Abstract
Anesthetics have been reported to promote Alzheimer’s disease (AD) neuropathogenesis by inducing β-amyloid protein accumulation and apoptosis. Neuroinflammation is associated with the emergence of AD. We therefore set out to determine the effects of the common anesthetic isoflurane on the levels of TNF-α, IL-6, and IL-1β, the proinflammatory cytokines, in vitro and in vivo, employing Western blot, immunohistochemistry, ELISA, and RT-PCR. Here, we show that a clinically relevant isoflurane anesthesia increased the protein and mRNA levels of TNF-α, IL-6, and IL-1β in the brain tissues of mice. The isoflurane anesthesia increased the amounts of TNF-α immunostaining positive cells in the brain tissues of mice, the majority of which were neurons. Furthermore, isoflurane increased TNF-α levels in primary neurons, but not microglia cells, of mice. Finally, isoflurane induced a greater degree of TNF-α increase in the AD transgenic mice than in the wild type mice. These results suggest that isoflurane may increase the levels of proinflammatory cytokines, which may cause neuroinflammation, leading to promotion of AD neuropathogenesis.
Keywords: Alzheimer’s disease, Anesthesia, Isoflurane, TNF-α, IL-6, IL-1β
Introduction
Neuroinflammation, which includes activation of microglia cells, participation of astrocytes, and involvement of neurons, has been suggested to play a role in neurodegenerative diseases, including Alzheimer’s disease (AD) [reviewed in (Akiyama et al., 2000; Cameron and Landreth, 2009; Heneka and O’Banion, 2007; Sastre et al., 2006)]. Specifically, a recent Framingham study by Tan et al. (Tan et al., 2007) suggests that elevated levels of tumor necrosis factor (TNF)-α and interleukin 1 (IL-1), the proinflammatory cytokines, in blood are associated with an increased risk of developing AD.
An estimated 200 million patients worldwide undergo anesthesia and surgery each year. Several studies have shown the potential association of previous general anesthesia/surgery with the development of AD (Bohnen et al., 1994; Lee et al., 2005). However, other studies have suggested different findings (Avidan et al., 2009; Gasparini et al., 2002; Knopman et al., 2005). More population studies, especially the adequately powered and multicenter human studies, are necessary to define the role of anesthesia and surgery in the development of AD (Harris and Eger, 2008).
Recent studies have suggested that a transient insult, e.g., ischemia, could lead to secondary and persistent brain injuries (van Groen et al., 2005). The inhalation anesthetics isoflurane and sevoflurane have been reported to induce such transient insults, including apoptosis, oligomerization of β-amyloid protein (Aβ), and Aβ accumulation (Dong et al., 2009; Eckenhoff et al., 2004; Wei et al., 2008; Xie et al., 2008; Xie et al., 2006a; Xie et al., 2006b; Xie et al., 2007), which may potentially contribute to AD neuropathogenesis. However, the effects of the inhalation anesthetics on neuroinflammation have not been determined. We therefore set out to assess the effects of isoflurane, one of the most commonly used inhalation anesthetics, on the levels of proinflammatory cytokine TNF-α, Interleukin-6 (IL-6) and IL-1β in primary neurons and in brain tissues of both wild type and AD transgenic mice [B6.Cg-Tg(APPswe, PSEN1dE9)85Dbo/J mice].
Experimental Procedures
Mice anesthesia
Mice were used to assess the potential in vivo effects of isoflurane on levels of proinflammatory cytokines. The protocol was approved by the Massachusetts General Hospital Standing Committee on Animals (Boston, Massachusetts) on the Use of Animals in Research and Teaching. Wild type mice (C57BL/6 mice) and AD transgenic mice [B6.Cg-Tg (APPswe, PSEN1dE9)85Dbo/J mice] (Jackson Laboratory, Bar Harbor, ME) at 5-8 months old were randomized by weight and gender into either an experimental group that received 1.4% isoflurane (a clinically relevant concentration) plus 100% oxygen for two hours or a control group that received 100% oxygen for two hours at an identical flow rate in identical anesthetizing chambers as previously described (Xie et al., 2008). We chose this anesthesia because the anesthesia with 2% isoflurane plus 100% oxygen for two hours is clinically relevant and has been shown to induce caspase activation and increase levels of BACE and Aβ without significant changes in blood pressure and blood gas in the mice at 5-8 month old (Xie et al., 2008). Moreover, the AD transgenic mice used in the experiment usually start to develop elevated Aβ levels during this age range (Garcia-Alloza et al., 2006). The mice breathed spontaneously, and the concentrations of isoflurane and oxygen were measured continuously (Datex, Tewksbury, MA). The temperature of the anesthetizing chamber was controlled to maintain rectal temperature of mice at 37 ± 0.5°C. Anesthesia was terminated by discontinuing isoflurane and placing animals in a chamber containing 100% oxygen until 20 minutes after the return of their righting reflex. They were then returned to individual home cages until they were humanely killed.
Primary neurons
Primary neurons were used to assess the potential in vitro effects of isoflurane on levels of proinflammatory cytokines. Wild type mice and AD transgenic mice with a gestation stage of day 15 were killed with carbon dioxide. We then performed a cesarean section to harvest the neurons as previously described (Zhen et al., 2009). Ten days after the harvest, the neurons were exposed to isoflurane.
Microglia cells
Microglia cells were used to compare the effects of isoflurane on the levels proinflammatory cytokines between primary neurons and microglia cells. Mice were one day old at the time of the harvest for microglia cells as described by Saura et al. (Saura et al., 2003) with modifications. Briefly, the harvested brains were put into F12 media on ice and centrifuged. The supernatant was aspirated. The brains were incubated in 0.25% trypsin for 30 minutes at 37°C. The cells were passed through 40 μm filters and were seeded to six well plates in DMEM-F12 with 10%FBS. The medium was replaced every three days for two weeks. Then, the microglia cells were obtained by mild trypsinization. Specifically, 0.25% trypsin (diluted 1:4 using serum-free DMEM-F12) was added to the cells and the mixture was incubated for 20 to 30 minutes at 37°C until the intact layer was detached. The supernatant after trypsinization was discarded and that cells remaining on the plate were the microglia cells. This is because the detachment of an intact cell layer will leave a population of cells firmly attached to the bottom of the well and these cells were identified as predominantly (>98%) microglia cells by the histochemical markers as described previously (Saura et al., 2003). Finally, the cells were used next day.
In vitro treatments of neurons and microglia cells
The primary neurons and microglia cells were treated with 2% isoflurane for six hours under 21% O2 and 5% CO2. This isoflurane treatment is clinically relevant and has been shown to induce apoptotic cell death and Aβ accumulation in H4 human neuroglia cells (Xie et al., 2006b; Xie et al., 2007) and primary neurons (Zhang et al., 2010). The inhalation anesthetic isoflurane was delivered from an anesthesia machine to a sealed plastic box in a 37°C incubator containing six-well plates seeded with 1 million cells, 0.25 million neurons, or 0.25 million microglia cells (80% confluent rate) in 1.5 ml culture media as described before (Zhang et al., 2010; Zhen et al., 2009). The control condition for the isoflurane treatment was 21% O2 plus 5% CO2, which has been shown not to induce cell death or Aβ accumulation (Xie et al., 2006a; Xie et al., 2006b; Xie et al., 2007).
Cell or tissue preparation
The cells and mouse brain tissues were prepared for Western blot analysis as previously descried (Xie et al., 2008; Zhang et al., 2010; Zhen et al., 2009). Specifically, primary neurons, microglia cells, or mouse cortices were homogenized in an immunoprecipitation buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.5% Nonidet P-40) plus protease inhibitors [(1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A) (Roche, Indianapolis, IN)]. The lysates were collected, centrifuged at 13,000 rpm for 15 minutes, and quantified for total proteins with a bicinchoninic acid protein assay kit (Pierce, Iselin, NJ).
Western blot analysis
The Western blot analysis was used to determine the effects of isoflurane on the protein levels of the proinflammatory cytokines TNF-α, IL-6 and IL-1β. The samples were subjected to Western blot analysis as previously described (Xie et al., 2008; Zhang et al., 2010; Zhen et al., 2009). Briefly, 40 μg (primary neurons or microglia cells) or 60 μg (mouse brain tissues) of each lysate was separated on SDS-PAGE gels and transferred to polyvinylidene difluoride blots (Bio-Rad, Hercules, CA) using a semi-dry electrotransfer system (Amersham Biosciences, San Francisco, CA). The blot was incubated overnight at 4°C with primary antibodies, followed by washes and incubation with an appropriate secondary antibody, and visualized with a chemoluminescence system. TNF-α, IL-6 and IL-1β levels were recognized by antibody ab9739 (17 kDa, 1:1,000, Abcam, Cambridge, MA), ab6672 (24 kDa, 1:1,000, Abcam) and ab13784 (17 kDa, 1:1,000, Abcam), respectively. Antibody to non-targeted protein β-Actin (42 kDa, 1:5,000, Sigma, St. Louis, MO) was used to control for loading differences in total protein amounts. The signal of the Western blot band was detected using Molecular Imager VersaDoc MP 5000 System (Bio-Rad Life Science Research, Hercules, CA). The intensity of signals was analyzed by using a Bio-Rad image program (Quantity One) and a NIH Image Version 1.37v (NIH, Bethesda, MD).
