Abstract
Accurate chromosome segregation depends on the proper attachment of sister kinetochores to microtubules emanating from opposite spindle poles. Merotelic kinetochore orientation is an error in which a single kinetochore is attached to microtubules emanating from both spindle poles. Despite correction mechanisms, merotelically attached kinetochores can persist until anaphase, causing chromatids to lag on the mitotic spindle and hindering their timely segregation. Recent studies showing that merotelic kinetochore attachment represents a major mechanism of aneuploidy in mitotic cells and is the primary mechanism of chromosomal instability in cancer cells have underlined the importance of studying merotely. Here, we highlight recent progress in our understanding of how cells prevent and correct merotelic kinetochore attachments.
Introduction
To segregate chromosomes properly, the cell must ensure that sister kinetochores attach to microtubules emanating from opposite spindle poles and prevent erroneous kinetochore attachment (Box 1 and Glossary). Merotelic kinetochore attachment is an error that occurs when a single kinetochore is attached to microtubules emanating from both spindle poles. Merotelically attached kinetochores are frequently observed in the early stages of mitosis but most are corrected [1,2]. If, however, they persist until anaphase, they cause chromatids to lag behind, hindering their segregation to spindle poles. Although the phenomenon of merotely has been known for decades, recent discoveries showing that merotelic kinetochore orientation represents a major mechanism of aneuploidy in mitotic cells [3] and is the primary mechanism of chromosomal instability (CIN) in cancer cells [4–7] have attracted the attention of scientists from various fields. In this review, we focus on mechanisms preventing and correcting merotelic kinetochore attachment and we outline some important areas of future studies in this field.
Box 1. Accurate chromosome segregation depends on the proper attachment of kinetochores to microtubules.
Chromosome segregation occurs thanks to the interaction between a microtubule-based bipolar spindle and kinetochores, proteinaceous complexes assembled on the centromeric heterochromatin of each chromosome [78]. The individual kinetochores of most eukaryotic cells are associated with multiple microtubules. For high-fidelity chromosome segregation, kinetochores must capture spindle microtubules and connect the sister chromatids of each chromosome to opposite spindle poles before anaphase onset. During anaphase, pulling forces of the spindle separate sister chromatids from each other to opposite spindle poles [79–83]. Thus, the attachment of sister kinetochores to microtubules emanating from opposite spindle poles (amphitelic attachment, Figure I) is necessary for accurate chromosome segregation. Commonly, at early mitotic stages, only one of the two sister kinetochores is attached to spindle microtubules (monotelic attachment) (Figure I) [84]. This is because the interaction between kinetochores and spindle microtubules is stochastic [85–88], and sister kinetochores rarely attach to microtubules simultaneously. In addition, two types of erroneous kinetochore attachments can occur during spindle assembly: syntelic attachment, where both sister kinetochores interact with microtubules that emanate from the same spindle pole (Figure I), and merotelic attachment, where a single kinetochore is connected to both spindle poles (Figure I, Figure II). If not corrected, erroneous kinetochore attachments might result in the mis-segregation of chromosomes during anaphase, leading to aneuploid progeny [89,90]. Therefore, it is important that the attachment of kinetochores to spindle microtubules is monitored by the SAC, which ensures that anaphase is triggered only after all kinetochores are attached to spindle microtubules [39,62,91]. In addition, correction mechanisms eliminate erroneous kinetochore attachments and promote correct (amphitelic) attachments [41,92–94]. This prevents the loss of unattached chromosomes and mis-segregation of incorrectly attached chromosomes during anaphase.
Preventing merotelic attachments
The number of merotelic attachments predicted by computer simulations highly exceeds those observed experimentally, suggesting that there are cellular mechanisms that prevent or correct such erroneous attachments [8]. Indeed, in addition to proteins that actively correct merotelic attachments (described below), many proteins are required to prevent the formation of merotelic attachments (Table 1). These are mostly proteins regulating chromosome or kinetochore structure and their absence leads to altered kinetochore architecture that would allow a single kinetochore to face both spindle poles, thereby favoring merotelic orientation. For example, it has been hypothesized that chromatin alterations induced by the inhibition of histone deacetylases lead to a loosely organized centromeric chromatin, which results in an increase in merotelically attached lagging chromosomes [9]. Such a mechanism would also explain the increase in lagging chromosomes observed after topoisomerase II inhibition [10]. Condensin, which has been proposed to regulate the stiffness of centromeres [11], has also been implicated in preventing merotelic attachments in human cells [12]. Caenorhabditis elegans hcp-6 mutant, which is unable to fully condense chromosomes, displays high frequencies of merotelic attachments [13]. However, an independent study concluded that condensin is not an obligate component of a system preventing merotelic attachments in chicken kinetochores [11]. It is not clear why condensin is required to prevent merotelic attachments in human and C. elegans cells, but is not in chicken cells. However, merotelic attachment might be less likely for kinetochores, such as those of chicken DT40 cells that bind only a few microtubules. Recent studies have shown that the depletion of the retinoblastoma protein (pRB) causes defects in chromosome condensation and the deformation of centromeric structure, which promote merotelic attachments [14–16]. Cohesion between sister chromatids is also important for preventing merotelic attachments. This might be because of the back-to-back arrangement of sister kinetochores that sterically hinders erroneous attachments [17,18]. Indeed, if single chromatids, rather than cohesed sister chromatids, enter anaphase their kinetochores are often merotelically attached [19–23]. Similarly, centromere fragments with single kinetochores or kinetochores detached from chromosomes establish merotelic attachments [24,25]. It is also possible that merotelic orientation is the only way to achieve the stable attachment of single kinetochores and thereby satisfy the spindle assembly checkpoint (SAC). Alternatively, the correction machinery might not be functional in the absence of sister chromatid cohesion. Finally, the importance of kinetochore morphology for proper microtubule attachment has also been shown by the effect of nocodazole treatment, which increases the size and alters the shape of the kinetochore [26]. This is believed to cause the massive increase of merotelic attachments observed in cells recovering from nocodazole treatment [3,27].
