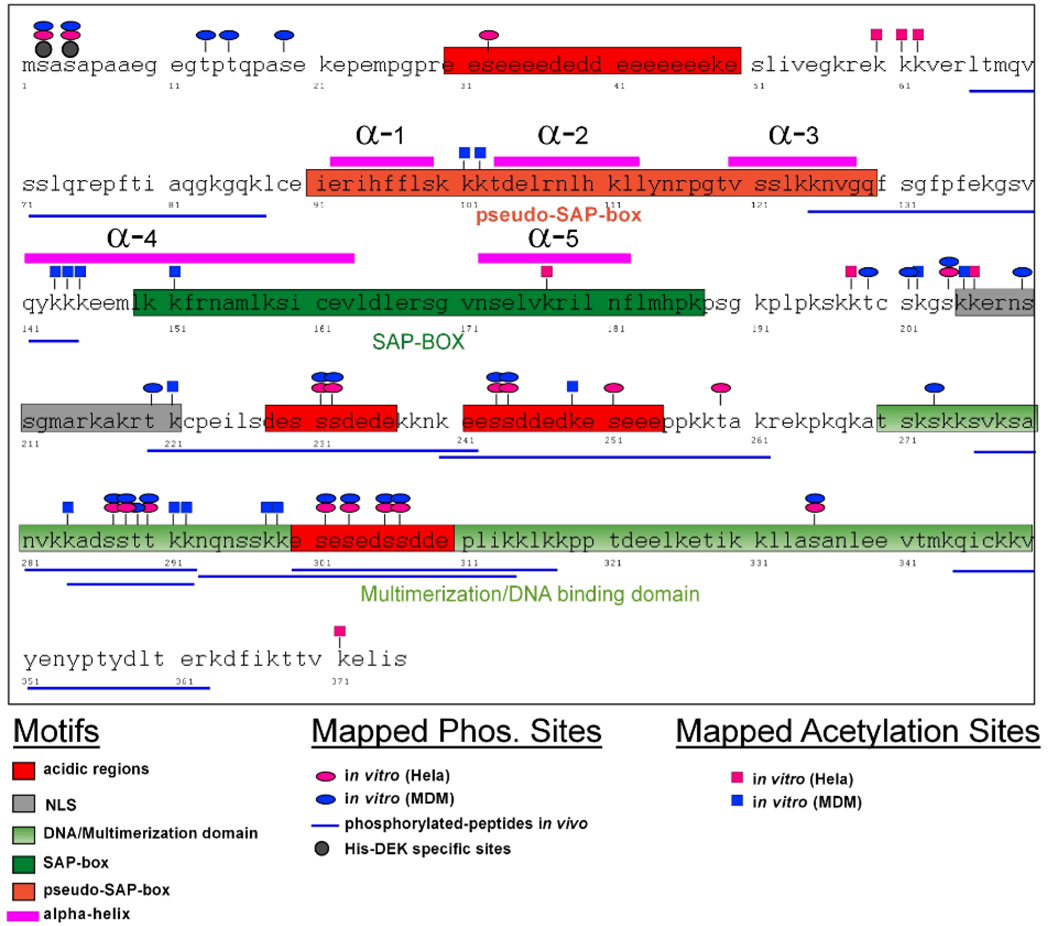
As shown above, DEK can be detected in SF in several different forms (see Figure 1 and Figure 3D). Posttranslational modifications of DEK, including acetylation and phosphorylation have been shown to regulate DEK’s DNA binding characteristics, nuclear localization, and secretion (22, 23, 28), and may account for the different forms of DEK noted in the experiments described above. For that reason, we created a modification map of DEK to identify potential phosphorylation and acetylation sites in recombinant His-DEK subjected to incubation with extracts derived from HeLa cells and MDM. His-DEK was dephosphorylated by λ phosphatase prior to incubation with cellular extracts. The modified protein was run on a gel and was subjected to in-gel tryptic digest prior to analysis by nano-LC-MS/MS as previously described (22). We identified 25 phosphorylation sites and an apparently unprecedented 22 acetylated lysines in the DEK molecule (Figure 5). Serine- and threonine-specific phosphorylation residues were found overlapping in MDM and HeLa cell extracts (Supplementary Table A2). Fifteen of the 67 lysine residues of DEK were found to be acetylated in DEK that was incubated with MDM cell extract, as compared to just 7 acetylated lysines on DEK incubated with HeLa cell extract (Supplementary Table A3). Most strikingly, comparison of acetylated residues in MDM vs. HeLa extract showed no overlapping residues, suggesting that acetylation plays an essential role in DEK function(s).
