Abstract
Objective
To determine whether the effect of neoadjuvant chemotherapy with methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) on the survival of patients with locally advanced urothelial carcinoma (UC) of the bladder treated with radical cystectomy varies with the presence of non-urothelial components in the tumour.
Patients and Methods
This is a secondary analysis of the Southwest Oncology Group-directed intergroup randomized trial S8710 of neoadjuvant MVAC followed by cystectomy versus cystectomy alone for treatment of locally advanced UC of the bladder. For the purpose of these analyses, tumours were classified based on the presence of non-urothelial components as either pure UC (n = 236) or mixed tumours (n = 59). Non-urothelial components included squamous and glandular differentiation. Cox regression models were used to estimate the effect of neoadjuvant MVAC on all-cause mortality for patients with pure UC and for patients with mixed tumours, with adjustment for age and clinical stage.
Results
There was evidence of a survival benefit from chemotherapy in patients with mixed tumours (hazard ratio 0.46; 95% CI 0.25–0.87; P = 0.02). Patients with pure UC had improved survival on the chemotherapy arm but the survival benefit was not statistically significant (hazard ratio 0.90; 95% CI 0.67–1.21; P = 0.48). There was marginal evidence that the survival benefit of chemotherapy in patients with mixed tumours was greater than it was for patients with pure UC (interaction P = 0.09).
Conclusion
Presence of squamous or glandular differentiation in locally advanced UC of the bladder does not confer resistance to MVAC and in fact may be an indication for the use of neoadjuvant chemotherapy before radical cystectomy.
Keywords: urothelial carcinoma, mixed histological features, neoadjuvant chemotherapy
Introduction
Bladder cancer (BC) is the fifth most commonly diagnosed malignancy in the USA, with more than 70 000 new cases and more than 14 000 deaths reported in 2009 [1,2]. Most deaths from BC occur among patients with an initial diagnosis of muscle-invasive disease (stages T2–T4). Standard therapy for resectable (T2–T4a) muscle-invasive BC without known metastases includes radical cystectomy with pelvic lymphadenectomy [3]. Unfortunately, many patients with apparently resectable muscle-invasive BC have undiagnosed micrometastatic disease at the time of definitive surgery. In a series of 1054 patients treated with radical cystectomy and pelvic lymphadenectomy between 1977 and 1997, with a median follow up of 10.2 years, BC recurred in 311 patients (30%) with a median time to recurrence of 12 months. Three-quarters of all patients with disease recurrence had distant metastases [4].
Early treatment of micrometastatic disease with neoadjuvant platinum-based combination chemotherapy (PBCC) administered before definitive local treatment (cystectomy and/or radiotherapy) has been compared with local treatment alone in several randomized trials. A meta-analysis of these trials showed that addition of a neo-adjuvant PBCC regimen to local treatment improves the average 5-year survival by 5% on the additive scale (from 45% to 50%) [5]. Several trials also reported that the use of neoadjuvant PBCC may increase the probability of pathological stage zero (pT0) at cystectomy from approximately 12–15% in the cystectomy-only arm to 33–38% in the PBCC plus cystectomy arm [6,7]. Although most patients who are treated with radical cystectomy for muscle-invasive BC have pure urothelial carcinoma (UC), tumours with mixed histological features (UC co-existing with non-urothelial histology) are also common. For example, in a series of 243 patients with clinical stage of at least T2, 96 patients (40%) had mixed histological features, most frequently UC with squamous and/or glandular differentiation [8].
It is currently unknown whether the benefit of neoadjuvant PBCC on pathological down-staging and survival of patients with apparently resectable muscle-invasive UC treated with radical cystectomy is influenced by the presence of a non-urothelial component in the tumour. Observational studies suggested that among patients with metastatic BC, patients with pure UC as well as patients with mixed tumours can achieve a complete clinical response to PBCC (disappearance of all clinical and radiographic evidence of disease) [9,10]. In one series of patients with metastatic BC, complete clinical response to PBCC was reported in 39% of 74 patients with pure UC and in 25% of 20 patients with mixed histology (UC with squamous, glandular or spindle cell components) [9]. In another series of patients with metastatic UC, any clinical response to PBCC (complete or partial) was observed in 44% of 389 patients with pure UC and in 34% of 42 patients with mixed urothelial and squamous histology [10].
