Abstract
The E2F1 transcription factor is post-translationally modified and stabilized in response to various forms of DNA damage to regulate the expression of cell cycle and pro-apoptotic genes. E2F1 also forms foci at DNA double-strand breaks (DSBs) but the function of E2F1 at sites of damage is unknown. Here we demonstrate that the absence of E2F1 leads to spontaneous DNA breaks and impaired recovery following exposure to ionizing radiation. E2F1 deficiency results in defective NBS1 phosphorylation and foci formation in response to DSBs but does not affect NBS1 expression levels. Moreover, an increased association between NBS1 and E2F1 is observed in response to DNA damage, suggesting that E2F1 may promote NBS1 foci formation through a direct or indirect interaction at sites of DNA breaks. E2F1 deficiency also impairs RPA and Rad51 foci formation indicating that E2F1 is important for DNA end resection and the formation of single-stranded DNA at DSBs. These findings establish new roles for E2F1 in the DNA damage response, which may directly contribute to DNA repair and genome maintenance.
Key words: E2F, NBS1, RPA, RAD51, DNA damage response
Introduction
Cellular DNA is constantly being damage by both exogenous and endogenous agents.1 Failure to repair DNA damage in a timely and efficient manner can lead to mutations, chromosomal instability and life threatening diseases such as cancer.2,3 Cells initiate an elaborate signaling network in response to DNA damage to activate cell cycle checkpoints and coordinate DNA repair mechanisms. 4 At the apex of the DNA damage response are several members of the PI3 kinase family, including ataxia telangiectasia mutated (ATM), which is activated in response to double-strand breaks (DSB) and ATM and Rad3 related (ATR), which is activated in response to stalled replication and transcription forks. Protein targets of these kinases include NBS1, SMC1, p53, Chk1 and 2, BRCA1 and 2, and many other proteins involved in cell cycle checkpoints, DNA repair, apoptosis and cellular senescence.4 Inherited genetic defects in the DNA damage response lead to chromosomal instability syndromes and a predisposition to cancer.2 For example, mutations in the ATM gene cause ataxia telagiectasia (AT), which is marked by immunodeficiency, progressive cerebellar ataxia and a predisposition to leukemia and lymphoma. Similarly, hypomorphic mutations in NBS1 cause Nijmegen breakage syndrome (NBS), which shares many characteristics with AT. Defects in other downstream targets of ATM, including p53, BRCA1 and BRCA2, are also strongly associated with cancer development.2
The E2F1 transcription factor is another component of the DNA damage response and a direct target of the ATM and ATR kinases.5 The E2F family regulates the expression of genes important for cell proliferation, apoptosis and DNA repair.6 Several members of the E2F family are now known to be responsive to DNA damage, the best characterized being E2F1.7–11 Stabilization of E2F1 in response to DNA damage involves phosphorylation of E2F1 on serine 31, a site not conserved in other E2F family members.5 Depending on the type of damage, this phosphorylation event is mediated by either the ATM or ATR kinases. Serine 31 phosphorylation induces an interaction between E2F1 and 14-3-3τ, which inhibits E2F1 ubiquitination and degradation.12 Other phosphorylation and acetylation events may also contribute to E2F1 stabilization and regulation in response DNA damage.13,14 DNA damage also enhances the interaction between E2F1 and the RB tumor suppressor protein and this contributes to the transcriptional repression of cyclin A and perhaps other cell cycle-related genes.15,16 Surprisingly, this E2F1-RB complex may also participate in the transcriptional activation of pro-apoptotic genes like p73 and caspase 7.15
In addition to RB, the TopBP1 protein also regulates E2F1 transcriptional activity in response to DNA damage.17,18 TopBP1 is known to be involved in the DNA damage response through its interaction with Rad9 and as an activator of ATR.19,20 TopBP1 contains eight BRCA1 C-terminal (BRCT) domains and phosphorylation of E2F1 on serine 31 creates a binding site for the sixth BRCT domain of TopBP1.17 This interaction with TopBP1 represses E2F1 transcriptional activity independent of RB and is critical for suppressing apoptosis and promoting cell survival following DNA damage. In addition, TopBP1 binding recruits E2F1 to sites of DSBs to form foci that overlap with foci formed by other DNA damage response proteins like BRCA1.17 The function of E2F1 at sites of DNA breaks is currently unknown.
In some instances, E2F1 stabilization contributes to DNA damage-induced apoptosis, which involves the transcriptional activation of p73.5,12,15,21 On the other hand, E2F1 can also have a pro-survival function under some DNA-damaging conditions.22,23 In the case of UV irradiation, enhanced survival mediated by E2F1 correlates with a stimulation of nucleotide excision repair.22 E2F1 appears to enhance nucleotide excision repair by increasing the accessibility of repair enzymes to sites of UV-induced DNA damage.24 Here we demonstrate that E2F1 is important for the recruitment and/or retention of several DNA repair factors to sites of DSBs. Evidence suggests that this function is independent of E2F1's role in regulating transcription and may explain the accumulation of DNA damage observed in cells lacking E2F1.
