Abstract
Utilizing the concept of synthetic lethality has provided new opportunities for the development of targeted therapies, by allowing the targeting of loss of function genetic aberrations. In cancer cells with BRCA1 or BRCA2 loss of function, which harbor deficiency of DNA repair by homologous recombination, inhibition of PARP1 enzymatic activity leads to an accumulation of single strand breaks that are converted to double strand breaks but cannot be repaired by homologous recombination. Inhibition of PARP has therefore been advanced as a novel targeted therapy for cancers harboring BRCA1/2 mutations. Preclinical and preliminary clinical evidence, however, suggests a potentially broader scope for PARP inhibitors. Loss of function of various proteins involved in double strand break repair other than BRCA1/2 has been suggested to be synthetically lethal with PARP inhibition. Inactivation of these genes has been reported in a subset of human cancers and might therefore constitute predictive biomarkers for PARP inhibition. Here we discuss the evidence that the clinical use of PARP inhibition may be broader than targeting of cancers in BRCA1/2 germ-line mutation carriers.
Key words: homologous recombination, PARP inhibitor, BRCA1, BRCA2, PTEN, PALB2, EMSY, double strand break repair
Introduction
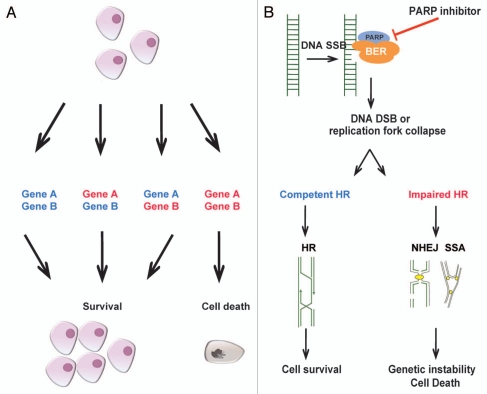
The concept of synthetic lethality was first described by Bridges in 1922,1 and the term of synthetic lethality was coined by Dobzhansky in 1946.2 This phenomenon occurs when two non-lethal mutations have no effect when they occur individually, but lead to death of the cell in combination.2 This concept is being exploited in cancer research aiming to identify single mutations that are present in cancer cells but not in normal cells by inhibiting the synthetic lethal ‘partner’ gene (Fig. 1A).
Figure 1.
The principle of synthetic lethality. (A) The principle of synthetic lethality proposes, which proposes that two genes display a synthetic lethal interaction if loss of function of either of them leads to a phenotype that is viable, whereas concurrent inhibition of both leads to cell death. (B) The mechanism of selective cell death caused by PARP inhibitors in cells with impaired homologous recombination (HR) DNA repair. DNA damage is a common phenomenon. Single strand breaks (SSBs) are normally repaired by base excision repair (BER ). PARP is a key component of the BER machinery. In the presence of a SSB, PARP localizes to the site of breakage and acts as a molecular beacon to the other components of the BER machinery. Inhibition of PARP leads to persistence of SSBs that when unrepaired lead to replication fork stalling and collapse and to the formation of DSBs during DNA replication. In cells with competent HR, these breaks are repaired by the HR DSB repair pathway. In the cells with deficient HR due to loss of function of BRCA1 or BRCA2, DNA DSB persist or are repair by alternative pathways that are highly error prone, for example, non-homologous end-joining (NHEJ) or single strand annealing (SSA). This results in genomic instability and cell death.
Hypothesis driven studies revealed that loss of function of BRCA1 or BRCA2 results in an exquisite sensitivity to silencing or chemical inhibition of the poly(ADP-ribose) polymerase (PARP)-1.3,4 The rationale for this synthetic lethal interactions stems from the fact that BRCA1 and BRCA2 are tumor suppressor genes that are involved in homologous recombination (HR) DNA repair of DNA double strand breaks (DSBs).5 The HR system is largely error free, whereas the other mechanisms of DNA double strand break repairs (i.e., nonhomologous end joining (NHEJ) and single strand annealing (SSA)) are error prone and lead to genomic instability. PARP1, a DNA repair enzyme, is responsible for the base excision repair of DNA single strand breaks.6 Inhibition of PARP activity thus leads to an accumulation of unrepaired single strand breaks that in proliferating cells result in stalling and collapse of replication forks and, consequently, to DSBs. These DSBs, if not repaired by HR, result in cell death due to mitotic catastrophe (Fig. 1B).6 In cells with loss of function of BRCA1/2, which are, therefore, HR-deficient, inhibition of PARP1 activity leads to an accumulation of single strand breaks that are converted to DSB but cannot be repaired by HR, resulting in increasingly high levels of genetic instability and, eventually, cell death.6
The synthetic lethal interactions between PARP1 and BRCA1/2 were confirmed with PARP inhibitors in vitro and in mouse models,3,7 and in a landmark phase I clinical trial.8 Phase II clinical trials demonstrated a significant response rate to monotherapy with the PARP inhibitor olaparib in 33% and 41% of patients with BRCA1 or BRCA2 mutant ovarian and breast cancer respectively.9,10 These results were particularly impressive as the participating patients had received several lines of standard chemotherapy prior to the administration of olaparib.