Figure 5. Schematic overview of acetylation and phosphorylation sites mapped in recombinant His-DEK after treatment with MDM or HeLa cell extract.
Summary of posttranslationally modified sites in the DEK molecule as identified by Nano-LC-MS/MS. Recombinant DEK was reacted with extracts from fully differentiated monocyte-derived macrophages (MDM) or HeLa cells in vitro under conditions that favor either acetylation or phosphorylation (see Materials and Methods section). Phosphorylated sites are marked by ovals and acetylated sites by rectangles (blue for MDM, red for HeLa). Modification sites shown here are additionally listed in the Supplemental Material (Tables A1, A2 and A3). Highlighted are the functional domains as indicated Figure 4. Positions of α-helices as revealed by NMR are indicated in pink (13). Phosphorylation of Serine-2 and Serine-4 were resistant to treatment with phosphatase and therefore represent background
Acetylation of DEK induces its recognition by antibodies in the SF of JIA patients
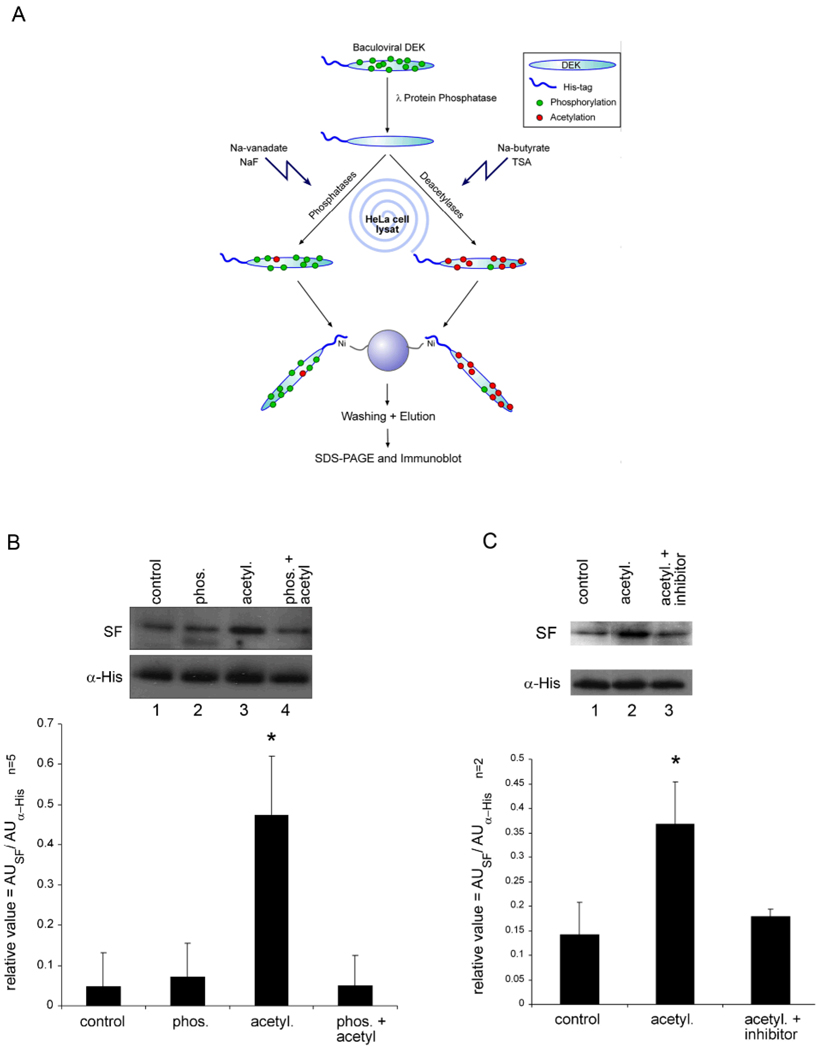
Other groups have previously demonstrated that posttranslational modification of proteins can elicit autoimmune responses (37, 38, 39). For example, increased citrullination of proteins and presence of antibodies to citrulinated proteins has recently been found in patients with rheumatoid arthritis (37). Noting the striking lack of overlap between DEK acetylation in MDM versus HeLa cell extracts, we chose to investigate whether acetylation of DEK might modify its antigenicity. To test this idea, we incubated dephosphorylated His-DEK protein with HeLa cell extract in the presence of either phosphatase inhibitors or deacetylase inhibitors with acetyl-CoA and ATP to enrich overall phosphorylation or acetylation, respectively (Figure 6A). Control and modified DEK species were analyzed for recognition by DEK-specific antibodies in SF from a JIA patient. As shown in Figure 6B, enhancing phosphorylation of DEK (lane 2) had no effect on its recognition by the patient’s autoantibodies, whereas increased acetylation of His-DEK (lane 3) favorably enhanced recognition by the patient’s autoantibodies as compared to control (lane 1). Addition of anarcardic acid, a potent inhibitor of acetyl transferases p300 and P/CAF, to the HeLa cell extract prior to modification of DEK reduced the recognition of DEK by the patient’s autoantibodies to control levels (Figure 6C). Enhancing phosphorylation and acetylation together led to the loss of the increased immunogenicity seen with acetylation alone, suggesting that there is interaction between acetylation and phosphorylation of DEK. Taken together, these data demonstrate that acetylation of DEK potentially enhances its effect as an autoantigen.
Figure 6. Acetylation increases recognition of DEK by JIA auto-antibodies.
