Abstract
Wnt/β-catenin pathway activation caused by APC mutations occurs in approximately 80% of sporadic colorectal cancers. The anti-helminth compound niclosamide downregulates components of the Wnt pathway, specifically Dishevelled-2 (Dvl2) expression, resulting in diminished downstream β-catenin signaling. In this study, we determined if niclosamide could inhibit the Wnt/ β-catenin pathway in human colorectal cancers and whether its inhibition might elicit antitumor effects in the presence of APC mutations. We found that niclosamide inhibited Wnt/ β-catenin pathway activation, downregulated Dvl2, decreased downstream β-catenin signaling and exerted anti-proliferative effects in human colon cancer cell lines and colorectal cancer cells isolated by surgical resection of metastatic disease, regardless of mutations in APC. In contrast, inhibition of NF-κB or mTOR did not exert similar anti-proliferative effects in these colorectal cancer model systems. In mice implanted with human colorectal cancer xenografts, orally administered niclosamide was well tolerated, achieved plasma and tumor levels associated with biologic activity and led to tumor control. Our findings support clinical explorations to reposition niclosamide for treatment of colorectal cancer.
INTRODUCTION
Currently available systemic therapies increase survival but do not cure advanced colorectal cancer (CRC). One explanation is the failure of these therapies to address the subset of malignant cells with stem cell-like characteristics. The Wnt signaling pathway, fundamental to embryonic tissue patterning, is also activated in stem-like cells (1-3). Binding of Wnt ligands, of which there are 19 subtypes in humans, to Frizzled receptors, of which there are at least ten (4,5), results in the recruitment of intracellular Dishevelled (Dvl), leading to Frizzled receptor internalization (6). Downstream signaling events include the stabilization of cytosolic β-catenin and translocation of the stabilized β-catenin to the nucleus which results in the activation of the transcription factor LEF/TCF (3,7). In the absence of pathway stimulation, a complex consisting of axin, GSK-3, and adenomatous polyposis coli (APC) promotes the proteolytic degradation of β-catenin.
The canonical Wnt pathway is activated in approximately 80% of sporadic CRC primarily due to mutations in the APC gene (8,9) and in a small proportion of cases, activating mutations of the β-catenin gene (CTNNB1)(9). Although one would expect that inhibition of the Wnt pathway would require drugs that inhibit β-catenin or its downstream intermediaries, recent observations reveal that Wnt ligands or inhibitors may affect the growth and survival of colon cancer cells in spite of the presence of APC or CTNNB1 mutations (10-12). This suggests that inhibition of upstream receptors, feasible drug targets compared with downstream protein-protein interactions, may provide therapeutic benefit in CRC (5, 13-16). We instituted a translational small molecule screening program to identify agents that inhibit Wnt/Frizzled signaling. Using libraries containing FDA-approved drugs, we discovered that the anti-helminthic niclosamide promotes Frizzled1 (Fzd1) endocytosis, downregulates Dishevelled-2 protein, and inhibits Wnt3A-stimulated β-catenin stabilization and downstream β-catenin signaling (LEF/TCF reporter activity). We noted that Fzd1 colocalizes in vesicles containing transferrin and agonist-activated beta(2)-adrenergic receptor following niclosamide-mediated internalization and could serve as a negative modulator of Wnt/Fzd1 signaling by depleting Fzd and Dvl (17).
We now extend our previous studies by demonstrating that niclosamide inhibits Fzd1 signaling in human colon cancer cell lines, and in human colorectal metastasectomy specimens. We found that niclosamide was well tolerated in experimental animal models and had anti-tumor effects in vitro and in vivo, despite mutations in APC.
MATERIALS and METHODS
Reagents
The following reagents were purchased for this study: Niclosamide, protease inhibitors (P8340), phenylmethanesulfonylfluoride (P7626), orthovanadate (220590), and the IκB kinase inhibitor PS-1145 (P6624) (Sigma-Aldrich, St. Louis, MO); 7-AAD and Annexin V-biotin kits (Immunotech, Marseille, France); TNF-α (R&D Systems, Minneapolis, MN); mTOR inhibitor everolimus (Novartis, East Hanover, NJ); oxaliplatin (Sanofi Aventis, Paris, France).
Mice
NOD.CB17-Prkdcscid/J (NOD/SCID) mice were purchased from Jackson Labs (Bar Harbor, ME) and bred in the Duke Comprehensive Cancer Center Isolation Facility. All work was performed under a Duke University IACUC-approved protocol.
Cell Lines
Colorectal cancer cell lines, HT29 (ATCC HTB-38), HCT116 (ATCC CCL-247), CaCO2 (ATCC HTB-37) and MCF-10A (ATCC CRL-10317) were purchased from the American Type Culture Collection, (ATCC, Manassas, VA). ATCC characterizes these cells by morphology, immunology, DNA fingerprint, and cytogenetics. HT29 and CaCO2 cells were cultured in Dulbecco’s Modified Eagle Medium, and HCT116 cells were cultured in McCoy’s 5A medium supplemented with 10% fetal bovine serum. MCF-10A cells were cultured in MEBM medium with 5% horse serum and MEGM growth factors (Lonza, Walkersville, MD). All cultures were monitored routinely and found to be free of contamination by mycoplasma or fungi. All cell lines were discarded after 4 months and new lines were propagated from frozen stocks.
Tumor cell isolation from patients’ colorectal cancer specimens and establishment of explants in NOD/SCID mice
Patients undergoing resection of colorectal cancer metastatic to the liver provided signed informed consent approved by the Duke University Medical Center Institutional Review Board to allow harvest of tumor remaining viable despite prior systemic chemotherapy. Colorectal cancer (CRC) cells were isolated from metastatic specimens as previously described (18), and CRC cells growing in vitro were used as target cells in subsequent assays.
APC and β-catenin Mutation Analyses
Genomic DNA was prepared from CRC explants growing in NOD/SCID mice using standard methods. H&E stains were initially performed on the CRC explants and areas of malignant epithelial cells were macrodissected. Genomic DNA was isolated, and used to PCR amplify the DNA fragments of mutation hotspot of APC (19) or β-catenin (20) gene. PCR fragments were agarose gel purified, and subjected for DNA sequencing. The sequences obtained were compared with the published DNA sequence of APC (NCBI accession: NG_008481) or β-catenin (NCBI accession: NG_013302) gene.)