A growing body of evidence suggests that PARP inhibitors might also play a role in the treatment of a subgroup of cancer patients without germ-line BRCA1 or BRCA2 mutation (hereafter in this review referred to as sporadic cancers). Despite having wild-type BRCA1 and BRCA2, these tumors may harbor other abnormalities in components of the HR DNA repair pathway leading to a dysfunction of this pathway of DNA repair and subsequently displaying clinical and biological characteristics similar to those of cancers developing in BRCA1 or BRCA2 germ-line mutation carriers. One of the main mechanisms leading to this phenomenon, which has been termed “BRCAness”,11 can be epigenetic silencing of BRCA1.12 The analysis of cell lines and xenograft models has revealed that BRCA1 gene promoter methylation may sensitize cancer cells to PARP inhibitors.12,13
Expression of BRCA1 is two-fold lower in sporadic triple-negative breast cancers compared to ER-positive cancers;14,15 however, promoter methylation is found only in the minority (14%) of sporadic triple-negative breast cancers.15,16 Other factors that might contribute to low BRCA1 expression are thought to be the upregulation of transcriptional suppressors of BRCA1, such as ID4,15 and HMG1.17 In addition, upregulation of microRNAs (miRNA) that target BRCA1 expression may also lead to BRCA1 pathway dysfunction.18 In particular, miR-182 negatively regulates BRCA1 expression and subsequently reduces the efficiency of HR DNA repair. In xenograft models, tumors with stably expressed miR-182 were shown to be more sensitive to olaparib than controls.18
Based on the hypothesis that sporadic triple-negative breast cancers often show significant BRCA1 downregulation, they may also harbor an impaired HR DNA repair. Hence, clinical trials have been started for this subgroup of breast cancer patients. A phase II clinical trial has recently demonstrated that the addition of iniparib, a drug that has modest PARP inhibitory activity, significantly increases the progression free survival and overall survival of patients with advanced triple-negative breast cancers.19 However, a small phase II study with single agent olaparib, a potent PARP inhibitor, in patients with advanced triple-negative breast cancer revealed no objective clinical responses.20 Hence, it remains to be determined whether potent PARP inhibitors used as single agents are of benefit for patients with sporadic triple-negative disease.
Recent findings from our group21–23 and others24 suggest that defects in genes other than BRCA1 and BRCA2 may also sensitize cancer cells to PARP inhibition. The scope of this Extra View is to discuss other potential synthetic lethal interactions between PARP inhibitors and genes defects in cancers (Table 1).
Table 1.
Evidence and mechanism of proposed genes beeing synthetic lethal to PARP inhibitors
| Gene | Abberation | Best available evidence | References |
| BRCA1 | Mutation | Clinical phase II trials | 8–10 |
| Methylation | In vitro and xenograft studies | 13 | |
| BRCA2 | Mutation | Clincal phase II trials | 8–10 |
| Methylation | In vitro and xenograft studies | 13 | |
| MRN-complex | Biallelic mutations of MRE11 | In vitro studies | 21, 30, 33 |
| NRS1 mutation | In vitro studies | 32 | |
| EMSY | Amplification | Hypothetical and circumstantial evidence | 58, 59 |
| PALB2 | Truncating Mutation | In vitro studies | 53 |
| PTEN | Loss of function | In vitro and xenograft studies; case report | 22, 23, 76, 78 |
| ATM | Mutations | In vitro and xenograft studies | 39, 40 |
| AURKA | Overexpression | In vitro and xenograft studies | 82 |
MRE11-RAD50-NBS1
The MRE11-RAD50-NBS1 (MRN) complex consists of three proteins and plays important roles in detection and signaling of DSBs, as well as the repair pathways of HR and NHEJ.25 MRN complex localizes in nuclear foci in response to DSB-inducing agents and links DSB recognition and repair, recruitment of ATM, cell cycle checkpoint signaling and regulation of chromatin remodeling.25
Mutations in any of these genes have been detected in various cancers. In particular within cancers with microsatellite instability such as colorectal and endometrial cancers, shortening of the noncoding poly (T) 11 tract of MRE11 was reported in 83.7% and 50% of cases, respectively.26 Although not all of these mutations are associated with loss of function of MRE11, monoallelic deletions of two or more nucleotides leads to reduced expression and functional impairment of the MRE11/NBS1/RAD50 complex by impaired MRE11 and NBS1 expression.26–28 Biallelic deletions or homozygous frameshift mutations, however, are associated with strong reduction of MRE11 transcript levels and protein expression, reduced functionality in cell lines assays as well as exquisite bleomycin sensitivity.29,30 Among MSI-positive colorectal cancer 82% of cancers harbored a MRE11 mutation and 32% of them were biallelic mutations. 30 Separately, biallelic mutations or deletions of two or more nucleotides were reported in 15% of MSI-positive endometrial cancers.31 It was first described by our group21 in in vitro studies and later confirmed by others30,32,33 that deficiency of HR caused by dysfunctional MRN complex may sensitize cancer cells to PARP inhibitors. MRE11 mutant colorectal cancer cell lines have been shown to be significantly more sensitive to olaparib and ABT-888 than MRE11 wild-type cells.30,33 However, only biallelic mutations leading to markedly reduced transcript levels were shown to be predictive for PARP-sensitivity.30 Likewise, NBS1 deficient human fibroblasts are also sensitive to various PARP inhibitors.21,32
Ataxia Telangectasia Mutated (ATM)
Ataxia telangiectasia mutated (ATM) is a serine threonine protein kinase with a crucial function in the response to DNA DSBs.34 Upon DNA damage, ATM is activated either by autophosphorylation or by the DNA damage sensor complex MRN. Activated ATM induces DNA damage signaling via Chk2 and p53 to induce DNA damage checkpoints and via H2AX phosphorylation, MRN complex, BRCA2 and RAD51 to initiate DNA repair by HR.34 ATM germ-line mutations are the underlying cause of ataxia telangiectasia, a severe syndrome characterized by neurodegeneration with increased susceptibility to develop leukemia and lymphomas. 35 Somatic mutations of ATM are common among hematological malignancies such as chronic lymphocytic leukemia and mantle cell lymphoma but also among some solid tumors such as lung adenocarcinomas.36
