Abstract
Objective:
To investigate the relationship between body mass index (BMI), waist circumference (WC) or waist–hip ratio (WHR) and all-cause mortality or cause-specific mortality.
Design:
Cross-sectional surveys linked to hospital admissions and death records.
Subjects:
In total, 20 117 adults (aged 18–86 years) from a nationally representative sample of the Scottish population.
Measurements:
Cox proportional hazards models were used to estimate hazard ratios (HRs) for all-cause, or cause-specific, mortality. The three anthropometric measurements BMI, WC and WHR were the main variables of interest. The following were adjustment variables: age, gender, smoking status, alcohol consumption, survey year, social class and area of deprivation.
Results:
BMI-defined obesity (⩾30 kg m−2) was not associated with increased risk of mortality (HR=0.93; 95% confidence interval=0.80–1.08), whereas the overweight category (25–<30 kg m−2) was associated with a decreased risk (0.80; 0.70–0.91). In contrast, the HR for a high WC (men⩾102 cm, women⩾88 cm) was 1.17 (1.02–1.34) and a high WHR (men⩾1, women⩾0.85) was 1.34 (1.16–1.55). There was an increased risk of cardiovascular disease (CVD) mortality associated with BMI-defined obesity, a high WC and a high WHR categories; the HR estimates for these were 1.36 (1.05–1.77), 1.41 (1.11–1.79) and 1.44 (1.12–1.85), respectively. A low BMI (<18.5 kg m−2) was associated with elevated HR for all-cause mortality (2.66; 1.97–3.60), for chronic respiratory disease mortality (3.17; 1.39–7.21) and for acute respiratory disease mortality (11.68; 5.01–27.21). This pattern was repeated for WC but not for WHR.
Conclusions:
It might be prudent not to use BMI as the sole measure to summarize body size. The alternatives WC and WHR may more clearly define the health risks associated with excess body fat accumulation. The lack of association between elevated BMI and mortality may reflect the secular decline in CVD mortality.
Keywords: body mass index, waist circumference, waist–hip ratio, mortality, epidemiology
Introduction
Obesity is a major health challenge of the 21st Century with rising worldwide prevalence in recent decades. One estimate is that 1 in 13 deaths in Europe is related to excess weight.1 A U- or J-shaped association between body mass index (BMI) and mortality has frequently been described.2, 3, 4, 5 BMI is a measure of overall obesity, but with known limitations, including the inability to reflect body fat distribution. This has prompted the use of waist circumference (WC) or waist–hip ratio (WHR), both alternative indices that are believed to be proxies for abdominal adiposity.6
Relatively few cross-sectional, nationally representative studies have been published regarding the potential impact on mortality of obesity, as identified using measured anthropometric indices, for example papers by Katzmarzyk et al.4 and Flegal et al.2, 7 The Scottish Health Surveys (SHeSs)-linked data set provides an opportunity to do this. According to the 2008 SHeS, the prevalence of obesity in Scotland was 26.0% in men and 27.5% in women,8 and one study using the 1998 SHeS identified that both obesity and underweight were associated with an increased risk of serious hospital admission.9 We aimed to investigate the relationship between body size and mortality, while adjusting for confounders such as smoking and alcohol consumption that might have an influence on mortality. The SHeSs also permitted the investigation of whether or not body size identifies additional risk to the accumulated risk factors reflected in socioeconomic status or area of deprivation. This was important because of the pronounced socioeconomic gradients for mortality10 and separately for obesity, especially for women.11 The objective of this study was to examine the association of body size with all-cause and cause-specific mortality using BMI, WC or WHR in a representative sample of the Scottish population using commonly used and understood categories.
Methods
Survey method and subjects
The SHeSs 1995, 1998 and 2003 are cross-sectional nationally representative surveys designed to provide reliable information on the health and health-related behaviours of people living in private households in Scotland; details of these surveys are described elsewhere.12, 13, 14, 15 The samples were selected using a multistage stratified clustered probability sampling design. The surveys were limited to 16–64 year olds in 1995 and 2–74 year olds in 1998. In 2003, all ages were surveyed. Data were collected during two household visits; first by an interviewer then by a nurse if consent was given. During the face-to-face interviews, baseline data such as age, gender, alcohol consumption, smoking status, occupational social class, height and weight were ascertained. Self-completed questionnaires were used at the interviewer's discretion to determine smoking and alcohol consumption behaviour in teenagers. Bodyweight was measured to the nearest 100 g using electronic scales. Respondents exceeding the scales' upper limit of 130 kg were invited to provide an estimated weight. Height was measured to the nearest millimetre using a stadiometer. Waist and hip circumferences were obtained during the nurse visit using a measuring tape with an insertion buckle. Both were taken at least twice and recorded to the nearest millimetre to provide a mean. The waist was defined as the midpoint between the lower rib and the upper margin of the iliac crest. The hips were measured at the widest circumference around the buttocks below the iliac crest. Pregnant women and participants who were chair bound were excluded from all the above measurements.