MTT Assay
Colorectal cancer cell lines (HT29, HCT116, CaCO2) and CRC explant cells growing in vitro (CRC007, CRC010, CRC020, CRC025, CRC028, CRC039, CRC057, CRC119) were put into 96-well flat-bottomed plates at 5,000 and 10,000 cells/well, respectively. Niclosamide (0.4, 2, 10 μM) was added and further incubated for 3 days. Oxaliplatin (10 μM) was added to control wells for comparison purposes. MTT assay was performed as described elsewhere (18). To examine the over time effect of niclosamide, cells were incubated with niclosamide (0.4, 2, 10 μM) for 24, 48, 72, and 96 h and MTT assays were performed at each time point. To examine the involvement of mTOR and NF-κB signaling in the cytotoxic effect of niclosamide on colorectal cancer cells, everolimus (0.1, 0.3, 1, 3, 9 μM) (21) or PS-1145 (0.4, 2, 10, 50 μM) (22) were added to the medium and MTT assays were performed after 72 h-incubation.
Flow-based Apoptosis Assay
Colon cancer cell lines and explants (1 × 105 tumor cells), normal mammary epithelial cells (MCF-10A), fibroblasts, and peripheral blood mononuclear cells (PBMCs) derived from normal donors were cultured in 12 well flat bottom plates with niclosamide (0.2 μM to 20 μM) for 3 days. All cells were harvested with 0.05% trypsin/EDTA, washed, labeled with biotin-conjugated Annexin V and then stained with 7-AAD and Streptavidin-APC. Samples were acquired on a FACSCalibur machine (BD Bioscience, San Jose, CA) and analyzed with CellQuest software for expression of Annexin V as a marker of apoptosis.
Luciferase Reporter Assay
Cancer cells (CaCO2, HCT116) were seeded in a 96 well plate and co-transfected with 30 ng/well TOPflash or FOPflash plasmid DNA with 3 ng/well pRL-TK Renilla Luciferase plasmid DNA (Promega, Madison, WI) using Lipofectamine LTX plus (Invitrogen, Carlsbad, CA). TOPflash and FOPflash were obtained from Dr. Randy Moon at University of Washington. Sixteen hours after transfection, cells were treated with DMSO or niclosamide at several concentrations and further incubated for 24 h. Luciferase activities were measured using Dual-Luciferase Reporter Assay System (Promega). The firefly luciferase activity was normalized to Renilla luciferase activity.
Western Blot Analysis
Cytosolic fractions of lysates were isolated as described elsewhere (23). Anti-Dvl2 (clone 10B5), anti-β-catenin (clone 7D11) and anti-β-actin (clone C-11) monoclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were used to probe the blots. To examine the NF-κB activation status of niclosamide-treated cancer cells, HT29, HCT116 and CaCO2 cell lines were treated with/without niclosamide (1 μM) or PS-1145 (10 μM) for 24 h and then with/without TNF-α (10 ng/mL) for 1 h. Whole cell lysates were analyzed by Western Blot with anti-phospho-NF-κB p65 (clone 93H1, Cell Signaling Technology, Danvers, MA) and anti- NF-κB p65 (clone F-6, Santa Cruz) antibodies.
Immunohistochemistry and Fluorescent Immunostaining
Tumors grown in the flank of NOD/SCID mice, treated with/without niclosamide, were analyzed by immunohistochemistry (24). Anti-β-catenin (clone 7D11, Santa Cruz) and anti-Dvl2 (clone 10B5, Santa Cruz) monoclonal antibodies were used at 1:100 and 1:50 dilution, respectively. An Olympus Vanox AHBS3 microscope (Olympus, Tokyo, Japan) was used for the analyses and pictures were taken with the Olympus DP-70 camera. To analyze niclosamide’s effect on NF-κB signaling, cancer cell lines (HT29, HCT116, CaCO2) were incubated with/without niclosamide (1 μM) or PS-1145 (10 μM) for 24 h, and then with/without TNF-α (10 ng/mL) for 1 h. Cells were fixed with 3% paraformaldehyde (Sigma), permeabilized with 0.01% Triton X-100 (Sigma) in PBS, and then incubated with FITC-conjugated anti-NF-κB p65 antibody (clone F-6, 1:100 dilution, Santa Cruz) for 1 h at room temperature. Nuclei were labeled with DAPI (4′,6-diamidino-2-phenylindole). An Axio Observer fluorescence microscope (Carl Zeiss, Thornwood, NY) was used and images were captured on a Hamamatsu ORCA ER CCD camera (Hamamatsu Photonics, Hamamatsu, Japan) and analyzed with MetaMorph Premier 7.6.5 software (Molecular Devices, Sunnyvale, CA). Additional methodologies are provided in the Supplementary sections.
Pharmacokinetics (PK) of Niclosamide in Plasma and Tumor
NOD/SCID mice (weighting 23 to 25 g) received oral administration of niclosamide (200 mg/kg of body weight). Blood samples were obtained at predose and at 0.25, 0.5, 0.75, 1, 1.5, 4, 8, 12, 24 h after drug administration. Plasma was isolated by centrifugation and stored at −20 °C until LC/MS/MS analysis.
In order to study tumor uptake of niclosamide, NOD/SCID mice were inoculated with HCT116 cells (5 × 106 cells), and on day 4, oral gavage of niclosamide (200 mg/kg body weight) or control solvent was initiated. After 3 weeks of treatment, mice were sacrificed 24 h after the last drug administration, and blood and tumor tissue were collected simultaneously. Tumor tissue was cryo-crushed in liquid nitrogen and homogenated with deionized water in the FastPrep apparatus (4 mm ceramic bead, 20 s, speed 4). Quantification of niclosamide in mouse plasma and tumor tissue was guided by a published LC/MS/MS method (25). A Shimadzu 20A series LC system and Applied Biosystems API 4000 QTrap tandem mass spectrometer were used.