The functional interconnection between PARP1 and ATM was first observed in mouse models where Parp1 and Atm double knockout mice were shown to be embryonically lethal.37 Inhibition of PARP was subsequently shown to cause selective cell death in ATM deficient cancer cell lines due to their impaired HR by our group where siRNA against ATM and the ATM inhibitor caffeine both resulted in sensitivity to the PARP inhibitor KU0058948 in HeLa cells.21 This link was confirmed by another study where PARP1 null cells showed activation of ATM, Chk2 and increased RAD51 foci formation and sensitivity to ATM inhibitors and ATM defective cells showed sensitivity to the PARP inhibitor 4-amino-1,8-naphtalamine.38 Two recent studies suggest a potential role for PARP inhibitors in treatment of mantle cell lymphoma as preclinical studies using olaparib in ATM deficient xenograft models increased survival and inhibited tumor growth.39,40 Furthermore, PARP inhibition sensitizes Atm−/− mouse fibroblasts to radiation therapy.41
Partner and Ligand of BRCA2 (PALB2)
The partner and localizer of BRCA2 (PALB2) maps to 16p12.2, a region associated with loss of heterozygosity in 12% of breast cancers.42 PALB2 now has an established role in the formation of the BRCA-complex with BRCA1 and BRCA2, which is necessary for competent HR (reviewed in ref. 43). However, emerging evidence suggests PALB2 can also bind D-loop DNA structures and enhances RAD51 recombinase activity.44 Controversy still exists in how PALB2 is regulated, with inconsistencies reported in how MRG15 (a major PALB2 binding partner45) interacts with PALB2 in regulating HR.45,46 It also remains unclear if PALB2 localization to sites of DNA DSBs is dependent on BRCA1 or not.47–49
While PALB2 biallelic mutations are known to cause Fanconi Anemia and predispose to pediatric malignancies, including medulloblastomas, Wilms' tumors and acute myeloid leukemia.50 Monoallelic truncating mutations of PALB2, on the other hand, have been shown to cause familial breast and pancreatic cancer,43,51 and to predispose to male breast cancer.51,52 Mutations in prostate and ovarian cancers are rarer events.43 Breast cancers arising in patients harboring PALB2 mutations appear to have phenotypic characteristics intermediate between those of tumors from BRCA1 and BRCA2 mutant cancers (i.e., 50% of histological grade III, 58% oestrogen receptor negative, 93% HER2 negative and 40% of triple-negative phenotype).43
Given its pivotal role in competent HR, PALB2 is likely to share a synthetic lethal interaction with DNA-damaging agents and PARP inhibitors. As proof of principle, an in vitro study demonstrated a dramatically increased sensitivity to olaparib in PALB2-deficient cells (EUFA1341) compared to control cells with wild-type PALB2.53 Further supporting evidence stems from a study where a personalized xenograft of a patient with a BRCA2 wild-type sporadic pancreatic adenocarcinoma showed a dramatic and persistent response to cisplatin and mitomycin C, but not gemcitabine.54 Exomic sequencing of the xenograft and germline DNA identified a somatically acquired point mutation in exon 10 and a germline frameshift deletion, resulting in biallelic inactivation of PALB2.54 These exciting preliminary findings clearly need corroboration in well designed in vitro and in vivo studies, but suggest that PALB2 mutation carriers are likely to represent a further subgroup of patients who may benefit from treatment with PARP inhibitors, either as monotherapy or in combination with platinum salts or interstand cross-linking agents.
EMSY
EMSY maps to the commonly amplified region at 11q13–q14 and has been identified as a BRCA2 binding partner.55 Using yeast two-hybrid screens, co-immunoprecipitation and site-directed mutagenesis, EMSY was shown to bind to the region encoded by exon 3 of BRCA2 (between residues 23 and 46) via its “EMSY N Terminal” (ENT) domain.55 Attributing other functions to this largely novel protein has proven challenging, however, there is evidence to suggest that EMSY mau play a role in global transcription control and chromatin remodeling.56,57 EMSY has also been reported have a role in HR-mediated repair of DNA DSBs,55 an assertion based on four observations: (1) EMSY co-localizes with phospho-γH2AX to sites of DNA DSBs following irradiation (though this phenomenon was shown only in mouse fibroblast cells). (2) EMSY shares its BRCA2 binding site with PALB2.42 (3) Overexpression of a truncated form of EMSY in human mammary epithelial cells (184-h Tert cells) was reported to produce a pattern of chromosomal instability akin to that found in BRCA2-null cells.58 (4) EMSY amplification has been identified in subsets of sporadic breast, ovarian and pancreatic cancer, with an association with reduced survival.59–63 Together these observations have lead to the suggestion that EMSY amplification is a mechanism of BRCA2 pathway inactivation in sporadic tumors. Some have also suggested that EMSY gene amplification could be used as a predictive biomarker for response to platinum salts,64 PARP inhibition,65 or neoadjuvant chemotherapy.66
Despite these interesting observations, the role of EMSY in carcinogenesis or of EMSY amplification in HR remains a controversial issue. The effects of re-expression of a full length EMSY construct have not been published, and direct evidence of EMSY amplification causing abrogation of HR is yet to be provided. Our own observations in cell line models suggest that EMSY amplification does not necessarily lead to abrogation of HR DNA repair of DSBs, nor does it confer sensitivity to platinum salts or potent PARP inhibitors (Wilkerson PM and Reis-Filho JS, unpublished observations). We would therefore advocate caution in the use of EMSY amplification as a predictive marker for response to PARP inhibitors.