(A) Schematic overview of His-DEK in vitro modification. Dephosphorylated His-DEK was incubated with protein extract serving as an enzyme source and inhibitors to either favor acetylation or phosphorylation. (B). His-DEK was modified to enhance phosphorylation (lane 2), acetylation (lane 3), or both (lane 4). As control (lane 1), samples were treated under conditions used to induce phosphorylation and acetylation. Immunogenicity of the modified DEK was detected by immunoblotting using crude JIA SF or His-tag-specific monoclonal antibodies (bottom panel), as a loading control. The intensity of the bands was quantified by densitometry (graph below the figure). *P = 0.0052 (compared to control) as calculated by student t-test, an average of 5 different experiments. (C). Acetylation of His-DEK was inhibited by the acetylase inhibitor anacardic acid (lane 3). DEK was detected as described above and intensity of the bands was quantified by densitometry. *P = 0.045, the data shown represent the average of 2 different experiments. Densitometry was done using image J, calculated as a relative value in arbitrary units (AU) in the Y axis, dividing the AU calculated from the SF detection by the AU values of the anti-His loading control.
Discussion
Juvenile arthritis is a disease that affects children mainly between the ages of 2–16 (1) leading to common arthritis-related complications, such as limitations in range of motion and function, significant changes in joint architecture that can predispose to early-onset osteoarthritis, and uveitis, a condition that frequently leads to decreased visual acuity and potential blindness (40). There are no specific diagnostic tests at the present time that can distinguish JIA from other chronic arthritides associated with psoriasis, systemic lupus erythematosus, sarcoidosis, or inflammatory bowel disease. Although both genetic and environmental factors have been implicated in the pathogenesis of JIA (1), its cause is still poorly understood. With the development of new biological drugs such as anti-cytokine agents, there has been substantial progress in disease management. However, none of the available drugs have curative potential, and most have significant side effects. For these reasons, improved understanding of immunologic mechanisms involved in inciting and perpetuating inflammatory joint disease is necessary for development of novel, more specific treatments for JIA.
In this paper, we have shown that human DEK protein is present in immune complexes in the synovial fluid from the inflamed joints of patients with JIA, and that its presence can be explained by secretion of DEK from local synovial macrophages in a free form and via exosomes (Figure 1). These results are in accordance with our previous report that used MDM as an experimental model (28). The presence of DEK in exosomes also suggests a mechanism by which DEK may become an auto-antigen (41, 42).