In vivo Anti-tumor Effect of Niclosamide
HCT116 cells were harvested from flasks with 0.05% Trypsin/EDTA, and resuspended with Hanks buffered solution (5 × 106 cells/100 μL). CRC explants (CRC039) cultured in vitro were harvested with the same procedure, and mixed with equal volume of Matrigel to make 1 × 106 cells/100 μL concentration. The cell suspensions (100 μL) were inoculated into the flanks of NOD/SCID mice. Four days later, niclosamide administration by gavage 6 days/week for 2 (HCT116) or 3 weeks (CRC039) began. Tumor size was measured 3 times a week until mice were euthanized.
Statistical Analysis
The Student’s t-test was used to analyze differences in tumor volumes for each niclosamide concentration compared to vehicle control. Differences at P < 0.05 were considered statistically significant.
RESULTS
Niclosamide inhibits proliferation of colorectal cancer cell lines and explants
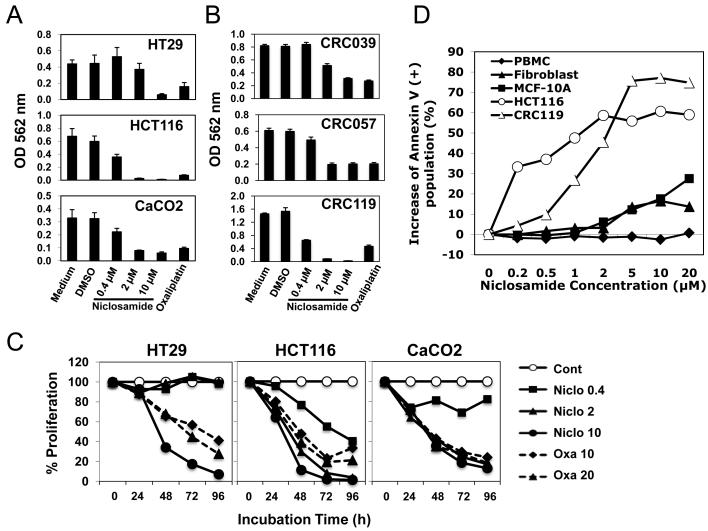
The CRC cell lines used in this study, HT29 and CaCO2 (APCmut , ß-cateninwt) and HCT116 (APCwt , ß-cateninmut), all express Fzd-1 and Fzd-2. These cell lines were incubated for 3 days with various concentrations of niclosamide (0.4~10 μM) or 10 μM of oxaliplatin, prior to analysis in an MTT assay. Niclosamide inhibited proliferation of all three cell lines with HCT116, most sensitive, and HT29, least sensitive (Figure 1A). The antiproliferative effect of niclosamide, as low as 2 μM concentration, was greater than 10 μM oxaliplatin against HCT116 and CaCO2 cells. The antiproliferative effect of niclosamide against cultured CRC explants derived from resection specimens (CRC007, CRC010, CRC020, CRC025, CRC028, CRC039, CRC057, CRC119) was similarly studied in MTT assays after 3 day-incubation with various concentrations of niclosamide. The mutational status of the APC gene and ß-catenin gene for these CRC explants is reported in Table 1. Niclosamide inhibited proliferation of all CRC explants tested in a dose-dependent fashion regardless of whether there were APC/ß-catenin mutations. While there was a trend for greater inhibition of proliferation of explants without mutations at low concentrations of niclosamide (0.4, 2.0 μM), there was equivalent inhibition of proliferation (of explants with and without APC/ ß-catenin mutations) at higher doses (Table 1; representative cases shown in Figure 1B). These data suggest that niclosamide has anticancer activity despite mutations in the downstream tumor suppressor APC.
Figure 1. Niclosamide inhibits the proliferation of colorectal cancer cell Lines and has minimal toxicity on normal fibroblasts, PBMCs, or immortalized mammary epithelial cells.
(A) Colorectal cancer cell lines, HT29, HCT116 and CaCO2, or (B) CRC explant cells growing in vitro (CRC039, CRC057, and CRC119) were cultured in 96-well flat-bottomed plates, and treated with several doses (0.4, 2, 10 μM) of niclosamide for 3 days. Oxaliplatin (10 μM) was used as a positive control. MTT assay was performed and the optical density (OD) at 562 nm was measured after cell lysis with DMSO. (C) HT29, HCT116, and CaCO2 cells were incubated with niclosamide (0.4, 2, 10 μM) for 24, 48, 72, and 96 h. Oxaliplatin (10, 20 μM) was used as a positive control. Percentages of proliferations (each OD 562nm value devided by OD 562nm value of the control (medium alone) of the same time point) are shown. (D) HCT116 and CRC119 tumor cells, fibroblasts, MCF-10A cells or PBMCs derived from normal donors, were incubated for 3 days with niclosamide at concentrations ranging from 0.2 μM to 20 μM. All cells were harvested, and labeled with annexin V. The percentage increase in annexin V-positive population (compared with untreated control) is shown.
Table 1. APC and β-catenin mutation status and effect of niclosamide on CRC explant cells in vitro.
APC and β-catenin mutations were analyzed as described in Materials and Methods. The antiproliferative effect of niclosamide against each CRC explant was analyzed by MTT assay and the percentage inhibition compared to medium alone is reported. Those conditions with >50% inhibition are presented in bold.
| APC mutation | β-catenin mutation |
% inhibition of proliferation |
|||
|---|---|---|---|---|---|
| Explant | 0.4 μM | 2 μM | 10 μM | ||
| CRC007 | Ser1356 to a stop codon | no | 1.9 | 39.8 | 47.2 |
| CRC010 | no | no | 14.2 | 63.8 | 59.3 |
| CRC020 | Glu1317 to Gln | no | 19.1 | 48.0 | 79.6 |
| CRC025 | no | no | 0.7 | 58.9 | 53.2 |
| CRC028 | Ser1356 to a stop codon | no | −2.4 | 64.1 | 50.0 |
| CRC039 | Ser1315 to a stop codon | no | −3.6 | 37.0 | 61.8 |
| CRC057 | no | no | 18.3 | 66.7 | 63.3 |
| CRC119 | no | no | 57.6 | 94.4 | 98.4 |
To assess the effect of niclosamide on colorectal cancers over time, the three cell lines were incubated with various concentrations of niclosamide or oxaliplatin for 24, 48, 72, or 96 h. As shown in Figure 1C, in general, the anti-proliferative effect increased over time.