Measures
Anthropometric
BMI was calculated as weight (kg) divided by the square of the height (m2). The World Health Organisation (WHO) principal cutoff points for BMI were used to create the categories: underweight (<18.5 kg m−2), desirable weight (18.5–<25 kg m−2), overweight (25–<30 kg m−2) and obese (⩾30 kg m−2).16 WC was defined in gender-specific low, reference, moderate and high categories for men equal to <79, 79–<94, 94–<102 and ⩾102 cm and women equal to <68, 68–<80, 80–<88 and ⩾88 cm.17, 18 WHR equalled WC divided by hip circumference and was categorized as low, reference, moderate and high separately for men <0.85, 0.85–<0.95, 0.95–<1 and ⩾1 and women <0.7, 0.7–<0.8, 0.8–<0.85 and ⩾0.85. The classifications for WC and WHR included cutoffs recommended by a WHO consultation.16
Covariates
Age was recorded as age at the time of interview. Smoking status was categorized as never smoker, ex-smoker, light smoker (0–<10 cigarettes per day), moderate smoker (10–<20 cigarettes per day) and heavy smoker (⩾20 cigarettes per day). Pipe and cigar smokers were included with light smokers. Alcohol consumption was defined as never or very occasional drinker, ex-drinker, 0–<7 units per week, 7–<14 units per week, 14–<21 units per week, 21–<28 units per week and ⩾28 units per week. A unit of alcohol is defined as 10 ml (∼8 g) ethanol. Occupational social class (Registrar General's classification) was divided into six categories I/II, III non-manual, III manual, IV, V and others (including armed forces/unknown/not able to be classified/undetermined). Postcode sectors of residence were categorized into quintiles based on the Carstairs score, an area-based deprivation measure derived from levels of male unemployment, manual social class, overcrowding and lack of car ownership and derived from the 2001 Census.19
Outcome measures
Respondents were asked for permission for their records to be linked to National Health Service administrative data. This enabled the linkage of these surveys to a database of deaths (to December 2007) and the Scottish Morbidity Records; both are maintained by the Information Services Division of National Health Service Scotland.15 The Scottish Morbidity Records are a patient-based database of acute and psychiatric hospital discharges from 1981 to December 2007 along with cancer registrations from 1981 to December 2005. Specific cause of death information was available using International Classification of Diseases, Ninth revision and International Classification of Diseases-10 codes. This was recoded, using a standard list of 113 causes of death,20 into three broad categories (each with subdivisions) of cardiovascular disease (CVD), cancer and all other causes (namely non-cancer and non-CVD deaths) as described by Flegal et al.7 (Table 1). An exception was that deaths due to diabetes and kidney disease were included in miscellaneous causes due to low numbers. Using their rationale, obesity-related cancers were defined as colon, breast, oesophageal, uterine, ovarian, kidney or pancreatic cancers. The SHeSs were linked to the Community Health Index (CHI) as at January 2008, to determine whether respondents had been registered with a Scottish general practice by the end of the follow-up period.
Table 1. Cause of death categorization for use in cause-specific mortality analyses.
| Cause of death | Corresponding numbers from 113 causes of death lista |
|---|---|
| CVD | 54–74 |
| Coronary heart disease | 58–63 |
| Other CVD | 55–57, 64–74 |
| Cancer | 19–43 |
| Lung cancer | 27 |
| Considered obesity relatedb | 21, 23, 25, 29, 31, 32, 34 |
| All other cancers | All other numbers within cancer category |
| Non-cancer, non-CVD disease | All other numbers |
| Chronic respiratory disease | 82–86 |
| Acute respiratory and infectious disease | 1–18, 76–81, 87–89 |
| Injuries | 112–135 |
| Miscellaneous—including diabetes and kidney disease | All other numbers within non-cancer, non-CVD categories |
Sample
Response for the interview stage of the survey declined from 81% in 1995 to 76% in 1998 and finally to 60% in 2003. The proportion of interviewees that progressed to a second interview with a nurse also declined from 88% in 1995 to 67% in 2003. Approximately 92% of respondents consented to data linkage reducing the combined sample size of the three surveys (for over 16 year olds) from 25 127 to 23 093; of these 1569 had died by December 2007. Only participants between the ages of 18 and 85 years were included in the analyses, resulting in a further reduction in sample size to 22 426 with 1523 deaths. The proportion of individuals with missing data was as follows: BMI=10.2%, WC=20.3%, WHR=20.4%, smoking status=0.1% and alcohol consumption=0.1% (Table 2). The missing anthropometric data included pregnant women (n=212) who were excluded from all analyses. The complete cases available for analyses were as follows: BMI=20 117 subjects (1280 deaths), WC=17 867 subjects (1220 deaths) and WHR=17 840 subjects (1218 deaths).
Table 2. Baseline characteristics for the linked 1995, 1998 and 2003 Scottish Health Surveys, individually, and combined.
|
Survey |
Combined surveys | |||
|---|---|---|---|---|
| 1995 | 1998 | 2003 | ||
| Sample size | 7170 | 8115 | 7141 | 22 426 |
| Number of deaths, n (%) | ||||
| All-cause | 503 (7.0) | 735 (9.1) | 285 (4.0) | 1523 (6.8) |
| CVD | 148 (2.1) | 245 (3.0) | 116 (1.6) | 509 (2.3) |
| Cancer | 185 (2.6) | 261 (3.2) | 91 (1.3) | 537 (2.4) |
| Non-cancer and non-CVD | 170 (2.4) | 229 (2.8) | 78 (1.1) | 477 (2.1) |
| Person-years of follow-up | 85 629 | 71 597 | 27 216 | 1 84 442 |
| Mean age (s.d.) | 40.9 (12.9) | 45.9 (15.5) | 50.1 (16.8) | 45.6 (15.6) |
| Gender, n (%) | ||||
| Women | 3961 (55.2) | 4551 (56.1) | 3990 (55.9) | 12 502 (55.7) |
| Men | 3209 (44.8) | 3564 (43.9) | 3151 (44.1) | 9924 (44.3) |
| Ethnicity, n (%) | ||||
| White | 7109 (99.1) | 8039 (99.1) | 7015 (98.2) | 22 163 (98.8) |
| Other | 59 (0.8) | 69 (0.9) | 112 (1.6) | 240 (1.1) |
| Missing | 2 (0.0) | 7 (0.1) | 14 (0.2) | 23 (0.1) |
| BMI (kg m−2), n (%) | ||||
| <18.5 (Underweight) | 99 (1.4) | 116 (1.4) | 80 (1.1) | 295 (1.3) |
| 18.5–<25 (Desirable weight) | 3004 (41.9) | 2802 (34.5) | 1973 (27.6) | 7779 (34.7) |
| 25–<30 (Overweight) | 2349 (32.8) | 2812 (34.7) | 2430 (34.0) | 7591 (33.8) |
| ⩾30 (Obese) | 1215 (16.9) | 1662 (20.5) | 1593 (22.3) | 4470 (19.9) |
| Missing | 503 (7.0) | 723 (8.9) | 1065 (14.1) | 2291 (10.2) |
| WC (cm), n (%) | ||||
| M <79 W <68 (Low) | 875 (12.2) | 618 (7.6) | 221 (3.1) | 1714 (7.6) |
| M 79–<94 W 68–<80 (Reference) | 2910 (40.6) | 2750 (33.9) | 1547 (21.7) | 7207 (32.1) |