Results
E2F1 inactivation leads to genomic instability and impaired recovery from IR.
Given that E2F1 protein stability, function and subcellular localization are regulated in response to DNA damage, we asked whether the absence of E2F1 would affect genome stability. Phosphorylation of the histone variant H2AX (referred to as γH2AX) occurs in chromatin flanking a DSB and is commonly used as a marker of DNA damage. Immunofluorescence (IF) staining for γH2AX foci was used to estimate the amount of DNA damage in wild type and E2f1−/− cells. On average, 60% of wild type primary adult mouse fibroblasts (MAFs) lacked visible γH2AX foci, while less than 35% of E2f1−/− MAFs lacked γH2AX foci (Fig. 1A). On the other hand, approximately 25% of primary E2f1−/− MAFs had over six γH2AX foci per cell while only 5% of wild type MAFs had greater than six foci. Western blot analysis confirmed increased levels of γH2AX in untreated E2f1−/− MAFs compared to untreated wild type MAFs (Fig. 1B and lanes 1 and 7).
Figure 1.

The absence of E2F1 causes spontaneous DNA breaks and impairs recovery from IR exposure. (A) Untreated primary wild type and E2f1−/− mouse adult fibroblasts (MAFs) were immunofluorescencently (IF) stained for γH2AX. Captured images were analyzed using the FociCounter software program.46 The percentage of cells showing different numbers of foci was calculated after scoring at least 85 cells for each genotype in three independent experiments. (B) Primary wild type (lanes 1–6) and E2f1−/− (lanes 7–12) MAFs were untreated (Un, lanes 1 and 7) or exposed to 5 Gy of IR and harvested immediately (0 h, lanes 2 and 8) or at various times post-IR as indicated. Western blot analysis was performed using antibody to γH2AX and β-tubulin. (C) Primary wild type and E2f1−/− MAFs were untreated (Un) or exposed to 10 Gy of IR and incubated for one or 24 h. Cells were subjected to the comet assay and the average Olive moment was determined for 50 cells per genotype. *Indicates statistically significant difference between genotypes treated the same as determind by the paired t test, p < 0.05.
E2F1 also appeared to be important for the recovery of cells from DNA damage caused by ionizing radiation (IR). Western blot analysis of γH2AX was used to indirectly monitor the generation of DNA damage and recovery over time. Following IR treatment, γH2AX was induced in both wild type and E2f1−/− MAFs although the levels in cells lacking E2F1 were higher (Fig. 1B). Moreover, while wild type cells lost γH2AX by 6 h post-irradiation, γH2AX persisted in E2f1−/− MAFs for up to 12 h. Similar results were obtained using the single cell gel electrophoresis (comet) assay to measure DNA damage. Consistent with the findings above, primary MAFs lacking E2F1 had significantly higher levels of DNA breaks before IR treatment compared to wild type MAFs (Fig. 1C). The absence of E2F1 also led to abnormally high levels of DNA damage at one h and the persistence of DNA breaks at 24 h post-IR treatment (Fig. 1C). Taken together, these results indicate that the absence of E2F1 leads to increased levels of spontaneous DNA damage and impaired repair of induced DSBs.
NBS1 foci formation is impaired in the absence of E2F1.
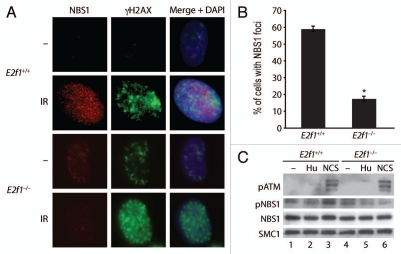
NBS1 is a component of the MRN (Mre11-Rad50-NBS1) complex involved in DNA end processing and is one of the first proteins to be recruited to sites of DSBs.25–29 To determine if E2F1 status would affect NBS1 localization to sites of DSBs, foci formation was examined by indirect IF in wild type and E2f1−/− MAFs. NBS1 foci formation in response to IR was much reduced in cells lacking E2F1 compared to wild type cells (Fig. 2A and B). In contrast, γH2AX foci formation was enhanced in the absence of E2F1.
Figure 2.
Lack of E2F1 impairs NBS1 foci formation and phosphorylation in response to DNA damage. (A) Primary wild type and E2f1−/− MAFs were untreated or treated with 2 Gy of IR. One h post-irradiation, IF staining was performed using antibodies to NBS1 and γH2AX and counterstained with DAPI. (B) Wild type and E2f1−/− MAFs were irradiated and stained as in (A) and the percentage of cells with NBS1 foci was calculated after counting 100 cells from three independent experiments. *Indicates statistically significant difference between genotypes as determined by the paired t test, p < 0.05. (C) Primary wild type and E2f1−/− MAFs were untreated or treated with HU (1.5 ng/ml) or NCS (50 ng/ml) for one h. Western blot analysis was performed on whole cell extracts using antibodies to phospo-ATM (serine 1981), total NBS1, phospho-NBS1 (serine 343) and SMC1 as a loading control.