These findings reported for metastatic UC may not be directly applicable to patients with apparently resectable BC because the biology of metastatic and locally advanced tumours may be different. In particular, complete clinical response of metastatic lesions (which is determined primarily by imaging studies) may not be equivalent to complete pathological response of the primary tumour in the bladder (stage pT0 at cystectomy). In addition, survival of patients with metastatic BC is usually very poor. While histological type may not have a strong impact on survival of patients with this very advanced form of disease, it may theoretically influence both response to chemotherapy and survival of patients with less advanced tumours treated with cystectomy and neoadjuvant PBCC with curative intent.
The purpose of the current study is to determine whether the effect of neoadjuvant PBCC on pathological down-staging and survival of patients with locally advanced UC of the bladder treated with radical cystectomy was influenced by the presence of non-urothelial components in the tumour.
Patients and Methods
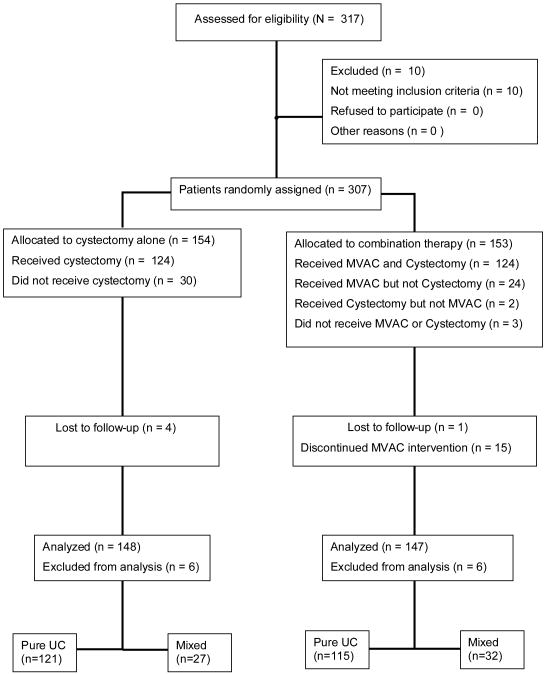
This is a secondary analysis of the Southwestern Oncology Group (SWOG) -directed intergroup trial of neo-adjuvant methotrexate, vinblastine, doxorubicin, cisplatin (MVAC) followed by cystectomy versus cystectomy alone (SWOG 8710) [6]. Eligibility criteria for the trial included clinical stage T2–T4a N0 M0 UC of the bladder, no previous pelvic radiation, adequate renal, hepatic and haematological function, and a SWOG performance status of 0 or 1 [6]. A total of 307 eligible patients were enrolled between 1987 and 1998 and they were randomized to either MVAC plus cystectomy (n = 153) or cystectomy alone (n = 154) (Fig. 1). According to the study protocol, two pathological reviews were planned for each patient: first, a central pathological review of the pre-registration biopsy (transurethral resection) specimen to confirm eligibility, and second, a review of the cystectomy specimen to determine the pathological stage. As was discussed in the original publication, the first review was not performed for 46 patients because slides were not submitted or were lost in shipment [6]. These patients were enrolled in the trial and underwent randomization. Of the remaining patients, four had missing information on histological type from the first review (because of inadequate specimen submission). For these 50 patients (46 + 4), histological type was determined from the institutional pathology reports. For all other patients, histological type was determined by the central pathological review (performed by a single expert genitourinary pathologist per case).
FIG. 1. CONSORT diagram for the SWOG 8710 trial: MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin; UC, urothelial carcinoma.