Table 1.
Selected proteins implicated in preventing or correcting merotelic attachments
| Protein name | Proposed roles | Organism studied | Reference |
|---|---|---|---|
| Pcs1, Mde4 | Clamping together microtubule attachment sites | Fission yeast (S. pombe) | [28–30] |
| Clr4, Swi6 | Establishment of centromeric heterochromatin | Fission yeast (S. pombe) | [29,30] |
| Rad21 | Mediates sister chromatid cohesion | Fission yeast (S. pombe) | [19] |
| Aurora B | Kinetochore assembly and correction of erroneous kinetochore attachments | Fission yeast (S. pombe), rat kangaroo (Potorous tridactylis) | [43,96,97] |
| MCAK, Kif2b | Depolymerization of microtubules | Man (Homo sapiens), rat kangaroo (P. tridactylis), Chinese hamster (Cricetulus griseus) | [4,36,98] |
| Hec1/Ndc80 | Mediates microtubule–kinetochore attachment | Rat kangaroo (P. tridactylis) | [52] |
| pRB | Negative regulator of cell proliferation, chromatin compaction | Man (H. sapiens) | [14] |
| Dido | Histone H3 lysine 4-binding protein | Mouse (Mus musculus) | [76] |
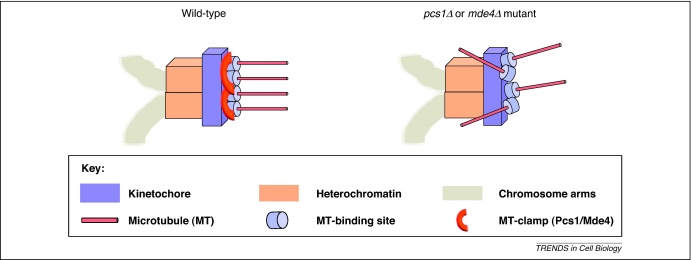
In the fission yeast Schizosaccharomyces pombe, both Clr4/Swi6-dependent centromeric heterochromatin and a putative microtubule site clamp Pcs1/Mde4 are required to prevent merotelic attachments [28–31]. It has been proposed that the Pcs1/Mde4 complex acts in the central kinetochore domain to clamp (or crosslink) microtubule-binding sites together, thereby ensuring that all microtubule attachment sites on a single kinetochore face the same pole [28,29] (Figure 1). Consistent with this model are recent structural studies of the Pcs1/Mde4 complex and its budding yeast counterpart Csm1/Lrs4, which have revealed the distinctive V-shaped structure of these complexes [31]. Although no orthologs of Pcs1 and Mde4 have been identified in higher eukaryotes, the recent discovery that the Pcs1/Mde4 complex shares similar features with the conserved kinetochore complex Spc24/Spc25 might provide important functional insights [30,31]. The molecular basis of how centromeric heterochromatin prevents merotelic attachments has not been studied in detail. However, it is probable that Pcs1/Mde4 clamps and centromeric heterochromatin suppress merotely in different ways because pcs1Δ and swi6Δ mutations are synthetically lethal [28]. Mutants defective in centromeric heterochromatin display a precocious separation of sister centromeres because of a lack of centromeric cohesin [32,33]. As discussed above, the lack of geometric constraint between sister kinetochores might increase the likelihood of merotelic orientations. In addition, it is also possible that centromeric heterochromatin provides rigidity to the kinetochore, ensuring that microtubule-binding sites are properly oriented (Figure 1). Defective centromeric heterochromatin might cause increased kinetochore flexibility, making it more prone to merotelic attachments.
Figure 1.
Putative microtubule site clamp Pcs1/Mde4 and centromeric heterochromatin are required to prevent merotelic attachments in fission yeast. Lagging chromosomes caused by merotelic attachment frequently occur in fission yeast cells lacking the components of the putative microtubule site clamp Pcs1/Mde4 or cells defective in centromeric heterochromatin [28–30]. The kinetochore proteins Pcs1 and Mde4 have been proposed to clamp together (or crosslink) microtubule-binding sites. Clr4/Swi6-dependent centromeric heterochromatin might provide rigidity to the kinetochore, which is necessary for the proper orientation of microtubule-binding sites. Disturbing either of these systems leads to high frequencies of merotelic attachments.
Finally, the proper assembly of a bipolar spindle is also important to minimize the occurrence of merotelic attachments. Indeed, delays in establishing spindle bipolarity or the presence of multipolar spindles induce merotelic attachments [1,5,6,8,34,35]. Although the mechanisms preventing merotelic attachments discussed here are important, additional mechanisms that actively correct merotelic attachments must operate to ensure the high fidelity of chromosome segregation.