It is well known that approximately 40–60% of all JIA patients have circulating DEK auto-antibodies (2), which have been detected mainly in children with oligoarticular JIA, and which are nearly omnipresent in children with JIA-associated iridocyclitis (3). Here, we provide evidence for the occurrence of both DEK protein and DEK-specific antibodies in the synovial fluid from children with active JIA, suggesting a direct role for DEK in the pathogenesis of this disease. We have purified DEK-specific antibodies, which are primarily of the IgG2 isotype or a mixed population of IgG1 and IgG2 isotypes, from the SF of nine different JIA patients with active synovitis. While IgG1 antibodies can potentially bind CD16 (Fcγ receptor) and CR3 (complement receptor), auto-antibodies of the IgG2 isotype predominantly bind complement receptors (43), suggesting that DEK-specific antibodies can be associated with immune complexes and can stimulate the complement system via the classical pathway. Serum levels of complement degradation products are elevated in patients with JIA (44–46), and correlate with disease activity. In addition, C1q, a soluble component of the complement defense mechanism that activates the classical pathway primarily through interactions with immune complexes, has been found in sera and synovial fluid immune complexes in JIA patients (46). We have identified DEK in immune complexes purified from synovial fluid, together with C1q (Figure 3), further supporting DEK-specific association with immune complexes.
To improve our understanding of the nature of DEK-specific antibodies, we screened synovial fluids from 8 patients with active JIA for their reactivity to specific regions of DEK. We found that DEK-specific antibodies recognize the C-terminal half of the DEK protein, and that loss of DEK’s C-terminal 25 amino acids appears to curtail its recognition by auto-antibodies, suggesting that this 25-amino acid domain contributes significantly to DEK’s antigenicity. Enhanced understanding of this region of DEK is likely to contribute to a more complete understanding of how DEK-specific antibodies develop and how they may contribute to the pathogenesis of JIA.
Post-translational modification of potential auto-antigens has recently been found to be an important factor in the induction of autoimmune diseases, including increased citrullination of proteins and production of antibodies to citrulinated proteins in almost 60% of adult rheumatoid arthritis (RA) patients (37–39). We have previously demonstrated that DEK’s cellular localization is regulated by posttranslational modifications, including acetylation, phosphorylation and poly(ADP-ribosyl)ation (13, 22–24). In addition, we have also shown that SF from JIA patients efficiently recognize poly(ADP-ribsyl)ated DEK forms, which are released by apoptotic T-cells (24). Here we show that multiple forms of DEK are associated with immune complexes (Figure 3C and 3D), suggesting that posttranslationally modified forms of DEK are found in SF. Similar results demonstrating variation in DEK phosphorylation patterns were also shown in 2-D gels by Tabbert et. al., (47). We have extended these studies by creating a comprehensive map of posttranslational modifications to DEK. We used HeLa cell extract, a system in which DEK has been extensively studied, and compared it with activated MDM cell extract (as a model of chronic inflammation). By assessing the phosphorylation or acetylation state of each individual amino acid of DEK, using a highly sensitive molecular approach (Nano-LC-MS/MS), we identified 22 acetylated lysines in the DEK molecule, suggesting that these amino acids might be also acetylated in vivo. (Studies are underway to examine which specific lysines are necessary for auto-antibody recognition). These results are in agreement with our previous findings (23) and further support the idea that acetylation of DEK can account for its antigenicity. While most of our current findings are in agreement with previously described phosphorylation sites of DEK (22) and Phosida phosphorylation site database, we also identified several novel phosphorylation sites that fully overlap in DEK modified by either MDM or HeLa cell extracts. In contrast, DEK’s acetylation pattern in MDM was distinctly different from that in HeLa cell extracts (Figure 5 and Supplementary Material section, Tables A2–A4). Additional studies have demonstrated that acetylation of DEK is involved in shaping DEK’s antigenicity, as DEK’s recognition by SF autoantibodies was enhanced almost 10-fold upon acetylation (Figure 6) and can be blocked by the acetylase inhibitor anarcardic acid (Figure 6B), which inhibits the activity of P/CAF and p300, but can have less specific effects at the concentration we used. For this reason, we concluded that DEK’s antigenicity is likely mediated by acetylases such as P/CAF or CBP, or by other indirect acetylation of a mediator protein(s) as previously shown for acetylated auto-antigens of histone H4 from lupus mice (48). Our results also imply interaction between phosphorylation and acetylation, as phosphorylation alone had no effect on DEK antigenicity but it eliminated the preferential recognition of acetylated DEK. This is similar to the phosphorylation/acetylation interdependence seen with histone H3 and p53 (49, 50).