| M 94–<102 W 80–<88 (Moderate) | 1292 (18.0) | 1532 (18.9) | 1285 (18.0) | 4109 (18.3) |
| M ⩾102 W ⩾88 (High) | 1212 (16.9) | 1818 (22.4) | 1824 (25.5) | 4854 (21.6) |
| Missing | 881 (12.3) | 1397 (17.2) | 2264 (31.7) | 4542 (20.3) |
| WHR, n (%) | ||||
| M<0.85 W <0.7 (Low) | 783 (10.9) | 678 (8.4) | 308 (4.3) | 1769 (7.9) |
| M 0.85–<0.95 W 0.7–<0.8 (Reference) | 3378 (47.1) | 3358 (41.4) | 2063 (28.9) | 8799 (39.2) |
| M 0.95–<1 W 0.8–<0.85 (Moderate) | 1262 (17.6) | 1501 (18.5) | 1184 (16.6) | 3947 (17.6) |
| M ⩾1 W ⩾0.85 (High) | 861 (12.0) | 1174 (14.5) | 1307 (18.3) | 3342 (14.9) |
| Missing | 886 (12.4) | 1404 (17.3) | 2279 (31.9) | 4569 (20.4) |
| Smoking status, n (%) | ||||
| Never smoker | 3074 (42.9) | 3236 (39.9) | 3039 (42.6) | 9349 (41.7) |
| Ex-smoker | 1282 (17.9) | 1916 (23.6) | 2043 (28.6) | 5241 (23.4) |
| Light smoker | 597 (8.3) | 650 (8.0) | 548 (7.7) | 1795 (8.0) |
| Moderate smoker | 1058 (14.8) | 1162 (14.3) | 792 (11.1) | 3012 (13.4) |
| Heavy smoker | 1159 (16.2) | 1142 (14.1) | 715 (10.0) | 3016 (13.4) |
| Missing | — | 9 (0.1) | 4 (0.1) | 13 (0.1) |
| Alcohol consumption, n (%) | ||||
| Never/occasional drinker | 345 (4.8) | 479 (5.9) | 471 (6.6) | 1295 (5.8) |
| Ex-drinker | 258 (3.6) | 397 (4.9) | 348 (4.9) | 1003 (4.5) |
| 0–<7 units per week | 3139 (43.8) | 3537 (43.6) | 3133 (43.9) | 9809 (43.7) |
| 7–<14 units per week | 1280 (17.9) | 1391 (17.1) | 1316 (18.4) | 3987 (17.8) |
| 14–<21 units per week | 853 (11.9) | 885 (10.9) | 749 (10.5) | 2487 (11.1) |
| 21–<28 units per week | 478 (6.7) | 494 (6.1) | 405 (5.7) | 1377 (6.1) |
| ⩾28 units per week | 817 (11.4) | 920 (11.3) | 711 (10.0) | 2448 (10.9) |
| Missing | — | 12 (0.1) | 8 (0.1) | 20 (0.1) |
| Individual social class, n (%) | ||||
| I/II-Professional/managerial | 1885 (26.3) | 2257 (27.8) | 2334 (32.7) | 6476 (28.9) |
| III-Skilled non-manual | 1577 (22.0) | 1750 (21.6) | 1555 (21.8) | 4882 (21.7) |
| III-Skilled manual | 1487 (20.7) | 1661 (20.5) | 1327 (18.6) | 4475 (20.2) |
| IV-Partly skilled | 1188 (16.6) | 1423 (17.5) | 1220 (17.1) | 3831 (17.0) |
| V-Unskilled | 578 (8.1) | 605 (7.5) | 471 (6.6) | 1654 (7.4) |
| Other | 455 (6.3) | 419 (5.2) | 234 (3.3) | 1108 (4.8) |
| Carstairs quintile, n (%) | ||||
| 1-Least deprived | 1292 (18.0) | 1431 (17.7) | 1336 (18.7) | 4059 (18.1) |
| 2 | 1496 (20.9) | 1640 (20.2) | 1716 (24.0) | 4852 (21.6) |
| 3 | 1468 (20.5) | 1950 (24.0) | 1532 (21.5) | 4950 (22.1) |
| 4 | 1297 (18.1) | 1501 (18.5) | 1287 (18.0) | 4085 (18.2) |
| 5-Most deprived | 1613 (22.5) | 1585 (19.5) | 1270 (17.8) | 4468 (19.9) |
| Missing | 4 (0.1) | 8 (0.1) | — | 12 (0.1) |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; M, men; W, women; WC, waist circumference; WHR, waist–hip ratio.
Statistical analyses
For each of the anthropometric measures, Cox proportional hazards models were used to estimate hazard ratios (HRs) for all-cause, or cause-specific, mortality in a data set consisting of the three surveys combined. They were fitted within a multilevel framework to account for the data hierarchy of individuals within postcodes. The timescale was taken as the time since interview (months) and censoring occurred on 31 December 2007. The alternative approach of using age as the timescale was also investigated using a single level model. The following covariates were included in all models: age (centred), gender, smoking status, alcohol consumption and survey cohort. These models were then further adjusted for either individual social class or Carstairs area of deprivation. The second lowest category was used as referent for the anthropometric covariates; all other categorical covariates used the first category. Interactions between all covariables were explored. Stratification by gender and age groups (18–39, 40–64 and ⩾65 years) was performed. Adherence to the proportional hazards assumption was investigated by incorporation of time-dependent covariates in the models and by plotting smoothed Schoenfeld residuals against time; no violations of the assumption were identified. All statistical tests were two tailed, and statistical significance was taken as P<0.05.
We assumed that the missing anthropometric data were missing at random, that is the probability that data were missing was dependent on observed variables. This assumption prompted us to investigate the association between missing data and observed variables. The relationship between missing data for BMI, WC and WHR and survival time was explored using Cox proportional hazards model adjusted for age, gender, survey year, smoking and alcohol consumption. Mutually adjusted logistic regression models were used to investigate the association of age, gender, death during follow-up, survey year, smoking and alcohol consumption with the binary outcome of missing or not missing BMI, WC or WHR. In both these exploratory analyses, cases with missing data for smoking or alcohol consumption were excluded (n=25). These cases were also excluded when multiple imputation was used to replace missing data for BMI, WC and WHR. Five imputed data sets were created using a logistic regression method for monotone missing data (PROC MI in SAS v9.2, SAS Institute Inc., Cary, NC, USA). The covariates included in the logistic regression model used to inform the imputation were outcome (censored or died), age, gender, survey cohort, smoking status and alcohol consumption. Estimates from multilevel, multivariable survival analyses using the imputed data sets were averaged to produce overall estimates and standard errors were combined using Rubin's rules.21
Sensitivity analyses involving sequential, and independent, exclusion of previous illness, all ever smokers, early death during follow-up and individuals >64 years were performed. Using the linked Scottish Morbidity Records data, previous illness was defined as having a diagnosis of cancer or CVD prior to interview. Early death was defined as death within 2 years of interview. Separate analyses were also restricted to those who had died or were linked to the CHI in order to assess the impact of emigration or other alternative reasons for loss to follow-up. The analyses were also repeated incorporating survey weights that accounted for disproportionate sampling, differing probabilities of selection and differential response. The main analyses were also repeated restricting the age range to that in the 1995 survey (18–64 years). All the aforementioned analyses were performed using SAS v9.2 (SAS Institute Inc., Cary, NC, USA) and MLwiN v2.1122 statistical software.