Correcting merotelic attachments
Merotelic kinetochore attachment represents a serious threat for dividing cells because it frequently occurs in the early stages of mitosis [1,2], it does not trigger SAC-dependent arrest in mitosis and uncorrected merotelically attached kinetochores can lead to chromosome mis-segregation and aneuploidy [2,3,20,36,37]. Therefore, mechanisms correcting merotelic attachments are crucial for the normal development and survival of an organism. Whereas monotelically attached chromosomes engage the SAC because of the presence of one unattached kinetochore on the sister pair (note that a single unattached kinetochore can trigger SAC-dependent arrest) [38], and syntelic attachment can be identified by low tension across sister kinetochores [39,40], it is rather difficult to distinguish merotelic attachment from the correct amphitelic attachment. Both merotelic and amphitelic attachments generate tension across sister kinetochores and do not produce unattached kinetochores. Nevertheless, mechanisms correcting merotelic kinetochore attachments clearly operate during mitosis, as best evidenced by much lower frequencies of merotelic attachments in anaphase cells compared with cells in prometaphase [1]. Thus, what are these mechanisms and how do they detect and correct merotelic attachments?
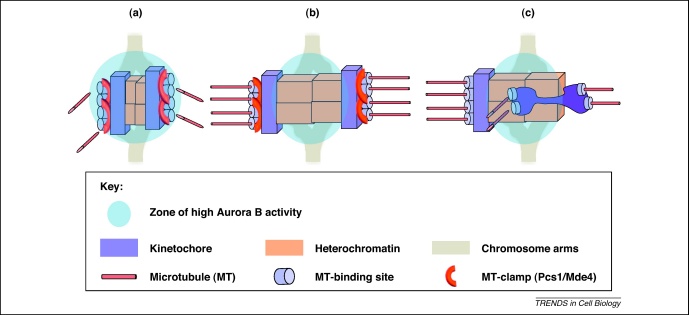
Several observations suggest that the centromeric pool of the Aurora B kinase plays a central role in correcting kinetochore attachment errors including merotelic attachments [41–47]. Aurora B is specifically enriched at merotelic attachment sites and its inhibition causes an increase in the number of merotelic attachments [42,43]. Aurora B localizes to the inner centromere, whereas its opposing phosphatase (protein phosphatase 1) localizes to the outer kinetochore [48,49]. This allows Aurora B to generate a phosphorylation gradient emanating from the inner centromere. In the absence of high tension across sister kinetochores (e.g. syntelic or merosyntelic kinetochore orientation), centromeric Aurora B is able to reach its kinetochore substrates, which leads to the destabilization of kinetochore microtubules. As soon as proper amphitelic attachment is established, spindle forces pull kinetochores away from the inner centromere, beyond the reach of Aurora B, but still within the zone of protein phosphatase 1 activity (Figure 2). Aurora B can directly modulate kinetochore–microtubule attachments by altering the activity of kinetochore proteins, including the microtubule-binding components of the KMN network (KNL1/Mis12 complex/Ndc80 complex) and microtubule depolymerizing kinesins (MCAK, Kif2b), and in this way efficiently detach incorrectly oriented kinetochore microtubules [50–54]. The Aurora B-dependent phosphorylation of the outer kinetochore proteins Ndc80, Dsn1 and KNL1 severely compromises the microtubule-binding activity of the KMN network, probably by introducing negative charges that prevent interaction with the negatively charged microtubules [53,55]. Both MCAK and Kif2b are required for the correction of merotelic orientations; however, the mechanism by which Aurora B regulates this process is less clear. Aurora B has a negative effect on the microtubule depolymerase activity of MCAK and it is required for the proper localization of Kif2b and MCAK [4,56–58]. The temporal control of kinetochore–microtubule dynamics mediated by Aurora B kinase activity seems to play a key role in eliminating merotelic kinetochore attachments. Reduction in the turnover of kinetochore microtubules by depleting microtubule depolymerizing kinesin Kif2b induces kinetochore misattachments, whereas stimulating the dynamics of kinetochore microtubules by overexpressing microtubule depolymerizing kinesin suppresses the incidence of erroneous kinetochore attachments [4]. Although this elegant mechanism explains the selective stabilization of amphitelic attachments and destabilization of erroneous attachments including syntelic and merosyntelic, it does not necessarily provide a satisfactory explanation for how meroamphitelic attachment is corrected. However, an interesting observation suggests how even meroamphitelic attachment might be selectively corrected via an Aurora B-dependent mechanism [43]. Both live and fixed cell analysis has shown that the portion of a merotelic kinetochore attached to the incorrect pole tends to be stretched toward the inner kinetochore region [1,2]. It has been proposed that this could bring the microtubule attachment sites bound to the incorrect pole within the region of high Aurora B concentration, resulting in the selective detachment of the misattached microtubules [43,59]. In addition, live cell analysis in PtK1 cells has shown the existence of meroamphitelically attached chromosomes persisting into anaphase segregated to the correct pole, indicating that this type of misattachment might not contribute to chromosome mis-segregation [2]. It is also possible that mechanisms correcting merotelic attachments operate during anaphase, relying on forces exerted by microtubules during spindle elongation [19], and it will be interesting to investigate this in future experiments.
Figure 2.
Model of the Aurora B-mediated correction of merotelic attachments. (a) In the absence of tension across sister kinetochores, centromeric Aurora B is in close proximity to, and thereby is able to phosphorylate, its kinetochore substrates. This leads to the destabilization of kinetochore microtubules [41–46]. (b) Upon the establishment of amphitelic attachment, microtubules pull the kinetochore away from the inner centromere and thereby out of reach of Aurora B. Consequently, kinetochore–microtubule attachment is stabilized. (c) Upon merotelic attachment (in this example, merotely occurs because of the absence of putative microtubule site clamp Pcs1/Mde4), the portion of a kinetochore attached to the incorrect pole tends to be stretched toward the inner kinetochore region. This could bring the microtubule attachment sites bound to the incorrect pole within the region of high Aurora B activity, resulting in the selective detachment of the misattached microtubules.