Phosphatase and Tensin Homolog Deleted on Chromosome 10 (PTEN)
The tumor suppressor gene PTEN (phosphatase and tensin homolog deleted on chromosome 10) has a well-established role in limiting PI3K-AKT signaling through its phosphatase activity.67 PTEN loss of function is a frequent event in different forms of human cancers and can occur through mutations, deletions or promoter hypermethylation.68 For example, mutation of PTEN occurs in up to 40% of glioblastomas,69 in 50% of endometrial cancers,70 and in up to 20% of colorectal cancers.71 Moreover, loss of PTEN expression is observed in 48% of breast cancers,72 and 20% of prostate cancers.73 Recent studies have demonstrated a distinct role for PTEN in the maintenance of chromosomal integrity and repair of DNA DSB.24,74,75 These and other studies showed that loss of PTEN leads to both genomic instability and impaired RAD51-mediated DNA DSB repair by HR.22–24,74,75 Specifically, in cells with PTEN loss, RAD51 does not appear at sites of DNA DSB to mediate repair by HR. The reason(s) for the absence of RAD51 at sites of DNA damage in cells with PTEN loss is still under investigation. Whilst some studies have reported that loss of PTEN leads to suppressed RAD51 expression,22,24 in other studies23,76 total RAD51 levels were shown to be similar in PTEN wild-type and mutant cancer cell lines. In our study in reference 23, however, although RAD51 expression remained unchanged, upon DNA damage RAD51 nuclear accumulation at sites of DNA DSB was reduced in PTEN mutant cell lines, suggesting an impaired ability of RAD51 to localize to the nucleus.23 Moreover, among PTEN mutant cell lines, McEllin et al. observed decreased transcript levels of the RAD51 paralogs RAD51B, RAD51C and RAD51D, which are all involved in multiple steps during the HR repair process.77 Given these discrepant findings regarding the exact mechanism of PTEN in HR DNA repair, further research is warranted to define the role of PTEN in DNA DSB repair. In three studies of PTEN mutant cell lines established from breast, prostate, melanoma, bladder, glioma and endometrioid endometrial cancer as well at PTEN−/− and PTEN+/+ astrocytes, PTEN loss of function was found to predict sensitivity to the PARP inhibitors olaparib and ABT-888.22,23,76 Moreover, we have recently reported a patient with endometrial adenocarcinoma harboring a PTEN gene mutation, lacking PTEN expression and not harboring germ-line BRCA1 or BRCA2 germ-line mutations, who experienced a clear objective response to single agent olaparib treatment.78 Additional evidence that PTEN null cancers may be more sensitive to DNA-damaging agents such as platinum-salts or anthracyclines comes from studies reporting that patients with PTEN-null advanced endometrial cancer treated with chemotherapy may respond better to chemotherapy than those with PTEN expression.79 Future clinical trials will determine if these promising findings will translate into patient benefit.
Aurora kinase A (AURKA)
Aurora-A kinase (AURKA) is a member of the Aurora serine/threonine protein kinase family, which plays an important role in mitosis and meiosis. AURKA is also involved in DNA damage response80,81 and modulates the repair of DSB.82 AURKA is frequently amplified and or overexpressed in various cancer types and associated with aggressive tumor behavior in tumors such as ovarian cancer83 pancreatic, breast and colon cancers.84
Overexpression of AURKA has recently been shown to result in reduced ability for HR DNA repair82 through PLK1-mediated inhibition of RAD51 recruitment to DNA DSBs82 and through suppression of BRCA2 expression.85 As expected, AURKA overexpression has been shown to result in sensitivity to the PARP inhibitor olaparib in in vitro experiments and in xenografts.82 Some conflicting evidence, however, exists on AURKA overexpression and its role in HR, as reports suggest that AURKA expression results in radio-resistance,85,86 and silencing of AURKA, either through short hairpin RNA or Aurora-A-inhibitors, leads to increased radiosensitivity in cancer cells.85,86 Furthermore, overexpression of AURKA assessed by immunohistochemistry in ovarian cancer tumors, which were treated with platinum-based chemotherapy, was associated with poor prognosis.83 Cancers with impaired HR DNA repair such as those with BRCA1 or BRCA2 mutations are well known to exhibit sensitivity to platinum-salts as well as irradiation87,88 and therefore, cancers with AURKA overexpression would be expected to be, based on the findings reporting its role in HR,82 radio-sensitive and/or responsive to platinum-based chemotherapy.
Conclusion
Several genes apart from BRCA1 and BRCA2 are involved in HR. It is well established that loss of BRCA1 or BRCA2, due to mutations6 and possibly due to promoter methylation,12,13 leads to impaired HR DNA repair. The loss of this type of DNA repair due to genetic/epigenetic aberrations in cancer cells provides a rational for the use of PARP inhibitors following the principles of synthetic lethality.
Recent studies focusing on genes involved in the DSB DNA repair machinery other than BRCA1 and BRCA2 have identified aberrations that are commonly found in various cancers and lead to defective HR DNA repair. Preclinical studies also support the rationale that some these aberrations are synthetic lethal with PARP inhibitors (e.g., PTEN loss of function); however, clinical trials are certainly needed to confirm these findings. The clinical use of PARP inhibitors will undoubtedly transcend the subgroup of cancer patients that harbor BRCA1 or BRCA2 germ-line mutations. Preliminary data from clinical trials testing PARP inhibitors in patients with sporadic cancers,8–10 as well a case report,78 indicate that tumors other than those from patients with BRCA1 or BRCA2 germline mutations may benefit from PARP inhibition. The main challenge, however, remains the identification of cancers with gene aberrations that result in impaired HR, which are the likeliest to respond to PARP inhibition.
The application of high-throughput functional genomic approaches, such as genome-wide shRNA and siRNA PARP inhibitor sensitization screens, and drug screens will likely continue to help identify the aberrations that are synthetically lethal with PARP inhibitors. In fact, such approaches have already proven successful in identifying novel synthetic lethal interactions,89,90 and one can envisage that additional hits will be identified in the near future.
Currently, different approaches for the identification of human cancers with impaired HR DNA repair are being explored. One is to generate classifiers based on microarray gene expression profiling65 or genomic profiling91,92 of BRCA1 and BRCA2 mutant cancers and to apply them to sporadic cancers in an attempt to identify those who share common transcriptomic and/or genomic characteristics with BRCA deficient tumors. The other is to assess surrogate markers of competent HR DNA repair, such as RAD51 foci formation upon DNA damage.93–96 This latter approach is likely to identify most if not all cancers harboring any aberrations in genes that control HR DNA repair and may constitute a biomarker for the selection of patients that are likely to benefit from PARP inhibitors. However, both approaches have their own limitations and still need to be further assessed and validated. With the rapid technological developments in the field of massively parallel sequencing that might lead to broader accessibility and accurate readouts, another approach would be to sequence gene sets that are involved in HR DNA repair to define a repertoire of mutations that predict PARP inhibitor sensitivity.