The anthropometric measures were also fitted as continuous variables using fractional polynomials in fully adjusted single level models for all-cause mortality.23 Command mfp in Stata/IC 10.1 (StataCorp LP., College Station, TX, USA) was used to fit the most appropriate first-degree, or second-degree, fractional polynomial to the data using the best power transformation(s) from, among others, the reciprocal, logarithm, square, cube and linear (no transformation). Age was simultaneously fitted using the same selection process.
Results
Descriptive data for the three survey cohorts are provided in Table 2. In all three surveys, there was a greater proportion of women sampled (55.7%). The minimal ethnic variation precluded investigation of its influence. The prevalence of obesity as defined by BMI increased from 16.9% in 1995, to 20.5% in 1998, to 22.3% in 2003 (P<0.0001). A similar trend was also visible for the high categories of WC (men⩾102 cm, women⩾88 cm) and WHR (men⩾1, women⩾0.85) (Table 2). In the combined data set, the prevalence of obesity according to BMI was 19.9%, whereas the prevalence of high WC and high WHR was 21.6 and 14.9%, respectively (Table 2). Gender differences in the distribution of individuals according to the different measures was very similar except for WHR where a greater proportion of women were classed as having a high WHR (20.2%) compared with men (8.2%) (data not shown).
Association between BMI and all-cause mortality
There was no increased risk of mortality associated with BMI-defined obesity (HR=0.93; 95% confidence interval=0.80–1.08) (Table 3). Underweight BMI was associated with elevated HR for all-cause mortality (2.66; 1.97–3.60), whereas there was a significantly decreased risk associated with being overweight (0.80; 0.70–0.91). There was a significant interaction between gender and year of survey. Relative to women in 1995, the HR (95% confidence interval) for mortality in men decreased from 1.75 (1.44–2.13) in 1995 to 0.92 (0.69–1.22) by 2003. An interaction between age and smoking status indicated that for a 10-year increase in age, the risk of mortality was higher for those individuals that smoked a greater number of cigarettes.
Table 3. Multilevel, multivariate hazard ratios and 95% CIs for all-cause mortality in different BMI categories within a model adjusted for age, gender, smoking status, alcohol consumption and year of survey.
|
Deaths |
Hazard ratio (95% CI) | |||
|---|---|---|---|---|
| n (%) | n (%) | |||
| Fixed | ||||
| Age (10-year increase) | —a | |||
| Gender | ||||
| Women | 11 075 (55.1) | 593 (46.3) | —b | |
| Men | 9042 (44.9) | 687 (53.7) | ||
| BMI (kg m−2) | ||||
| <18.5 (Underweight) | 294 (1.5) | 49 (3.8) | 2.66 (1.97–3.60) | |
| 18.5–<25 (Desirable weight) | 7765 (38.6) | 437 (34.1) | 1 | |
| 25–<30 (Overweight) | 7589 (37.7) | 474 (37.0) | 0.80 (0.70–0.91) | |
| ⩾30 (Obese) | 4469 (22.2) | 320 (25.0) | 0.93 (0.80–1.08) | |
| Smoking status | ||||
| Never smoked | 8406 (41.8) | 277 (21.6) | —a | |
| Ex-smoker | 4662 (23.2) | 373 (29.1) | ||
| Light smoker | 1610 (8.0) | 119 (9.3) | ||
| Moderate smoker | 2716 (13.5) | 221 (17.3) | ||
| Heavy smoker | 2723 (13.5) | 290 (22.7) | ||
| Alcohol consumption | ||||
| Never/occasional drinker | 1089 (5.4) | 102 (8.0) | 1 | |
| Ex-drinker | 846 (4.2) | 112 (8.8) | 1.22 (0.92–1.60) | |
| 0–<7 units per week | 8783 (43.7) | 557 (43.5) | 0.95 (0.76–1.17) | |
| 7–<14 units per week | 3613 (18.0) | 180 (14.1) | 0.84 (0.65–1.07) | |
| 14–<21 units per week | 2289 (11.4) | 106 (8.3) | 0.79 (0.59–1.04) | |
| 21–<28 units per week | 1266 (6.3) | 73 (5.7) | 0.94 (0.68–1.28) | |
| ⩾28 units per week | 2231 (11.1) | 150 (11.7) | 1.01 (0.77–1.32) | |
| SHeSs | ||||
| 1995 | 6667 (33.1) | 448 (35.0) | —b | |
| 1998 | 7380 (36.7) | 626 (48.9) | ||
| 2003 | 6070 (30.2) | 206 (16.1) | ||
| Interactions | ||||
| Gender*SHeSs | ||||
| Women*1995 | 3634 (18.1) | 189 (14.8) | 1 | |
| Women*1998 | 4100 (20.4) | 300 (23.4) | 1.01 (0.83–1.23) | |
| Women*2003 | 3341 (16.6) | 104 (8.1) | 0.82 (0.63–1.08) | |
| Men*1995 | 3033 (15.1) | 259 (20.2) | 1.75 (1.44–2.13) | |
| Men*1998 | 3280 (16.3) | 326 (25.5) | 1.50 (1.23–1.84) | |
| Men*2003 | 2729 (13.6) | 102 (8.0) | 0.92 (0.69–1.22) | |
| Age (10-year increase)*smoking | ||||
| Age (10 years increase)*never smoked | — | — | 2.53 (2.30–2.79) | |
| Age (10 years increase)*ex-smoker | — | — | 3.65 (3.35–3.99) | |
| Age (10 years increase)*light smoker | — | — | 5.38 (4.71–6.15) | |
| Age (10 years increase)*moderate smoker | — | — | 6.75 (6.06–7.52) | |
| Age (10 years increase)*heavy smoker | — | — | 8.24 (7.36–9.23) | |
| Random | Estimate | s.e. | ||
| Variance at postcode sectors level | 0.03 | 0.03 | ||
| Total, n | 20 117 | 1280 | ||
Abbreviations: BMI, body mass index; CI, confidence interval; SHeSs, Scottish Health Surveys.