The fact that merotelic kinetochore attachments do not trigger a checkpoint-dependent delay of anaphase onset [2,20,37,38] indicates that the pre-anaphase correction of merotelic attachments might not involve the detachment of the kinetochore from all its kinetochore microtubules. Otherwise, the exposure of the unattached kinetochore would inevitably trigger a checkpoint-dependent response. Therefore, a likely scenario is that only kinetochore microtubules emanating from the incorrect pole are detached and possibly replaced by microtubules emanating from the correct pole. The correction mechanism must be efficient enough to ensure that before the onset of anaphase most, if not all, sister kinetochores attain proper amphitelic, or occasionally meroamphitelic, attachment. This ensures the high fidelity of chromosome segregation during cell division.
Finally, it is noteworthy that mechanisms correcting erroneous kinetochore attachments can be overwhelmed (or bypassed) if the frequency of erroneous attachments is elevated. For example, chromosomes often mis-segregate in cells transiently treated with the microtubule poison nocodazole, which induces various kinetochore misattachments including merotelic [3]. Similarly, increasing the number of merotelic attachments by acquisition of extra centrosomes leads to a high rate of chromosome mis-segregation [5,6]. This indicates that there is a thin line between the faithful transmission of chromosomes and mis-segregation, and that dividing cells might be particularly vulnerable to factors inducing errors in kinetochore attachment. Thus, the machinery correcting erroneous kinetochore attachments can be seen as the Achilles’ heel of dividing cells.
Future directions and concluding remarks
The functional disruption of many genes leads to high frequencies of merotelic attachments (Table 1). In future experiments, it will be important to dissect which of these proteins have a direct role in the correction mechanism and which proteins are more likely to have an indirect role by altering kinetochore structure. These studies will be complicated by the fact that some proteins (e.g. Aurora B) might be involved in both correcting erroneous kinetochore attachments and kinetochore assembly. It will be important to address the possible relation between the putative microtubule site clamp Pcs1/Mde4 and Aurora B. Both Pcs1 and Mde4 are phosphorylated, but it is not known if they are substrates of the Aurora B kinase [30,60]. Merotely also occurs during meiosis, when the dramatic rearrangements of kinetochore architecture occur [21,45,61]. It will be interesting to investigate if the mechanisms preventing and correcting merotelic attachments characterized in mitotic cells also operate during meiosis. Finally, it is probable that the list of proteins required to prevent or correct merotelic attachments is still incomplete. Identifying these proteins and defining the mechanisms by which they function is an important aim for future research.
The molecular machinery involved in correcting merotelic attachment is beginning to emerge. Future experiments should address whether error correction mechanisms promoting amphitelic attachments and the SAC are independent or constitute two parts of the same pathway. Similarly, it will be important to determine what the SAC monitors because these issues are still a matter of debate [39,40,62,63]. This will also help us understand why merotelic attachment does not trigger SAC-dependent arrest. It also remains to be tested whether the correction mechanism based on the Aurora B gradient is sufficient for efficient error correction or whether other correction mechanisms are involved (Box 2).
Box 2. Outstanding questions.
To fully understand the mechanisms correcting merotelic attachments it will be important to address the following questions:
-
•
How does the timing of merotely formation impact the efficiency of repair?
-
•
Does the stretched shape of the merotelic kinetochore increase the probability of additional (secondary) erroneous attachments on the same kinetochore?
-
•
How much time is needed for efficient correction and does this depend on the number of erroneously attached microtubules?
-
•
Does the presence of multiple merotelic attachments slow the rate of correction at individual kinetochores?
Recent work has provided many important details about the internal architecture of the kinetochore [64,65]. However, little work has been performed to determine how the kinetochore structure changes upon merotelic attachment. A combination of light and electron microscopy has revealed dramatic changes in the shape of the merotelic kinetochore. Microtubules can stretch a merotelic kinetochore laterally from its normal width of approximately 0.4 μm to more than 2 μm. Sometimes, the kinetochore is extended laterally into two domains that remain connected by a thin kinetochore extension [3]. The molecular identity of these domains is not known. To fully understand how the cell prevents and corrects merotelic attachments, it will be important to determine the molecular architecture of the merotelic kinetochore. This might allow the identification of kinetochore proteins whose localization patterns are altered upon merotelic attachment, thereby providing insight into the molecular components potentially acting as effectors in the biochemical pathway responsible for correcting merotelic attachments.
Given that relatively subtle changes in kinetochore size or shape in organisms with localized kinetochores can lead to increased frequencies of merotelic attachments [9,10,13,19,23,26,29], it will also be interesting to study how organisms with holocentric chromosomes, which assemble kinetochores along the entire length of each sister chromatid (e.g. C. elegans), deal with merotelic attachments.