Immunohistochemistry
The immunohistochemistry was used to assess the effects of isoflurane on the number of TNF-α positive cells in mouse brain tissue. The immunohistochemistry was performed as described by Wang et al. (Wang et al., 2009) with modifications. Specifically, mice were anesthetized with 2% isoflurane for 5 to 10 seconds, which has been shown not to alter TNF-α levels (data not shown), and transcardially perfused with heparinized saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer with a pH of 7.4. Mice brain tissues were removed and kept at 4°C over night in 4% paraformaldehyde. The sections for stainings were cut at 5 μm intervals, deparaffinized, and washed. The sections were then incubated with TNF-α antibody (1:200, ab6671, Abcam) and biotinylated secondary antibody. The TNF-α immunostaining positive cells in the mouse cortex were counted manually in five randomly selected areas under a 20× objective microscope by an investigator who was blinded to the experiments. The cryosections were prepared as described by Wang et al. (Wang et al., 2009) with modifications. The mice were decapitated and the brain tissues were obtained. 5 μm sections were cut using a cryostat. Sections were incubated with the first primary antibody TNF-α (Abcam, ab6671, 1:400) and the second primary antibody NeuN (mab377, 1:500, Millipore, Billerica, MA) dissolved in 1% BSA in PBS at 4°C overnight. Next day, the sections were exposed to donkey anti-mouse IgG-FITC (1:1,000, Jackson ImmunoResearch Inc., West Grove, PA) and Alexa Fluor®594 goat anti-rabbit IgG (1:1,000, Invitrogen, Carlsbad, CA) secondary antibodies for one hour at 37°C in a dark chamber followed by counterstaining with 10 μg/ml Hoechst 33342 at room temperature for 10 minutes. Finally, the sections were wet mounted and viewed immediately using a fluorescence microscope. The double immunostaining of TNF-α [primary antibody: ab6671, 1:200, Abcam; secondary antibody: DyLight 549 AffiniPure Mouse Anti-Rabbit IgG (H+L), 1:1000, Jackson ImmunoResearch, West Grove, PA] and GFAP [primary antibody: ab53554, 1:200, Abcam; secondary antibody: DyLight 488 AffiniPure Mouse Anti-Goat IgG (H+L) 1:1000, Jackson ImmunoResearch] were performed with a similar approach.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
The RT-PCR was used to assess the effects of isoflurane on the mRNA levels of TNF-α, IL-6 and IL-1β in mouse brain tissue. The RNA was obtained as described in the protocol of RNeasy Mini Kit (Qiagen Inc., Valencia, CA). The RNA concentration was determined using NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE). Primers of Mouse TNF-α (ID No., QT00104006), Mouse IL-6 (ID No., QT00098875), Mouse IL-1β (ID No. QT01048355), Mouse β-actin (ID No. QT01136772) and Mouse GAPDH (ID No., QT01658692) were purchased from Qiagen (Valencia, CA). Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was carried out using the QuantiTect SYBR Green RT-PCR Kit (Qiagen Inc., Valencia, CA). TNF-α, IL-6 and IL-1β mRNA levels were determined and standardized with both GAPDH and β-actin as internal controls. The quantification of RT-PCR was performed as described in previous studies (Livak and Schmittgen, 2001; Peng et al., 2007). Specifically, the delta-delta method was used to calculate the difference of gene expression. There were six samples in each control group. Each control was valued against the mean of the control group. The CT number of each control (e.g., 22) was subtracted by the mean (e.g., 21) of CT numbers from the control group to have a delta CT value (e.g., 1) for each control. Then, the fold value of each control was obtained by the 2 delta CT (e.g., 2 1 = 2). Finally, the mean of the fold value from each control was used as the changes in the gene expression. The data were presented as changes in fold in the target gene expression normalized to two endogenous reference genes (GAPDH and β-actin). Then t-test was used to determine the difference of the changes (in fold) between isoflurane-treated cells and control condition-treated cells.
Quantification of TNF-α with Sandwich Enzyme-linked immunosorbant assay (ELISA)
The ELISA was used to assess the effects of isoflurane on extracellular TNF-α protein levels in primary neurons. TNF-α levels in the media of neurons were measured with a Quantikine HS (High Sensitivity ELISA) Human TNF-α/TNFSF1A immunoassay kit (R&D System, Minneapolis, MN) as described by the protocol provided by the company. Specifically, the polyclonal antibody specific for mouse TNF-α was coated on 96-well polystyrene microplates. The wells were incubated for two hours at room temperature with test samples of conditioned cell culture media, and then a polyclonal antibody against mouse TNF-α conjugated to horseradish peroxidase was added. The plates were then developed with tetramethylbenzidine reagent, terminated by stop solution, and the well absorbance was measured at 450 nm. TNF-α levels in the test samples were determined by comparing results with signals from unconditioned media spiked with known quantities of TNF-α. Statistics- Data were expressed as mean ± SD. The number of samples varied from 3 to 9, and the samples were normally distributed. ANOVA with post-hoc adjustment or a t-test was used to compare the differences from the control group. P-values less than 0.05 (* or #) and 0.01 (** or ##) were considered statistically significant. The significance testing was two-tailed, and SAS software (Cary, NC) was used to analyze the data.
Results
Isoflurane increased levels of proinflammatory cytokines in brain tissues of wild type mice
Neuroinflammation has been reported to cause cognitive impairment in humans (Hauss-Wegrzyniak et al., 2000; Hoogman et al., 2007; Langdon et al., 2008) and learning/memory impairment in animals (Liu et al., 2007; Pikis et al., 1996; Schmidt et al., 2006). TNF-α, IL-6, and IL-1β are proinflammatory cytokines, which have been reported to be associated with learning/memory impairment (Reichenberg et al., 2001; Sparkman et al., 2006; Weaver et al., 2002). Moreover, it has been reported that AD patients have elevated blood levels of TNF-α and IL-1 (Tan et al., 2007). We therefore set out to determine the effects of the commonly used inhalation anesthetic isoflurane on the levels of TNF-α, IL-6 and IL-1β.
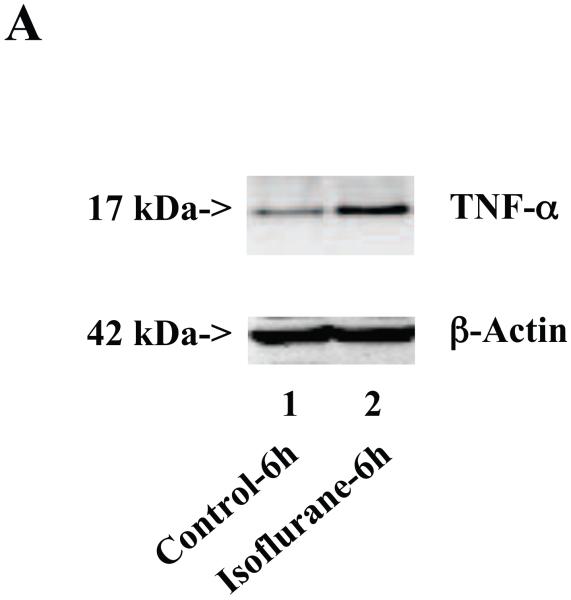
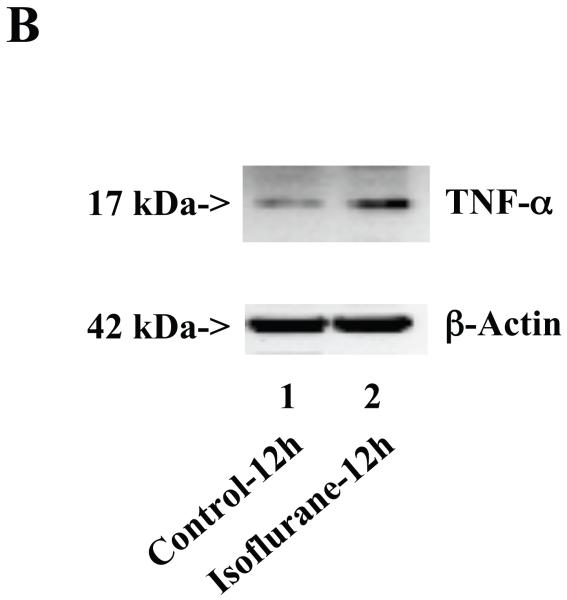
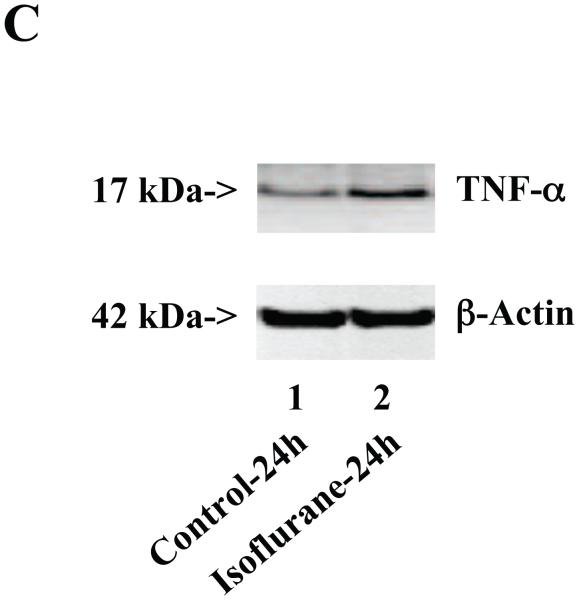
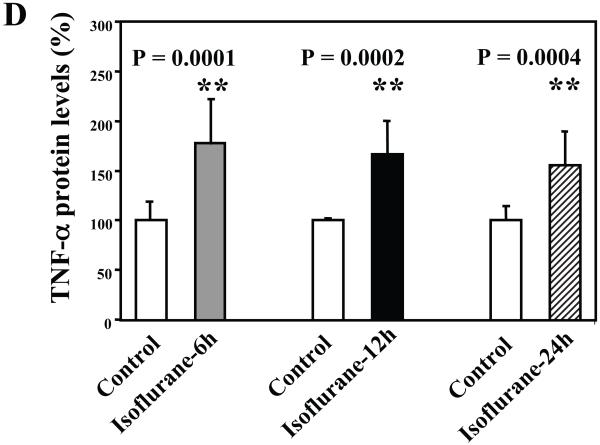
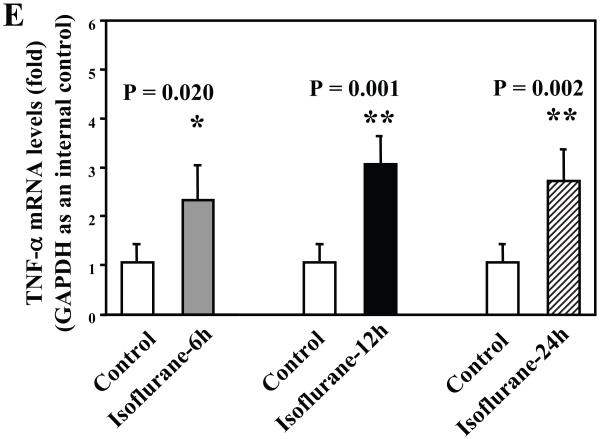
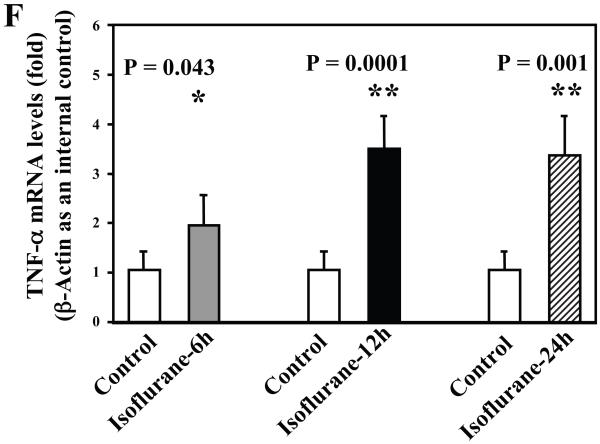
Wild type mice were subjected to anesthesia with 1.4% isoflurane for two hours and the brain tissues were harvested at the end of the anesthesia as previously described (Xie et al., 2008). TNF-α immunoblotting revealed that the anesthesia with 1.4% isoflurane for two hours yielded visible increases in the levels of TNF-α at 6 (Figure 1A), 12 (Figure 1B) and 24 (Figure 1C) hours after the anesthesia; quantification of the results showed that the isoflurane anesthesia led to a 178% (P = 0.0001), 167% (P = 0.0002) and 156% (P = 0.0004) increase in TNF-α levels 6, 12 and 24 hours after the anesthesia, respectively (Figure 1D).