Western blot analysis was performed to determine if impaired NBS1 foci formation in the absence of E2F1 was related to NBS1 expression levels. Total NBS1 protein levels were similar in wild type and E2f1−/− MAFs and did not change in response to DNA damage (Fig. 2C). As expected, treatment with the radiomimetic drug neocarzinostatin (NCS), which induces DSBs, induced the autophosphorylation and activation of ATM in both wild type and E2f1−/− MAFs. On the other hand, treatment with hydroxyurea (HU), which induces replication stress and activates ATR, did not induce ATM autophosphorylation. Consistent with this, the phosphorylation of NBS1 on a site targeted by ATM also increased in response to NCS but not HU in wild type MAFs. However, the induction of NBS1 phosphorylation in response to NCS was impaired in MAFs lacking E2F1.
To confirm these findings, NBS1 foci formation and phosphorylation were also examined in normal human fibroblasts (NHFs) depleted for E2F1 using short interfering RNA (siRNA). Treatment with IR or NCS induced numerous bright foci of both NBS1 and γH2AX in NHFs transfected with control siRNA (Fig. 3A and B). As in the E2f1−/− MAFs, E2F1 deficiency in NHFs impaired the formation of NBS1 foci, which were either nonexistent or reduced in brightness and numbers in cells depleted for E2F1. In sharp contrast, knock down of E2F1 appeared to enhance γH2AX foci formation in response to DNA damage (Fig. 3C). Western blot analysis demonstrated that E2F1 depletion did not affect total NBS1 protein levels either before or after IR treatment (Fig. 3C). However, similar to what was observed in E2f1−/− MEFs, E2F1 knock down impaired the phosphorylation of NBS1 following IR treatment.
Figure 3.
E2F1 deficiency impairs NBS1 foci formation and phosphorylation in normal human cells. (A) NHFs were transfected with control siRNA or E2F1 siRNA 48 h before treatment. Cells were untreated or treated with IR (10 Gy) or NCS (50 ng/ml) for 15 min and then incubated for one h. IF staining was then performed on cells using antibodies to NBS1 and γH2AX and counterstained with DAPI. (B) NHFs were treated and stained as in (A) and the percentage of cells containing NBS1 foci was calculated after counting 100 cells from three independent experiments. *Indicicates statistically significant difference between genotypes treated the same as determined by the paired t test, p < 0.05. (C) NHFs were transfected with control siRNA or E2F1 siRNA as above. 48 h after transfection, cells were untreated or exposed to 10 Gy of IR and incubated for one h. Western blot analysis was performed on whole cell extracts using antibodies to E2F1, total NBS1, phospho-NBS1 (serine 343) and SMC1.
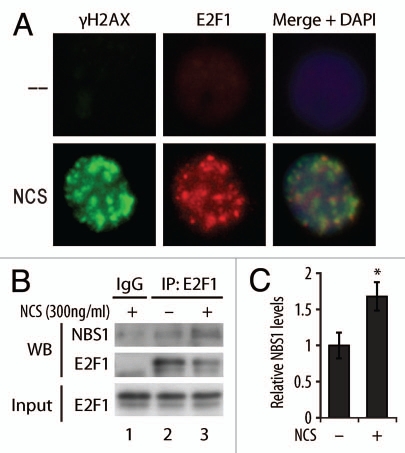
It was previously demonstrated that like NBS1, E2F1 accumulates at foci that represent sites of DNA DSBs.17 We confirmed this result and demonstrate that E2F1 and γH2AX foci partially overlap (Fig. 4A). An earlier study also suggested that E2F1 and NBS1 physically interact and that this occurs near origins of DNA replication.30 To determine if the association between NBS1 and E2F1 is regulated in response to DNA damage, a co-immunoprecipitation (co-IP) experiment was performed. Following DNA damage, an increased association between E2F1 and NBS1 was observed (Fig. 4B and C). Taken together, these findings raise the possibility that E2F1 promotes NBS1 recruitment and/or retention at sites of DSBs through a DNA damage-inducible interaction with NBS1. This in turn may be important for activated ATM to phosphorylate NBS1 at sites of DSBs.
Figure 4.
E2F1 localizes to DSB foci and associates with NBS1. (A) HCT116 cells were treated with 2 Gy of IR and IF staining was performed using antibodies to E2F1 and γH2AX and counterstained with DAPI. (B) NHFs were untreated (lane 2) or treated with NCS (300 ng/ml) for 3 h (lanes 1 and 3) before harvesting. Cell lysate was subjected to IP using control IgG (lane 1) or antibody to E2F1 (lanes 2 and 3). Western blot analysis was performed to detect NBS1 and E2F1 in the precipitate (WB) or the input cell lysate as a control (Input). (C) Relative levels of NBS1 protein immunoprecipitated with E2F1 were measured using ImageJ software from three independent experiments. *indicates statistically significant difference between untreated and NCS treated samples (student's t test, p < 0.05) and bars represent standard deviation.