Acknowledgements
K.J.D., P.M.W., D.W., A.A. and J.S.R.F. are funded in part by Breakthrough Breast Cancer. A.A. is funded in part by The American Association of Cancer Research, The Breast Cancer Campaign, the Breast Cancer Research Foundation and Stand Up to Cancer. K.J.D. is the recipient of Swiss National Science Foundation fellowship and P.M.W. is the recipient of a Wellcome Trust Foundation Clinical Research Fellowship. B.W. is the recipient of a Cancer Research UK post-doctoral fellowship.
Conflicts of Interest
A.A. may benefit financially from the development of PARP inhibitors through patents held jointly with AstraZeneca through the Institute of Cancer Research ‘rewards to inventors’ scheme. J.S.R.F. received honoraria for the delivery of talks in meetings organized by Sanofi Aventis. The remaining authors declare no conflicts of interest.
References
- 1.Bridges CB. The origin of variation. Amer Nat. 1922;56:51–63. [Google Scholar]
- 2.Dobzhansky T. Genetics of natural populations. Xiii. Recombination and variability in populations of Drosophila pseudoobscura. Genetics. 1946;31:269–290. doi: 10.1093/genetics/31.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar-Quesada R, Munoz-Gamez JA, Martin-Oliva D, Peralta A, Valenzuela MT, Matinez-Romero R, et al. Interaction between ATM and PARP-1 in response to DNA damage and sensitization of ATM deficient cells through PARP inhibition. BMC Mol Biol. 2007;8:29. doi: 10.1186/1471-2199-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagaraju G, Scully R. Minding the gap: The underground functions of BRCA1 and BRCA2 at stalled replication forks. DNA Repair (Amst) 2007;6:1018–1031. doi: 10.1016/j.dnarep.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26:3785–3790. doi: 10.1200/JCO.2008.16.0812. [DOI] [PubMed] [Google Scholar]
- 7.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 8.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 9.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 10.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 11.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 12.Veeck J, Ropero S, Setien F, Gonzalez-Suarez E, Osorio A, Benitez J, et al. BRCA1 CpG island hypermethylation predicts sensitivity to poly(adenosine diphosphate)-ribose polymerase inhibitors. J Clin Oncol. 2010;28:563–564. doi: 10.1200/JCO.2010.30.1010. [DOI] [PubMed] [Google Scholar]
- 13.Drew Y, Mulligan EA, Vong WT, Thomas HD, Kahn S, Kyle S, et al. Therapeutic potential of poly(ADP-ribose) polymerase inhibitor AG014699 in human cancers with mutated or methylated BRCA1 or BRCA2. J Natl Cancer Inst. 2011;103:334–346. doi: 10.1093/jnci/djq509. [DOI] [PubMed] [Google Scholar]
- 14.Turner NC, Reis-Filho JS. Basal-like breast cancer and the BRCA1 phenotype. Oncogene. 2006;25:5846–5853. doi: 10.1038/sj.onc.1209876. [DOI] [PubMed] [Google Scholar]
- 15.Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 16.Matros E, Wang ZC, Lodeiro G, Miron A, Iglehart JD, Richardson AL. BRCA1 promoter methylation in sporadic breast tumors: Relationship to gene expression profiles. Breast Cancer Res Treat. 2005;91:179–186. doi: 10.1007/s10549-004-7603-8. [DOI] [PubMed] [Google Scholar]
- 17.Baldassarre G, Battista S, Belletti B, Thakur S, Pentimalli F, Trapasso F, et al. Negative regulation of BRCA1 gene expression by HMGA1 proteins accounts for the reduced BRCA1 protein levels in sporadic breast carcinoma. Mol Cell Biol. 2003;23:2225–2238. doi: 10.1128/MCB.23.7.2225-2238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moskwa P, Buffa FM, Pan Y, Panchakshari R, Gottipati P, Muschel RJ, et al. miR-182-mediated downregulation of BRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Mol Cell. 2011;41:210–220. doi: 10.1016/j.molcel.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 20.Gelmon KA, Hirte HW, Robidoux A, Tonkin KS, Tischkowitz M, Swenerton K, et al. Can we define tumors that will respond to PARP inhibitors? A phase II correlative study of olaparib in advanced serous ovarian cancer and triple-negative breast cancer. J Clin Oncol. 2010:28. [Google Scholar]
- 21.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 22.Mendes-Pereira AM, Martin SA, Brough R, McCarthy A, Taylor JR, Kim JS, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1:315–322. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dedes KJ, Wetterskog D, Mendes-Pereira AM, Natrajan R, Lambros MB, Geyer FC, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med. 2010;2:53–75. doi: 10.1126/scitranslmed.3001538. [DOI] [PubMed] [Google Scholar]
- 24.Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 25.Stracker TH, Petrini JH. The MRE11 complex: Starting from the ends. Nat Rev Mol Cell Biol. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giannini G, Rinaldi C, Ristori E, Ambrosini MI, Cerignoli F, Viel A, et al. Mutations of an intronic repeat induce impaired MRE11 expression in primary human cancer with microsatellite instability. Oncogene. 2004;23:2640–2647. doi: 10.1038/sj.onc.1207409. [DOI] [PubMed] [Google Scholar]
- 27.Ottini L, Falchetti M, Saieva C, De Marco M, Masala G, Zanna I, et al. MRE11 expression is impaired in gastric cancer with microsatellite instability. Carcinogenesis. 2004;25:2337–2343. doi: 10.1093/carcin/bgh257. [DOI] [PubMed] [Google Scholar]