Figure 1. Anesthesia with 1.4% isoflurane for two hours increases the protein and mRNA levels of TNF-α in brain tissues of wild type mice.
A. The anesthesia with 1.4% isoflurane for two hours (lane 2) increases TNF-α levels as compared to control condition (lane 1) in the brain tissues of wild type mice 6 hours after the anesthesia. B. The anesthesia with 1.4% isoflurane for two hours (lane 2) increases TNF-α levels as compared to control condition (lane 1) in the brain tissues of wild type mice 12 hours after the anesthesia. C. The anesthesia with 1.4% isoflurane for two hours (lane 2) increases TNF-α levels as compared to control condition (lane 1) in the brain tissues of wild type mice 24 hours after the anesthesia. D. Quantification of the Western blot shows that the isoflurane anesthesia increases TNF-α levels as compared to control condition (white bar) in the brain tissues of wild type mice 6 (gray bar), 12 (black bar) and 24 (net bar) hours after the anesthesia, normalized to β-Actin levels. E. RT-PCR (GAPDH as an internal control) shows that the isoflurane anesthesia increases TNF-α mRNA levels as compared to the control condition (white bar) in the brain tissues of wild type mice 6 (gray bar), 12 (black bar) and 24 (net bar) hours after the anesthesia. F. RT-PCR (β-Actin as an internal control) shows that the isoflurane anesthesia increases TNF-α mRNA levels as compared to the control condition (white bar) in the brain tissues of wild type mice 6 (gray bar), 12 (black bar) and 24 (net bar) hours after the anesthesia. Data are means ± S.D., n = 6 for each experimental group. A t-test was used to compare the difference between control group and the isoflurane anesthesia group (* p<0.05; ** p<0.01).
The isoflurane-induced increases of TNF-α levels could be due to the increase in generation or decrease in degradation of TNF-α. We therefore assessed the effects of the isoflurane anesthesia on the mRNA levels of TNF-α. We were able to show that the isoflurane anesthesia increased the mRNA levels of TNF-α in the brain tissues of the mice 6 (2.2 fold, P = 0.011), 12 (2.3 fold, P = 0.004) and 24 (2.6 fold, P = 0.006) hours after the anesthesia, respectively (Figure 1E), using GAPDG as an internal control. Similar results were found when β-actin was used as an internal control (Figure 1F). Collectively, these results suggest that isoflurane may induce neuroinflammation by increasing the levels of proinflammatory cytokine TNF-α in the brain tissues of mice. Moreover, the findings that the isoflurane anesthesia can increase the mRNA levels of TNF-α in mouse brain tissue suggest that isoflurane may increase the TNF-α levels by increasing TNF-α generation.
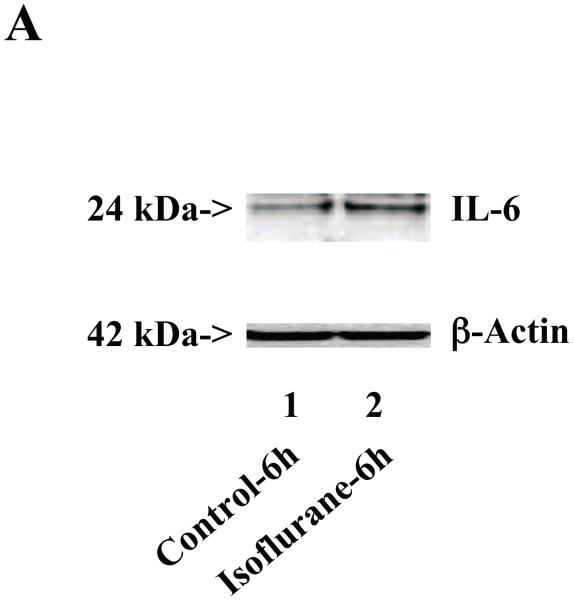
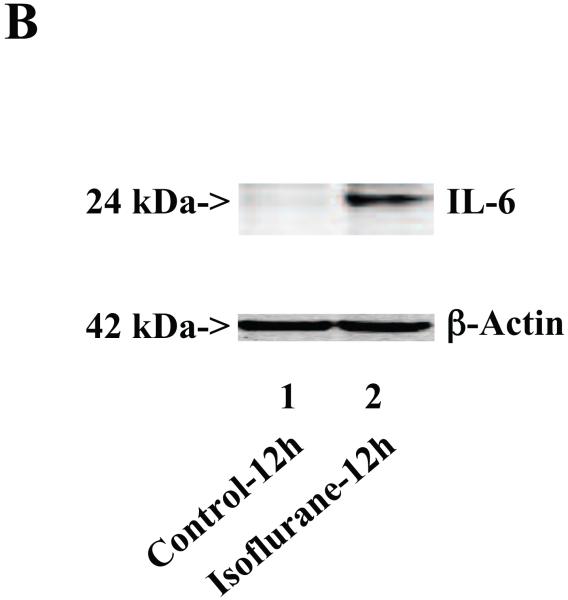
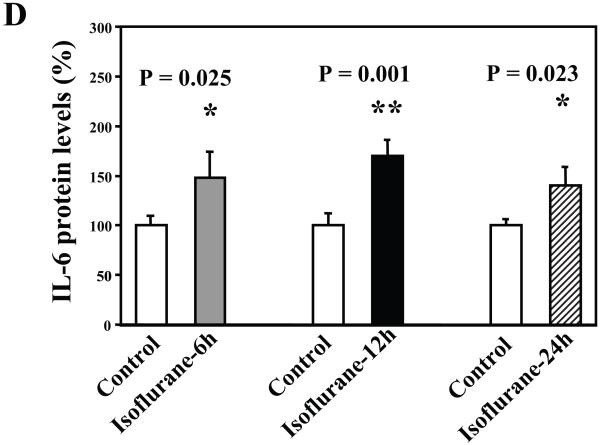
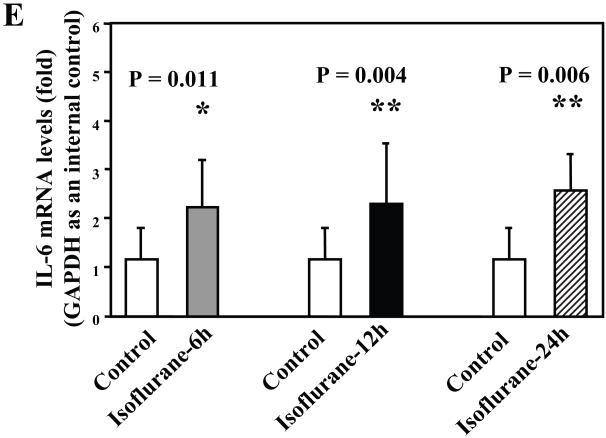
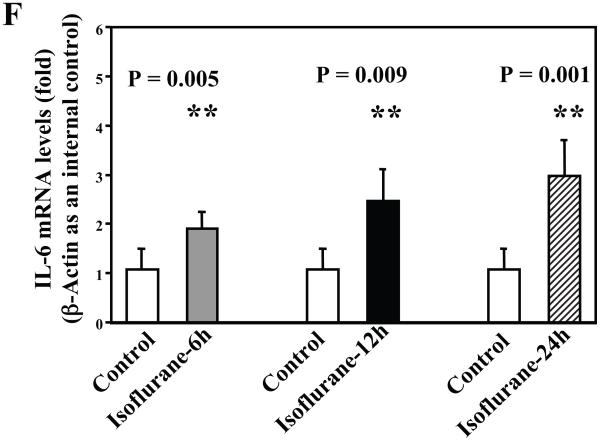
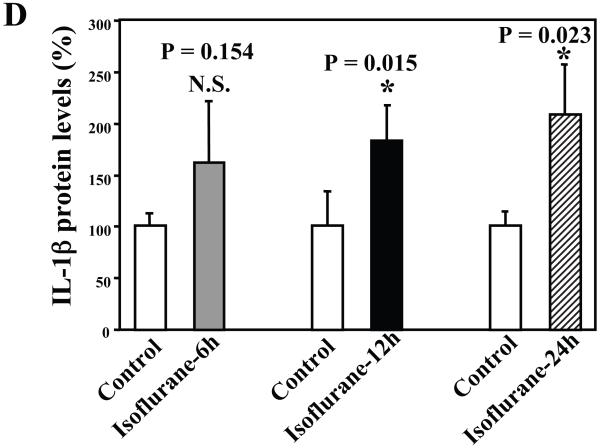
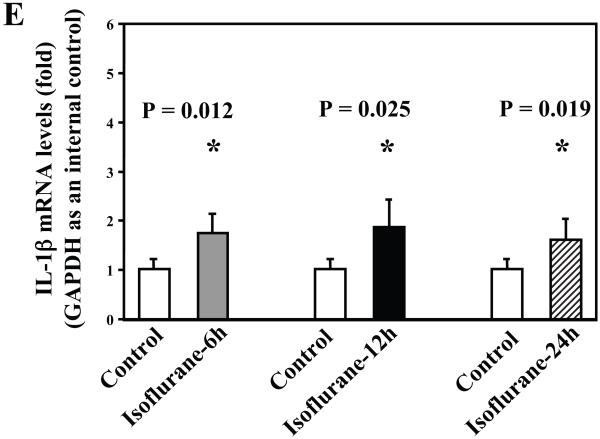
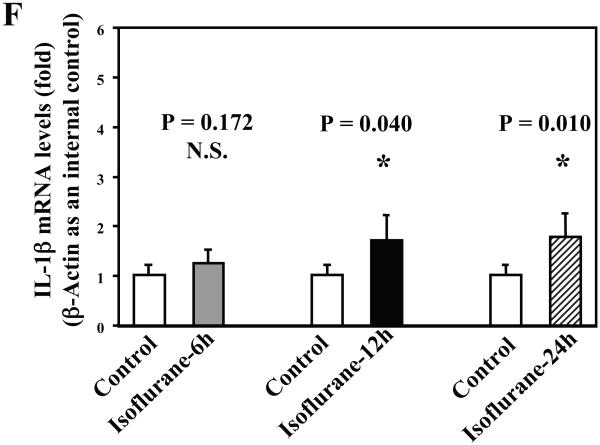
Next, we asked whether isoflurane can increase levels of other proinflammatory cytokines. We therefore set out to determine the effects of the isoflurane anesthesia on both protein and mRNA levels of proinflammatory cytokine IL-6 and IL-1β in the brain tissues of wild type mice. We were able to show that the isoflurane anesthesia increased protein levels of IL-6 (Figure 2A – 2D) and IL-1β (Figure 3A – 3D). Moreover, we were able to show that the isoflurane anesthesia increased the mRNA levels of IL-6 (Figure 2E) and IL-1β (Figure 3E) in the brain tissues of the mice 6, 12 and 24 hours after the anesthesia, using GAPDG as an internal control. Similar results were found when β-actin was used as an internal control (Figure 2F and 3F). These findings suggest that isoflurane may induce neuroinflammation by increasing the levels of proinflammatory cytokine IL-6 and IL-1β in vivo.