E2F1 promotes the formation of RPA and Rad51 foci.
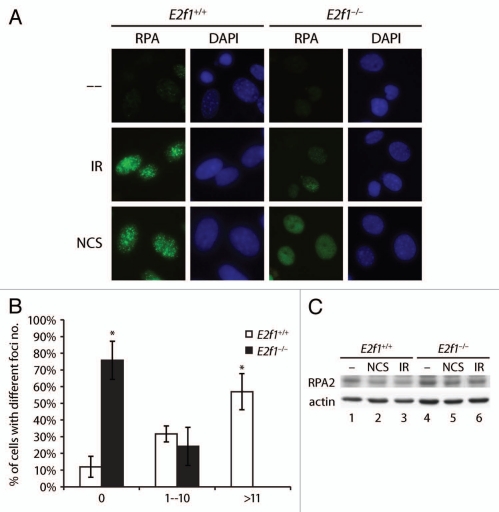
NBS1, as part of the MRN complex, is involved in DNA end processing to create single-stranded DNA that is required for both homologous recombination and microhomology-mediated end joining.25–29 Single-stranded DNA generated at sites of DSBs is stabilized by binding to the three subunit factor RPA (replication protein A). To determine if E2F1 is important for DNA end processing and the generation of single-stranded DNA, RPA foci formation was examined in MAFs. Robust RPA foci formation was observed in wild type MAFs following treatment with IR or NCS (Fig. 5A and B). In contrast, MAFs lacking E2F1 were significantly impaired for RPA foci formation in response to these DNA damaging agents. While the majority of wild type cells treated with 5 Gγ of IR displayed greater than 10 RPA foci per cell, no E2f1−/− cells displayed over 10 foci (Fig. 5B). As with NBS1, impairment of RPA foci formation in the absence of E2F1 was not related to RPA expression levels (Fig. 5C).
Figure 5.
Lack of E2F1 impairs RPA foci formation. (A) Primary wild type and E2f1−/− MAFs were untreated or exposed to IR (5 Gy) or NCS (50 ng/ml) for one h. IF staining was performed for RPA2 and cells were counterstained with DAPI. (B) Primary wild type and E2f1−/− MAFs were untreated or exposed to IR (5 Gy) and IF stained using antibody to RPA2 one h after exposure. Images of at least 55 cells per group were analyzed using the FociCounter software program46 and the average percent of foci number per cell from three independent experiments is presented. *Indicates statistically significant difference between genotypes treated the same as determined by the paired t test, p < 0.05. (C) Western blot analysis was performed on primary wild type (lanes 1–3) and E2f1−/− (lanes 4–6) MAFs untreated (lanes 1 and 4), exposed to NCS (50 ng/ml) for one h (lanes 2 and 5) or exposed to IR (5 Gy) and incubated for one h (lanes 3 and 6) using antibodies to RPA2 and actin.
The Rad51 protein plays an important role in homologous recombination (HR) repair of DSBs by functioning as the main recombinase. Rad51 is loaded on single-stranded DNA flanking DSBs by BRCA2 and displaces RPA.31,32 In cells lacking E2F1, the number of Rad51 foci per cell and the brightness of foci were decreased compared to wild type cells (Fig. 6A). In contrast, 53BP1 foci formation was unaffected by the absence of E2F1. Unlike NBS1 and RPA, Rad51 protein levels increased following treatment with IR or NCS and this was inhibited in cells lacking E2F1 (Fig. 6B). However, Rad51 mRNA levels did not change in response DNA damage and was similar between wild type and E2f1−/− MAFs (Fig. 6C). This suggests that the increase in Rad51 levels in response to damage, while partially dependent on E2F1, is not regulated at the level of transcription.
Figure 6.
Lack of E2F1 impairs Rad51 foci formation. (A) NHFs were transfected with control siRNA (siCon) or E2F1 siRNA for 48 h and then were untreated or exposed to IR (5 Gy) or NCS (50 ng/ml) for 15 min. One h following treatment, IF staining was performed for Rad51 and 53BP1 and cells were counter stained with DAPI. (B) Primary wild type and E2f1−/− MAFs were untreated (lanes 1 and 4), treated with the radiomemitic drug NCS (50 ng/ml, lanes 2 and 5) or IR (10 Gy, lanes 3 and 6). Cells were harvested 1 h post treatments and subjected to western blot analysis using whole cell extracts and antibody to Rad51 and β-tubulin. (C) Primary wild type and E2f1−/− MAFs were untreated or exposed to 5 Gy of IR and harvested one h post-treatment. Real-time PCR was performed to examine relative Rad51 mRNA levels.
