Abstract
Objectives
To compare the new Patient Rule Induction Method(PRIM) Score and common clinical practice with the Framingham Point Score for classification of individuals with respect to coronary heart disease(CHD) risk.
Methods and Results
PRIM Score and the Framingham Point Score were estimated for 11,444 participants from the Copenhagen City Heart Study. Gender specific cumulative incidences and 10 year absolute CHD risks were estimated for subsets defined by age, total cholesterol, high-density lipoprotein(HDL) cholesterol, blood pressure, diabetes and smoking categories. PRIM defined seven mutually exclusive subsets in women and men, with cumulative incidences of CHD from 0.01 to 0.22 in women, and from 0.03 to 0.26 in men. PRIM versus Framingham Point Score found 11% versus 4% of all women, and 31% versus 35% of all men to have 10 year CHD risks >20%. Among women ≥65 years with hypertension and/or with diabetes, 10 year CHD risk >20% was found for 100% with PRIM scoring but for only 18% with the Framingham Point Score.
Conclusion
Compared to the PRIM Score, common clinical practice with the Framingham Point Score underestimates CHD risk in women, especially in women ≥65 years with hypertension and/or with diabetes.
Keywords: coronary heart disease, risk factors, risk scoring
Introduction
Coronary heart disease (CHD) is a major cause of morbidity and mortality in affluent societies (1). Because half of all first coronary events occur in asymptomatic individuals (2), an important clinical task is to accurately identify high risk individuals in order to implement primary prevention therapy and lifestyle changes. For the clinical assessment of CHD risk, cardiovascular risk factors have been transformed into risk scores (3–5); e.g. the Framingham Point Score (3) has since 2001 been incorporated into the National Cholesterol Education Program (Adult Treatment Panel III) to assess specific low-density lipoprotein (LDL) cholesterol treatment goals (6). It is thus of great importance that such risk scoring systems estimate the individual CHD risk accurately, as any systematic over- or underestimation results in an inappropriate number of people being recommended for statin treatment.
CHD risk scores used in clinical practice today classify all individuals using one model to define the contribution of risk variables (3–5;7). This approach assumes that the estimate of the contribution of a risk factor is appropriate for any person in the population, irrespective of presence or absence of other risk factors. This assumption is inconsistent with the observed heterogeneity of the pathobiology of CHD. The Patient Rule Induction Method (PRIM) is an alternative prediction strategy that constructs multiple models defined by subsets of risk factors that are appropriate for subsets of individuals in the population at large (8).
We compared the PRIM derived Score and common clinical practice with the Framingham Point Score for classification of individuals with respect to CHD risk. For this purpose, the PRIM was applied to model the cumulative incidence of CHD in a prospective study of 11,444 individuals from the Danish general population, the Copenhagen City Heart Study. Cumulative incidences for each mutually exclusive subset of individuals identified by the PRIM were converted into 10 year absolute CHD risk scores. Finally, 10 year absolute CHD risks using the Framingham Point Score and the PRIM derived Score were compared.
Methods
Participants
Participants were from the 1981–1983 examination of the Copenhagen City Heart Study (9, 10). Individuals were randomly selected based on the national Danish Civil Registration System to reflect the adult Danish general population aged 20–80+ years. Of the 18,089 individuals invited, 12,698 participated (70% response rate) (11). Among these, we included the 11,444 white Danish individuals with no prior history of CHD, and with complete clinical and laboratory baseline data available.
We categorized risk factors according to Wilson et al. (3) to produce the Framingham Point Score for each individual studied. For the application of the PRIM we collapsed Framingham categories as follows: age <55, 55–64, or ≥65 years; total cholesterol <200, 200–239, or ≥240 mg/dL; HDL cholesterol, <45, 45–59, or ≥60 mg/dL; blood pressure, hypertension stage II–IV (diastolic blood pressure≥100 mmHg and/or systolic blood pressure ≥160 mmHg), hypertension stage I (diastolic blood pressure 90–99 mmHg and/or systolic blood pressure 140–159 mmHg) and not hypertension stage II–IV, or normal (diastolic blood pressure<90 mmHg and systolic blood pressure<140 mmHg); diabetes, presence (self-reported disease, use of insulin, use of oral hypoglycemic drugs and/or nonfasting plasma glucose>200 mg/dL, or absence; and smoker, current smokers or non-smokers (never or former smoker). Participants were assigned to blood pressure/hypertension groups irrespective of receiving antihypertensive medication.