Interaction between age and smoking status.
Interaction between gender and year of SHeS.
Association between BMI and all-cause mortality after adjustment for socioeconomic status
Socioeconomic status was associated with mortality using either the individual or area-based measures (Table 4). Inclusion of socioeconomic status in the models attenuated the HR of the other variables to a limited extent, most noticeably for smoking status and for an underweight BMI. The limited variation at the postcode sector level was partly explained by adjustment for individual social class, whereas a greater proportion was explained by the separate inclusion of area of deprivation (Table 4). Stratification by gender revealed a steeper social class gradient for men than women, but there was no evidence of an interaction between BMI and social class (data not shown).
Table 4. Multilevel, multivariate adjusted hazard ratios for all-cause mortality in different body mass index categories adjusted separately by social class and area of deprivation.
|
Hazard ratio (95% CI) |
||||||
|---|---|---|---|---|---|---|
| BMI modela | BMI model adjusted for social classa | BMI model adjusted for deprivationa | ||||
| BMI (kg m−2) | ||||||
| <18.5 (underweight) | 2.63 (1.95–3.55) | 2.60 (1.93–3.51) | 2.56 (1.90–3.46) | |||
| 18.5–<25 (desirable weight) | 1 | 1 | 1 | |||
| 25–<30 (overweight) | 0.80 (0.70–0.91) | 0.79 (0.70–0.91) | 0.80 (0.70–0.91) | |||
| ⩾30 (obese) | 0.93 (0.80–1.07) | 0.91 (0.78–1.05) | 0.91 (0.79–1.06) | |||
| Individual social class | ||||||
| I/II-professional/managerial | — | 1 | — | |||
| III-skilled non-manual | — | 1.21 (1.00–1.46) | — | |||
| III-skilled manual | — | 1.60 (1.36–1.89) | — | |||
| IV-partly skilled | — | 1.61 (1.35–1.92) | — | |||
| V-unskilled | — | 1.53 (1.23–1.89) | — | |||
| Other | — | 1.55 (1.14–2.10) | — | |||
| Carstairs area of deprivation | ||||||
| 1-Least deprived | — | — | 1 | |||
| 2 | — | — | 1.07 (0.88–1.31) | |||
| 3 | — | — | 1.09 (0.90–1.33) | |||
| 4 | — | — | 1.37 (1.12–1.68) | |||
| 5-Most deprived | — | — | 1.63 (1.35–1.97) | |||
| Random | Estimate | s.e. | Estimate | s.e. | Estimate | s.e. |
| Variance at postcode sectors level | 0.03 | 0.03 | 0.02 | 0.03 | 0.003 | 0.03 |
| n | 20 117 | 20 117 | 20 106 | |||
| Deaths | 1280 | 1280 | 1279 | |||
Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; s.e., standard error.
BMI model: BMI categories adjusted for age, gender, smoking status, alcohol consumption and survey year.
Association between WC, or WHR, and all-cause mortality
The HR for subjects in the highest categories of WC was 1.17 (1.02–1.34) and of WHR was 1.34 (1.16–1.55) (Table 5). Other estimates for covariates in the adjusted model were very similar to those in the BMI-adjusted model (data not shown). Further adjusting these two measures for social class or area of deprivation had minimal effect on the estimates (data not shown). Any variation at the postcode sector level appeared to be fully explained by adjustment for area of deprivation. There were no significant interactions in these two models. There were no significant interactions between gender, or age, with any of the anthropometric measures. Age stratification did not significantly alter estimates for any of the measures (data not shown).
Table 5. Multilevel, multivariate adjusted hazard ratios for cause specific mortalities for categories of (a) body mass index, (b) waist circumference and (c) waist-hip ratio.
| (a) Body mass index | ||||
|---|---|---|---|---|
| Cause of death |
Hazard ratio (95% CI) BMI (kg m−2)a |
|||
| <18.5 (underweight) | 18.5–<25 (desirable weight) | 25–<30 (overweight) | ⩾30 (obese) | |
| All-cause | 2.63 (1.95–3.55) | 1 | 0.80 (0.70–0.91) | 0.93 (0.80–1.07) |
| Cardiovascular disease | 1.33 (0.62–2.87) | 1 | 1.08 (0.85–1.37) | 1.36 (1.05–1.77) |
| Coronary heart disease | 1.53 (0.62–3.80) | 1 | 1.09 (0.81–1.48) | 1.33 (0.96–1.85) |
| Other cardiovascular disease | 1.01 (0.24–4.19) | 1 | 1.05 (0.71–1.56) | 1.41 (0.93–2.14) |
| Cancer | 1.97 (1.13–3.42) | 1 | 0.75 (0.60–0.94) | 0.91 (0.72–1.16) |
| Lung cancer | 1.94 (0.81–4.63) | 1 | 0.72 (0.49–1.05) | 0.70 (0.44–1.11) |
| Obese related cancer | 1.54 (0.48–4.96) | 1 | 0.92 (0.63–1.33) | 1.01 (0.67–1.51) |
| Other cancer | 2.20 (0.88–5.51) | 1 | 0.66 (0.45–0.97) | 1.04 (0.71–1.54) |
| Non-cancer, non-CVD | 4.51 (3.00–6.80) | 1 | 0.65 (0.51–0.82) | 0.63 (0.48–0.84) |
| Chronic respiratory disease | 3.17 (1.39–7.21) | 1 | 0.43 (0.26–0.73) | 0.25 (0.12–0.55) |
| Acute respiratory disease | 11.68 (5.01–27.21) | 1 | 0.59 (0.30–1.14) | 0.97 (0.49–1.90) |
| Injury | 1.46 (0.19–11.18) | 1 | 0.71 (0.37–1.38) | 0.57 (0.24–1.36) |
| Miscellaneous | 3.82 (2.01–7.27) | 1 | 0.80 (0.58–1.10) | 0.80 (0.55–1.17) |
| (b) Waist circumference | ||||
| Cause of death |
Hazard ratio (95% CI) WC (cm)a |
|||
| |
M <79 W <68 (low) |
M 79–<94 W 68–<80 (reference) |
M 94–<102 W 80–<88 (moderate) |
M ⩾102 W ⩾88 (high) |