Niclosamide is non-toxic to normal fibroblasts and PBMC
To assess the toxicity of niclosamide on non-malignant tissues, PBMCs from a normal donor, fibroblasts isolated from colorectal cancer patient’s tumor tissue and mammary epithelial cells (MCF-10A) were compared with the colorectal explants for their sensitivity to niclosamide using an annexin V-based flow cytometric assay (Figure 1D). CRC explants and HCT116 cells showed steep increases in annexin V-positive cells at 1 μM or 0.2 μM of niclosamide, respectively, reaching 60 to 80 %, suggesting a significant induction of apoptotic cell death. However, neither fibroblasts, MCF-10A cells, nor PBMCs from a normal donor showed a significant increase in apoptotic cell death. Thus, niclosamide does not have significant toxicity against non-tumor cells.
Niclosamide inhibits Wnt/ß-catenin pathway activation and decreases the cytosolic expression of endogenous Dishevelled-2 and ß-catenin in colorectal cancer cells
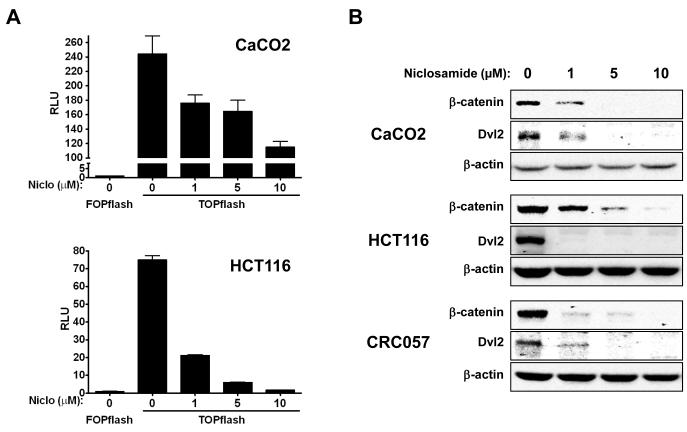
To analyze the effect of niclosamide on Wnt/ ß-catenin signaling, we performed TOPflash assay with CRC cell lines (CaCO2, HCT116) and could demonstrate that niclosamide inhibits the activation of this pathway in dose-dependent manner (Figure 2A). In our previous study, we reported the downregulation of Dvl2 and ß-catenin expression in U2OS cells by niclosamide. In the present study, we also analyzed Dvl2 and ß-catenin expression in CRC explants and cell lines and observed that these cancer cells show similar downregulation of these molecules in response to niclosamide treatment (Figure 2B). Interestingly, HT29 cells, assessed as less sensitive to niclosamide based on the MTT assay (Figure 1A), showed only moderate changes in cytosolic expression of Dvl2/ß-catenin (data not shown), while sensitive CRC explants and cell lines (CRC057, CRC119, HCT116, and CaCO2) showed more evident downregulation, suggesting a correlation of the cytotoxic effect and Wnt/Fzd1 signaling inhibition.
Figure 2. Niclosamide inhibits Wnt/β-catenin signaling and downregulates dishevelled 2 and β-catenin expression by colorectal cancer cells in vitro.
(A) CRC cell lines (CaCO2, HCT116) were transiently co-transfected with TOPflash or FOPflash plasmid DNA with pRL-TK Renilla Luciferase plasmid DNA. Cells were treated with different concentrations of niclosamide (0, 1, 5, 10 μM) for 24 h. Activity of Wnt/β-catenin signaling pathway was quantified by measuring relative firefly luciferase activity units (RLUs) normalized to Renilla luciferase. (B) CRC explants and colorectal cancer cell lines were treated with different concentrations (0, 1, 5, 10 μM) of niclosamide overnight (18 h). After washing cells with PBS, cell lysates were made with hypotonic lysis buffer. Cytosolic fractions of lysates were isolated and analyzed by Western Blot with anti-Dishevelled 2 (clone 10B5), anti-β-catenin (clone 7D11) and anti-β-actin (clone: C-11) monoclonal antibodies.
Colorectal cancer cells not affected by NF-kB or mTOR inhibitors are inhibited by niclosamide under conditions in which it inhibits Wnt pathway signaling
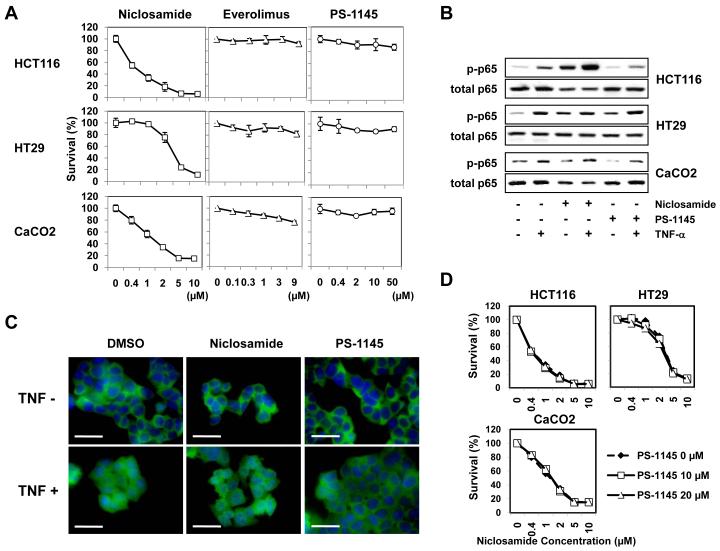
Others have reported that niclosamide inhibits the NF-κB pathway in leukemia cell lines (26) or mTOR signaling in MCF-7 breast cancer cells (27). Therefore, we wished to examine this effect in our model of colorectal cancer cells. First, we examined if specific inhibitors of the NF-κB or mTOR pathways would affect the viability of colorectal cancer cells sensitive to the effects of niclosamide. The pan-IκB kinase (IKK) inhibitor PS-1145 was used to block NF-κB signaling (22) and everolimus (21) was used to block mTOR signaling. As shown in Figure 3A, the three cell lines, under conditions where niclosamide causes inhibition of cell proliferation, were not sensitive to PS-1145 or everolimus suggesting that neither the NF-κB or mTOR pathway inhibition was a mechanism of action for niclosamide in our models. We also examined if the NF-κB pathway could be perturbed by niclosamide. When exposed to TNF-α, these cells demonstrated activations in the NF-κB pathway (elevated phospho-p65 and translocation of NF-κB p65 to the nucleus) (Figure 3B, 3C). In these same cell lines, TNF-α-induced activation of NF-κB could not be blocked by niclosamide whereas they were blocked by the IKK (IκB-kinase) inhibitor PS-1145 at least in HCT116 and CaCO2 cells (Figure 3B, 3C). Furthermore, to determine whether niclosamide-induced killing of colorectal cancers could be prevented by blocking the NF-κB pathway, we exposed these cell lines to PS-1145 during niclosamide treatment. PS-1145 could not inhibit the niclosamide-induced killing of these cancer cells (Figure 3D). Therefore, we concluded neither NF-κB nor mTOR pathway inhibition was a dominant mechanism of action in our colorectal cancer model system and niclosamide is exerting its effect primarily through the Wnt pathway.