Discussion
E2F1 can function as both an oncogene and tumor suppressor gene depending on the context.33 E2F1's ability to stimulate cell proliferation likely underlies its oncogenic activity while tumor suppression by E2F1 is widely assumed to involve its proapoptotic activity. However, in at least some cases tumor suppression by E2F1 appears to be unrelated to the regulation of apoptosis.34,35 Here we demonstrate that E2F1 plays a role in maintaining genomic stability and the repair of DNA DSBs. In the absence of E2F1, primary cells display increased γH2AX foci, spontaneous DNA breaks and impaired recovery from IR treatment. Taken together with our earlier finding that E2F1 deficiency impairs nucleotide excision repair,22,24 these findings indicate that E2F1 may function as a tumor suppressor by contributing to genome maintenance.
In response to DNA DSBs, E2F1 was shown to both transcriptionally activate some pro-apoptotic genes and transcriptionally repress some cell cycle progression genes.15,18,21 Thus, one mechanism by which E2F1 could contribute to genome integrity is through the transcriptional regulation of DNA repair genes. Indeed, some repair genes, such as BRCA1 and Rad51, have been shown to be responsive to E2F transcriptional activity.36–40 However, these previous studies examined the regulation of Rad51 and BRCA1 by E2F in the context of cell cycle progression or hypoxia. At present there is no evidence that E2F1 regulates the expression of DNA repair genes in response to DNA damage. Indeed, we find that E2F1 deficiency has no effect on the expression of NBS1 or RPA2, at least at early time points. Rad51 protein levels increase in response to DNA damage and this is dependent on E2F1. However, this is likely an indirect effect on the increased stability of the Rad51 protein when incorporated into nucleoprotein filaments on single-stranded DNA since E2F1 depletion did not affect Rad51 mRNA levels.
In addition to regulating the expression of DNA repair genes, E2F1 may also promote genome stability through nontranscriptional mechanisms at sites of DNA damage. It was previously demonstrated that E2F1 accumulates at sites of DSBs and forms foci that overlap with foci formed by BRCA1.17 This involves E2F1 binding to the TopBP1 protein, which is stimulated by the ATM-mediated phosphorylation of E2F1 on serine 31.17 Moreover, we have recently shown that E2F1 also localizes to sites of UV-induced DNA damage, which involves the ATR-mediated phosphorylation of E2F1 on serine 31.24 In the case of UV damage, E2F1 stimulates DNA repair through a nontranscriptional mechanism involving increased recruitment of nucleotide excision repair factors, such as XPA and XPC, to sites of damage.24 We suggest that E2F1 plays a similar role in DSB repair by promoting the recruitment and/or retention of repair factors at sites of DNA breaks. E2F1 may be particularly important for repair of DSBs in heterochromatic regions given the role of ATM in this process and the finding that ATM is important for the repair of breaks only in heterochromatin.41
In support of this model we demonstrate that E2F1 deficiency impairs NBS1 foci formation but does not affect NBS1 protein levels. The lack of E2F1 also impairs NBS1 phosphorylation by ATM, which may be related to the defective recruitment of NBS1 to sites of damage. We also find that RPA and Rad51 foci formation is defective in cells lacking E2F1. RPA and Rad51 bind and coat single-stranded, which occurs at sites of DSBs during the process of repair. The MRN complex is critical for the processing of DSB ends to create single-stranded DNA.25–29 Thus, defective RPA and Rad51 foci formation in the absence of E2F1 may reflect impaired MRN recruitment and function at DSBs.
Several potential mechanisms may be involved in the promotion of NBS1 foci formation by E2F1. NBS1, as part of the MRN complex, is recruited to sites of DSBs through several mechanisms, including direct binding to the ends of broken DNA.42 However, the majority of NBS1 is thought to be recruited through a phospho-specific interaction with the MDC1 (mediator of DNA-damage checkpoint 1) protein, which in turn is recruited to chromatin flanking DSBs through a phospho-specific interaction with γH2AX.43 We confirmed a previous study demonstrating a physical association between E2F1 and NBS1,30 and further show that this interaction is enhanced following exposure to a DNA-damaging agent. While we cannot rule out the possibility that E2F1 regulates the expression of a gene that in turn is important for NBS1 foci formation, the fact that E2F1 associates with NBS1 raises the possibility that E2F1, through its interaction with TopBP1, cooperates with MDC1 to recruit and/or retain NBS1 at sites of DSBs.