For the purpose of analyses reported in this paper, tumours were classified based on the presence of non-urothelial components as either pure UC (n = 236) or mixed tumours (n = 61). For 10 patients, tumours could not be definitively classified as either pure or mixed based on available information. These 10 patients were excluded from our analysis. Non-urothelial components included squamous histology (n = 37), adenocarcinoma (n = 20), squamous histology with adenocarcinoma (n = 2) and other histological types (n = 2). The two patients with other histological types (one small cell and one signet ring) were also excluded to make the mixed histology group a more homogeneous pathological entity (UC with squamous and/or glandular differentiation). None of the tumours in this study had a documentation of micropapillary features. The relative frequencies of cases contributed by different study centres were similar for the two histology groups (pure UC and mixed tumours).
The primary covariates of interest in the current analyses were ‘treatment’ (MVAC plus cystectomy vs cystectomy alone) and ‘histological type’ (pure UC vs mixed tumours). Other candidate covariates included age at randomization (in years), clinical stage (T2 vs T3–T4a), gender (male or female), and race (white or other race). The clinical stages were defined according to the fourth edition of the American Joint Committee on Cancer staging manual [6,11]. Two outcome measures were examined in the current study: the probability of no residual tumour in the cystectomy specimen (stage pT0 at cystectomy), and the hazard of death from all causes (all-cause mortality). All-cause mortality was the primary endpoint of the trial according to the original study protocol. Survival time was measured from randomization until death from any cause. Patients were surveyed at their last contact date. All patients provided written informed consent, and the study was approved by the ethics committees of participating institutions. The proportions of patients with stage pT0 at cystectomy were compared between the two treatment arms separately for patients with pure UC and for patients with mixed tumours using the chi-squared test or the Fisher's exact test (where appropriate). The same methods were used to compare the proportions of pT0s at cystectomy between patients with pure UC and patients with mixed tumours within each treatment arm. Direct standardization was used to adjust the estimated down-staging effects of treatment and histological type for clinical stage and to test for treatment-by-histological type interaction with stage-adjustment [12–14]. The statistical interaction between treatment and histological type would indicate that the effect of treatment in patients with mixed tumours is different in magnitude from the effect of treatment in patients with pure UC. In the analysis of tumour down-staging, we made a conservative assumption that patients who did not undergo cystectomy, regardless of reason, had residual disease (they were considered not to have stage pT0).
The effect of MVAC on all-cause mortality was estimated separately for patients with mixed tumours and for patients with pure UC using the Cox model [15]. This model was also used to estimate the effect of histological type (mixed tumours vs pure UC) on all-cause mortality within each treatment arm and to test for treatment-by-histological type interaction. All regression models were stratified on clinical stage and included age as a continuous covariate. Hence, the estimated effects of treatment and histological type on the hazard of death from all causes were controlled for age and clinical stage at randomization in all comparisons reported in this paper. The assumptions of proportional hazards and of linearity of age (the only continuous covariate) with respect to the log-hazard were checked and no major model violations were observed. Product terms were used to test for treatment-by-histological type interaction. All analyses were performed in SAS version 9.2 (SAS Institute, Cary, NC, USA). All reported P values are two-sided.
Results
The distribution of patient characteristics for each combination of treatment and histological type is shown in Table 1. Table 2 shows numbers and percentages of patients who had stage pT0 based on pathological examination of the cystectomy specimen and numbers and percentages of patients whose pT0 status was not known. The pT0 status was known for 266 patients who either received cystectomy (n = 243) or had surgery cancelled or aborted because of overt disease progression/unresectable disease. The pT0 status was not known for 29 patients who did not undergo cystectomy for reasons other than overt disease progression (e.g. refused cystectomy for personal reasons).
TABLE 1. Patient characteristics for each combination of treatment with histological type.
| Characteristic | Mixed tumours | Pure UC | ||
|---|---|---|---|---|
| MVAC + cystectomy | Cystectomy alone | MVAC + cystectomy | Cystectomy alone | |
| N | 32 | 27 | 115 | 121 |
| Mean age (years) | 60 | 65 | 63 | 62 |
| cT3–T4a*, % | 59 | 70 | 59 | 57 |
| Women, % | 31 | 15 | 14 | 21 |
| White, % | 91 | 78 | 96 | 96 |
cT3–T4a, clinical stage T3 or T4a.
UC, urothelial carcinoma; MVAC, methotrexate, vinblastine, doxorubicin and cisplatin.