In summary, we have found both DEK protein and DEK-specific antibodies in the SF of JIA patients. Free DEK can be found in the synovium as a result of active release by synovial macrophages and/or apoptosis of T-cells. Mechanisms by which DEK may provoke local inflammation includes chemoattraction of neutrophils and T-cells into the joint (28) and formation of immune complexes with DEK antibodies or other IgGs. DEK-specific antibodies in the SF recognize the C-terminal half of DEK, and acetylation appears to enhance DEK recognition by SF autoantibodies, suggesting that this post-translational modification of DEK can induce the generation of DEK auto-antibodies and/or enhance immune complex formation in the inflamed joint of JIA patients. Taken together, the findings presented here further elucidate the biology of the DEK autoantigen and its role in the pathogenesis of JIA.
Supplementary Material
Acknowledgments
We thank Drs. David Fox and Alisa Koch for their advice and intellectual support and Donna Gschwend for help in manuscript preparation. We also acknowledge Dr. David Fox for supplying the OA and RA synovial samples and Dr. David Glass for the orthopedic surgery samples. This work was supported by grants R01AI062248 and R01AI087128 to D.M.M. from the National Institutes of Health and by a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research. N.M.-V. was supported by NIH grant T32CA88784-03 through the University of Michigan Tumor Immunology Training Program, the Rheumatic Disease Core Center of the University of Michigan (5 P30 AR048310-07), the Post-Doctoral Translational Scholars Program (UL1RR024986), and R03 AR056748-01 and K01 AR055620 from the NIH. F.K. was supported by a William D. Robinson Fellowship from the Michigan Chapter of the Arthritis Foundation and is a recipient of a Post-Doctoral Fellowship from the Arthritis Foundation. E.F.M. was supported by a MA2385/2–3 grant from the DFG (Deutsche Forschungsgemeinschaft). A.E.D. was supported by the Bayer Science and Education Foundation. R.K. was supported by R01 DK067102 and funds from the A. Alfred Taubman Medical Research Institute, University of Michigan. B.S.A. is supported by a grant from the University of Michigan Health System (UMHS) Faculty Group Practice Board of Directors.
References
- 1.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007 Mar 3;369(9563):767–778. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 2.Murray KJ, Szer W, Grom AA, Donnelly P, Levinson JE, Giannini EH, et al. Antibodies to the 45 kDa DEK nuclear antigen in pauciarticular onset juvenile rheumatoid arthritis and iridocyclitis: selective association with MHC gene. J Rheumatol. 1997;24(3):560–567. [PubMed] [Google Scholar]
- 3.Szer IS, Sierakowska H, Szer W. A novel autoantibody to the putative oncoprotein DEK in pauciarticular onset juvenile rheumatoid arthritis. J Rheumatol. 1994 Nov;21(11):2136–2142. [PubMed] [Google Scholar]
- 4.von Lindern M, Fornerod M, van Baal S, Jaegle M, de Wit T, Buijs A, et al. The translocation (6;9), associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA. Mol Cell Biol. 1992 Apr;12(4):1687–1697. doi: 10.1128/mcb.12.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daibata M, Matsuo Y, Machida H, Taguchi T, Ohtsuki Y, Taguchi H. Differential gene-expression profiling in the leukemia cell lines derived from indolent and aggressive phases of CD56+ T-cell large granular lymphocyte leukemia. Int J Cancer. 2004 Mar 1;108(6):845–851. doi: 10.1002/ijc.11647. [DOI] [PubMed] [Google Scholar]