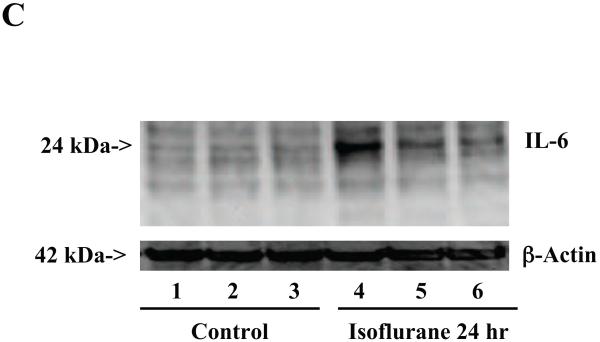
Figure 2. Anesthesia with 1.4% isoflurane for two hours increases the protein and mRNA levels of IL-6 in brain tissues of wild type mice.
A. The anesthesia with 1.4% isoflurane for two hours (lane 2) increases IL-6 levels as compared to control condition (lane 1) in the brain tissues of wild type mice 6 hours after the anesthesia. B. The anesthesia with 1.4% isoflurane for two hours (lane 2) increases IL-6 levels as compared to control condition (lane 1) in the brain tissues of wild type mice 12 hours after the anesthesia. C. The anesthesia with 1.4% isoflurane for two hours (lanes 4 to 6) increases IL-6 levels as compared to control condition (lanes 1 to 3) in the brain tissues of wild type mice 24 hours after the anesthesia. D. Quantification of the Western blot shows that the isoflurane anesthesia increases IL-6 levels as compared to control condition (white bar) in the brain tissues of wild type mice 6 (gray bar), 12 (black bar) and 24 (net bar) hours after the anesthesia, normalized to β-Actin levels. E. RT-PCR (GAPDH as an internal control) shows that the isoflurane anesthesia increases IL-6 mRNA levels as compared to the control condition (white bar) in the brain tissues of wild type mice 6 (gray bar), 12 (black bar) and 24 (net bar) hours after the anesthesia. F. RT-PCR (β-Actin as an internal control) shows that the isoflurane anesthesia increases IL-6 mRNA levels as compared to the control condition (white bar) in the brain tissues of wild type mice 6 (gray bar), 12 (black bar) and 24 (net bar) hours after the anesthesia. Data are means ± S.D., n = 6 for each experimental group. A t-test was used to compare the difference between control group and the isoflurane anesthesia group (* p<0.05; ** p<0.01).
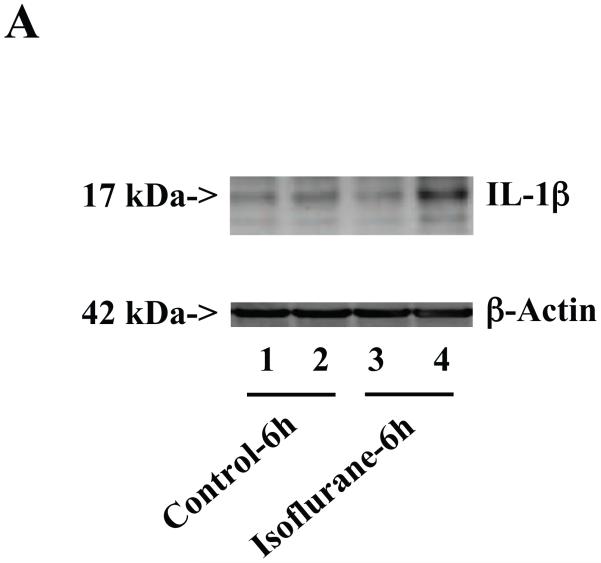
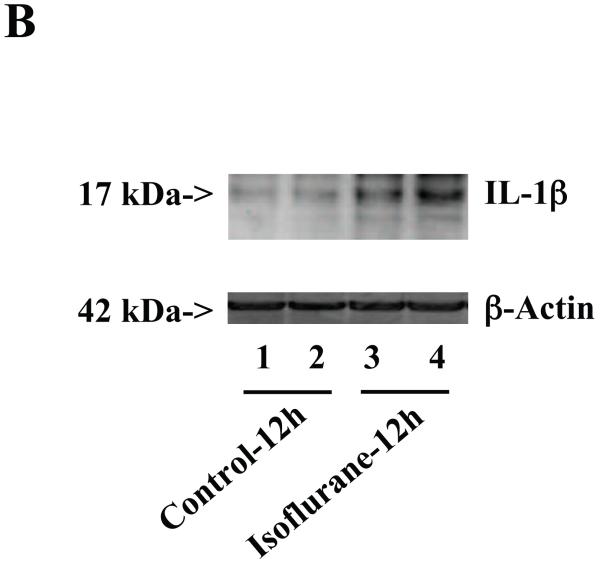
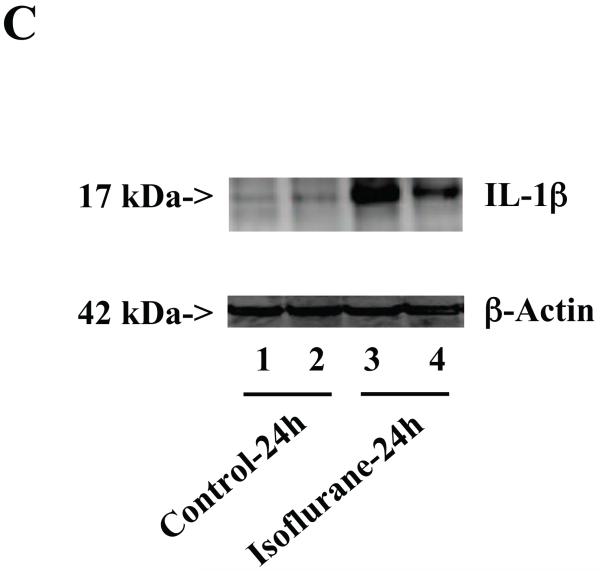
Figure 3. Anesthesia with 1.4% isoflurane for two hours increases the protein and mRNA levels of IL-1β in brain tissues of wild type mice.
A. The anesthesia with 1.4% isoflurane for two hours (lanes 3 and 4) moderately increases IL-6 levels as compared to control condition (lanes 1 and 2) in the brain tissues of wild type mice 6 hours after the anesthesia. B. The anesthesia with 1.4% isoflurane for two hours (lanes 3 and 4) increases IL-6 levels as compared to control condition (lanes 1 and 2) in the brain tissues of wild type mice 12 hours after the anesthesia. C. The anesthesia with 1.4% isoflurane for two hours (lanes 3 and 4) increases IL-6 levels as compared to control condition (lanes 1 to 2) in the brain tissues of wild type mice 24 hours after the anesthesia. D. Quantification of the Western blot shows that the isoflurane anesthesia significantly increases IL-6 levels as compared to control condition (white bar) in the brain tissues of wild type mice 12 (black bar) and 24 (net bar), but not 6 (gray bar), hours after the anesthesia, normalized to β-Actin levels. E. RT-PCR (GAPDH as an internal control) shows that the isoflurane anesthesia increases IL-1β mRNA levels as compared to the control condition (white bar) in the brain tissues of wild type mice 6 (gray bar), 12 (black bar) and 24 (net bar) hours after the anesthesia. F. RT-PCR (β-Actin as an internal control) shows that the isoflurane anesthesia increases IL-1β mRNA levels as compared to the control condition (white bar) in the brain tissues of wild type mice 12 (black bar) and 24 (net bar), but not 6 (gray bar), hours after the anesthesia. Data are means ± S.D., n = 6 for each experimental group. A t-test was used to compare the difference between control group and the isoflurane anesthesia group (* p<0.05).