- 28.Giannini G, Ristori E, Cerignoli F, Rinaldi C, Zani M, Viel A, et al. Human MRE11 is inactivated in mismatch repair-deficient cancers. EMBO Rep. 2002;3:248–254. doi: 10.1093/embo-reports/kvf044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li HR, Shagisultanova EI, Yamashita K, Piao Z, Perucho M, Malkhosyan SR. Hypersensitivity of tumor cell lines with microsatellite instability to DNA double strand break producing chemotherapeutic agent bleomycin. Cancer Res. 2004;64:4760–4767. doi: 10.1158/0008-5472.CAN-04-0975. [DOI] [PubMed] [Google Scholar]
- 30.Vilar E, Bartnik CM, Stenzel SL, Raskin L, Ahn J, Moreno V, et al. MRE11 deficiency increases sensitivity to poly(ADP-ribose) polymerase inhibition in microsatellite instable colorectal cancers. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-1120. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilbao C, Ramirez R, Rodriguez G, Falcon O, Leon L, Diaz-Chico N, et al. Double strand break repair components are frequent targets of microsatellite instability in endometrial cancer. Eur J Cancer. 2010;46:2821–2827. doi: 10.1016/j.ejca.2010.06.116. [DOI] [PubMed] [Google Scholar]
- 32.Horton JK, Stefanick DF, Zeng JY, Carrozza MJ, Wilson SH. Requirement for NBS1 in the S phase checkpoint response to DNA methylation combined with PARP inhibition. DNA Repair (Amst) 2011;10:225–234. doi: 10.1016/j.dnarep.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knights C, Chresta C, Riches L, Mangena R, Avis T, O'Shaughnessy A, et al. Preclinical evaluation of the PARP inhibitor olaparib in homologous recombination deficient (HRD) MRE11 mutant microsatellite instable (MSI) colorectal cancer. Mol Cancer Ther. 2009:8. [Google Scholar]
- 34.Lavin MF. Ataxia-telangiectasia: From a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 35.Gumy-Pause F, Wacker P, Sappino AP. ATM gene and lymphoid malignancies. Leukemia. 2004;18:238–242. doi: 10.1038/sj.leu.2403221. [DOI] [PubMed] [Google Scholar]
- 36.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menisser-de Murcia J, Mark M, Wendling O, Wynshaw-Boris A, de Murcia G. Early embryonic lethality in PARP-1 Atm double-mutant mice suggests a functional synergy in cell proliferation during development. Mol Cell Biol. 2001;21:1828–1832. doi: 10.1128/MCB.21.5.1828-1832.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryant HE, Helleday T. Inhibition of poly (ADP-ribose) polymerase activates ATM which is required for subsequent homologous recombination repair. Nucleic Acids Res. 2006;34:1685–1691. doi: 10.1093/nar/gkl108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williamson CT, Muzik H, Turhan AG, Zamo A, O'Connor MJ, Bebb DG, et al. ATM deficiency sensitizes mantle cell lymphoma cells to poly(ADP-ribose) polymerase-1 inhibitors. Mol Cancer Ther. 2010;9:347–357. doi: 10.1158/1535-7163.MCT-09-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weston VJ, Oldreive CE, Skowronska A, Oscier DG, Pratt G, Dyer MJ, et al. The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood. 2010;116:4578–4587. doi: 10.1182/blood-2010-01-265769. [DOI] [PubMed] [Google Scholar]
- 41.Loser DA, Shibata A, Shibata AK, Woodbine LJ, Jeggo PA, Chalmers AJ. Sensitization to radiation and alkylating agents by inhibitors of poly(ADP-ribose) polymerase is enhanced in cells deficient in DNA double-strand break repair. Mol Cancer Ther. 2010;9:1775–1787. doi: 10.1158/1535-7163.MCT-09-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, et al. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell. 2006;22:719–729. doi: 10.1016/j.molcel.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 43.Tischkowitz M, Xia B. PALB2/FANCN: Recombining cancer and Fanconi anemia. Cancer Res. 2010;70:7353–7359. doi: 10.1158/0008-5472.CAN-10-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dray E, Etchin J, Wiese C, Saro D, Williams GJ, Hammel M, et al. Enhancement of RAD51 recombinase activity by the tumor suppressor PALB2. Nat Struct Mol Biol. 2010;17:1255–1259. doi: 10.1038/nsmb.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sy SM, Huen MS, Chen J. MRG15 is a novel PALB2-interacting factor involved in homologous recombination. J Biol Chem. 2009;284:21127–21131. doi: 10.1074/jbc.C109.023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayakawa T, Zhang F, Hayakawa N, Ohtani Y, Shinmyozu K, Nakayama J, et al. MRG15 binds directly to PALB2 and stimulates homology-directed repair of chromosomal breaks. J Cell Sci. 2010;123:1124–1130. doi: 10.1242/jcs.060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang F, Ma J, Wu J, Ye L, Cai H, Xia B, et al. PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr Biol. 2009;19:524–529. doi: 10.1016/j.cub.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang F, Fan Q, Ren K, Andreassen PR. PALB2 functionally connects the breast cancer susceptibility proteins BRCA1 and BRCA2. Mol Cancer Res. 2009;7:1110–1118. doi: 10.1158/1541-7786.MCR-09-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sy SM, Huen MS, Chen J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc Natl Acad Sci USA. 2009;106:7155–7160. doi: 10.1073/pnas.0811159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reid S, Schindler D, Hanenberg H, Barker K, Hanks S, Kalb R, et al. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet. 2007;39:162–164. doi: 10.1038/ng1947. [DOI] [PubMed] [Google Scholar]