Information on diagnoses of CHD (World Health Organization; International Classification of Diseases, 8th edition: codes 410–414; 10th edition: codes I20–I25) was collected and verified from study entry in 1981–83 through July 2007 by reviewing all hospital admissions and diagnoses entered in the national Danish Patient Registry, all causes of death entered in the national Danish Causes of Death Registry, and medical records from hospitals and general practitioners. CHD included myocardial infarction or characteristic symptoms of stable angina pectoris (12). A diagnosis of myocardial infarction required the presence of at least two of the following criteria: characteristic chest pain, elevated cardiac enzymes, and electrocardiographic changes indicative of myocardial infarction. The diagnosis of myocardial infarction was confirmed from discharge records by cardiologists up until 1994. Because a sample of 200 cases of MI then showed that at least 99.5% were correctly diagnosed in the national Danish Patient registry, further individual confirmation of diagnoses was no longer performed. Follow-up time was up to 10 years and was 100% complete. In 1990, approximately at the end of follow-up for this study, the autopsy rate in Denmark was 16% (13).
The study was approved by institutional review boards and the Danish ethical committee no. KF.V.100.2039/91, and was conducted according to the Declaration of Helsinki. Written informed consent was obtained from participants.
Biochemical Analyses
Colorimetric assays were used to measure baseline plasma levels of total cholesterol, and HDL cholesterol after precipitation of apolipoprotein B containing lipoproteins (Boehringer Mannheim GmbH, Mannheim, Germany).
Statistical Analyses
Patient Rule Induction Method
The PRIM was introduced by Friedman and Fisher (14) and modified by Dyson et al. (8) for use in identifying mutually exclusive subsets (partitions) of individuals with varying cumulative incidences of CHD. The subsets are defined by terms (selected values of predictor variables), and are created through repeated implementations of the peeling and pasting algorithms. Peeling is an iterative process that creates a subset by excluding individuals with particular values of predictor variables, while pasting iteratively amends individuals to the subset, also based upon values of predictor variables, after the peeling stage has been completed. Only when pasting terms added a significant increase in the cumulative incidence to the subset, was the pasting risk factor category included in the subset. For the present implementation of PRIM, three newly described methodological techniques were incorporated (15): first, each possible term was tested for statistical significance employing a permutation test to determine if the resultant cumulative incidence of the constructed subset was larger than would be expected by chance alone, second the resultant p-values were adjusted for multiple testing using α/(2+3α) as the critical p-value, where α is the desired level of experiment-wise error, and third a confidence interval for the estimated cumulative incidence for each subset was produced by bootstrap resampling of exchangeable observations.
Ten year absolute CHD risk
To facilitate a comparison between the PRIM derived Score and common clinical practice with the Framingham Point Score, we converted cumulative incidences to percent. A direct conversion was possible because the cumulative incidences were obtained over a 10 year follow-up period. For comparison, the Framingham Point Score 10 year absolute CHD risks were calculated for all 11,444 individuals as described (3).
Results
Characteristics of the 6,387 women and 5,057 men sampled from the general Copenhagen population are shown in Supplementary Table. The distribution of individuals in categories of age, total cholesterol, HDL cholesterol and blood pressure differed between genders (χ2: P<0.0001 for all comparisons). Women more frequently had total cholesterol ≥240 mg/dL and HDL cholesterol levels ≥60 mg/dL compared to men. Men more frequently had hypertension stage I or hypertension stage II–IV compared to women. More men than women had diabetes and were smokers (χ2: P<0.0001 for both comparisons). None of the study participants were on lipid lowering medication.