Isoflurane increased the amount of TNF-α immunostaining positive cells in brain tissues of wild type mice
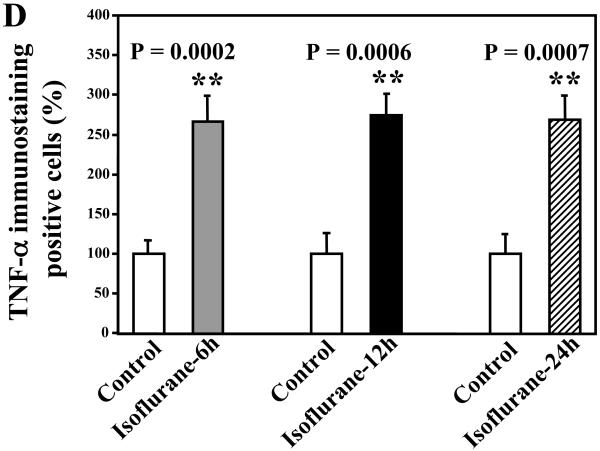
Given that isoflurane can increase the protein levels of TNF-α in vivo, we then asked whether the isoflurane anesthesia can also increase the amount of TNF-α immunostaining positive cells in brain tissues of wild type mice. Wild type mice were subjected to anesthesia with 1.4% isoflurane for two hours as previously described (Xie et al., 2008). The mice were perfused and euthanized 6, 12, and 24 hours after the anesthesia, and the brain tissues were harvested for immunohistochemistry studies. TNF-α immunohistochemistry showed that the isoflurane anesthesia increased the amounts of TNF-α immunostaining positive cells 6 (Figure 4A), 12 (Figure 4B), and 24 (Figure 4C) hours after the isoflurane anesthesia. Quantification of the immunohistochemistry sections showed that the isoflurane anesthesia led to a 266% (P = 0.0002), 274% (P = 0.00006), and 269% (P = 0.0007) increases in TNF-α immunostaining positive cells as compared to control conditions 6, 12, and 24 hours after the anesthesia, respectively (Figure 4D). These results further suggest that isoflurane may increase the levels of proinflammatory cytokine TNF-α in the brain tissues of wild type mice.
Figure 4. Anesthesia with 1.4% isoflurane for two hours enhances the amounts of TNF-α immunostaining positive cells in brain tissues of wild type mice.
A. Immunohistochemistry studies show that the isoflurane anesthesia enhances the amounts of TNF-α immunostaining positive cells in the brain tissues of wild type mice as compared to the control condition 6 hours after the anesthesia. B. Immunohistochemistry studies show that the isoflurane anesthesia enhances the amounts of TNF-α immunostaining positive cells in the brain tissues of wild type mice as compared to the control condition 12 hours after the anesthesia. C. Immunohistochemistry studies show that the isoflurane anesthesia enhances the amounts of TNF-α immunostaining positive cells in the brain tissues of wild type mice as compared to the control condition 24 hours after the anesthesia. D. Quantification of the immunohistochemistry image shows that the isoflurane anesthesia enhances the amounts of TNF-α immunostaining positive cells compared with control condition (white bar) in the brain tissues of wild type mice 6 (gray bar), 12 (black bar) and 24 (net bar) hours after the anesthesia. Data are means ± S.D., n = 5 for each experimental group. A t-test was used to compare the difference between control group and the isoflurane anesthesia group (** p<0.01). (Original magnification × 200).
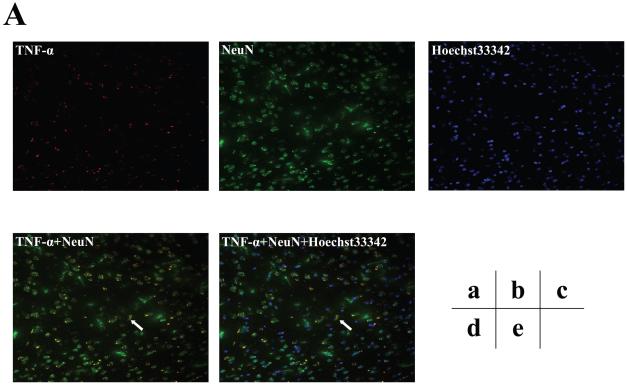
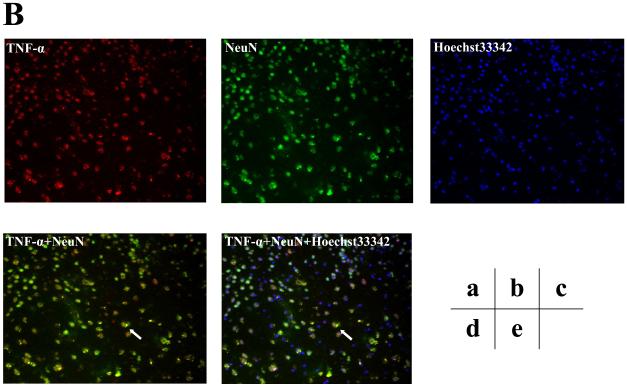
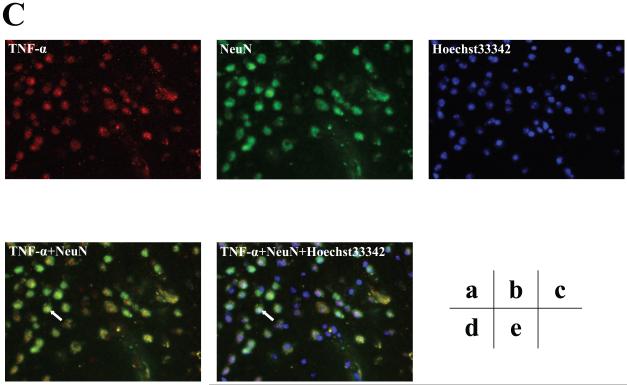
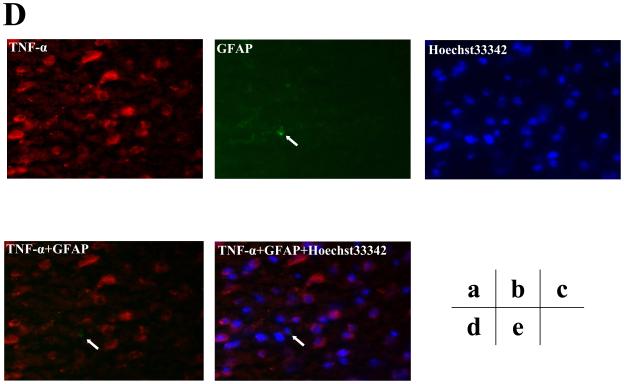
Furthermore, we assessed the cell types of these TNF-α immunostaining positive cells by double immunohistochemistry staining. As can be seen in figures 5B and 5C (intensified figure 5B), panel a shows immunohistochemistry staining of TNF-α (red) in the brain cortex of mice 12 hours after the isoflurane anesthesia, panel b shows the immunohistochemistry staining of neurons (green) detected by NeuN antibody, panel c shows the immunohistochemistry staining of nuclei (blue) detected by Hoechst33342. Panel d shows the immunohistochemistry staining of the combined images of TNF-α (red) and NeuN (green), and panel e shows the combined image of TNF-α (red), NeuN (green), and Hoechst33342 (blue). These images suggest that the majority (rather than entire) of the isoflurane-induced TNF-α immunostaining positive cells (indicated by the white bar) in the brain tissues were neurons (Figure 5B and 5C). In addition, the double immunohistochemistry staining of TNF-α and GFAP (detecting astrocytes) showed that the majority of the TNF-α immunostaining positive cells in the brain tissues of mice following the isoflurane anesthesia were not astrocytes (Figure 5D).
Figure 5. Isoflurane-induced TNF-α immunostaining positive cells are mainly neurons.
A. Immunofluorescence photomicrographs of TNF-α immunostaining positive cells in mouse cortex 12 hours after control condition. a. Immunofluorescence photomicrographs of TNF-α (red). b. Immunofluorescence photomicrographs of NeuN (green) to detect neurons. c. Hoechst33342 (blue) as a nuclear counterstaining. d. Merged TNF-α (red) and NeuN (green) immunofluorescence photomicrographs. The white arrow indicates that the overlap of TNF-α immunostaining positive cell and NeuN positive cells (yellow). e. Merged TNF-α (red), NeuN (green) and nuclei (blue) immunofluorescence photomicrographs, the white arrow indicates that the overlap of TNF-α immunostaining positive cell, NeuN positive cells and nuclei. (Original magnification × 200). B. Immunofluorescence photomicrographs of TNF-α immunostaining positive cells in mouse cortex 12 hours after an anesthesia with 1.4% isoflurane for two hours with same immunostaining (a to e) as those in Figure 5A. C. Intensified image (x2) of Figure 5B. D. Immunofluorescence photomicrographs of TNF-α immunostaining positive cells in mouse cortex 12 hours after an anesthesia with 1.4% isoflurane for two hours. a. Immunofluorescence photomicrographs of TNF-α (red). b. Immunofluorescence photomicrographs of GFAP (green) to detect astrocytes. c. Hoechst33342 (blue) as a nuclear counterstaining. d. Merged TNF-α (red) and GFAP (green) immunofluorescence photomicrographs. The white arrow indicates that the GFAP immunostaining positive cell is not TNF-α immunostaining positive cell. e. Merged TNF-α (red), GFAP (green) and nuclei (blue) immunofluorescence photomicrographs, the white arrow indicates that the GFAP immunostaining positive cell is not TNF-α immunostaining positive cell. (Original magnification × 200, intensified image x2).
Isoflurane increased TNF-α levels in primary neurons, but not in microglia cells
Given the findings that the majority of the isoflurane-induced TNF-α immunostaining positive cells in the brain tissues were neurons, we set out to confirm these findings by assessing the effects of isoflurane on TNF-α levels in both primary neurons and microglia cells from mice.