- 6.Evans AJ, Gallie BL, Jewett MA, Pond GR, Vandezande K, Underwood J, et al. Defining a 0.5-mb region of genomic gain on chromosome 6p22 in bladder cancer by quantitative-multiplex polymerase chain reaction. Am J Pathol. 2004 Jan;164(1):285–293. doi: 10.1016/S0002-9440(10)63118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grottke C, Mantwill K, Dietel M, Schadendorf D, Lage H. Identification of differentially expressed genes in human melanoma cells with acquired resistance to various antineoplastic drugs. Int J Cancer. 2000 Nov 15;88(4):535–546. doi: 10.1002/1097-0215(20001115)88:4<535::aid-ijc4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Kondoh N, Wakatsuki T, Ryo A, Hada A, Aihara T, Horiuchi S, et al. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res. 1999 Oct 1;59(19):4990–4996. [PubMed] [Google Scholar]
- 9.Kroes RA, Jastrow A, McLone MG, Yamamoto H, Colley P, Kersey DS, et al. The identification of novel therapeutic targets for the treatment of malignant brain tumors. Cancer Lett. 2000 Aug 11;156(2):191–198. doi: 10.1016/s0304-3835(00)00462-6. [DOI] [PubMed] [Google Scholar]
- 10.Wise-Draper TM, Morreale RJ, Morris TA, Mintz-Cole RA, Hoskins EE, Balsitis SJ, et al. DEK proto-oncogene expression interferes with the normal epithelial differentiation program. Am J Pathol. 2009 Jan;174(1):71–81. doi: 10.2353/ajpath.2009.080330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khodadoust MS, Verhaegen M, Kappes F, Riveiro-Falkenbach E, Cigudosa JC, Kim DS, et al. Melanoma proliferation and chemoresistance controlled by the DEK oncogene. Cancer Res. 2009 Aug 15;69(16):6405–6413. doi: 10.1158/0008-5472.CAN-09-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohm F, Kappes F, Scholten I, Richter N, Matsuo H, Knippers R, et al. The SAF-box domain of chromatin protein DEK. Nucleic Acids Res. 2005;33(3):1101–1110. doi: 10.1093/nar/gki258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devany M, Kappes F, Chen KM, Markovitz DM, Matsuo H. Solution NMR structure of the N-terminal domain of the human DEK protein. Protein Sci. 2008 Feb;17(2):205–215. doi: 10.1110/ps.073244108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu GK, Grosveld G, Markovitz DM. DEK, an autoantigen involved in a chromosomal translocation in acute myelogenous leukemia, binds to the HIV-2 enhancer. Proc Natl Acad Sci U S A. 1997;94(5):1811–1815. doi: 10.1073/pnas.94.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faulkner NE, Hilfinger JM, Markovitz DM. Protein phosphatase 2A activates the HIV-2 promoter through enhancer elements that include the pets site. J Biol Chem. 2001;276(28):25804–25812. doi: 10.1074/jbc.M006454200. [DOI] [PubMed] [Google Scholar]
- 16.Adams BS, Cha HC, Cleary J, Haiying T, Wang H, Sitwala K, et al. DEK binding to class II MHC Y-box sequences is gene- and allele-specific. Arthritis Res Ther. 2003;5(4):R226–R233. doi: 10.1186/ar774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas JP, Kimura A, Truckenbrodt H, Suschke J, Sasazuki T, Volgger A, et al. Early-onset pauciarticular juvenile chronic arthritis is associated with a mutation in the Y-box of the HLA-DQA1 promoter. Tissue Antigens. 1995;45(5):317–321. doi: 10.1111/j.1399-0039.1995.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 18.Le Hir H, Izaurralde E, Maquat LE, Moore MJ. The spliceosome deposits multiple proteins 20–24 nucleotides upstream of mRNA exon-exon junctions. Embo J. 2000;19(24):6860–6869. doi: 10.1093/emboj/19.24.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGarvey T, Rosonina E, McCracken S, Li Q, Arnaout R, Mientjes E, et al. The acute myeloid leukemia-associated protein, DEK, forms a splicing- dependent interaction with exon-product complexes. J Cell Biol. 2000;150(2):309–320. doi: 10.1083/jcb.150.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soares LM, Zanier K, Mackereth C, Sattler M, Valcarcel J. Intron removal requires proofreading of U2AF/3' splice site recognition by DEK. Science. 2006 Jun 30;312(5782):1961–1965. doi: 10.1126/science.1128659. [DOI] [PubMed] [Google Scholar]