Subsets with different CHD cumulative incidences
The risk factor categories described in Supplementary Table 1 were used to build subsets using the PRIM in each gender separately. Seven mutually exclusive subsets were defined in both women and men.
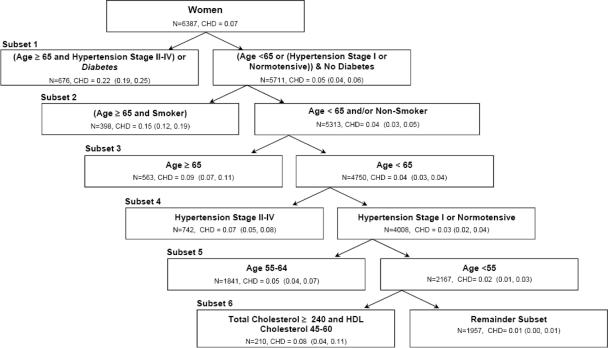
In the sample of women, the cumulative incidences of CHD in the seven sequentially identified subsets were 0.22 (95% confidence interval (CI): 0.19–0.25) for age ≥65 years and hypertension stage II–IV and/or diabetes, 0.15 (95% CI: 0.12–0.19) for age ≥65 and smoker, 0.09 (95% CI: 0.07–0.11) for age ≥65 years, 0.07 (95% CI: 0.05–0.08) for hypertension stage II–IV, 0.05 (95% CI: 0.04–0.07) for age 55–64 years, 0.08 (95% CI: 0.04–0.11) for total cholesterol ≥240 mg/dL and HDL cholesterol 45–60 mg/dL, and 0.01 (95% CI: 0.00–0.01) for the remainder subset (Figure 1).
Figure 1.
Consecutively identified, mutually exclusive subsets with decreasing cumulative incidences of coronary heart disease using PRIM Score in women from the Copenhagen City Heart Study.
The dataset contained 6,387 women, with an overall 10 year cumulative incidence of coronary heart disease of 0.07. The first subset was defined by two peeling (Age ≥65 and Hypertension Stage II–IV) and one pasting term (Diabetes) (pasting terms in italic). The process of producing a new subset based on the unassigned individuals from the previous partition continued until all individuals were assigned to a subset. The individuals that were not included in any of the statistically significant subsets were assigned to the remainder subset.
N=number of individuals; CHD=cumulative incidence of coronary heart disease; HDL=high-density lipoprotein. Parentheses after CHD indicate the 95% confidence interval for the cumulative incidence. Age is in years and total cholesterol and HDL cholesterol are in mg/dL.
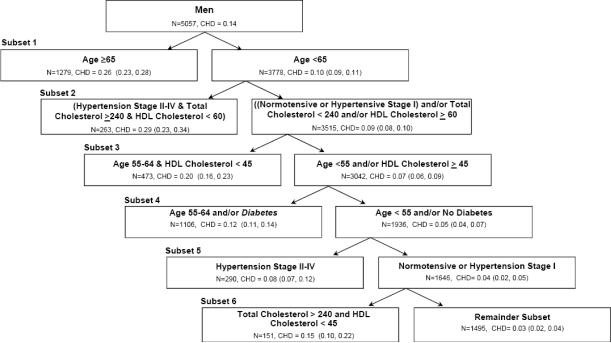
In the sample of men, the cumulative incidences of CHD in the seven sequentially identified subsets were 0.26 (95% CI: 0.23–0.28) for age ≥65 years, 0.29 (95% CI: 0.23–0.34) for hypertension stage II–IV and total cholesterol ≥240 mg/dL and HDL cholesterol <60 mg/dL, 0.20 (95% CI: 0.16–0.23) for age 55–64 years and HDL cholesterol <45 mg/dL, 0.12 (95% CI: 0.11–0.14) for age 55–64 years and/or diabetes, 0.08 (95% CI: 0.07–0.12) for hypertension stage II–IV, 0.15 (95% CI: 0.10–0.22) for total cholesterol ≥240 mg/dL and HDL cholesterol <45 mg/dL, and 0.03 (95% CI: 0.02–0.04) for the remainder subset (Figure 2).