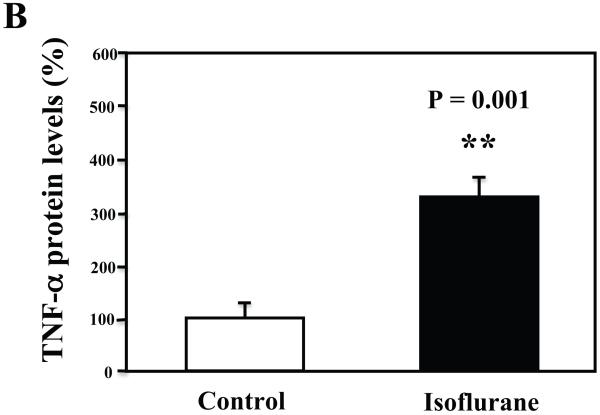
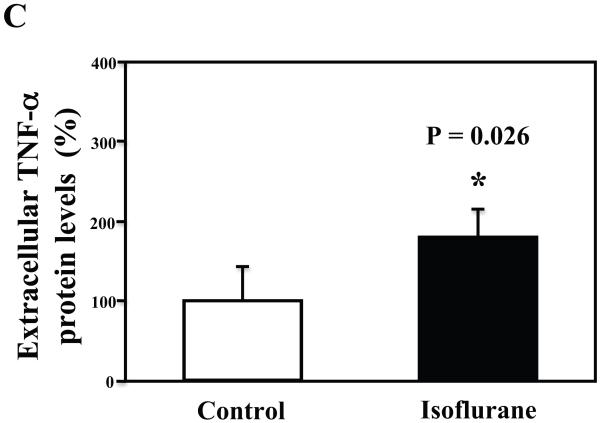
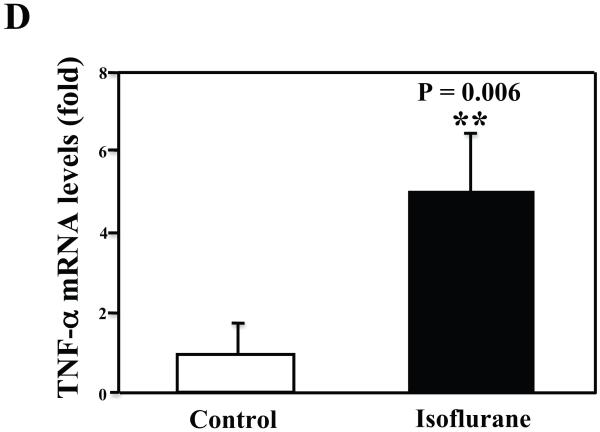
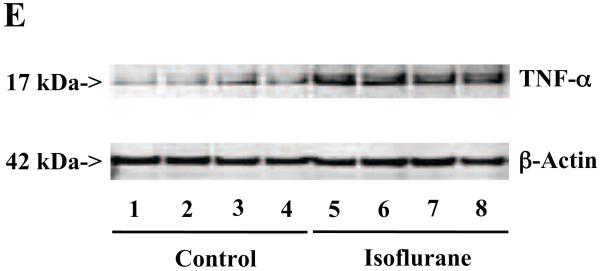
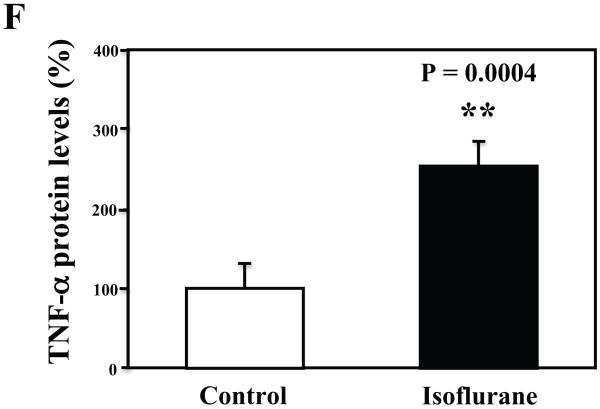
The primary neurons were subjected to anesthesia with 2% isoflurane for six hours as previously described (Xie et al., 2006a; Xie et al., 2006b; Xie et al., 2007). The neurons were harvested at the end of the anesthesia. We assessed the effects of isoflurane on TNF-α levels in the neurons using quantitative Western blots analyses. The treatment with 2% isoflurane for six hours increased levels of TNF-α in the primary neurons from wild type mice (Figure 6A). Quantification of the Western blot revealed that the isoflurane treatment led to a 333% (Figure 6B, P = 0.001) increase in the TNF-α levels as compared to the control condition. Moreover, we were able to show that the isoflurane treatment increased the extracellular TNF-α levels, detected by ELISA sandwich, as compared to the control condition: 100% versus 179% (Figure 6C, P = 0.026). In addition, we showed that the isoflurane treatment increased the mRNA levels of TNF-α as compared to the control condition (Figure 6D): one versus five fold, P = 0.006. Taken together, these results suggest that isoflurane may increase the generation of TNF-α (in contrast to facilitating the release of the TNF-α) in the neurons. Finally, the isoflurane treatment also increased TNF-α levels in the primary neurons from AD transgenic mice [B6.Cg-Tg(APPswe, PSEN1dE9)85Dbo/J]: 100% versus 266% (Figure 6E and 6F, P = 0.0004).
Figure 6. Treatment with 2% isoflurane for six hours increases TNF-α levels in the primary neurons from wild type and AD transgenic mice.
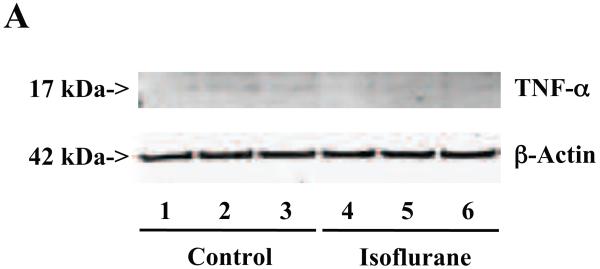
A. The treatment with 2% isoflurane for six hours (lanes 4 to 6) increases TNF-α levels as compared to control condition (lanes 1 to 3) in the primary neurons from wild type mice. B. Quantification of the Western blot shows that the isoflurane treatment (black bar) increases TNF-α levels as compared to control condition (white bar), normalized to β-Actin levels. C. ELISA sandwich assay shows that the isoflurane treatment (black bar) increases soluble TNF-α levels in the conditioned media as compared to control condition (white bar) in the primary neurons from wild type mice. D. RT-PCR shows that the isoflurane treatment (black bar) increases mRNA levels of TNF-α as compared to control condition (white bar) in the primary neurons of wild type mice. E. The treatment with 2% isoflurane for six hours (lanes 5 to 8) increases TNF-α levels as compared to control conditions (lanes 1 to 4) in the primary neurons of AD transgenic. F. Quantification of the Western blot shows that the isoflurane treatment (black bar) increases TNF-α levels as compared to control condition (white bar) in the primary neurons of AD transgenic mice, normalized to β-Actin levels. Data are means ± S.D., n = 4 to 8 for each experimental group. A t-test was used to compare the difference between control group and the treatment group (* p<0.05; ** p<0.01).
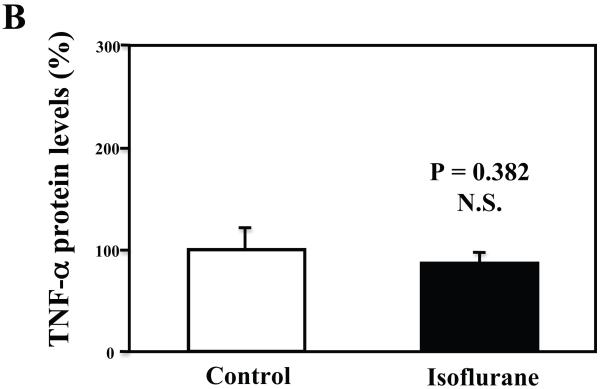
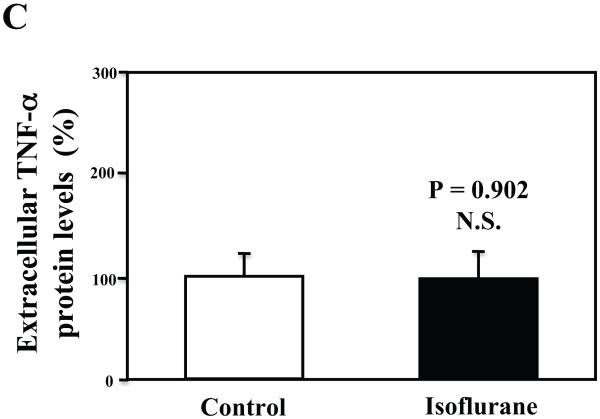
Furthermore, we found that the isoflurane treatment did not increase TNF-α levels in the microglia cells (Figures 7A, 7B, and 7C). These findings suggest that the isoflurane-induced increases in TNF-α levels could be cell-type dependent, and isoflurane may specifically enhance the generation of TNF-α in neurons, leading to increases in the levels of TNF-α in the brain tissues of mice, and consequently neuroinflammation.
Figure 7. Treatment with 2% isoflurane for six hours does not increase TNF-α levels in the microglia cells from the wild type mice.
A. The treatment with 2% isoflurane for six hours (lanes 4 to 6) does not increase TNF-α levels as compared to control condition (lanes 1 to 3) in the microglia cells. B. Quantification of the Western blot shows that the isoflurane treatment does not increase TNF-α levels as compared to control condition (white bar) in the microglia cells, normalized to β-Actin levels. C. ELISA sandwich assay shows that the isoflurane treatment (black bar) does not increase soluble TNF-α levels in the conditioned media as compared to control condition (white bar) in the microglia cells. Data are means ± S.D., n = 6 for each experimental group. A t-test was used to compare the difference between control group and the isoflurane anesthesia group.