- 51.Casadei S, Norquist BM, Walsh T, Stray SM, Mandell JB, Lee MK, et al. Contribution to familial breast cancer of inherited mutations in the BRCA2-interacting protein PALB2. Cancer Res. 2011;71:2222–2229. doi: 10.1158/0008-5472.CAN-10-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding YC, Steele L, Kuan CJ, Greilac S, Neuhausen SL. Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Res Treat. 2011;126:771–778. doi: 10.1007/s10549-010-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buisson R, Dion-Cote AM, Coulombe Y, Launay H, Cai H, Stasiak AZ, et al. Cooperation of breast cancer proteins PALB2 and piccolo BRCA2 in stimulating homologous recombination. Nat Struct Mol Biol. 2010;17:1247–1254. doi: 10.1038/nsmb.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, De Jesus-Acosta A, Jones S, Maitra A, et al. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol Cancer Ther. 2011;10:3–8. doi: 10.1158/1535-7163.MCT-10-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hughes-Davies L, Huntsman D, Ruas M, Fuks F, Bye J, Chin SF, et al. EMSY links the BRCA2 pathway to sporadic breast and ovarian cancer. Cell. 2003;115:523–535. doi: 10.1016/s0092-8674(03)00930-9. [DOI] [PubMed] [Google Scholar]
- 56.Chavali GB, Ekblad CM, Basu BP, Brissett NC, Veprintsev D, Hughes-Davies L, et al. Crystal structure of the ENT domain of human EMSY. J Mol Biol. 2005;350:964–973. doi: 10.1016/j.jmb.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 57.Garapaty S, Xu CF, Trojer P, Mahajan MA, Neubert TA, Samuels HH. Identification and characterization of a novel nuclear protein complex involved in nuclear hormone receptor-mediated gene regulation. J Biol Chem. 2009;284:7542–7552. doi: 10.1074/jbc.M805872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raouf A, Brown L, Vrcelj N, To K, Kwok W, Huntsman D, et al. Genomic instability of human mammary epithelial cells overexpressing a truncated form of EMSY. J Natl Cancer Inst. 2005;97:1302–1306. doi: 10.1093/jnci/dji254. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez C, Hughes-Davies L, Valles H, Orsetti B, Cuny M, Ursule L, et al. Amplification of the BRCA2 pathway gene EMSY in sporadic breast cancer is related to negative outcome. Clin Cancer Res. 2004;10:5785–5791. doi: 10.1158/1078-0432.CCR-03-0410. [DOI] [PubMed] [Google Scholar]
- 60.Brown LA, Irving J, Parker R, Kim H, Press JZ, Longacre TA, et al. Amplification of EMSY, a novel oncogene on 11q13, in high grade ovarian surface epithelial carcinomas. Gynecol Oncol. 2006;100:264–270. doi: 10.1016/j.ygyno.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 61.Brown LA, Johnson K, Leung S, Bismar TA, Benitez J, Foulkes WD, et al. Co-amplification of CCND1 and EMSY is associated with an adverse outcome in ER-positive tamoxifen-treated breast cancers. Breast Cancer Res Treat. 2010;121:347–354. doi: 10.1007/s10549-009-0479-x. [DOI] [PubMed] [Google Scholar]
- 62.Kirkegaard T, Nielsen KV, Jensen LB, Campbell FM, Muller S, Tovey SM, et al. Genetic alterations of CCND1 and EMSY in breast cancers. Histopathology. 2008;52:698–705. doi: 10.1111/j.1365-2559.2008.03007.x. [DOI] [PubMed] [Google Scholar]
- 63.van Hattem WA, Carvalho R, Li A, Offerhaus GJ, Goggins M. Amplification of EMSY gene in a subset of sporadic pancreatic adenocarcinomas. Int J Clin Exp Pathol. 2008;1:343–351. [PMC free article] [PubMed] [Google Scholar]
- 64.Konstantinopoulos PA, Spentzos D, Karlan BY, Taniguchi T, Fountzilas E, Francoeur N, et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol. 2010;28:3555–3561. doi: 10.1200/JCO.2009.27.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bast RC, Jr, Mills GB. Personalizing therapy for ovarian cancer: BRCAness and beyond. J Clin Oncol. 2010;28:3545–3548. doi: 10.1200/JCO.2010.28.5791. [DOI] [PubMed] [Google Scholar]
- 66.Lips EH, Mulder L, Hannemann J, Laddach N, Vrancken Peeters MT, van de Vijver MJ, et al. Indicators of homologous recombination deficiency in breast cancer and association with response to neoadjuvant chemotherapy. Ann Oncol. 2011;22:870–876. doi: 10.1093/annonc/mdq468. [DOI] [PubMed] [Google Scholar]
- 67.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 68.Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 69.Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, et al. PTEN mutation, EGFR amplification and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246–1256. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- 70.Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011 doi: 10.1038/nrclinonc.2010.216. In press. [DOI] [PubMed] [Google Scholar]
- 71.Nassif NT, Lobo GP, Wu X, Henderson CJ, Morrison CD, Eng C, et al. PTEN mutations are common in sporadic microsatellite stable colorectal cancer. Oncogene. 2004;23:617–628. doi: 10.1038/sj.onc.1207059. [DOI] [PubMed] [Google Scholar]
- 72.Depowski PL, Rosenthal SI, Ross JS. Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol. 2001;14:672–676. doi: 10.1038/modpathol.3880371. [DOI] [PubMed] [Google Scholar]
- 73.McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59:4291–4296. [PubMed] [Google Scholar]
- 74.Gupta A, Yang Q, Pandita RK, Hunt CR, Xiang T, Misri S, et al. Cell cycle checkpoint defects contribute to genomic instability in PTEN deficient cells independent of DNA DSB repair. Cell Cycle. 2009;8:2198–2210. doi: 10.4161/cc.8.14.8947. [DOI] [PubMed] [Google Scholar]
- 75.Meyn RE. Linking PTEN with genomic instability and DNA repair. Cell Cycle. 2009;8:2322–2323. [PubMed] [Google Scholar]