TABLE 2. Numbers and percentages of patients who had stage pT0 at cystectomy and numbers and percentages of patients whose pT0 status was not known, by treatment–histology combinations.
| Histological type | Treatment arm | N | No. (%) with pT0 | No. (%) with pT0 status unknown |
|---|---|---|---|---|
| Mixed tumours | MVAC + cystectomy | 32 | 11 (34) | 4 (13) |
| Mixed tumours | Cystectomy alone | 27 | 1 (4) | 3 (11) |
| Pure UC | MVAC + cystectomy | 115 | 33 (29) | 15 (13) |
| Pure UC | Cystectomy alone | 121 | 17 (14) | 7 (6) |
UC, urothelial carcinoma; MVAC, methotrexate, vinblastine, doxorubicin and cisplatin.
The percentages of patients with stage pT0 in Table 2 were calculated by dividing the number of patients with pT0 by the total number of patients in the corresponding combination of treatment with histological type, with the conservative assumption that all patients whose pT0 status was not known had residual disease (did not have stage pT0). In the crude analysis, the additive down-staging effect (ADE) of chemotherapy estimated with this conservative assumption was equal to 34% minus 4% (i.e. 30%; P = 0.004) for patients with mixed tumours, and 29% minus 14% (i.e. 15%; P = 0.006) for patients with pure UC. The estimated ADE of mixed histology was equal to 34% minus 29% (i.e. 5%; P = 0.53) in the MVAC-plus-cystectomy arm, and 4% minus 14% (i.e. − 10%; P = 0.20) in the cystectomy-only arm.
The ADEs of treatment and histological type estimated with adjustment for clinical stage are shown in Table 3. These effects were very similar to the crude effects in terms of their magnitude and statistical significance. Evidence of tumour down-staging to pT0 after chemotherapy was clearly present among patients with mixed tumours (ADE = 28%, P = 0.004) and among patients with pure UC (ADE = 15%, P = 0.004; interaction P = 0.15).
TABLE 3. Estimated down-staging effects.
| Subset | Patients included in the subset | N | Contrast | ADE*, % | 95% CI, % | P value |
|---|---|---|---|---|---|---|
| 1 | Mixed tumours | 59 | MVAC vs cystectomy-only | 28 | (11–44) | 0.004 |
| 2 | Pure UC | 236 | MVAC vs cystectomy-only | 15 | (5–25) | 0.004 |
| 3 | MVAC + cystectomy | 147 | Mixed vs pure UC | 6 | (− 11 to 23) | 0.51 |
| 4 | Cystectomy-only | 148 | Mixed vs pure UC | − 8 | (− 20 to 3) | 0.27 |
ADE, additive down-staging effect, directly standardized to the distribution of clinical stages among all patients included in the analysis.
95% CI, 95% confidence intervals; UC, urothelial carcinoma; MVAC, methotrexate, vinblastine, doxorubicin and cisplatin.
Among patients with mixed tumours randomized to MVAC plus cystectomy, stage pT0 at the time of definitive surgery was observed in 6 of 20 patients with urothelial and squamous differentiation and in 5 of 10 patients with urothelial and glandular differentiation. Hence, pathological down-staging after chemotherapy was observed in both sub-types of mixed tumours, and was clearly not limited to only one sub-type (squamous or glandular).
Table 4 shows the estimated hazard ratios (HR) for the effect of MVAC on all-cause mortality among patients with mixed tumours (model 1) and among patients with pure UC (model 2). Also included in Table 4 are the estimated HR for the effect of mixed histology on all-cause mortality among patients randomized to MVAC-plus-cystectomy (model 3) and among patients randomized to cystectomy alone (model 4).