| All-cause | 1.63 (1.30–2.04) | 1 | 0.90 (0.77–1.05) | 1.17 (1.02–1.34) |
| Cardiovascular disease | 0.85 (0.50–1.46) | 1 | 1.08 (0.82–1.41) | 1.41 (1.11–1.79) |
| Coronary heart disease | 1.13 (0.61–2.10) | 1 | 1.06 (0.75–1.50) | 1.59 (1.18–2.16) |
| Other cardiovascular disease | 0.43 (0.13–1.38) | 1 | 1.11 (0.73–1.68) | 1.15 (0.79–1.70) |
| Cancer | 1.96 (1.38–2.78) | 1 | 0.97 (0.75–1.26) | 1.22 (0.97–1.54) |
| Lung cancer | 2.44 (1.38–4.31) | 1 | 0.97 (0.61–1.54) | 0.99 (0.64–1.53) |
| Obese related cancer | 1.86 (0.99–3.48) | 1 | 1.05 (0.67–1.64) | 1.45 (0.98–2.15) |
| Other cancer | 1.53 (0.81–2.89) | 1 | 0.93 (0.60–1.44) | 1.26 (0.86–1.84) |
| Non-cancer, non-CVD | 1.91 (1.34–2.72) | 1 | 0.66 (0.49–0.89) | 0.91 (0.70–1.17) |
| Chronic respiratory disease | 2.39 (1.18–4.84) | 1 | 0.68 (0.36–1.28) | 0.59 (0.33–1.06) |
| Acute respiratory disease | 2.75 (1.12–6.74) | 1 | 0.79 (0.37–1.68) | 1.17 (0.62–2.20) |
| Injury | 1.95 (0.79–4.80) | 1 | 0.48 (0.18–1.32) | 1.07 (0.49–2.34) |
| Miscellaneous | 1.33 (0.77–2.31) | 1 | 0.67 (0.45–1.01) | 0.98 (0.70–1.39) |
| (c) Waist-hip ratio | ||||
| Cause of death |
Hazard ratio (95% CI) WHRa |
|||
| |
M <0.85 W <0.7 (low) |
M 0.85–<0.95 W 0.7–<0.8 (reference) |
M 0.95–<1 W 0.8–<0.85 (moderate) |
M ⩾1 W ⩾0.85 (high) |
| All-cause | 1.23 (0.95–1.58) | 1 | 1.10 (0.95–1.26) | 1.34 (1.16–1.55) |
| Cardiovascular disease | 0.66 (0.37–1.16) | 1 | 1.22 (0.95–1.55) | 1.44 (1.12–1.85) |
| Coronary heart disease | 0.63 (0.30–1.29) | 1 | 1.24 (0.91–1.69) | 1.45 (1.06–2.00) |
| Other cardiovascular disease | 0.71 (0.28–1.77) | 1 | 1.17 (0.79–1.75) | 1.42 (0.95–2.12) |
| Cancer | 1.61 (1.09–2.38) | 1 | 1.09 (0.86–1.38) | 1.26 (0.99–1.61) |
| Lung cancer | 1.29 (0.63–2.66) | 1 | 0.75 (0.48–1.19) | 1.15 (0.75–1.77) |
| Obese related cancer | 1.39 (0.65–2.94) | 1 | 1.39 (0.93–2.08) | 1.56 (1.04–2.36) |
| Other cancer | 1.94 (1.07–3.51) | 1 | 1.18 (0.80–1.75) | 1.12 (0.74–1.70) |
| Non-cancer, non-CVD | 1.39 (0.91–2.12) | 1 | 0.98 (0.75–1.28) | 1.33 (1.02–1.74) |
| Chronic respiratory disease | 2.14 (0.80–5.75) | 1 | 1.23 (0.66–2.28) | 1.74 (0.96–3.16) |
| Acute respiratory disease | 1.91 (0.70–5.23) | 1 | 1.55 (0.83–2.90) | 1.06 (0.51–2.19) |
| Injury | 0.87 (0.32–2.37) | 1 | 0.59 (0.24–1.46) | 1.14 (0.47–2.78) |
| Miscellaneous | 1.05 (0.55–1.99) | 1 | 0.87 (0.60–1.27) | 1.36 (0.95–1.95) |
Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; WC, waist circumference; WHR, waist-hip ratio; CVD, cardiovascular disease; M, men; W, women.
Adjusted for age, gender, smoking status, alcohol consumption and survey year.
Association between the three anthropometric measures and cause-specific mortalities
The highest categories of all three measures were associated with an increased hazard of death caused by CVD (Table 5). For WC and WHR, the association with mortality was linear across categories with a positive gradient. The obese category of BMI was not associated with a significantly increased risk of coronary heart disease mortality. For deaths caused by other CVDs, that included stroke, there were no significant associations for any measure.
The pattern of association for all the anthropometric measures with cancer-related mortality was broadly similar to that identified for all-cause mortality. A significant increased risk was associated with the lowest categories. For the subcategories of cancer mortality, this significant association was only maintained in relation to lung cancer mortality for WC. For deaths caused by obesity-related cancers, only WHR demonstrated an increased risk in the highest category.
For the remaining causes of death that were non-cancer and non-CVD in aetiology participants in the lowest categories were associated with an increased risk of death for BMI and WC only. For WHR, there was only a significant increased risk for those in the high category. Underweight BMI was associated with elevated HR for acute (11.68; 5.01–27.21) and chronic respiratory disease mortality (3.17; 1.39–7.21); a similar but less extreme pattern was identified for the low category of WC but not for WHR. For overweight and obesity as defined by BMI, there was a significant protective association for chronic respiratory disease mortality.
Multiple imputation and sensitivity analyses
The proportion of missing values for BMI increased from 7% in 1995 to 14% in 2003; for WC and WHR, this proportion had increased to 32% by 2003 (Table 2). The participants with missing data for BMI, WC or WHR had significantly worse survival times than those with complete data, after adjusting for age, gender, survey year, smoking and alcohol consumption (Supplementary Table 1). In the adjusted logistic regression models, the missingness for BMI, WC and WHR was significantly associated with all the variables considered, except for smoking in the case of BMI (Supplementary Table 1). The estimates from the logistic regression models used for imputation are available in Supplementary Tables 2, 4 and 6.