Recent studies showing that merotelic kinetochore orientation is the primary mechanism of CIN in cancer cells [4–7] have attracted wide attention. Understanding the mechanism of CIN is important because it can drive tumorigenesis through the tumor suppressor gene loss of heterozygosity and can promote tumor relapse [66–68]. Previous models have assumed that the chromosome mis-segregation phenotype of CIN cells resulted mainly from a defective SAC [69,70] or multipolar cell division [71]. However, recent studies have revealed that the SAC in many CIN cells is normal [7,72,73] and that progeny from multipolar divisions are usually inviable [5]. A recently proposed model for chromosome mis-segregation in CIN cells with supernumerary centrosomes suggested that multipolar spindles are assembled only transiently because of centrosome clustering before anaphase onset, and this allows for the frequent formation of merotelic attachments, but bipolar cell division. This model provides an elegant explanation for the high rates of lagging chromosomes observed in CIN cells (Figure 3). Although this mechanism does not rule out other factors contributing to CIN, it largely explains the chromosome mis-segregation typical of CIN cells and links it to centrosome amplification, another common feature of cancer cells. However, this model is based on analyses of CIN cell lines. Thus, it will be important to address whether this model can be extended to in vivo tumorigenesis models and identify the additional changes that allow CIN cells to tolerate aneuploidy. Although the relation between aneuploidy and tumorigenesis remains highly complex and controversial, a mounting body of evidence suggests that CIN contributes to tumor initiation and progression [74,75]. In this respect, it is encouraging that recent work showed that CIN can be suppressed in tumor cells [4]. It will also be interesting to further analyze the recently proposed model that merotelic attachments give rise to chromosome breakage at the centromere, which might activate DNA damage repair pathways and promote carcinogenesis [76]. However, it is important to mention that an independent study found no evidence of DNA damage on lagging chromosomes [77].
Figure 3.
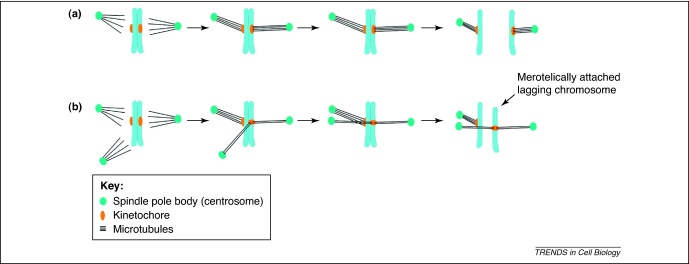
Multipolar spindle geometry promotes merotelic kinetochore attachments. (a) In normal unperturbed mitosis, sister kinetochores are attached amphitelically and segregate to opposite poles of the bipolar spindle during anaphase. (b) CIN cells with supernumerary centrosomes assemble multipolar spindles, which allows for the frequent formation of merotelic attachments. This is followed by the clustering of centrosomes into two poles and division in a bipolar fashion. During anaphase, merotelically attached kinetochores give rise to lagging chromosomes, which might lead to mis-segregation [5,6].
Taken together, the work summarized in this review shows that there are multiple cellular mechanisms for preventing or correcting merotelic kinetochore attachments. Recent studies have provided important functional insights into some of these mechanisms and highlighted the key role of the Aurora B kinase. Deciphering how cells orchestrate individual components to efficiently suppress merotelic attachments and ensure the faithful segregation of chromosomes will be an important aim of future studies. Given the recent discoveries showing that merotely represents a major mechanism of aneuploidy in mitotic cells [3] and is the primary mechanism of CIN in cancer cells [4–7], it is probable that this will continue to be an area of intense research.
Figure I.
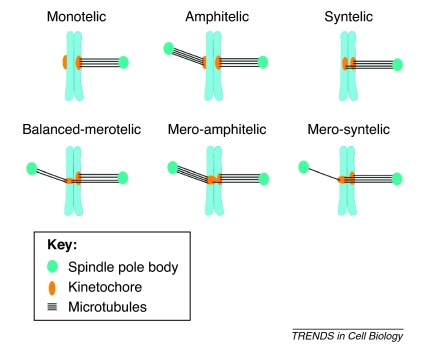
Types of kinetochore attachments during mitosis. Whereas only one of the two sister kinetochores is attached to spindle microtubules in monotelic attachment, sister kinetochores are attached to microtubules emanating from opposite spindle poles in amphitelic attachment. Monotelic kinetochore attachment is an intermediate state preceding proper amphitelic attachment. There are two types of erroneous kinetochore attachments: syntelic attachment, where both sister kinetochores interact with microtubules emanating from the same spindle pole, and merotelic attachment, where a single kinetochore is connected to both spindle poles. There are 15–30 microtubule attachment sites at vertebrate kinetochores, thereby providing considerable opportunity for generating merotelic attachments. Three types of merotelic attachments have been observed: i) balanced merotelic (similar number of kinetochore microtubules attached from both poles), ii) mero-amphitelic (more kinetochore microtubules emanating from the pole opposite to that of the sister kinetochore) and iii) mero-syntelic (more kinetochore microtubules emanating from the pole to which the sister kinetochore is attached) [36,95]. Chromosomes with monotelic or syntelic attachments are also referred to as mono-oriented, whereas those with amphitelic or merotelic attachments are referred to as bioriented. To segregate chromosomes properly, erroneous kinetochore attachments should be corrected and amphitelic attachments stabilized.
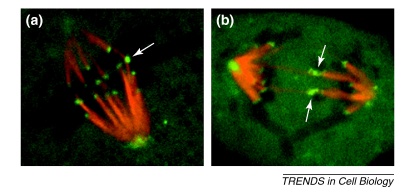
Figure II.
Merotelically attached kinetochores in PtK1 cells. PtK1 cells are ideal for studying kinetochores because of the small number of chromosomes and the fact that the cells remain flat throughout mitosis, making them amenable for high-resolution light microscopy. The images show live PtK1 cells microinjected with X-rhodamine-labeled tubulin and Alexa 488-labeled CENP-F antibodies to fluorescently label kinetochore fibers (red) and kinetochores/spindle poles (green), respectively. (a) Late prometaphase PtK1 cell with one merotelic kinetochore (arrow) aligned at the metaphase plate. (b) Anaphase PtK1 cell with two merotelically attached lagging chromosomes (arrows).