- 76.McEllin B, Camacho CV, Mukherjee B, Hahm B, Tomimatsu N, Bachoo RM, et al. PTEN loss compromises homologous recombination repair in astrocytes: Implications for glioblastoma therapy with temozolomide or poly(ADP-ribose) polymerase inhibitors. Cancer Res. 2010;70:5457–5464. doi: 10.1158/0008-5472.CAN-09-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 78.Forster MD, Dedes KJ, Sandhu S, Frentzas S, Kristeleit R, Ashworth A, et al. Response to olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat Rev Clin Oncol. 2011 doi: 10.1038/nrclinonc.2011.42. In press. [DOI] [PubMed] [Google Scholar]
- 79.Mackay HJ, Gallinger S, Tsao MS, McLachlin CM, Tu D, Keiser K, et al. Prognostic value of microsatellite instability (MSI) and PTEN expression in women with endometrial cancer: results from studies of the NCIC Clinical Trials Group (NCIC CTG) Eur J Cancer. 2010;46:1365–1373. doi: 10.1016/j.ejca.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 80.Cazales M, Schmitt E, Montembault E, Dozier C, Prigent C, Ducommun B. CDC25B phosphorylation by Aurora-A occurs at the G2/M transition and is inhibited by DNA damage. Cell Cycle. 2005;4:1233–1238. doi: 10.4161/cc.4.9.1964. [DOI] [PubMed] [Google Scholar]
- 81.Krystyniak A, Garcia-Echeverria C, Prigent C, Ferrari S. Inhibition of Aurora A in response to DNA damage. Oncogene. 2006;25:338–348. doi: 10.1038/sj.onc.1209056. [DOI] [PubMed] [Google Scholar]
- 82.Sourisseau T, Maniotis D, McCarthy A, Tang C, Lord CJ, Ashworth A, et al. Aurora-A expressing tumour cells are deficient for homology-directed DNA double strand-break repair and sensitive to PARP inhibition. EMBO Mol Med. 2010;2:130–142. doi: 10.1002/emmm.201000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lassus H, Staff S, Leminen A, Isola J, Butzow R. Aurora-A overexpression and aneuploidy predict poor outcome in serous ovarian carcinoma. Gynecol Oncol. 2011;120:11–17. doi: 10.1016/j.ygyno.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 84.Yang G, Chang B, Yang F, Guo X, Cai KQ, Xiao XS, et al. Aurora kinase A promotes ovarian tumorigenesis through dysregulation of the cell cycle and suppression of BRCA2. Clin Cancer Res. 2010;16:3171–3181. doi: 10.1158/1078-0432.CCR-09-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tao Y, Leteur C, Calderaro J, Girdler F, Zhang P, Frascogna V, et al. The aurora B kinase inhibitor AZD1152 sensitizes cancer cells to fractionated irradiation and induces mitotic catastrophe. Cell Cycle. 2009;8:3172–3181. doi: 10.4161/cc.8.19.9729. [DOI] [PubMed] [Google Scholar]
- 86.Wang X, Dong L, Xie J, Tong T, Zhan Q. Stable knockdown of Aurora-A by vector-based RNA interference in human esophageal squamous cell carcinoma cell line inhibits tumor cell proliferation, invasion and enhances apoptosis. Cancer Biol Ther. 2009;8:1852–1859. doi: 10.4161/cbt.8.19.9550. [DOI] [PubMed] [Google Scholar]
- 87.Ernestos B, Nikolaos P, Koulis G, Eleni R, Konstantinos B, Alexandra G, et al. Increased chromosomal radiosensitivity in women carrying BRCA1/BRCA2 mutations assessed with the G2 assay. Int J Radiat Oncol Biol Phys. 2010;76:1199–1205. doi: 10.1016/j.ijrobp.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 88.Fedier A, Steiner RA, Schwarz VA, Lenherr L, Haller U, Fink D. The effect of loss of Brca1 on the sensitivity to anticancer agents in p53-deficient cells. Int J Oncol. 2003;22:1169–1173. [PubMed] [Google Scholar]
- 89.Lord CJ, McDonald S, Swift S, Turner NC, Ashworth A. A high-throughput RNA interference screen for DNA repair determinants of PARP inhibitor sensitivity. DNA Repair (Amst) 2008;7:2010–2019. doi: 10.1016/j.dnarep.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 90.Wiltshire TD, Lovejoy CA, Wang T, Xia F, O'Connor MJ, Cortez D. Sensitivity to poly(ADPribose) polymerase (PARP) inhibition identifies ubiquitin-specific peptidase 11 (USP11) as a regulator of DNA double-strand break repair. J Biol Chem. 2010;285:14565–14571. doi: 10.1074/jbc.M110.104745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vollebergh MA, Lips EH, Nederlof PM, Wessels LF, Schmidt MK, van Beers EH, et al. An aCGH classifier derived from BRCA1-mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann Oncol. 2010 doi: 10.1093/annonc/mdq624. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Joosse SA, van Beers EH, Tielen IH, Horlings H, Peterse JL, Hoogerbrugge N, et al. Prediction of BRCA1-association in hereditary non-BRCA1/2 breast carcinomas with array-CGH. Breast Cancer Res Treat. 2009;116:479–489. doi: 10.1007/s10549-008-0117-z. [DOI] [PubMed] [Google Scholar]
- 93.Asakawa H, Koizumi H, Koike A, Takahashi M, Wu W, Iwase H, et al. Prediction of breast cancer sensitivity to neoadjuvant chemotherapy based on status of DNA damage repair proteins. Breast Cancer Res. 2010;12:17. doi: 10.1186/bcr2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Graeser M, McCarthy A, Lord CJ, Savage K, Hills M, Salter J, et al. A marker of homologous recombination predicts pathologic complete response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2010;16:6159–6168. doi: 10.1158/1078-0432.CCR-10-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mukhopadhyay A, Elattar A, Cerbinskaite A, Wilkinson SJ, Drew Y, Kyle S, et al. Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADPribose) polymerase inhibitors. Clin Cancer Res. 2010;16:2344–2351. doi: 10.1158/1078-0432.CCR-09-2758. [DOI] [PubMed] [Google Scholar]
- 96.Willers H, Taghian AG, Luo CM, Treszezamsky A, Sgroi DC, Powell SN. Utility of DNA repair protein foci for the detection of putative BRCA1 pathway defects in breast cancer biopsies. Mol Cancer Res. 2009;7:1304–1309. doi: 10.1158/1541-7786.MCR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]