TABLE 4. Estimated hazard ratios.
| Model | Patients included in the model | N | Contrast | HR | 95% CI | P value |
|---|---|---|---|---|---|---|
| 1 | Mixed tumours | 59 | MVAC vs cystectomy-only | 0.46 | (0.25–0.87) | 0.02 |
| 2 | Pure UC | 236 | MVAC vs cystectomy-only | 0.90 | (0.67–1.21) | 0.48 |
| 3 | MVAC + cystectomy | 147 | Mixed vs pure UC | 0.69 | (0.42–1.13) | 0.14 |
| 4 | Cystectomy-only | 148 | Mixed vs pure UC | 1.28 | (0.80–2.06) | 0.30 |
HR, hazard ratio, adjusted for age and clinical stage; 95% CI, 95% confidence intervals; UC, urothelial carcinoma; MVAC, methotrexate, vinblastine, doxorubicin and cisplatin.
There was evidence of a survival benefit from chemotherapy in patients with mixed tumours (HR = 0.46; 95% CI 0.25–0.87; P = 0.02). Patients with pure UC had improved survival on the chemotherapy arm; however, the survival benefit was not statistically significant (HR = 0.90; 95% CI 0.67–1.21; P = 0.48). There was marginal evidence that the survival benefit of chemotherapy in patients with mixed tumours was greater than it was for patients with pure UC (statistical interaction, P = 0.09). These analyses also suggested that compared with pure UC, mixed tumours may be associated with increased mortality when treated with cystectomy alone and with decreased mortality when treated with MVAC plus cystectomy, although the estimated HR were relatively imprecise in these two comparisons (rows 3 and 4 of Table 4). Table 5 shows the age-standardized 5-year survival estimates by treatment, histological type and clinical stage obtained from the Cox model. The estimated improvement in 5-year survival associated with MVAC was much greater in magnitude among patients with mixed tumours than among patients with pure UC.
Table 5. Estimated five-year survival probabilities.
| Stage | Treatment | Pure UC | Mixed Tumors | ||
|---|---|---|---|---|---|
| 5-yr survival† | 95% CI | 5-yr survival† | 95% CI | ||
| cT2 | Cystectomy-only | 0.61 | (0.52,0.72) | 0.54 | (0.39,0.74) |
| cT2 | MVAC + cystectomy | 0.64 | (0.55,0.74) | 0.73 | (0.62,0.86) |
| cT3-T4a | Cystectomy-only | 0.42 | (0.34,0.53) | 0.34 | (0.21,0.55) |
| cT3-T4a | MVAC + cystectomy | 0.46 | (0.37,0.56) | 0.58 | (0.45,0.75) |
adjusted for age by conditional standardization (conditioned on the average age of all patients in the study); cT2 = clinical stage T2, cT3-T4a = clinical stage T3-T4a
UC, urothelial carcinoma; MVAC, methotrexate, vinblastine, doxorubicin and cisplatin
All estimates in Tables 4 and 5 were controlled for age and clinical stage. These covariates were pre-specified in the original study protocol as stratification factors for survival analysis. Because the covariates gender and race also appeared to be somewhat unbalanced between the comparison sub-groups (Table 1), we performed sensitivity analyses by including these covariates in the model. Gender and race were not independently associated with all-cause mortality in these analyses (after adjusting for age, clinical stage and histological type) and had no substantial impact on reported findings.
Discussion
The purpose of this study was to determine whether the effect of neoadjuvant MVAC on pathological down-staging and survival of patients with locally advanced UC of the bladder treated with radical cystectomy is influenced by the presence of squamous and/or glandular components in the tumour. This question is important because squamous and/or glandular differentiation co-existing with malignant urothelial histology is a fairly common finding in muscle-invasive BC. To our knowledge, it has never been clearly showed that neoadjuvant PBCC can induce complete pathological response (stage pT0) and improve survival of patients with these mixed tumours. Before results of the current analyses became available, we considered it possible that the presence of non-urothelial components could render UC resistant to MVAC. If this was confirmed, then patients with mixed tumours could potentially benefit from immediate cystectomy (without neoadjuvant chemotherapy), especially as delaying cystectomy for more than 3 months from diagnosis of muscle invasion has been associated with decreased survival in some patient populations [16].