It is also possible that E2F1 indirectly stimulates NBS1 foci formation by modifying chromatin structure to facilitate access of NBS1 to sequences flanking a DSB. This would be analogous to E2F1's function in transcriptional regulation, in which E2F1 recruits histone modifying enzymes to gene promotes to alter chromatin structure and facilitate access to the basal transcription machinery. Indeed, we have recently found that E2F1 recruits the GCN5 histone acetyl-transferase to sites of UV-induced DNA damage and promotes the acetylation of H3K9 to stimulate nucleotide excision repair.44
Whatever the molecular mechanism, this role for E2F1 in promoting the recruitment of DNA repair factors to sites of damage is likely important for maintaining genomic stability in response to both endogenous and exogenous sources of DNA breaks. In addition to its function in DNA repair, NBS1 has also been shown to play roles in DNA damage checkpoint signaling and apoptosis in response to agents that cause DSBs. Future experiments will examine the role of E2F1 in these NBS1-dependent processes and determine under which conditions E2F1 may enhance cell survival by contributing to DNA repair or cell death by promoting DNA-damage induced apoptosis.
Materials and Methods
Cell culture and treatments.
Primary mouse adult fibroblasts (MAFs) were isolated from the peritoneal fascia of mice at least 5 weeks old mice as previously described in reference 45. MAFs and human normal fibroblasts obtained from Coriell Institute were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The HCT116 colon carcinoma cell line was obtained from ATCC and maintained in McCoy's 5A Medium (Gibco) with 10% FBS. Cells were exposed to the indicated Gy of IR using a RS-2000 Biological Irradiator (Rad Source). Alternatively, cells were exposed to the radiomimetic drug NCS (50 ng/ml) for the indicated times to induce DSBs.
SiRNA and antibodies.
SiRNAs for E2F1 and control were obtained from Santa Cruz. Transfections were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA USA). Sources for the antibodies/antisera used are NBS1, phospho-NBS1 ser343 and SMC1 (Cell signaling), E2F1, actin, β-tubulin and Rad51 (Santa Cruz), RPA2/p34 (NeoMarker), phospho-ATM ser1981 (Rockland) and γH2AX (Millipore).
Comet assay.
The single cell gel electrophoresis (comet) assay was performed using the CometAssay kit from Trevigen. Briefly, cells were harvested after treatments, embedded in low melting agarose on a glass slide and incubated overnight at 4°C in lysis buffer. After washing, samples were electrophoresed at 19 V for 5–20 minutes in TBE and stained with SYBR Green. Nuclei were visualized and images captured using a fluorescent microscope. Tail length and Olive moment of 50 nuclei per slide were calculated using COMETSCORE software (Tritek).
Immunofluorescence.
For immunofluorescent staining, fixed cells were incubated with 3% BSA, washed and then incubated with appropriate primary antibodies. Slides were washed, incubated with fluorescently tagged secondary antibody and then stained with DAPI before visualization. Appropriate fluorescent images were captured using a Nikon eclipse 80i microscope equipped with an X-cite 120 fluorescence illumination system and Metamorph image analysis software. The FociCounter software program was used to analyze images.46
Coimmunoprecipitation assay.
Coimmunoprecipitation was performed as described previously in reference 47. Briefly, whole cell lyaste (600 µg) was incubated with protein-A-agarose beads coupled with anti-E2F1 antibodies (Novagen) for 2 h in 4°C. Immunoblotting followed standard procedure with anti-E2F1 and anti-NBS1 antibodies.
Acknowledgements
We thank Jennifer Smith, Pamela Blau and Jennifer McKinney for technical assistance, Becky Brooks for preparation of the manuscript, Chris Brown and Joi Holcomb for graphics and Lezlee Coghlan, Dale Weiss and coworkers for animal care. This research was performed in partial fulfillment of the requirements for the Ph.D. degree from The University of Texas Graduate School of Biomedical Sciences at Houston. This research is supported in part by grants from the National Institutes of Health (CA079648 to D.G.J., through MD Anderson's Cancer Center Support Grant CA016672 and P30ES007784).