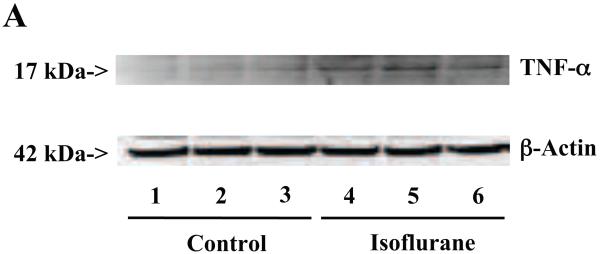
Isoflurane anesthesia led to a greater degree of increase in TNF-α levels in AD transgenic mice
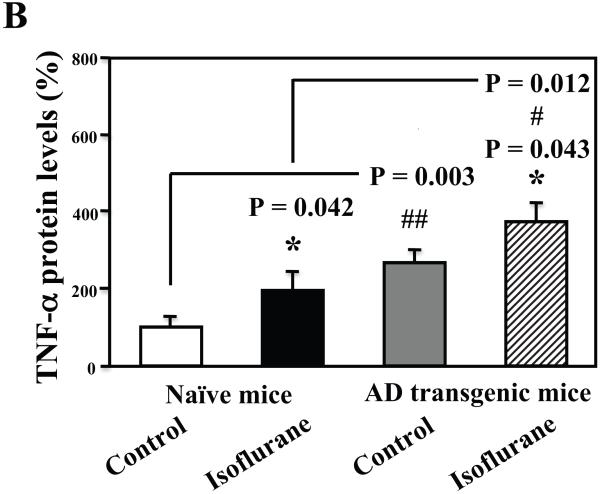
Finally, we were able to show that the isoflurane anesthesia induced a greater degree of increases in TNF-α levels in the brain tissues of AD transgenic mice as compared to that in the wild type mice: 373% versus 195%, P = 0.012 (Figure 8A and 8B). These results suggest that the higher Aβ levels in the AD transgenic mice may potentiate the isoflurane-induced increases in TNF-α levels.
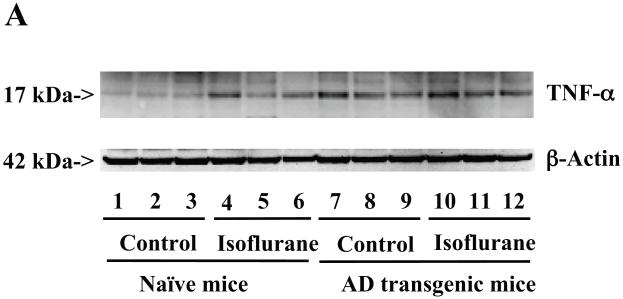
Figure 8. Isoflurane induces a greater increase of TNF-α levels in the brain tissues of AD transgenic mice than wild type mice.
A. The anesthesia with 1.4% isoflurane for two hours (lanes 4 to 6 and 10 to 12) increases TNF-α protein levels as compared to control conditions (lanes 1 to 3 and 7 to 9) in the wild type mice and the AD transgenic mice, respectively. The TNF-α levels following the isoflurane anesthesia in the AD transgenic mice (lanes 10 to 12) are higher than those in the wild type mice (lanes 4 to 6). There is no significant difference in the amounts of β-Actin in control conditions or isoflurane-treated wild type or AD transgenic mice. B. Quantification of the Western blot shows that the isoflurane anesthesia (black bar and net bar) increases TNF-α levels as compared to control conditions (white bar and gray bar) in the wild type mice and the AD transgenic mice, respectively. The TNF-α levels following the isoflurane anesthesia in the AD transgenic mice (net bar) are higher than those in the wild type mice (black bar). Data are means ± S.D., n = 3 for each experimental group. ANOVA was used to compare the difference in TNF-α levels between control condition and the isoflurane anesthesia (* p<0.05), and the differences in TNF-α levels between the wild type mice and the AD transgenic mice (# p<0.05; ## p<0.01).
Discussion
We have previously shown that the commonly used inhalation anesthetic isoflurane can induce cellular apoptosis and increase Aβ generation, parts of the AD neuropathogenesis, in vitro and in vivo (Xie et al., 2008; Xie et al., 2006a; Xie et al., 2006b; Xie et al., 2007). Recent studies have suggested that AD neuropathogenesis may also include neuroinflammation [reviewed in (Akiyama et al., 2000; Cameron and Landreth, 2009; Heneka and O’Banion, 2007; Sastre et al., 2006)]. We therefore set out to determine the effects of isoflurane on levels of proinflammatory cytokine TNF-α IL-6 and IL-1β in vitro and in vivo.
We have shown for the first time that a clinically relevant concentration of isoflurane may induce neuroinflammation by enhancing levels of proinflammatory cytokines TNF-α, IL-6, and IL-1β in the primary neurons and brain tissues of both wild type and AD transgenic mice [B6.Cg-Tg(APPswe, PSEN1dE9)85Dbo/J]. Isoflurane may increase these proinflammatory cytokine levels by enhancing their generations. Furthermore, isoflurane may mostly (but not uniquely) act on neurons to increase TNF-α levels. Finally, isoflurane can induce a greater degree of TNF-α increase in the AD transgenic mice than in the wild type mice.
It is interesting that isoflurane increased the TNF-α levels in the neurons, but not in the microglia cells, in our current experiments. These results suggest that the isoflurane-induced increases in TNF-α levels could be dependent on the cell type. Traditionally, it has been suggested that neurons are not involved in neuroinflammation. However, recent studies have revealed that neurons can also produce inflammatory factors [reviewed in (Cameron and Landreth, 2009; Heneka and O’Banion, 2007)]. Specifically, it has been shown that neurons can produce proinflammatory cytokines including IL-6 and TNF-α [(Botchkina et al., 1997; Gong et al., 1998; Grammas and Ovase, 2001; Hoozemans et al., 2004; Murphy et al., 1999; Orzylowska et al., 1999; Suzuki et al., 1999; Szczepanik et al., 2001); reviewed in (Cameron and Landreth, 2009; Heneka and O’Banion, 2007)], which could lead to neuroinflammation. Taken together, the findings from our studies suggest that environmental factors, e.g., anesthesia, may affect neurons, leading to increases in proinflammatory cytokines and induction of neuroinflammation.
The underlying molecular mechanism by which isoflurane selectively affects neurons to increase TNF-α levels remains to be determined. It has been shown that nuclear factor kappa B (NFκB)-dependent pathway is required for cytokine gene transcription (Combs et al., 2001). Receptor for advanced glycation end products (RAGE) is involved in the generation of cytokine (Fang et al., 2009; Yan et al., 1998). Isoflurane may regulate the NFκB-dependent pathway and RAGE in neurons and microglia cells differently, and thus increase the TNF-α levels in neurons, but not in microglia cells. It is therefore important to further investigate whether isoflurane may have different effects on the NFκB-dependent pathway and RAGE between neurons and microglia cells in the future studies. The results from these studies would help us to understand not only the effects of isoflurane on TNF-α metabolism, but also the molecular mechanisms of anesthetics-induced neurotoxicity, e.g., neuroinflammation.
We have found that the isoflurane anesthesia can induce a greater degree of TNF-α elevation in the AD transgenic mice as compared to that in the wild type mice, which is likely due to the higher baseline levels of TNF-α in the AD transgenic mice. These finding suggest that AD transgenic mice could be more susceptible to develop neuroinflammation following the isoflurane anesthesia. Given that increasing evidence suggest the role of neuroinflammation in the neuropathogenesis of AD and other neurodegenerative diseases [reviewed in (Cameron and Landreth, 2009; Heneka and O’Banion, 2007)], the findings from the current experiments may raise novel concerns regarding the use of isoflurane, a commonly used anesthetic, in patients with or at risk for neurodegenerative diseases, e.g., AD. A study by Groen et al. (van Groen et al., 2005) showed that a transient ischemia insult can increase levels of APP and Aβ in the ischemia area, and persistent APP and Aβ deposits in a different region of the brain 9 months after the initial insult. The inhalation anesthetic isoflurane has been shown to induce acute apoptosis, Aβ accumulation, and neuroinflammation, which may lead to long-term damage to brain.
The increases in the TNF-α levels detected in the conditioned medium of neuronal cultures by ELISA (Figure 6C) were relatively modest as compared to those detected by Western blot analysis (Figure 6A and 6B). This is likely due to the fact that we did not use a denaturation step to liberate the TNF-α from soluble receptors in these ELISA studies. Moreover, in the present experiment we were unable to obtain cerebrospinal fluid from the mice for the measurement of the soluble TNF-α, IL-6 and IL-1β because the amount of cerebrospinal fluid in mice is small. We plan to develop microdialysis technology and to use it to assess the effects of isoflurane on the levels of soluble cytokines, which actually matter in term of mediating inflammatory processes and toxicity of neurons.
The identification of single nucleotide polymorphisms in the TNF-α gene that are linked to AD (Collins et al., 2000; Lio et al., 2006; Ramos et al., 2006) and the epidemiology study (Tan et al., 2007) strongly support the hypothesis that TNF-α contributes to AD neuropathogenesis. However, whether the isoflurane-induced elevation of TNF-α in the present experiment is a transient or long-term effect remains to be determined. Moreover, TNF-α has also been suggested to have neuroprotective effects (Orellana et al., 2007). Therefore, it is premature to conclude that the isoflurane-induced elevation of TNF-α, IL-6, and IL-1β in the present studies will lead only to deleterious outcomes. The studies to determine the potential association between the isoflurane-induced learning/memory impairment and isoflurane-induced elevation of proinflammatory cytokines will be the next step to further test the hypothesis that isoflurane can induce neuroinflammation, leading to neurotoxicity.
The current studies are also limited by missing the behavioral data which would show that isoflurane can induce learning and memory impairments as described in other studies (Bianchi et al., 2008; Culley et al., 2004). In the future studies we should assess whether the isoflurane anesthesia (1.4% isoflurane for two hours) can lead to learning and memory impairment, e.g., increase in the time to complete the radical arm maze; and whether these behavioral effects are associated with the isoflurane-induced biochemistry changes, e.g., Aβ accumulation and neuroinflammation.
Even our in vitro and in vivo studies together with the findings from other laboratories have suggested that isoflurane may affect AD neuropathogenesis, the conclusion that the inhalational anesthetic isoflurane promotes AD neuropathogenesis cannot be made at this moment. It is necessary to perform further studies, especially the in vivo relevance of these effects of isoflurane in humans, to either rule in or rule out the contribution of isoflurane in the AD neuropathogenesis.
In conclusion, we have found that isoflurane can increase the levels of proinflammatory cytokine TNF-α, IL-6, and IL-1α, which would lead to neuroinflammation. Isoflurane may increase the TNF-α levels by enhancing its generation. Moreover, isoflurane may specifically affect neurons to increase the TNF-α levels. Finally, isoflurane may induce a greater degree of neuroinflammation in the AD transgenic mice. These results reveal several new insights regarding the neurotoxicity of anesthesia and AD neuropathogenesis, and will hopefully advance the field in determining the role of anesthesia in AD neuropathogenesis. Ultimately, these efforts will lead to safer and better anesthesia care for patients, especially senior and AD patients.