Our current analyses have showed that the presence of squamous and/or glandular differentiation does not make a locally advanced UC of the bladder resistant to neoadjuvant chemotherapy with MVAC. Evidence of tumour down-staging to pT0 after chemotherapy was clearly present among patients with pure UC (ADE = 15%, P = 0.004) and among patients with mixed tumours (ADE = 28%, P = 0.004) (Table 3). The actual proportions of pT0s reported in these analyses for each combination of treatment with histological type (Table 2) are slightly less than the proportions originally reported for all histological types combined (15% in the cystectomy-only arm and 38% in the MVAC-plus-cystectomy arm) [6]. In the original analyses, the proportions of pT0s were computed using only those patients who received cystectomy. Because some patients did not undergo cystectomy because of disease progression/unresectable disease and others refused surgery or did not receive it for unknown reasons, in the current analyses of tumour down-staging we made a conservative assumption that patients who did not undergo cystectomy, regardless of reason, had residual disease (they were considered not to have stage pT0). This is the most conservative approach to the analysis of tumour down-staging. Indeed, it is possible that some patients who refused cystectomy did so because of complete clinical response to chemotherapy. If some of these patients in fact had no residual disease, then the down-staging effect of chemotherapy reported in this paper could be underestimated. This down-staging effect (estimated under the most conservative assumptions) was nevertheless fairly large in magnitude, especially for patients with mixed tumours (28% on the additive scale or 28 extra pT0s per 100 patients treated, P = 0.004). The estimated survival benefit of chemotherapy among patients with mixed tumours was also fairly large (HR = 0.46, P = 0.02). For patients with pure UC, the estimated improvement in survival after chemotherapy was not as substantial (HR = 0.90, P = 0.48).
The strengths of this study include randomized treatment allocation, central pathological review (performed for most patients), the use of the standard treatment protocol (MVAC as the only form of neoadjuvant chemotherapy, no previous pelvic irradiation, etc.), and rigorous follow up. However, some limitations of this study exist. First, the results of the current analyses may be difficult or impossible to generalize to mixed urothelial tumours with non-urothelial components other than squamous cell or adenocarcinoma. For example, there is evidence to suggest that small cell tumours of the bladder may respond better to neuroendocrine regimens than to urothelial regimens such as MVAC [17]. Second, even for squamous and glandular components, survival could not be analysed separately in the current study because the estimated HR would be highly imprecise because of the small sample size for individual histological sub-types. However, our analyses suggested that pathological down-staging after chemotherapy occurs in both sub-types of mixed tumours (squamous and glandular), and clearly is not limited to only one sub-type. Hence, it is unlikely that improvement in survival after chemotherapy is limited to only one of the two sub-types.
It must also be recognized that definition and documentation of mixed histology may vary between pathologists. In our study, histological type of 50 patients was determined by institutional pathology report because slides were not available for the central review (these patients contributed 12 of the 59 mixed histology cases). Hence some potential misclassification of histological types in our study could occur, and this must be recognized as a limitation. Similarly, proportions of non-urothelial components in mixed histology tumours could not be obtained from this analysis (it was rarely reported) and so the impact of this proportion on response to MVAC or outcome in the cystectomy-only arm could not be ascertained. Another question which needs to be considered is whether results reported in this paper are fully applicable to PBCC regimens other than MVAC. In the setting of metastatic disease, MVAC seems to result in the same survival as a less toxic regimen composed of gemcitabine and cisplatin [18]. However, the two regimens have never been directly compared in the setting of apparently resectable BC. Hence, the ability of gemcitabine and cisplatin to induce complete pathological response and improve survival of patients with locally advanced UC of the bladder with non-urothelial components remains uncertain.
Some of the questions that could not be fully answered in this study may potentially be examined in secondary analyses of other trials of neoadjuvant PBCC for locally advanced BC, and possibly in pooled analyses of two or more trials. For example, a European trial of CMV (cisplatin, methotrexate and vinblastine) enrolled more than 900 patients with stage T2–T4a N0/NX M0 BC [7]. To our knowledge, the effect of CMV in that trial has not been examined separately for patients with pure UC and for patients with mixed tumours. A pooled analysis of two or more trials would improve statistical power and increase the precision of estimation. This would be particularly beneficial for the formal test of statistical interaction between treatment and histological type. Unfortunately, interaction effects are difficult to detect in studies powered for the analysis of the main effects [19]. Even large interactions often produce P values above α = 0.05 (the conventional level of significance for the main effects) in moderate-sized studies. Secondary analyses of larger trials and pooled analyses of two or more trials may be particularly beneficial in these situations.