- 21.Waldmann T, Eckerich C, Baack M, Gruss C. The ubiquitous chromatin protein DEK alters the structure of DNA by introducing positive supercoils. J Biol Chem. 2002 Jul 12;277(28):24988–24994. doi: 10.1074/jbc.M204045200. [DOI] [PubMed] [Google Scholar]
- 22.Kappes F, Damoc C, Knippers R, Przybylski M, Pinna LA, Gruss C. Phosphorylation by protein kinase CK2 changes the DNA binding properties of the human chromatin protein DEK. Mol Cell Biol. 2004 Jul;24(13):6011–6020. doi: 10.1128/MCB.24.13.6011-6020.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleary J, Sitwala KV, Khodadoust MS, Kwok RP, Mor-Vaknin N, Cebrat M, et al. p300/CBP-associated Factor Drives DEK into Interchromatin Granule Clusters. J Biol Chem. 2005 Sep 9;280(36):31760–31767. doi: 10.1074/jbc.M500884200. [DOI] [PubMed] [Google Scholar]
- 24.Kappes F, Fahrer J, Khodadoust MS, Tabbert A, Strasser C, Mor-Vaknin N, et al. DEK is a poly(ADP-ribose) acceptor in apoptosis and mediates resistance to genotoxic stress. Mol Cell Biol. 2008 May;28(10):3245–3257. doi: 10.1128/MCB.01921-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kappes F, Scholten I, Richter N, Gruss C, Waldmann T. Functional domains of the ubiquitous chromatin protein DEK. Mol Cell Biol. 2004 Jul;24(13):6000–6010. doi: 10.1128/MCB.24.13.6000-6010.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sierakowska H, Williams KR, Szer IS, Szer W. The putative oncoprotein DEK, part of a chimera protein associated with acute myeloid leukaemia, is an autoantigen in juvenile rheumatoid arthritis. Clin Exp Immunol. 1993 Dec;94(3):435–439. doi: 10.1111/j.1365-2249.1993.tb08214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kappes F, Burger K, Baack M, Fackelmayer FO, Gruss C. Subcellular localization of the human proto-oncogene protein DEK. J Biol Chem. 2001 Jul 13;276(28):26317–26323. doi: 10.1074/jbc.M100162200. [DOI] [PubMed] [Google Scholar]
- 28.Mor-Vaknin N, Punturieri A, Sitwala K, Faulkner N, Legendre M, Khodadoust MS, et al. The DEK nuclear autoantigen is a secreted chemotactic factor. Mol Cell Biol. 2006 Dec;26(24):9484–9496. doi: 10.1128/MCB.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B- lymphocytes. J Biol Chem. 1998;273(32):20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 30.Sexton PS, Cenedella RJ. Immunomagnetic capture of lens membrane fractions containing steroid binding protein. Biochem Biophys Res Commun. 2002 Jul 26;295(4):1027–1031. doi: 10.1016/s0006-291x(02)00770-2. [DOI] [PubMed] [Google Scholar]
- 31.Van Hoeyveld E, Willebrods L, Bossuyt X. Protein G ELISA for the detection of circulating immune complexes. An alternative to the solid-phase radioimmunoassay for the monoclonal rheumatoid factor. Clin Chem Lab Med. 2001 Jun;39(6):562–564. doi: 10.1515/CCLM.2001.092. [DOI] [PubMed] [Google Scholar]
- 32.Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 33.Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol Dis. 2006 Mar-Apr;36(2):315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal A, Bhardwaj A, Alam S, Misra R. Evidence for activation of the alternate complement pathway in patients with juvenile rheumatoid arthritis. Rheumatology (Oxford) 2000 Feb;39(2):189–192. doi: 10.1093/rheumatology/39.2.189. [DOI] [PubMed] [Google Scholar]
- 35.Khalkhali-Ellis Z, Bulla GA, Schlesinger LS, Kirschmann DA, Moore TL, Hendrix MJ. C1q-containing immune complexes purified from sera of juvenile rheumatoid arthritis patients mediate IL-8 production by human synoviocytes: role of C1q receptors. J Immunol. 1999 Oct 15;163(8):4612–4620. [PubMed] [Google Scholar]