Figure 3. The mTOR and NF-κB pathway were not targets of niclosamide.
(A) Colorectal cancer cell lines (HCT116, HT29, CaCO2) were treated with niclosamide (0-10 μM), everolimus (0-9 μM), or PS-1145 (0-50 μM) for 72 h, and cytotoxicity was analyzed in MTT assays. The relative ratio (percentages) of OD 562 nm values were calculated for each reagent by setting the OD 562 nm value at concentration 0 μM as 100%. (B, C) To examine if the NF-κB pathway could be perturbed by niclosamide, cancer cells were treated with niclosamide (1 μM) or PS-1145 (10 μM) for 24 h, and further incubated with TNF-α (10 ng/mL) for 1 h. Whole cell lysates were made and analyzed with anti-phospho-NF-κB p65 and anti-p65 antibodies by Western Blot (B). Cells were fixed, permeabilized and stained with FITC-conjugated anti-NF-κB p65 antibody (green) for 1 h. Nuclei were stained with DAPI (blue). Staining of HCT116 cells is shown. Unclear morphology of nuclei (blue) indicates nuclear translocation of p65 protein (C). Scale bars: 30 μm. (D) To determine whether niclosamide-induced cytotoxicity of colorectal cancers could be prevented by blocking the NF-κB pathway, cancer cells were treated with niclosamide (0-10 μM) in the presence/absence of PS-1145 (10, 20 μM) for 72 h and an MTT assay was performed.
Additive anti-proliferative activity of niclosamide combined with oxaliplatin
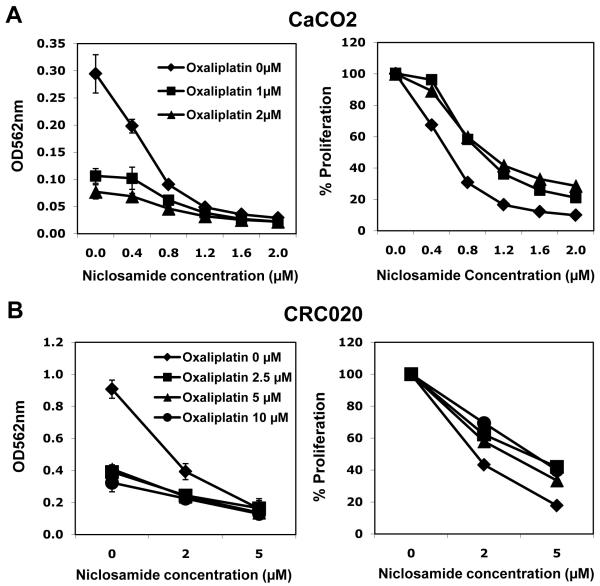
We sought to assess the combination of niclosamide with oxaliplatin, commonly used to treat CRC. The addition of oxaliplatin, even at lower concentration (1-2 μM), induced more killing of CaCO2 cells compared to niclosamide alone (Figure 4A). Percent proliferation value (right panel) was also calculated by adjusting OD562 nm values of niclosamide 0 μM at each oxaliplatin concentration to 100%. The effect of the two drugs was at least additive (right panel). Similar additive effects were observed also with CRC explant cells at low niclosamide concentrations (Figure 4B). These data suggest that niclosamide enhances the anti-tumor effect of oxaliplatin.
Figure 4. Combination effect of niclosamide and oxaliplatin on colorectal cancer cell lines and explants.
(A) Colorectal cancer cell line (CaCO2) and (B) CRC explant cells (CRC020) were cultured in 96 well plates at 5,000 cells or 10,000 cells per well, and treated with various combinations of niclosamide and oxaliplatin (CaCO2: niclosamide 0-2.0 μM, oxaliplatin 0-2.0 μM, CRC020: niclosamide 0-5.0 μM, oxaliplatin 0-10.0 μM). An MTT assay was performed after 72 h incubation. After cell lysis with DMSO, the optical density (OD) at 562 nm was measured.