In summary, presence of squamous or glandular differentiation in locally advanced UC of the bladder does not confer resistance to MVAC and in fact may be an indication for the use of neoadjuvant chemotherapy before radical cystectomy.
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA21115, CA35421, CA46441, CA22433, CA42777, CA58861, CA59416, CA46282, CA27057, CA14028, CA46113, CA20319, CA46136, CA45377, CA128567, CA45560, CA35431, CA32734, CA35261, CA35090, CA16385, CA58882, CA76447, CA46368, CA68183, CA28862, CA58415, CA35281, CA63844, CA35192, CA35117, CA35084, CA58686 and CA35262; and by Ashley Family Foundation.
Abbreviations
- BC
bladder cancer
- PBCC
platinum-based combination chemotherapy
- UC
urothelial carcinoma
- SWOG
Southwestern Oncology Group
- MVAC
methotrexate, vinblastine, doxorubicin, cisplatin
- ADE
additive down-staging effect
- HR
hazard ratio
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2009. Available at: www.cdc.gov/uscs. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Stenzl A, Cowan NC, De Santis M, et al. Guidelines on Bladder Cancer: Muscle-Invasive and Metastatic. Arnheim, the Netherlands: European Association of Urology; 2008. p. 56. [Google Scholar]
- 4.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 5.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol. 2005;48:202–5. doi: 10.1016/j.eururo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 7.International collaboration of triallists on behalf of the Medical Research Council Advanced Bladder Cancer Working Party. Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet. 1999;354:533–40. [PubMed] [Google Scholar]
- 8.Wasco M, Daignault Y, Zhang L, et al. Urothelial carcinoma with divergent histologic differentiation (mixed histologic features) predicts the presence of locally advanced bladder cancer when detected at transurethral resection. Urology. 2007;70:69–74. doi: 10.1016/j.urology.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Logothetis CJ, Dexeus FH, Chong C, et al. Cisplatin, cyclophosphomide and doxorubicin chemotherapy for unresectable urothelial tumors: the M.D. Anderson experience. J Urol. 1989;141:33–7. doi: 10.1016/s0022-5347(17)40578-7. [DOI] [PubMed] [Google Scholar]
- 10.Kastritis E, Dimopoulos N, Antoniou C, et al. The outcome of patients with advanced pure squamous or mixed squamous and transitional urothelial carcinomas following platinum-based chemotherapy. Anticancer Res. 2006;26:3865–9. [PubMed] [Google Scholar]
- 11.Beahrs OH, Henson DE, Hutter RVP, editors. Manual for Staging of Cancer. 4th. Philadelphia: Lippincott; 1992. pp. 195–200. [Google Scholar]
- 12.Rothman K, Greenland S, Lash T. Modern Epidemiology. 3rd. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 266. [Google Scholar]
- 13.Szklo M, Nieto F. Epidemiology – Beyond the Basics. 2nd. Sudbury, MA: Jones and Bartlet; 2007. p. 422. [Google Scholar]
- 14.Woodward M. Epidemiology – Study Design and Data Analysis. Boca Raton, FL: CRC Press; 2005. pp. 184–5.pp. 207 [Google Scholar]
- 15.Cox D. Regression models and life tables. J Royal Stat Soc. 1972;B34:187. [Google Scholar]
- 16.Gore JL, Lai J, Setodji CM, et al. Mortality increases when radical cystectomy is delayed more than 12 weeks. Cancer. 2009;115:988–96. doi: 10.1002/cncr.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black PC, Brown GA, Colin PN, et al. The impact of variant histology on the outcome of bladder cancer treated with curative intent. Urol Oncol. 2009;27:3–7. doi: 10.1016/j.urolonc.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–77. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 19.Greenland S. Tests for interaction in epidemiologic studies: a review and a study of power. Stat Med. 1983;2:243–51. doi: 10.1002/sim.4780020219. [DOI] [PubMed] [Google Scholar]