- 36.Reichert VL, Le Hir H, Jurica MS, Moore MJ. 5' exon interactions within the human spliceosome establish a framework for exon junction complex structure and assembly Genes & Development. 2002;16(21):2778–2791. doi: 10.1101/gad.1030602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderton SM. Post-translational modifications of self antigens: implications for autoimmunity. Curr Opin Immunol. 2004 Dec;16(6):753–758. doi: 10.1016/j.coi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Doyle H, Mamula MJ. Posttranslational protein modifications: new flavors in the menu of autoantigens. Curr Opin Rheumatol. 2002;14:244–249. doi: 10.1097/00002281-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Eggleton P, Haigh R, Winyard PG. Consequence of neo-antigenicity of the 'altered self'. Rheumatology (Oxford) 2008 May;47(5):567–571. doi: 10.1093/rheumatology/ken014. [DOI] [PubMed] [Google Scholar]
- 40.Ravelli A, Felici E, Magni-Manzoni S, Pistorio A, Novarini C, Bozzola E, et al. Patients with antinuclear antibody-positive juvenile idiopathic arthritis constitute a homogeneous subgroup irrespective of the course of joint disease. Arthritis Rheum. 2005 Mar;52(3):826–832. doi: 10.1002/art.20945. [DOI] [PubMed] [Google Scholar]
- 41.Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, et al. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002 Jul;14(7):713–722. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- 42.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001 Mar;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 43.Voice JK, Lachmann PJ. Neutrophil Fc gamma and complement receptors involved in binding soluble IgG immune complexes and in specific granule release induced by soluble IgG immune complexes. Eur J Immunol. 1997 Oct;27(10):2514–2523. doi: 10.1002/eji.1830271008. [DOI] [PubMed] [Google Scholar]
- 44.Mollnes TE, Paus A. Complement activation in synovial fluid and tissue from patients with juvenile rheumatoid arthritis. Arthritis Rheum. 1986 Nov;29(11):1359–1364. doi: 10.1002/art.1780291108. [DOI] [PubMed] [Google Scholar]
- 45.Olds LC, Miller JJ., 3rd C3 activation products correlate with antibodies to lipid A in pauciarticular juvenile arthritis. Arthritis Rheum. 1990 Apr;33(4):520–524. doi: 10.1002/art.1780330409. [DOI] [PubMed] [Google Scholar]
- 46.Jarvis JN, Taylor H, Iobidze M, Krenz M. Complement activation and immune complexes in children with polyarticular juvenile rheumatoid arthritis: a longitudinal study. J Rheumatol. 1994 Jun;21(6):1124–1127. [PubMed] [Google Scholar]
- 47.Tabbert A, Kappes F, Knippers R, Kellermann J, Lottspeich F, Ferrando-May E. Hypophosphorylation of the architectural chromatin protein DEK in death-receptor-induced apoptosis revealed by the isotope coded protein label proteomic platform. Proteomics. 2006 Nov;6(21):5758–5772. doi: 10.1002/pmic.200600197. [DOI] [PubMed] [Google Scholar]
- 48.Dieker JW, Fransen JH, van Bavel CC, Briand JP, Jacobs CW, Muller S, et al. Apoptosis-induced acetylation of histones is pathogenic in systemic lupus erythematosus. Arthritis Rheum. 2007 Jun;56(6):1921–1933. doi: 10.1002/art.22646. [DOI] [PubMed] [Google Scholar]
- 49.Edmondson DG, Davie JK, Zhou J, Mirnikjoo B, Tatchell K, Dent SY. Site-specific loss of acetylation upon phosphorylation of histone H3. J Biol Chem. 2002;277(33):29496–29502. doi: 10.1074/jbc.M200651200. [DOI] [PubMed] [Google Scholar]
- 50.Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, et al. DNA damage activates p53 through a phosphorylation-acetylation cascade Genes & Development. 1998;12(18):2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.