Figure 2.
Consecutively identified, mutually exclusive subsets with decreasing cumulative incidences of coronary heart disease using the PRIM Score in men from the Copenhagen City Heart Study.
The dataset contained 5,057 men, with an overall 10 year cumulative incidence of coronary heart disease of 0.14. The first subset was defined by one peeling (Age ≥65) term. The process of producing a new subset based on the unassigned individuals from the previous partition continued until all individuals were assigned to a subset. The individuals that were not included in any of the statistically significant subsets were assigned to the remainder population subset.
N=number of individuals; CHD=cumulative incidence of coronary heart disease; HDL=high-density lipoprotein. Parentheses after CHD indicate the 95% confidence interval for the cumulative incidence. Age is in years and total cholesterol and HDL cholesterol are in mg/dL.
10 year absolute CHD risk
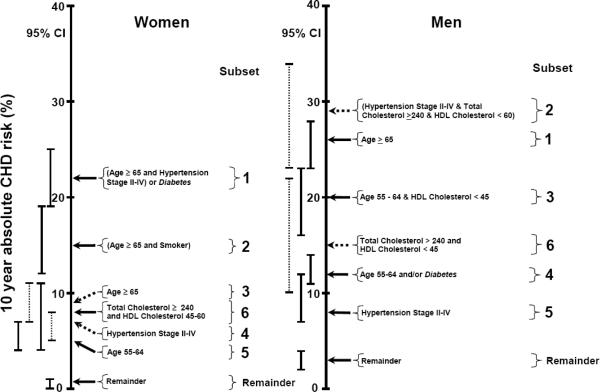
Because cumulative incidences were calculated over a 10 year follow-up period, they correspond to 10 year absolute risks as depicted in Figure 3. Each subset was sorted according to increasing 10 year absolute CHD risk. Ten year absolute CHD risk in women increased from 1% (95% CI: 0–1%) in the remainder subset, to 5% (95% CI: 4–7%) in subset 5, to 7% (95% CI: 5–8%) in subset 4, to 8% (95% CI: 4–11%) in subset 6, to 9% (95% CI: 7–11%) in subset 3, to 15% (95% CI: 12–19%) in subset 2, and to 22% (95% CI: 19–25%) in subset 1. For men, 10 year absolute CHD risk increased from 3% (95% CI: 2–4%) in the remainder subset, to 8% (95% CI: 7–12%) in subset 5, to 12% (95% CI: 11–14%) in subset 4, to 15% (95% CI: 10–22%) in subset 6, to 20% (95% CI: 16–23%) in subset 3, to 26% (95% CI: 23–28%) in subset 1, and to 29% (95% CI: 23–34%) in subset 2.
Figure 3.
Ten year absolute coronary heart disease (CHD) risk in consecutively identified, mutually exclusive subsets identified using PRIM Score in women and men from the Copenhagen City Heart Study.
Cumulative incidences (Figure 1 and 2) were transformed into 10 year CHD risks for the seven subsets in both women and men. Pasting terms are in italic. HDL=high-density lipoprotein. Age is in years and total cholesterol and HDL cholesterol are in mg/dL.
CI= confidence interval; CHD=coronary heart disease.
Common clinical practice versus PRIM Scoring
Individuals in the Copenhagen City Heart Study cohort were categorized as having <10%, 10–20%, and >20% 10 year CHD risks using PRIM Score and Framingham Point Score (Table 1), because these are the categories used by the National Cholesterol Education Program to suggest specific LDL cholesterol levels at which to consider statin or other drug therapy (6). PRIM versus the Framingham Point Score found 11% versus 4% of all women, and 31% versus 35% of all men, to have 10 year CHD risks >20%. In women, PRIM Score and Framingham Point Score agreed on 55%, 5%, and 2% of all women in the Copenhagen City Heart Study cohort to have 10 year CHD risks of <10%, 10–20%, and >20%, respectively (Table 1). In men, PRIM Score and Framingham Point Score agreed on 19%, 19%, and 22% of all men in the Copenhagen City Heart Study cohort to have 10 year CHD risks of <10%, 10–20%, and >20%, respectively (Table 1).