Phamacokinetic analysis of niclosamide following oral administration in NOD/SCID mice
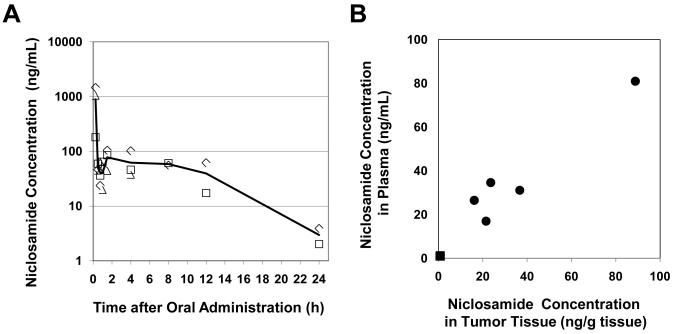
Niclosamide is administered orally for the treatment of intestinal helminthic infections and is reported to have little systemic absorption. However, we wished to determine if niclosamide was sufficiently absorbed to attain the inhibitory concentrations required in vitro. Plasma concentrations of niclosamide were measured in mice following oral administration of 200 mg/kg mixed into PEG (Figure 5A). Win-Nonlin software was used for the noncompartmental pharmacokinetic analysis of the rather complex concentration-time profile observed. Within the first hour, the concentration-time data showed a sharp increase (tmax = 15 min, Cmax = 893.7 ng/mL) and decrease (30-45 min, C = 50-40 ng/mL) followed by a small but definite “rebound” (1.5 hr, C = 78 ng/mL) after which plasma concentration decreased gradually through at least two exponential decay processes. Elimination rate (λZ = 0.217 h−1) and the half-life (t1/2 = 3.2 h) were calculated from the slope of the 2-point line (12 and 24 h) and the area under the curve between t = 0 and t = 24 h was calculated as AUClast = 1011 mg hr /L. The simplest explanation for the complex profile observed is that the initial “burst” in niclosamide plasma concentration is caused by a smaller portion of the drug in more soluble form (AUC0-1 h = 206 mg hr /L) and that the “rebound” is the final portion of the absorption profile of the main and less soluble fraction of the drug (AUC1-24 h = 805 mg hr /L). The two-phase profile at later times cannot be explained by saturation of liver enzymes since in mammals niclosamide is not metabolized by the liver. The behavior can be explained either by differing absorption profiles at different regions of the gastrointestinal tract or, less likely, by enterohepatic recycling (reabsorption from gall bladder). The overall complex behavior is most probably the consequence of high doses administered or by the specificity of the solvent system (simple pharmacokinetics observed in rats at 5 mg/kg, DMSO/cremophor EL (25)). It is important that from 0.5 h to 12 h after oral intake, plasma concentrations were relatively stable at the range of 39.5 to 77.6 ng/ml (approx. 0.1 – 0.2 μM). In order to study the niclosamide uptake by solid tumors, we measured the niclosamide concentrations in tumor tissue (ng/g tissue) obtained from mice implanted with HCT116 tumor cells and treated with niclosamide (200 mg/kg body weight/day) or control solvent for 3 weeks. At 24 h after the final administration, niclosamide concentrations in tumor tissue (37 ng/g tissue) were similar to those in plasma (38 ng/mL) (Figure 5B), suggesting the efficient and reliable distribution of niclosamide from blood to tumor tissue. Considering that the vascular volume accounts for only a small fraction of the tissue volume, our data indicate that niclosamide is retained in the tissue and that its concentration at the site of action, may be several-fold higher than in plasma (i.e. in the μM range).
Figure 5. Phamacokinetic analysis of niclosamide following oral administration in NOD/SCID mice.
(A) NOD/SCID mice received oral administration of niclosamide (200 mg/kg of body weight). Blood samples were obtained at predose and at 0.25, 0.5, 0.75, 1, 1.5, 4, 8, 12, 24 h after drug administration. Quantification of niclosamide in mouse plasma was performed by LC/MS/MS method (19) and reported as ng/ml. (B) NOD/SCID mice were inoculated with HCT116 tumor cells (5 × 106 cells), and on day 4, oral gavage of niclosamide (200 mg/kg body weight) or control solvent was initiated. After 3 weeks of treatment, mice were sacrificed 24 h after the last oral administration, and blood and tumor tissue were collected simultaneously. Tumor tissue was cryo-crushed in liquid nitrogen and homogenated with three volumes of deionized water. Quantification of niclosamide in mouse plasma and tumor tissue was performed by LC/MS/MS. Circle: niclosamide-treated (n=5), Square: control-treated (n=5).
Niclosamide has antitumor activity in vivo
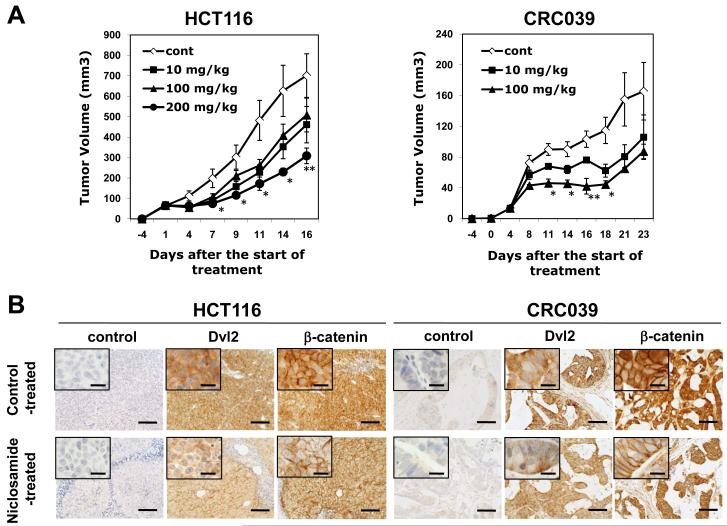
In order to study the in vivo anti-tumor effect of niclosamide, HCT116 and CRC039. were inoculated subcutaneously into mice and four days later, oral gavage of niclosamide was initiated 6 times a week. During the course of the treatment with niclosamide, no obvious side effects were observed in the mice. As shown in Figure 6A, niclosamide significantly inhibited the growth of both HCT116 and CRC039 tumors. In the more rapidly growing tumor (HCT116), a dose of 200 mg/kg of body weight was needed to suppress the tumor growth; however, 100 mg/kg of niclosamide could suppress the growth of the relatively slow-growing tumor (CRC039) to the same level. We analyzed another CRC explant (CRC028), and observed the similar inhibition of tumor growth with niclosamide (25 mg/kg per day) compared to control-treated mice. Thus, niclosamide was confirmed to inhibit the growth of human CRCs in NOD/SCID mice.
Figure 6. Niclosamide inhibits the growth of colorectal cancers in NOD/SCID mice and downregulates dishevelled 2 and β-catenin expression.