Acknowledgments
We thank R. Allshire, A. Pidoux, A. Khodjakov and C. Rumpf for helpful discussions, and William T. Silkworth for help with Figure II. This work was supported by Austrian Science Fund grants (P21437, P20444 and F3403) to J.G., NSF grant MCB-0842551 to D.C. and HFSP grant RGY0069/2010 to J.G, D.C. and I.M.T. S.P. was supported by an EMBO long-term fellowship. We apologize to our colleagues whose work could not be cited here because of space limitations.
Glossary
- Kinetochore
Protein complex assembled on centromeric chromatin. Site of microtubule attachment during cell division.
- Microtubule
Filament composed of the protein tubulin.
- Kinetochore microtubules
Subset of spindle microtubules that attach to kinetochores.
- Kinetochore fiber
Bundle of kinetochore microtubules.
- Mitotic spindle
Apparatus consisting of microtubule arrays and many other associated components, which segregates chromosomes during mitosis (mitotic spindle) and meiosis (meiotic spindle).
- Spindle assembly checkpoint (SAC)
Biochemical pathway that monitors the attachment state of kinetochores. Delays anaphase onset until all kinetochores are attached to spindle microtubules.
- Chromosome mis-segregation
Segregation of a whole chromosome to the incorrect daughter cell during cell division. This leads to the formation of aneuploid daughter cells.
- Chromosomal instability (CIN)
Elevated rate of chromosome mis-segregation during mitosis. Hallmark of many types of cancer cells.
- Aneuploidy
Abnormal number of chromosomes. Common characteristic of cancer cells.
References
- 1.Cimini D. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 2003;116:4213–4225. doi: 10.1242/jcs.00716. [DOI] [PubMed] [Google Scholar]
- 2.Cimini D. Anaphase spindle mechanics prevent mis-segregation of merotelically oriented chromosomes. Curr. Biol. 2004;14:2149–2155. doi: 10.1016/j.cub.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 3.Cimini D. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 2001;153:517–527. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakhoum S.F. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat. Cell Biol. 2009;11:27–35. doi: 10.1038/ncb1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganem N.J. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silkworth W.T. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS ONE. 2009;4:e6564. doi: 10.1371/journal.pone.0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson S.L., Compton D.A. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 2008;180:665–672. doi: 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul R. Computer simulations predict that chromosome movements and rotations accelerate mitotic spindle assembly without compromising accuracy. Proc. Natl. Acad. Sci. U.S.A. 2009;106:15708–15713. doi: 10.1073/pnas.0908261106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cimini D. Histone hyperacetylation in mitosis prevents sister chromatid separation and produces chromosome segregation defects. Mol. Biol. Cell. 2003;14:3821–3833. doi: 10.1091/mbc.E03-01-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cimini D. Topoisomerase II inhibition in mitosis produces numerical and structural chromosomal aberrations in human fibroblasts. Cytogenet. Cell Genet. 1997;76:61–67. doi: 10.1159/000134517. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro S.A. Condensin regulates the stiffness of vertebrate centromeres. Mol. Biol. Cell. 2009;20:2371–2380. doi: 10.1091/mbc.E08-11-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samoshkin A. Human condensin function is essential for centromeric chromatin assembly and proper sister kinetochore orientation. PLoS ONE. 2009;4:e6831. doi: 10.1371/journal.pone.0006831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stear J.H., Roth M.B. Characterization of HCP-6, a C. elegans protein required to prevent chromosome twisting and merotelic attachment. Genes Dev. 2002;16:1498–1508. doi: 10.1101/gad.989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning A.L. Loss of pRB causes centromere dysfunction and chromosomal instability. Genes Dev. 2010;24:1364–1376. doi: 10.1101/gad.1917310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coschi C.H. Mitotic chromosome condensation mediated by the retinoblastoma protein is tumor-suppressive. Genes Dev. 2010;24:1351–1363. doi: 10.1101/gad.1917610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Harn T. Loss of Rb proteins causes genomic instability in the absence of mitogenic signaling. Genes Dev. 2010;24:1377–1388. doi: 10.1101/gad.580710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loncarek J. The centromere geometry essential for keeping mitosis error free is controlled by spindle forces. Nature. 2007;450:745–749. doi: 10.1038/nature06344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Indjeian V.B., Murray A.W. Budding yeast mitotic chromosomes have an intrinsic bias to biorient on the spindle. Curr. Biol. 2007;17:1837–1846. doi: 10.1016/j.cub.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 19.Courtheoux T. Ase1/Prc1-dependent spindle elongation corrects merotely during anaphase in fission yeast. J. Cell Biol. 2009;187:399–412. doi: 10.1083/jcb.200902093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khodjakov A. Chromosome fragments possessing only one kinetochore can congress to the spindle equator. J. Cell Biol. 1997;136:229–240. doi: 10.1083/jcb.136.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H.G., Dawe R.K. Functional redundancy in the maize meiotic kinetochore. J. Cell Biol. 2000;151:131–142. doi: 10.1083/jcb.151.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenney R.D., Heald R. Essential roles for cohesin in kinetochore and spindle function in Xenopus egg extracts. J. Cell Sci. 2006;119:5057–5066. doi: 10.1242/jcs.03277. [DOI] [PubMed] [Google Scholar]
- 23.Parry D.H. Cyclin B destruction triggers changes in kinetochore behavior essential for successful anaphase. Curr. Biol. 2003;13:647–653. doi: 10.1016/s0960-9822(03)00242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brinkley B.R. Movement and segregation of kinetochores experimentally detached from mammalian chromosomes. Nature. 1988;336:251–254. doi: 10.1038/336251a0. [DOI] [PubMed] [Google Scholar]