Table 1.
Ten year absolute coronary heart disease risks among 11,444 individuals from the general population, the Copenhagen City Heart Study.
| Framingham | PRIM Score | |||
|---|---|---|---|---|
| Point Score | <10% | 10–20% | >20% | Total |
| Women | ||||
| <10% | 3,537 (55) | 89 (1) | 136 (2) | 3,762 (59) |
| 10–20% | 1,681 (26) | 300 (5) | 418 (7) | 2,399 (38) |
| >20% | 95 (2) | 9 (0.1) | 122 (2) | 226 (4) |
| Total | 5,313 (83) | 398 (6) | 676 (11) | 6,387 (100) |
| Men | ||||
| <10% | 965 (19) | 147 (3) | 19 (0.4) | 1,131 (22) |
| 10–20% | 783 (16) | 959 (19) | 416 (8) | 2,158 (43) |
| >20% | 37 (0.7) | 624 (12) | 1,107 (22) | 1,768 (35) |
| Total | 1,785 (35) | 1,730 (34) | 1,542 (31) | 5,057 (100) |
Values are N (%).
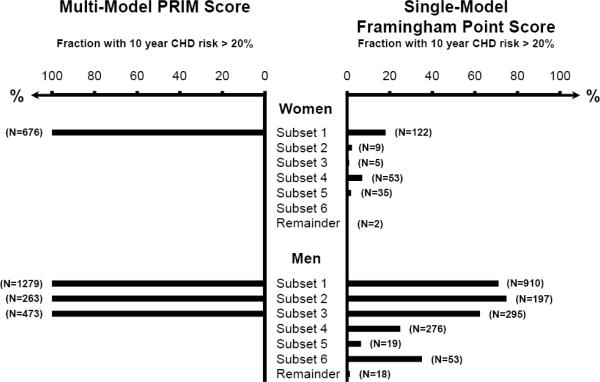
Among women ≥65 years and hypertension stage II–IV and/or with diabetes (subset 1), 10 year CHD risk >20% was found for 100% with PRIM scoring but for only 18% with the Framingham Point Score; corresponding values in men ≥65 years were 100% and 71% (Figure 4). Also, among men in subset 2 (hypertension stage II–IV and total cholesterol >240 mg/dL and HDL cholesterol <60 mg/dL) and 3 (age 55–64 years and HDL cholesterol <45 mg/dL), 10 year CHD risk >20% was found for 100% with PRIM Score, but only for 75% and 62% with Framingham Point Score.
Figure 4.
Fraction of women and men with 10 year coronary heart disease risk >20% according to PRIM Score and common clinical practice with the Framingham Point Score.
Comparison is shown for the different subsets identified with PRIM Score on the Copenhagen City Heart Study cohort with 11,444 individuals followed for 10 years (Figures 1 and 2).
CHD=coronary heart disease.
Discussion
The principal finding of this study is that common clinical practice with the Framingham Point Score underestimates CHD risk in high risk women compared to the new PRIM derived Score, particularly in women ≥65 years and with hypertension stage II–IV and/or with diabetes. This was observed in a prospective study of 11,444 individuals followed for 10 years from the Danish general population, the Copenhagen City Heart Study.