(A) HCT116 colon cancer cells were harvested from flasks with 0.05% Trypsin/EDTA, resuspended with Hanks buffered solution at 5 × 106 cells/100 μL concentration. CRC explants (CRC039) cultured in vitro were harvested with the same procedure, and mixed with equal volume of Matrigel to make 1 × 106 cells/100 μL concentration. The cell suspension (100 μL) was inoculated into the flank of NOD/SCID mice 4 days before the start of treatment. Niclosamide (10, 100, 200 mg/kg body weight) was administered by gavage for 6 days /week for 2 (HCT116) or 3 weeks (CRC039). Tumor size was measured 3 times a week until mice were euthanized. * p < 0.05, ** p < 0.01. (B) HCT116 and CRC039 tumors grown in the flank of NOD/SCID mice were treated with/without niclosamide (200 mg/kg body weight) for 2 or 3 weeks. β-catenin and Dvl2 immunohistochemistry was performed for tumor sections as described in Materials and Methods. Scale bars: 100 μm. Inlets show higher magnification (Scale bars: 20 μm).
Dishevelled-2 and ß-catenin expression decline in niclosamide treated colorectal cancer tumors in vivo
We wished to document that Wnt pathway intermediaries would decline after in vivo treatment with niclosamide. Colorectal cancer masses (HCT116, CRC028, CRC039) from mice treated with niclosamide (25 or 100 mg/kg per day) for 2 or 3 weeks were excised, formalin-fixed, and analyzed by immunohistochemistry for the expression of Dvl2 and ß-catenin. Niclosamide treated tumors showed decreased levels of cytoplasmic expression of Dvl2 and ß-catenin compared to control treated tumors (Figure 6B). This result indicates the prolonged inhibitory effect of niclosamide in vivo for Wnt/ß-catenin signaling.
DISCUSSION
The importance of inappropriate Wnt signaling to the development and progression of advanced cancers such as colorectal cancer has been well documented. Therefore, components of the Wnt pathway are prime drug development targets, particularly the accessible, plasma membrane receptors such as Frizzled (5, 13-16). However, there are currently no FDA-approved drugs that regulate Wnt signaling at the level of the Frizzled receptor. We had previously found that niclosamide promotes Fzd1 internalization and inhibits Wnt/Frizzled function, suggesting it may have anti-tumor effects. Indeed, in the current study, we demonstrated that niclosamide has anti-tumor effects in vitro against CRC cell lines and CRC cells obtained from patient resection specimens at low micromolar concentrations, but has no significant toxicity against non-tumor cells, including PBMCs.
In the current study, we could demonstrate by TOPflash assay that niclosamide can inhibit Wnt pathway activation in CRC. The mechanism of action of the niclosamide in our studies is thought to be through internalization of Fzd1 and downregulation of Wnt pathway intermediaries as we reported previously (17). In its common usage as an antihelminthic (28,29), is believed to uncouple oxidative phosphorylation (30). It has recently been found to be effective at low micromolar concentrations in preventing the synthesis of corona virus proteins in a tissue culture model of severe acute respiratory syndrome (SARS) (31). Its mechanism of action in this instance has not been well defined, but niclosamide can interact with DNA (31). We do not believe that either of these mechanisms are the operative mechanism in the tumor models, because we would have expected much greater toxicity to normal tissue if there were uncoupling of oxidative phosphorylation or DNA interacting effects in mammalian tissues. Recently, Jin et al. (26) reported that niclosamide inhibited the NF-κB pathway and increased reactive oxygen species levels to induce apoptosis in AML cells. In contrast, we did not observe any inhibitory effect of niclosamide on NF-κB signaling in our CRC model (Figure 3). Balgi (27) reported mTORC1 inhibition and autophagosome accumulation leading to autophagy in response to niclosamide exposure. Although we did not formally study the mTOR pathway in our models, it should be noted that the cell lines tested in our studies did not have mTOR pathway dysregulation and were not sensitive to the potent mTOR inhibitor everolimus (Figure 3A).
We were encouraged to find that niclosamide had activity despite the presence of APC mutations in some of the cell lines and CRC explants. Because APC mutations which impair the process of targeting ß-catenin for elimination are a common finding in CRCs, we were concerned that niclosamide, exerting its effect through upstream molecules would not have activity in APC-mutated tumors. Nonetheless, we saw similar activity regardless of the APC mutational status suggesting that inhibition of upstream molecules could impact downstream molecules as reported by He et al. (11). Since functions of both Dvl2 and ß-catenin are shown to be controlled by ubiquitination and degradation by the proteasome (32), another possible mechanism of niclosamide function might be its effect on either the ubiquitination or a proteolytic pathway to cause degradation of Dvl-2 and ß-catenin, independent of APC and ß-catenin mutation status.
One potential concern for the use of niclosamide as an anticancer therapy is the poor absorption of this drug. Indeed, we required higher doses (100 ~ 200 mg/kg body weight) of niclosamide in order to demonstrate significant inhibition of tumor growth in NOD/SCID mice. Nonetheless, we confirmed relatively stable concentrations of niclosamide in the plasma at the range of 39.5 to 77.6 ng/ml (approx. 0.1 – 0.2 μM) from 0.5 h to 12 h after oral intake (200 mg/kg). Importantly, niclosamide concentrations in tumor tissue showed good correlation with those in plasma, suggesting the efficient distribution of niclosamide from blood to tumor tissue. Furthermore, we observed downregulation of Dvl2 and ß-catenin cytosolic expression in niclosamide-treated tumor cells in vivo. Thus, oral administration of niclosamide does result in sufficient distribution of the drug into tumor tissue, to prove a prolonged inhibitory effect on Wnt/ß-catenin signaling, resulting in tumor growth inhibition.
In summary, we demonstrated down regulation of Wnt/β-catenin signaling and anti-tumor effects of niclosamide in vitro and in vivo. No significant toxicity was shown against non-tumor cells in vitro and no obvious side effects were observed in niclosamide-treated mice. We now propose to study niclosamide in phase I human studies for patients with CRC. Also, since niclosamide is minimally absorbable from gastrointestinal tract, we are studying modifications that might permit greater absorption of the drug. Finally, high niclosamide exposure should occur in the intestinal lumen, which support the use of niclosamide to prevent induction, progression, or recurrence of pre-malignant lesions and/or adenocarcinomas of the gastrointestinal tract. The relatively poor absorption, and low systemic levels of niclosamide may make this an attractive agent for cancer prevention.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Karrie Comatas and Amanda Summers for their technical assistance in this study. This work was supported in part by Susan G. Komen for the Cure (W.C.). W. C. is a V Foundation and American Cancer Society scholar. This work was also supported in part by a grant from NIH National Cancer Institute (K12-CA100639 Clinical Oncology Research Career Development Program).