The distribution of imputed BMI, WC and WHR values was very similar to that of observed values. Analyses using these data sets generated very similar estimates to the complete case models. The largest change in the models was lower HRs for the lowest categories of BMI (2.38; 1.72–3.27), WC (1.49; 1.20–1.84) and WHR (1.17; 0.93–1.49) (Supplementary Tables 3, 5 and 7). From the CHI, it was determined that 6.6% of participants had either migrated outwith Scotland, were untraceable or had no current link to the index. Analyses restricted to individuals that were registered on the CHI generated estimates comparable to those from the complete case analyses. Use of survey weights in complete case models for BMI, WC and WHR again provided estimates that were almost identical except for underweight BMI where the HR was reduced to 2.30 (1.66–3.18).
Analyses using age as the timescale generated estimates similar to those using time since interview (Supplementary Tables 8–11). For the main BMI model (Supplementary Table 8), the interaction between gender and survey year, identified in the time since interview model, was only just non-significant for inclusion. Sensitivity analyses sequentially excluding previous illness, ever smokers, early follow-up and individuals >64 years increased, rather than decreased, the HR estimates for underweight BMI (Table 6). Generally, sequential exclusions had the effect of reducing HR estimates towards unity for the lowest category of WC whereas risks increased for the highest category, although these estimates were almost all non-significant. This was also the case for WHR, and following exclusion of the first 2 years of follow-up, the relationship assumed a positive linear association. The estimates for the highest category increased in magnitude and remained significant. Overall, changes in estimates for the lowest categories for all three measures were minimal after exclusion of pre-existing disease and smoking; major alterations only occurred following exclusion of over half the subjects and almost 90% of the deaths. Therefore, results are reported for all participants with complete cases in this study. The independent exclusion of ever smokers generated a similar magnitude of change in estimates as when it was applied sequentially. When only respondents >64 years of age were excluded, estimates in the main models were largely unaltered (Supplementary Tables 12–16). The principal alteration was an increase in the HR estimate for underweight BMI in almost all models. Independent exclusion of the other two restrictions had minimal influence on estimates (data not shown).
Table 6. Sensitivity analyses involving multilevel, multivariable adjusted hazard ratios for categories of (a) body mass index, (b) waist circumference and (c) waist-hip ratio following sequential exclusion of 1. individuals with a diagnosis prior to interview of cancer or cardiovascular disease, 2. smokers and ex-smokers, 3. individuals who died within two years of interview and 4. individuals over 64 years of age.
| (a) body mass index | ||||||
|---|---|---|---|---|---|---|
| n | Deaths |
Hazard ratio (95% CI) BMI (kg m−2)a |
||||
| <18.5 (underweight) | 18.5–<25 (desirable weight) | 25–<30 (overweight) | ⩾30 (obese) | |||
| Full sample | 20 117 | 1280 | 2.63 (1.95–3.55) | 1 | 0.80 (0.70–0.91) | 0.93 (0.80–1.07) |
| Sequential exclusions: | ||||||
| Cancer and CVD | 18 826 | 978 | 2.54 (1.82–3.55) | 1 | 0.75 (0.65–0.87) | 0.87 (0.74–1.03) |
| Ever smokers | 8024 | 214 | 2.71 (0.84–8.70) | 1 | 0.68 (0.49–0.95) | 0.94 (0.67–1.32) |
| Death within 2 yrs | 7995 | 185 | 3.85 (1.19–12.49) | 1 | 0.83 (0.58–1.19) | 1.07 (0.73–1.56) |
| Individuals >64 yrs | 7268 | 126 | 4.52 (1.06–19.18) | 1 | 1.02 (0.66–1.59) | 1.09 (0.67–1.78) |
| (b) waist circumference | ||||||
| n | Deaths |
Hazard ratio (95% CI) WC (cm)a |
||||
| |
|
|
M <79 W<68 (low) |
M 79–<94 W 68–<80 (reference) |
M 94–<102 W 80–<88 (moderate) |
M ⩾102 W⩾88 (high) |
| Full sample | 17 867 | 1220 | 1.63 (1.30–2.04) | 1 | 0.90 (0.77–1.05) | 1.17 (1.02–1.34) |
| Sequential exclusions: | ||||||
| Cancer and CVD | 15 730 | 921 | 1.64 (1.28–2.10) | 1 | 0.92 (0.77–1.10) | 1.12 (0.96–1.31) |
| Ever smokers | 7024 | 204 | 1.24 (0.64–2.43) | 1 | 0.91 (0.63–1.34) | 1.34 (0.96–1.88) |
| Death within 2 yrs | 7024 | 180 | 1.00 (0.45–2.21) | 1 | 0.98 (0.66–1.47) | 1.46 (1.03–2.09) |
| Individuals >64 yrs | 6520 | 123 | 0.87 (0.34–2.22) | 1 | 1.04 (0.64–1.68) | 1.63 (1.06–2.50) |
| (c) waist-hip ratio | ||||||
| n | Deaths |
Hazard ratio (95% CI) WHRa |
||||
| |
|
|
M <0.85 W<0.7 (low) |
M 0.85–<0.95 W 0.7–<0.8 (reference) |
M 0.95–<1 W 0.8–<0.85 (moderate) |
M ⩾1 W⩾0.85 (high) |
| Full sample | 17 840 | 1218 | 1.22 (0.95–1.58) | 1 | 1.10 (0.95–1.26) | 1.34 (1.16–1.55) |
| Sequential exclusions: | ||||||
| Cancer and CVD | 15 708 | 919 | 1.19 (0.89–1.58) | 1 | 1.14 (0.96–1.34) | 1.34 (1.13–1.58) |
| Ever smokers | 7013 | 203 | 1.18 (0.62–2.23) | 1 | 1.43 (1.01–2.03) | 1.86 (1.30–2.66) |
| Death within 2 yrs | 7013 | 180 | 0.81 (0.37–1.76) | 1 | 1.44 (1.00–2.07) | 1.83 (1.26–2.67) |
| Individuals >64 yrs | 6511 | 123 | 0.73 (0.31–1.72) | 1 | 1.29 (0.83–2.01) | 1.76 (1.10–2.80) |
Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; WC, waist circumference; WHR, waist-hip ratio; CVD, cardiovascular disease; M, men; W, women.
Adjusted for age, gender, smoking status, alcohol consumption and survey year.