It is often recognized that the Framingham Point Score (3) and the European SCORE system (16) may underestimate the risk of CHD, especially in women (17). It is widely accepted that the Framingham Point Score can be used broadly in white populations in the United States, whereas a simple calibration adjustment is employed for populations with a lower CHD incidence (18, 19). The traditional approach to improve the performance of a risk prediction algorithm is to incorporate additional risk factors, e.g. the Reynolds Score (7) and QRISK (20). All approaches to risk assessment used in clinical practice at present assume that each risk factor has the same relative importance for every individual in the population at large. Our PRIM approach relaxes this assumption by identifying mutually exclusive subsets of individuals whose risk is determined by selected values of particular combinations of risk factors.
In our analyses, 4% of women had 10 year CHD risks of >20% based on Framingham Point Score whereas a total of 11% of all women had 10 year CHD risks of >20% when the new PRIM derived Score was applied. This reclassification in women is potentially of major clinical relevance, because current treatment guidelines are based upon 10 year absolute CHD risks obtained from the Framingham Point Score. According to US treatment guidelines, statin treatment is considered mandatory when the 10 year CHD risk is above 20%, and the LDL cholesterol goal is set to 100 mg/dL (6). Consequently, reclassification of large proportions of the female population into 10 year CHD risk >20% leads to a substantial increase in the number of women eligible for statin treatment. Application of the current National Cholesterol Education Program treatment guidelines (6) to the PRIM derived Score risk assignments obtained in our study results in an LDL cholesterol goal of 100 mg/dL for all women ≥65 years with hypertension stage II–IV and/or with diabetes regardless of age, for all men ≥65 years, for all men with both hypertension stage II–IV and cholesterol ≥240 mg/dL and HDL cholesterol <60 mg/dL, and for all men aged 55–64 years with HDL cholesterol <45 mg/dL. Compared to the Framingham Point Score, application of the PRIM derived Score increased the total number of women having a 10 year absolute CHD risk >20% from 226 (4%) to 676 (11%). The number decreased slightly in men from 1768 (35%) to 1542 (31%).
The 2007 updated US heart disease and stroke statistics document that despite similar prevalence of cardiovascular disease in men and women, women have a 6% higher cardiovascular mortality than men (2). Many different reasons for such higher cardiovascular mortality in women versus in men are likely to exist; however, imprecise risk scoring systems and thus preventive treatment especially in women may partly explain such a gender difference. The Framingham Point Score was originally based on only 227 coronary incident events among 2,856 women (3), and therefore may suffer from lack of statistical power for accurate risk estimates.
Standard statistical analyses, as applied in the Framingham Point Score and in the European SCORE system (16), develop a single prediction model that generates an average risk estimate obtained from a large pooled sample of cases. Consequently, this average risk estimate will most likely be inaccurate for individual persons because most individuals do not represent an average risk due to the influences of compensatory or detrimental genetic or environmental factors (21). This corresponds with the experience of most physicians that patients very often do not fit into average risk schemes. There is thus a strong need for alternative strategies that identify several prediction models, each appropriate for subsets of the population at large, in order to give a more accurate CHD risk estimate for the individual person (21–23). Our present approach to addressing this concern using a PRIM derived Score is the first published attempt to do so.
Through the pasting process of PRIM, infrequent risk factors, such as diabetes in the Copenhagen City Heart Study, are included in the definition of the subsets in the population at large. This feature is of high value in clinical practice, because valid risk estimates for infrequent conditions in the general population are lacking. An example of this is the European SCORE system for fatal CHD (16) that does not include diabetes in the algorithm because it is infrequent, but instead assigns diabetes patients to higher age- and gender risk groups. Furthermore, a large patient group will be influenced by extensive environmental and genetic heterogeneity (21) that is not likely to be captured with the application of a standard statistical strategy derived from the average effects of risk factors. The PRIM derived score constructs subsets of risk factors that are appropriate for subsets of individuals. In contrast to the Framingham Risk Score, SCORE, and other scoring systems, the physician does not need to calculate a point score when using PRIM. The physician should measure total cholesterol, HDL cholesterol, and blood pressure and obtain information on age, sex, diabetes, and smoking. If the patient falls into a subset with more than 20% 10 year absolute CHD risk, the consequence is to consider treatment with a statin and aim at the LDL cholesterol goal of 100 mg/dL. PRIM is an entirely new way of addressing risk factor stratification applying a multiple model approach instead of a single model approach used in all current risk scoring systems. Such a new strategy naturally needs further testing in several different populations before its final utility in clinical practice can be determined.