References
- 1.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khanna KK, Jackson SP. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 4.Kastan MB, Bartek J. Cell cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 5.Lin WC, Lin FT, Nevins JR. Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev. 2001;15:1833–1844. [PMC free article] [PubMed] [Google Scholar]
- 6.DeGregori J, Johnson DG. Distinct and Overlapping Roles for E2F Family Members in Transcription, Proliferation and Apoptosis. Curr Mol Med. 2006;6:739–748. doi: 10.2174/1566524010606070739. [DOI] [PubMed] [Google Scholar]
- 7.Blattner C, Sparks A, Lane D. Transcription factor E2F-1 is upregulated in response to DNA damage in a manner analogous to that of p53. Molecular Cellular Biololgy. 1999;19:3704–3713. doi: 10.1128/mcb.19.5.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofferer M, Wirbelauer C, Humar B, Krek W. Increased levels of E2F-1-dependent DNA binding activity after UV- or gamma-irradiation. Nucleic Acids Res. 1999;27:491–495. doi: 10.1093/nar/27.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Freeman SN, Cress WD. E2F4 deficiency promotes drug-induced apoptosis. Cancer Biol Ther. 2004;3:1262–1269. doi: 10.4161/cbt.3.12.1239. [DOI] [PubMed] [Google Scholar]
- 10.Martinez LA, Goluszko E, Chen HZ, Leone G, Post S, Lozano G, et al. E2F3 is a mediator of DNA damage-induced apoptosis. Mol Cell Biol. 30:524–536. doi: 10.1128/MCB.00938-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zalmas LP, Zhao X, Graham AL, Fisher R, Reilly C, Coutts AS, et al. DNA-damage response control of E2F7 and E2F8. EMBO Rep. 2008;9:252–259. doi: 10.1038/sj.embor.7401158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Liu K, Lin FT, Lin WC. A role for 14-3-3 tau in E2F1 stabilization and DNA damage-induced apoptosis. J Biol Chem. 2004;279:54140–54152. doi: 10.1074/jbc.M410493200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ianari A, Gallo R, Palma M, Alesse E, Gulino A. Specific role for PCAF acetylytransferase activity in E2F1 stabilization in response to DNA damage. J Biol Chem. 2004;279:20830–20835. doi: 10.1074/jbc.M402403200. [DOI] [PubMed] [Google Scholar]
- 14.Stevens C, Smith L, La Thangue NB. Chk2 activates E2F-1 in response to DNA damage. Nat Cell Biol. 2003;5:401–409. doi: 10.1038/ncb974. [DOI] [PubMed] [Google Scholar]
- 15.Ianari A, Natale T, Calo E, Ferretti E, Alesse E, Screpanti I, et al. Proapoptotic function of the retinoblastoma tumor suppressor protein. Cancer Cell. 2009;15:184–194. doi: 10.1016/j.ccr.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue Y, Kitagawa M, Taya Y. Phosphorylation of pRB at Ser612 by Chk1/2 leads to a complex between pRB and E2F-1 after DNA damage. EMBO J. 2007;26:2083–2093. doi: 10.1038/sj.emboj.7601652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K, Lin FT, Ruppert JM, Lin WC. Regulation of E2F1 by BRCT domain-containing protein TopBP1. Mol Cell Biol. 2003;23:3287–3304. doi: 10.1128/MCB.23.9.3287-3304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu K, Luo Y, Lin FT, Lin WC. TopBP1 recruits Brg1/Brm to repress E2F1-induced apoptosis, a novel pRb-independent and E2F1-specific control for cell survival. Genes Dev. 2004;18:673–686. doi: 10.1101/gad.1180204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greer DA, Besley BD, Kennedy KB, Davey S. hRad9 rapidly binds DNA containing double-strand breaks and is required for damage-dependent topoisomerase II beta binding protein 1 focus formation. Cancer Res. 2003;63:4829–4835. [PubMed] [Google Scholar]
- 20.Kumagai A, Lee J, Yoo HY, Dunphy WG. TopBP1 activates the ATR-ATRIP complex. Cell. 2006;124:943–955. doi: 10.1016/j.cell.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 21.Pediconi N, Ianari A, Costanzo A, Belloni L, Gallo R, Cimino L, et al. Differential regulation of E2F1 apoptotic target genes in response to DNA damage. Nat Cell Biol. 2003;5:552–558. doi: 10.1038/ncb998. [DOI] [PubMed] [Google Scholar]
- 22.Berton TR, Mitchell DL, Guo R, Johnson DG. Regulation of epidermal apoptosis and DNA repair by E2F1 in response to ultraviolet B radiation. Oncogene. 2005;24:2449–2460. doi: 10.1038/sj.onc.1208462. [DOI] [PubMed] [Google Scholar]
- 23.Wikonkal NM, Remenyik E, Knezevic D, Zhang W, Liu M, Zhao H, et al. Inactivating E2f1 reverts apoptosis resistance and cancer sensitivity in Trp53-deficient mice. Nat Cell Biol. 2003;5:655–660. doi: 10.1038/ncb1001. [DOI] [PubMed] [Google Scholar]