- 25.Wise D.A., Brinkley B.R. Mitosis in cells with unreplicated genomes (MUGs): spindle assembly and behavior of centromere fragments. Cell Motil. Cytoskeleton. 1997;36:291–302. doi: 10.1002/(SICI)1097-0169(1997)36:3<291::AID-CM9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman D.B. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol. Biol. Cell. 2001;12:1995–2009. doi: 10.1091/mbc.12.7.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladrach K.S., LaFountain J.R., Jr. Malorientation and abnormal segregation of chromosomes during recovery from colcemid and nocodazole. Cell Motil. Cytoskeleton. 1986;6:419–427. doi: 10.1002/cm.970060407. [DOI] [PubMed] [Google Scholar]
- 28.Rabitsch K.P. Kinetochore recruitment of two nucleolar proteins is required for homolog segregation in meiosis I. Dev. Cell. 2003;4:535–548. doi: 10.1016/s1534-5807(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 29.Gregan J. The kinetochore proteins Pcs1 and Mde4 and heterochromatin are required to prevent merotelic orientation. Curr. Biol. 2007;17:1190–1200. doi: 10.1016/j.cub.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumpf C. Laser microsurgery provides evidence for merotelic kinetochore attachments in fission yeast cells lacking Pcs1 or Clr4. Cell Cycle. 2010;9:3997–4004. doi: 10.4161/cc.9.19.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corbett K.D. The monopolin complex crosslinks kinetochore components to regulate chromosome-microtubule attachments. Cell. 2010;142:556–567. doi: 10.1016/j.cell.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernard P. Fission yeast Bub1 is essential in setting up the meiotic pattern of chromosome segregation. Nat. Cell Biol. 2001;3:522–526. doi: 10.1038/35074598. [DOI] [PubMed] [Google Scholar]
- 33.Nonaka N. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol. 2002;4:89–93. doi: 10.1038/ncb739. [DOI] [PubMed] [Google Scholar]
- 34.Heneen W.K. Kinetochores and microtubules in multipolar mitosis and chromosome orientation. Exp. Cell Res. 1975;91:57–62. doi: 10.1016/0014-4827(75)90140-8. [DOI] [PubMed] [Google Scholar]
- 35.Sluder G. The checkpoint control for anaphase onset does not monitor excess numbers of spindle poles or bipolar spindle symmetry. J. Cell Sci. 1997;110(Pt. 4):421–429. doi: 10.1242/jcs.110.4.421. [DOI] [PubMed] [Google Scholar]
- 36.Kline-Smith S.L. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol. Biol. Cell. 2004;15:1146–1159. doi: 10.1091/mbc.E03-08-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cimini D. Merotelic kinetochore orientation versus chromosome mono-orientation in the origin of lagging chromosomes in human primary cells. J. Cell Sci. 2002;115:507–515. doi: 10.1242/jcs.115.3.507. [DOI] [PubMed] [Google Scholar]
- 38.Rieder C.L. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nezi L., Musacchio A. Sister chromatid tension and the spindle assembly checkpoint. Curr. Opin. Cell Biol. 2009;21:785–795. doi: 10.1016/j.ceb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Pinsky B.A., Biggins S. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 2005;15:486–493. doi: 10.1016/j.tcb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Carmena M. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr. Opin. Cell Biol. 2009;21:796–805. doi: 10.1016/j.ceb.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knowlton A.L. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr. Biol. 2006;16:1705–1710. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 43.Cimini D. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr. Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka T.U. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 45.Hauf S. Aurora controls sister kinetochore mono-orientation and homolog bi-orientation in meiosis-I. EMBO J. 2007;26:4475–4486. doi: 10.1038/sj.emboj.7601880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuller B.G. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature. 2008;453:1132–1136. doi: 10.1038/nature06923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lampson M.A., Cheeseman I.M. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011;21:133–140. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu D. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu D. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J. Cell Biol. 2010;188:809–820. doi: 10.1083/jcb.201001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X. Aurora B phosphorylates multiple sites on mitotic centromere-associated kinesin to spatially and temporally regulate its function. Mol. Biol. Cell. 2007;18:3264–3276. doi: 10.1091/mbc.E07-01-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheeseman I.M. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 52.DeLuca J.G. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 53.Welburn J.P. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol. Cell. 2010;38:383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alushin G.M. The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature. 2010;14:805–810. doi: 10.1038/nature09423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guimaraes G.J. Kinetochore-microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr. Biol. 2008;18:1778–1784. doi: 10.1016/j.cub.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lan W. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 2004;14:273–286. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 57.Andrews P.D. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell. 2004;6:253–268. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- 58.Ohi R. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol. Biol. Cell. 2004;15:2895–2906. doi: 10.1091/mbc.E04-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cimini D. Detection and correction of merotelic kinetochore orientation by Aurora B and its partners. Cell Cycle. 2007;6:1558–1564. doi: 10.4161/cc.6.13.4452. [DOI] [PubMed] [Google Scholar]