Despite advantages of large sample size and statistical power in our analyses, limitations need to be called to attention. For the application of PRIM we used similar cutpoints for risk factor categories as in the Framingham Point Score, but collapsed these into larger groups, in order to enable generation of large clinically relevant partitions. Because our study, like the Framingham Heart Study, is limited to whites only, caution should be taken before generalizing our results to other ethnic groups, or to white populations with different rates of CHD and risk factor distributions. Previous studies of the Copenhagen City Heart Study have established that when standard strategies (24–29) for risk assessment are employed, the association of risk of CHD with the classical risk factors is similar to that in other western European populations. It is not known how the outcome of a PRIM analysis will vary among populations of comparable ethnicities; however, it can be anticipated that differences in distributions of risk factors among populations will impact on the selection of mutually exclusive subsets identified through PRIM. In comparable populations we might expect to find a few invariant high risk subsets of individuals, but also several lower risk subsets that depend upon the specific risk factor distribution in that specific population of inference. These latter lower risk groups could be regarded as context dependent subsets, that reflect the heterogeneity of the pathobiology of CHD among ethnically homogeneous populations.
In conclusion, we observed that the Framingham Point Score used in common clinical practice underestimates CHD risk in women compared to the new PRIM derived Score, particularly in women ≥65 years with hypertension stage II–IV and/or with diabetes.
Supplementary Material
Acknowledgements
We are indebted to the staff and participants of the Copenhagen City Heart Study for their important contributions. In particular, we thank Kenneth G. Weiss at the University of Michigan for his dedicated attention to the details of the data analyses.
Funding This work was supported by The Danish Heart Foundation, The Research Fund at Rigshospitalet, Copenhagen University Hospital, by a Specific Targeted Research Project grant from the European Union, Sixth Framework Programme Priority [FP-2005-LIFESCIHEALTH-6, contract # 037631], and in part by National Institutes of Health [GM065509 and HL072905, Charles F. Sing, principle investigator, University of Michigan, Ann Arbor, MI 48109].
Footnotes
Conflict of interest None declared.
References
- 1.Murray CJL, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–76. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y, The American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart Disease and Stroke Statistics - 2007 Update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 4.Graham I. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Atherosclerosis. 2007;194:1–45. doi: 10.1016/j.atherosclerosis.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ. 2007;335:136. doi: 10.1136/bmj.39261.471806.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA. 2007;297:611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 8.Dyson G, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A, Sing CF. An application of the Patient Rule Induction Method for evaluating the contribution of the apolipoprotein E and lipoprotein lipase genes to predicting ischemic heart disease. Genet Epidemiol. 2007;31:515–27. doi: 10.1002/gepi.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjærg-Hansen A. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299:2524–32. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- 10.Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 11.Appleyard M, Hansen AT, Jensen G, Schnohr P, Nyboe J. The Copenhagen City Heart Study. Østerbroundersøgelsen. A book of tables with data from the first examination (1976–78) and a five year follow-up (1981–83). The Copenhagen City Heart Study Group. Scand J Soc Med Suppl. 1989;41:1–160. [PubMed] [Google Scholar]