Acknowledgements
This research was supported by K08NS048140, R21AG029856 and R01 GM088801 (National Institutes of Health), Jahnigen Career Development Award (American Geriatrics Society), Investigator Initiated Research Grant (Alzheimer’s Association) (to Z. X.); K08GM077057 (National Institutes of Health) (to D. J. C.); RO1 GM088817 (National Institutes of Health) (to G.C.); R37MH 60009 (National Institutes of Health) and the Cure Alzheimer’s Fund (to R. E. T.). The cost of anesthetic isoflurane was generously provided by the Department of Anesthesia, Critical Care and Pain Medicine in Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts.The authors thank Dr. Hui Peng in the University of Nebraska Medical Center for the technical advice and useful discussion, especially in regard to the RT-PCR studies in the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have no conflict of interest to disclose.
Reference
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidan MS, Searleman AC, Storandt M, Barnett K, Vannucci A, Saager L, Xiong C, Grant EA, Kaiser D, Morris JC, Evers AS. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111:964–970. doi: 10.1097/ALN.0b013e3181bc9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, Eckenhoff RG, Eckenhoff MF. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29:1002–1010. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Warner MA, Kokmen E, Beard CM, Kurland LT. Alzheimer’s disease and cumulative exposure to anesthesia: a case-control study. J Am Geriatr Soc. 1994;42:198–201. doi: 10.1111/j.1532-5415.1994.tb04952.x. [DOI] [PubMed] [Google Scholar]
- Botchkina GI, Meistrell ME, 3rd, Botchkina IL, Tracey KJ. Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med. 1997;3:765–781. [PMC free article] [PubMed] [Google Scholar]
- Cameron B, Landreth GE. Inflammation, microglia, and alzheimer’s disease. Neurobiol Dis. 2009;37:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JS, Perry RT, Watson B, Jr., Harrell LE, Acton RT, Blacker D, Albert MS, Tanzi RE, Bassett SS, McInnis MG, Campbell RD, Go RC. Association of a haplotype for tumor necrosis factor in siblings with late-onset Alzheimer disease: the NIMH Alzheimer Disease Genetics Initiative. Am J Med Genet. 2000;96:823–830. doi: 10.1002/1096-8628(20001204)96:6<823::aid-ajmg26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100:309–314. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zhang G, Zhang B, Moir RD, Xia W, Marcantonio ER, Culley DJ, Crosby G, Tanzi RE, Xie Z. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66:620–631. doi: 10.1001/archneurol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, Walker DG, Stern DM, Schmidt AM, Chen JX, Yan SS. RAGE-dependent signaling in microglia contributes to neuroinflammation, Aβ accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J. 2009 doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Gasparini M, Vanacore N, Schiaffini C, Brusa L, Panella M, Talarico G, Bruno G, Meco G, Lenzi GL. A case-control study on Alzheimer’s disease and exposure to anesthesia. Neurol Sci. 2002;23:11–14. doi: 10.1007/s100720200017. [DOI] [PubMed] [Google Scholar]
- Gong C, Qin Z, Betz AL, Liu XH, Yang GY. Cellular localization of tumor necrosis factor alpha following focal cerebral ischemia in mice. Brain Res. 1998;801:1–8. doi: 10.1016/s0006-8993(98)00489-2. [DOI] [PubMed] [Google Scholar]
- Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging. 2001;22:837–842. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- Harris RA, Eger EI., 2nd Alzheimer’s disease and anesthesia: out of body, out of mind...or not? Ann Neurol. 2008;64:595–597. doi: 10.1002/ana.21575. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vannucchi MG, Wenk GL. Behavioral and ultrastructural changes induced by chronic neuroinflammation in young rats. Brain Res. 2000;859:157–166. doi: 10.1016/s0006-8993(00)01999-5. [DOI] [PubMed] [Google Scholar]
- Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Hoogman M, van de Beek D, Weisfelt M, de Gans J, Schmand B. Cognitive outcome in adults after bacterial meningitis. J Neurol Neurosurg Psychiatry. 2007;78:1092–1096. doi: 10.1136/jnnp.2006.110023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans JJ, Veerhuis R, Rozemuller AJ, Arendt T, Eikelenboom P. Neuronal COX-2 expression and phosphorylation of pRb precede p38 MAPK activation and neurofibrillary changes in AD temporal cortex. Neurobiol Dis. 2004;15:492–499. doi: 10.1016/j.nbd.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Petersen RC, Cha RH, Edland SD, Rocca WA. Coronary artery bypass grafting is not a risk factor for dementia or Alzheimer disease. Neurology. 2005;65:986–990. doi: 10.1212/01.wnl.0000171954.92119.c7. [DOI] [PubMed] [Google Scholar]
- Langdon KD, Granter-Button S, Corbett D. Persistent behavioral impairments and neuroinflammation following global ischemia in the rat. Eur J Neurosci. 2008;28:2310–2318. doi: 10.1111/j.1460-9568.2008.06513.x. [DOI] [PubMed] [Google Scholar]
- Lee TA, Wolozin B, Weiss KB, Bednar MM. Assessment of the emergence of Alzheimer’s disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J Alzheimers Dis. 2005;7:319–324. doi: 10.3233/jad-2005-7408. [DOI] [PubMed] [Google Scholar]
- Lio D, Annoni G, Licastro F, Crivello A, Forte GI, Scola L, Colonna-Romano G, Candore G, Arosio B, Galimberti L, Vergani C, Caruso C. Tumor necrosis factor-alpha -308A/G polymorphism is associated with age at onset of Alzheimer’s disease. Mech Ageing Dev. 2006;127:567–571. doi: 10.1016/j.mad.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Murphy PG, Borthwick LS, Johnston RS, Kuchel G, Richardson PM. Nature of the retrograde signal from injured nerves that induces interleukin-6 mRNA in neurons. J Neurosci. 1999;19:3791–3800. doi: 10.1523/JNEUROSCI.19-10-03791.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana DI, Quintanilla RA, Maccioni RB. Neuroprotective effect of TNFalpha against the beta-amyloid neurotoxicity mediated by CDK5 kinase. Biochim Biophys Acta. 2007;1773:254–263. doi: 10.1016/j.bbamcr.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Orzylowska O, Oderfeld-Nowak B, Zaremba M, Januszewski S, Mossakowski M. Prolonged and concomitant induction of astroglial immunoreactivity of interleukin-1beta and interleukin-6 in the rat hippocampus after transient global ischemia. Neurosci Lett. 1999;263:72–76. doi: 10.1016/s0304-3940(99)00043-9. [DOI] [PubMed] [Google Scholar]
- Peng H, Kolb R, Kennedy JE, Zheng J. Differential expression of CXCL12 and CXCR4 during human fetal neural progenitor cell differentiation. J Neuroimmune Pharmacol. 2007;2:251–258. doi: 10.1007/s11481-007-9081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikis A, Kavaliotis J, Tsikoulas J, Andrianopoulos P, Venzon D, Manios S. Long-term sequelae of pneumococcal meningitis in children. Clin Pediatr (Phila) 1996;35:72–78. doi: 10.1177/000992289603500204. [DOI] [PubMed] [Google Scholar]
- Ramos EM, Lin MT, Larson EB, Maezawa I, Tseng LH, Edwards KL, Schellenberg GD, Hansen JA, Kukull WA, Jin LW. Tumor necrosis factor alpha and interleukin 10 promoter region polymorphisms and risk of late-onset Alzheimer disease. Arch Neurol. 2006;63:1165–1169. doi: 10.1001/archneur.63.8.1165. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int J Dev Neurosci. 2006;24:167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–189. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Heimann B, Djukic M, Mazurek C, Fels C, Wallesch CW, Nau R. Neuropsychological sequelae of bacterial and viral meningitis. Brain. 2006;129:333–345. doi: 10.1093/brain/awh711. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Tanaka K, Nagata E, Ito D, Dembo T, Fukuuchi Y. Cerebral neurons express interleukin-6 after transient forebrain ischemia in gerbils. Neurosci Lett. 1999;262:117–120. doi: 10.1016/s0304-3940(99)00051-8. [DOI] [PubMed] [Google Scholar]
- Szczepanik AM, Rampe D, Ringheim GE. Amyloid-beta peptide fragments p3 and p4 induce pro-inflammatory cytokine and chemokine production in vitro and in vivo. J Neurochem. 2001;77:304–317. doi: 10.1046/j.1471-4159.2001.t01-1-00240.x. [DOI] [PubMed] [Google Scholar]
- Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- van Groen T, Puurunen K, Maki HM, Sivenius J, Jolkkonen J. Transformation of diffuse beta-amyloid precursor protein and beta-amyloid deposits to plaques in the thalamus after transient occlusion of the middle cerebral artery in rats. Stroke. 2005;36:1551–1556. doi: 10.1161/01.STR.0000169933.88903.cf. [DOI] [PubMed] [Google Scholar]
- Wang S, Lim G, Mao J, Sung B. Regulation of the trigeminal NR1 subunit expression induced by inflammation of the temporomandibular joint region in rats. Pain. 2009;141:97–103. doi: 10.1016/j.pain.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff RG. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–260. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–627. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006a;104:988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Maeda U, Moir R, Inouye SK, Culley DJ, Crosby G, Tanzi RE. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006b;61:1300–1306. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Stern D, Kane MD, Kuo YM, Lampert HC, Roher AE. RAGE-Abeta interactions in the pathophysiology of Alzheimer’s disease. Restor Neurol Neurosci. 1998;12:167–173. [PubMed] [Google Scholar]
- Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T, Xie Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem. 2010;285:4025–4037. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Dong Y, Wu X, Xu Z, Lu Y, Zhang Y, Norton D, Tian M, Li S, Xie Z. Nitrous oxide plus isoflurane induces apoptosis and increases beta-amyloid protein levels. Anesthesiology. 2009;111:741–752. doi: 10.1097/ALN.0b013e3181b27fd4. [DOI] [PMC free article] [PubMed] [Google Scholar]