- 60.Choi S.H. Phosphorylation state defines discrete roles for monopolin in chromosome attachment and spindle elongation. Curr. Biol. 2009;19:985–995. doi: 10.1016/j.cub.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janicke M.A. Chromosome malorientations after meiosis II arrest cause nondisjunction. Mol. Biol. Cell. 2007;18:1645–1656. doi: 10.1091/mbc.E06-10-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khodjakov A., Pines J. Centromere tension: a divisive issue. Nat. Cell Biol. 2010;12:919–923. doi: 10.1038/ncb1010-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khodjakov A., Rieder C.L. The nature of cell-cycle checkpoints: facts and fallacies. J. Biol. 2009;8:88. doi: 10.1186/jbiol195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wan X. Protein architecture of the human kinetochore microtubule attachment site. Cell. 2009;137:672–684. doi: 10.1016/j.cell.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alushin G.M. The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature. 2010;467:805–810. doi: 10.1038/nature09423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sotillo R. Mad2- induced chromosome instability leads to lung tumour relapse after oncogene withdrawal. Nature. 2010;464:436–440. doi: 10.1038/nature08803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baker D.J. Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity. Cancer Cell. 2009;16:475–486. doi: 10.1016/j.ccr.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geigl J.B. Defining ‘chromosomal instability’. Trends Genet. 2008;24:64–69. doi: 10.1016/j.tig.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Suijkerbuijk S.J., Kops G.J. Preventing aneuploidy: the contribution of mitotic checkpoint proteins. Biochim. Biophys. Acta. 2008;1786:24–31. doi: 10.1016/j.bbcan.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 70.Cahill D.P. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 71.Nigg E.A. Centrosome aberrations: cause or consequence of cancer progression? Nat. Rev. Cancer. 2002;2:815–825. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- 72.Gascoigne K.E., Taylor S.S. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–122. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Tighe A. Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO Rep. 2001;2:609–614. doi: 10.1093/embo-reports/kve127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schvartzman J.M. Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nat. Rev. Cancer. 2010;10:102–115. doi: 10.1038/nrc2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holland A.J., Cleveland D.W. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat. Rev. Mol. Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guerrero A.A. Centromere-localized breaks indicate the generation of DNA damage by the mitotic spindle. Proc. Natl. Acad. Sci. U.S.A. 2010;107:4159–4164. doi: 10.1073/pnas.0912143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thompson S.L., Compton D.A. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J. Cell Biol. 2010;188:369–381. doi: 10.1083/jcb.200905057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walczak C.E., Heald R. Mechanisms of mitotic spindle assembly and function. Int. Rev. Cytol. 2008;265:111–158. doi: 10.1016/S0074-7696(07)65003-7. [DOI] [PubMed] [Google Scholar]
- 79.Maiato H. The dynamic kinetochore-microtubule interface. J. Cell Sci. 2004;117:5461–5477. doi: 10.1242/jcs.01536. [DOI] [PubMed] [Google Scholar]
- 80.Yanagida M. Clearing the way for mitosis: is cohesin a target? Nat. Rev. Mol. Cell Biol. 2009;10:489–496. doi: 10.1038/nrm2712. [DOI] [PubMed] [Google Scholar]
- 81.Nasmyth K., Haering C.H. Cohesin: its roles and mechanisms. Annu. Rev. Genet. 2009;43:525–558. doi: 10.1146/annurev-genet-102108-134233. [DOI] [PubMed] [Google Scholar]
- 82.Westermann S. Structures and functions of yeast kinetochore complexes. Annu. Rev. Biochem. 2007;76:563–591. doi: 10.1146/annurev.biochem.76.052705.160607. [DOI] [PubMed] [Google Scholar]
- 83.Vagnarelli P. Centromeres: old tales and new tools. FEBS Lett. 2008;582:1950–1959. doi: 10.1016/j.febslet.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 84.Rieder C.L., Salmon E.D. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 1998;8:310–318. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirschner M., Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 86.Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 87.Hayden J.H. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J. Cell Biol. 1990;111:1039–1045. doi: 10.1083/jcb.111.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rieder C.L., Alexander S.P. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J. Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tanaka K., Hirota T. Chromosome segregation machinery and cancer. Cancer Sci. 2009;100:1158–1165. doi: 10.1111/j.1349-7006.2009.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Torres E.M. Aneuploidy: cells losing their balance. Genetics. 2008;179:737–746. doi: 10.1534/genetics.108.090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Musacchio A., Salmon E.D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 92.Liu D., Lampson M.A. Regulation of kinetochore-microtubule attachments by Aurora B kinase. Biochem. Soc. Trans. 2009;37:976–980. doi: 10.1042/BST0370976. [DOI] [PubMed] [Google Scholar]
- 93.Kelly A.E., Funabiki H. Correcting aberrant kinetochore microtubule attachments: an Aurora B-centric view. Curr. Opin. Cell Biol. 2009;21:51–58. doi: 10.1016/j.ceb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hauf S., Watanabe Y. Kinetochore orientation in mitosis and meiosis. Cell. 2004;119:317–327. doi: 10.1016/j.cell.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 95.Torosantucci L. Aneuploidy in mitosis of PtK1 cells is generated by random loss and nondisjunction of individual chromosomes. J. Cell Sci. 2009;122:3455–3461. doi: 10.1242/jcs.047944. [DOI] [PubMed] [Google Scholar]
- 96.Lampson M.A. Correcting improper chromosome-spindle attachments during cell division. Nat. Cell Biol. 2004;6:232–237. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- 97.Hauf S. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maney T. Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J. Cell Biol. 1998;142:787–801. doi: 10.1083/jcb.142.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]