Footnotes
The work was performed at: Duke University Medical Center, Durham, NC 27710.
Conflict of Interest: The authors have declared that no conflict of interest exists.
REFERENCE
- 1.Fuerer C, Nusse R, Ten Berge D. Wnt signalling in development and disease. Max Delbruck Center for Molecular Medicine meeting on Wnt signaling in Development and Disease. EMBO Rep. 2008;9:134–138. doi: 10.1038/sj.embor.7401159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chien AJ, Conrad WH, Moon RT. A Wnt Survival Guide: From Flies to Human Disease. J Invest Dermatol. 2009;129:1614–27. doi: 10.1038/jid.2008.445. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 4.Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–45. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 5.Katoh M. WNT signaling in stem cell biology and regenerative medicine. Curr Drug Targets. 2008;9:565–70. doi: 10.2174/138945008784911750. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, et al. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–94. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 7.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 8.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–37. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- 9.Schneikert J, Behrens J. The canonical Wnt signalling pathway and its APC partner in colon cancer development. Gut. 2007;56:417–425. doi: 10.1136/gut.2006.093310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 11.He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, et al. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005;24:3054–58. doi: 10.1038/sj.onc.1208511. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–22. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 13.Ding S, Wu TY, Brinker A, Peters EC, Hur W, Gray NS, et al. Synthetic small molecules that control stem cell fate. Proc Natl Acad Sci U S A. 2003;100:7632–37. doi: 10.1073/pnas.0732087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 15.Shan J, Shi DL, Wang J, Zheng J. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry. 2005;44:15495–503. doi: 10.1021/bi0512602. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Major MB, Takanashi S, Camp ND, Nishiya N, Peters EC, et al. Small-molecule synergist of the Wnt/beta-catenin signaling pathway. Proc Natl Acad Sci U S A. 2007;104:7444–48. doi: 10.1073/pnas.0702136104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M, Wang J, Lu J, Bond MC, Ren XR, Lyerly HK, et al. The anti-helminthic niclosamide inhibits Wnt/Frizzled1 signaling. Biochemistry. 2009;48:10267–74. doi: 10.1021/bi9009677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osada T, Hsu D, Hammond S, Hobeika A, Devi G, Clay TM, et al. Metastatic colorectal cancer cells from patients previously treated with chemotherapy are sensitive to T-cell killing mediated by CEA/CD3-bispecific T-cell-engaging BiTE antibody. Br J Cancer. 2010;102:124–133. doi: 10.1038/sj.bjc.6605364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groves C, Lamlum H, Crabtree M, Williamson J, Taylor C, Bass S, et al. Mutation cluster region, association between germline and somatic mutations and genotype-phenotype correlation in upper gastrointestinal familial adenomatous polyposis. Am J Pathol. 2002;160:2055–61. doi: 10.1016/S0002-9440(10)61155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakheja D, Cunningham JC, Mitui M, Patel AS, Tomlinson GE, Weinberg AG. A subset of cranial fasciitis is associated with dysregulation of the Wnt/beta-catenin pathway. Mod Pathol. 2008;21:1330–6. doi: 10.1038/modpathol.2008.112. [DOI] [PubMed] [Google Scholar]
- 21.Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, O’reilly T, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–59. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 22.Yemelyanov A, Gasparian A, Lindholm P, Dang L, Pierce JW, Kisseljov F, et al. Effects of IKK inhibitor PS1145 on NF-κB function, proliferation, apoptosis and invasion activity in prostate carcinoma cells. Oncogene. 2006;25:387–398. doi: 10.1038/sj.onc.1209066. [DOI] [PubMed] [Google Scholar]
- 23.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Osada T, Wang Y, Yu L, Sakakura K, Katayama A, et al. CSPG4 protein as a new target for antibody-based immunotherapy of triple-negative breast cancer. J Natl Cancer Inst. 2010;102:1–17. doi: 10.1093/jnci/djq343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang YW, Yeh TK, Lin KT, Chen WC, Yao HT, Lan SJ, et al. Pharmacokinetics of Anti-SARS-CoV Agent Niclosamide and Its Analogs in Rats. J. Food Drug Anal. 2006;14:329–333. [Google Scholar]
- 26.Jin Y, Lu Z, Ding K, Li J, Du X, Chen C, et al. Antineoplastic Mechanisms of Niclosamide in Acute Myelogenous Leukemia Stem Cells: Inactivation of the NF-{kappa}B Pathway and Generation of Reactive Oxygen Species. Cancer Res. 2010;70:2516–27. doi: 10.1158/0008-5472.CAN-09-3950. [DOI] [PubMed] [Google Scholar]
- 27.Balgi AD, Fonseca BD, Donohue E, Tsang TC, Lajoie P, Proud CG, et al. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS One. 2009;4:e7124. doi: 10.1371/journal.pone.0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson RD, Hewlett EL. Niclosamide therapy for tapeworm infections. Ann Intern Med. 1985;102:550–51. doi: 10.7326/0003-4819-102-4-550. [DOI] [PubMed] [Google Scholar]
- 29.Wu ZS, Wang TG, Zhang XS, Zhong B, Xu L, Gao GB, et al. Snail control by using soil pasting mixed with niclosamide. Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42:569–73. [PubMed] [Google Scholar]
- 30.Weinbach EC, Garbus J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature. 1969;221:1016–18. doi: 10.1038/2211016a0. [DOI] [PubMed] [Google Scholar]
- 31.Wu CJ, Jan JT, Chen CM, Hsieh HP, Hwang DR, Liu HW, et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob Agents Chemother. 2004;48:2693–96. doi: 10.1128/AAC.48.7.2693-2696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, MacCoss MJ, Moon RT. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-ß-catenin pathway by targeting Dishevelled for degradation. Nature Cell Biology. 2006;8:348–357. doi: 10.1038/ncb1381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.