- 24.Guo R, Chen J, Zhu F, Biswas AK, Berton TR, Mitchell DL, et al. E2F1 localizes to sites of UV-induced DNA damage to enhance nucleotide excision repair. J Biol Chem. 2010;285:19308–19315. doi: 10.1074/jbc.M110.121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paull TT, Gellert M. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 1999;13:1276–1288. doi: 10.1101/gad.13.10.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paull TT, Gellert M. A mechanistic basis for Mre11-directed DNA joining at microhomologies. Proc Natl Acad Sci USA. 2000;97:6409–6414. doi: 10.1073/pnas.110144297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor EM, Cecillon SM, Bonis A, Chapman JR, Povirk LF, Lindsay HD. The Mre11/Rad50/Nbs1 complex functions in resection-based DNA end joining in Xenopus laevis. Nucleic Acids Res. 2010;38:441–454. doi: 10.1093/nar/gkp905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trujillo KM, Yuan SS, Lee EY, Sung P. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11 and p95. J Biol Chem. 1998;273:21447–21450. doi: 10.1074/jbc.273.34.21447. [DOI] [PubMed] [Google Scholar]
- 29.Zhuang J, Jiang G, Willers H, Xia F. Exonuclease function of human Mre11 promotes deletional nonhomologous end joining. J Biol Chem. 2009;284:30565–30573. doi: 10.1074/jbc.M109.059444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maser RS, Mirzoeva OK, Wells J, Olivares H, Williams BR, Zinkel RA, et al. Mre11 complex and DNA replication: Linkage to E2F and sites of DNA synthesis. Mol Cell Biol. 2001;21:6006–6016. doi: 10.1128/MCB.21.17.6006-6016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carreira A, Hilario J, Amitani I, Baskin RJ, Shivji MK, Venkitaraman AR, et al. The BRC repeats of BRCA2 modulate the DNA-binding selectivity of RAD51. Cell. 2009;136:1032–1043. doi: 10.1016/j.cell.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sung P, Krejci L, Van Komen S, Sehorn MG. Rad51 recombinase and recombination mediators. J Biol Chem. 2003;278:42729–42732. doi: 10.1074/jbc.R300027200. [DOI] [PubMed] [Google Scholar]
- 33.Johnson DG, Degregori J. Putting the Oncogenic and Tumor Suppressive Activities of E2F into Context. Curr Mol Med. 2006;6:731–738. doi: 10.2174/1566524010606070731. [DOI] [PubMed] [Google Scholar]
- 34.Rounbehler RJ, Rogers PM, Conti CJ, Johnson DG. Inactivation of E2f1 enhances tumorigenesis in a Myc transgenic model. Cancer Res. 2002;62:3276–3281. [PubMed] [Google Scholar]
- 35.Russell JL, Weaks RL, Berton TR, Johnson DG. E2F1 suppresses skin carcinogenesis via the ARF-p53 pathway. Oncogene. 2006;25:867–876. doi: 10.1038/sj.onc.1209120. [DOI] [PubMed] [Google Scholar]
- 36.Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ, et al. Hypoxia-induced downregulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–11604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- 37.Bindra RS, Glazer PM. Repression of RAD51 gene expression by E2F4/p130 complexes in hypoxia. Oncogene. 2007;26:2048–2057. doi: 10.1038/sj.onc.1210001. [DOI] [PubMed] [Google Scholar]
- 38.Bindra RS, Schaffer PJ, Meng A, Woo J, Maseide K, Roth ME, et al. Downregulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24:8504–8518. doi: 10.1128/MCB.24.19.8504-8518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwanaga R, Komori H, Ohtani K. Differential regulation of expression of the mammalian DNA repair genes by growth stimulation. Oncogene. 2004;23:8581–8590. doi: 10.1038/sj.onc.1207976. [DOI] [PubMed] [Google Scholar]
- 40.Wang A, Schneider-Broussard R, Kumar AP, MacLeod MC, Johnson DG. Regulation of BRCA1 expression by the Rb-E2F pathway. J Biol Chem. 2000;275:4532–4536. doi: 10.1074/jbc.275.6.4532. [DOI] [PubMed] [Google Scholar]
- 41.Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M, et al. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31:167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Lee JH, Ghirlando R, Bhaskara V, Hoffmeyer MR, Gu J, Paull TT. Regulation of Mre11/Rad50 by Nbs1: Effects on nucleotide-dependent DNA binding and association with ataxia-telangiectasia-like disorder mutant complexes. J Biol Chem. 2003;278:45171–45181. doi: 10.1074/jbc.M308705200. [DOI] [PubMed] [Google Scholar]
- 43.Chapman JR, Jackson SP. Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage. EMBO Rep. 2008;9:795–801. doi: 10.1038/embor.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo R, Chen J, Mitchell DL, Johnson DG. GCN5 and E2F1 stimulate nucleotide excision repair by promoting H3K9 acetylation at sites of damage. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powers JT, Hong S, Mayhew CN, Rogers PM, Knudsen ES, Johnson DG. E2F1 uses the ATM signaling pathway to induce p53 and Chk2 phosphorylation and apoptosis. Mol Cancer Res. 2004;2:203–214. [PubMed] [Google Scholar]
- 46.Jucha A, Wegierek-Ciuk A, Koza Z, Lisowska H, Wojcik A, Wojewodzka M, et al. FociCounter: A freely available PC programme for quantitative and qualitative analysis of gammaH2AX foci. Mutat Res. 2010;696:16–20. doi: 10.1016/j.mrgentox.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Zhu F, Xia X, Liu B, Shen J, Hu Y, Person M. IKKalpha shields 14-3-3sigma, a G(2)/M cell cycle checkpoint gene, from hypermethylation, preventing its silencing. Mol Cell. 2007;27:214–227. doi: 10.1016/j.molcel.2007.05.042. [DOI] [PubMed] [Google Scholar]