- 12.Julian DG, Bertrand ME, Hjalmarson A, Fox K, Simoons ML, Ceremuzyndki O, Maseri A, Meinertz T, Meyer J, Pyrörälä K, Rehnqvist N, Tavazzi L, Toutouzas P, Treasure T. Management of stable angina pectoris - recommendations of the Task Force of the European Society of Cardiology. Eur Heart J. 1997;18:394–413. doi: 10.1093/oxfordjournals.eurheartj.a015259. [DOI] [PubMed] [Google Scholar]
- 13.Petri CN. Decrease in the frequency of autopsies in Denmark after the introduction of a new autopsy act. Qual Assur Health Care. 1993;5:315–8. doi: 10.1093/intqhc/5.4.315. [DOI] [PubMed] [Google Scholar]
- 14.Friedman JH, Fisher NI. Bump hunting in high-dimensional data. Statistics and Computing. 1999;9:123–43. [Google Scholar]
- 15.Dyson G, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A, Sing CF. Modifications to 5the Patient Rule-Induction Method that utilize non-additive combinations of genetic and environmental effects to define partitions that predict ischemic heart disease. Genet Epidemiol. 2009;33:317–24. doi: 10.1002/gepi.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetiere P, Jousilahti P, Keil U, Njølstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 17.Blumenthal RS, Michos ED, Nasir K. Further improvements in CHD risk prediction for women. JAMA. 2007;297:641–3. doi: 10.1001/jama.297.6.641. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, D'Agostino RB, Sullivan L, Wilson PWF. Concept and usefulness of cardiovascular risk profiles. Am Heart J. 2004;148:16–26. doi: 10.1016/j.ahj.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 19.D'Agostino RB, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary Heart Disease Prediction Scores. Results of a multiple ethnic group investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 20.Hippisley-Cox J, Couplan C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ. 2007;335:136. doi: 10.1136/bmj.39261.471806.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sing CF, Stengard JH, Kardia SLR. Genes, Environment, and Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2003;23:1190–6. doi: 10.1161/01.ATV.0000075081.51227.86. [DOI] [PubMed] [Google Scholar]
- 22.Clark AG, Boerwinkle E, Hixson JE, Sing CF. Determinants of the success of whole-genome association testing. Genome Res. 2005;15:1463–7. doi: 10.1101/gr.4244005. [DOI] [PubMed] [Google Scholar]
- 23.Benfey PN, Mitchell-Olds T. From genotypes to phenotype: systems biology meets natural variation. Science. 2008;320:495–7. doi: 10.1126/science.1153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnohr P, Jensen JS, Scharling H, Nordestgaard BG. Coronary heart disease risk factors ranked by importance for the individual and community. A 21 year follow-up of 12000 men and women from The Copenhagen City Heart Study. Eur Heart J. 2002;23:620–6. doi: 10.1053/euhj.2001.2842. [DOI] [PubMed] [Google Scholar]
- 25.Ducimetiere P, Richard JL, Cambien F, Rakotovao R, Claude R. Coronary heart disease in middle-aged Frenchmen. Comparisons between Paris Prospective Study, Seven Countries Study, and Pooling Project. Lancet. 1980;1:1346–50. doi: 10.1016/s0140-6736(80)91796-1. [DOI] [PubMed] [Google Scholar]
- 26.Shaper AG, Pocock SJ, Walker M, Cohen NM, Wale CJ, Thomsen AG. British Regional Heart Study: cardiovascular risk factors in middle-aged men in 24 towns. BMJ. 1981;283:179–86. doi: 10.1136/bmj.283.6285.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilhelmsen L, Berglund G, Elmfeldt D, Tibblin G, Wedel H, Pennert K, Vedin A, Wilhelmsson C, Werkö L. The multifactor primary prevention trial in Goteborg, Sweden. Eur Heart J. 1986;7:279–88. doi: 10.1093/oxfordjournals.eurheartj.a062065. [DOI] [PubMed] [Google Scholar]
- 28.Njolstad I, Arnesen E, Lund-Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction: a 12-year follow-up of the Finnmark Study. Circulation. 1996;93:450–6. doi: 10.1161/01.cir.93.3.450. [DOI] [PubMed] [Google Scholar]
- 29.Vartiainen E, Jousilahti P, Alfthan G, Sundvall J, Pietinen P, Puska P. Cardiovascular risk factor changes in Finland, 1972–1997. Int J Epidemiol. 2000;29:49–56. doi: 10.1093/ije/29.1.49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.