Fractional polynomials
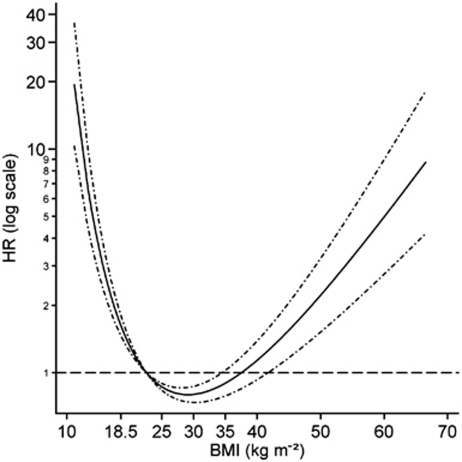
The association between continuous BMI and all-cause mortality was U shaped (fitted using two-term fractional polynomials), the nadir was in the region of 25–30 kg m−2 (Figure 1; Supplementary Table 18). HR point estimates (95% confidence interval) for the midpoints of the BMI categories, with the mean of the desirable weight category as reference, were: underweight (14.25 kg m−2)=4.80 (3.45–6.67), overweight (27.50 kg m−2)=0.81 (0.76–0.86) and obese (45.00 kg m−2)=1.56 (1.18–2.08) (Supplementary Table 17). The analyses for WC and WHR were stratified by gender, as significant interactions were identified between these variables. For both genders, a U-shaped relationship was identified for the association of WC with all-cause mortality (Supplementary Figure 1; Supplementary Tables 19 and 20). For both, the nadir was at the upper limit of the reference categories used in the categorical analyses. For WHR the association, for both men and women, was best described using a linear relationship; a steeper gradient was identified for women, whereas for men, there was no significant association (Supplementary Figure 2; Supplementary Tables 21 and 22). A linear relationship for age was deemed most appropriate in all these models.
Figure 1.
Functional form of the association of BMI with the relative hazard of death estimated in a Cox proportional hazards model adjusted for age, gender, smoking, alcohol consumption and survey year. The function was fitted using two-term fractional polynomial functions with powers (log, log). The function was standardized such that the HR was 1 at the mean of the desirable weight category for BMI (18.5–<25 kg m−2)=22.57 kg m−2. Dot-dash lines indicate the 95% confidence interval.
Discussion
We investigated the association of three proxy measures of adiposity with mortality using the SHeSs-linked data. It is widely accepted from epidemiological studies that obesity, as defined by BMI, is associated with increased mortality.3, 5, 24 It was therefore surprising that this association was not identified in this study; in fact, it was suggestive of a slightly protective relationship. The use of fractional polynomials in our data identified that the risk of death did not substantially increase until BMI>40 kg m−2 (morbid obesity). It has been recognized that even among those classed as obese, the excess mortality is principally confined to those who are morbidly obese.25 A number of studies that, like the SHeSs, have used cohorts recruited in more recent times have identified an attenuation of the mortality risk associated with BMI-defined obesity2, 18, 26, 27 compared with those with historically older cohorts.3, 24 In Flegal and colleagues' study, the obesity associated mortality risk was higher in the earliest recruited cohort than in later cohorts. Mortality among the obese category of BMI, and the high categories of WC and WHR, was largely restricted to CVD in our study, and in many others.3, 5, 7, 28 There has been a secular decline in CVD mortality as a result of changes in CVD risk factors, for example reduced smoking, and advances in medical care.2, 29 In Scotland, the mortality rate from ischemic heart disease and stroke has fallen from 593 per 1 00 000 (42%) of deaths in 1981 to 306 (31%) in 2001 among men; for women, the rate has reduced from 343 (40%) to 191 (30%).30 This would go some way to explain the weakening in the association between obesity and all-cause mortality when comparing recent and historic studies. Some researchers have refuted such a secular trend31 but based their evidence on a single cohort, not on a number of independent cohorts.32
Historically, recent studies will also tend to have shorter length of follow-up; in the current study, the maximum was 12 years 9 months. The burden of obesity might take a relatively long time to demonstrate a detrimental association with mortality, thus requiring a long follow-up.33 The ideal length of follow-up is difficult to determine as a long time between baseline and follow-up can result in increased misclassification error. It has been proposed that ⩾15 years is required before obesity will have its full effect on CVD mortality.34 One study, with an average of 5.7 years of follow-up, identified no association using BMI but did using other measures of fat proportion.26 The highest categories of WC or WHR were associated with increased all-cause mortality in this study. These two indices are thought to identify individuals with increased risk from obesity-related illness.16 According to Lean et al.,17 the highest category of WC identifies individuals requiring intervention to prevent health problems. This association with increased mortality may reflect the ability of these measures, unlike BMI, to assess the distribution of body fat and in particular visceral fat accumulation.35, 36 It has been proposed that the increased mortality risk associated with excess body fat is to some degree due to visceral adiposity.37 Abdominal obesity is associated with metabolic syndrome and the consequent elevation in CVD risk factors.38 WC and WHR have been identified as being stronger predictors of all-cause mortality than BMI.18, 26, 28 There is evidence that both are superior to BMI in the identification of CVD risk factors35, 39 and in the prediction of CVD events.40, 41 If these circumferential measures better reflect CVD mortality risks then it could be expected that these measures would also have superior performance for all-cause mortality.
Reis et al.42 identified a high correlation between WC and BMI and suggested this could imply WC is not only an indicator of abdominal adiposity but also overall obesity; WHR had a weaker correlation with BMI, perhaps indicating that it provides more unique information on fat distribution. WC reflects abdominal or intra-abdominal fat, whereas hip circumference reflects gluterofemerol muscle, bone and fat mass. It is the biological significance of the latter that is hard to deduce and consequently the exact meaning of WHR is unclear.43 In addition, WHR can be the same for the lean and the very obese. In our study, the highest category of WHR provided the strongest association with all-cause mortality. The relatively strong linear relationship for continuous WHR with all-cause mortality for women has been reported elsewhere.18, 26 Whether WC or WHR is the better measure is often debated; frequently, there is only a marginal difference in performance. Comparison is difficult as studies have often concentrated on certain age or gender groups as well as used different methodology, including different covariate categorization. Both WC and WHR have the potential for greater measurement error than BMI, although WC requires only one measurement. These two indices on their own, or in combination with BMI, could better capture the health risks of increased adiposity.44
Overweight as defined by BMI was not associated with an increased risk of mortality. This finding is similar to a number of other studies.2, 27, 45 BMI is believed to be a surrogate measure of general adiposity, but it is unable to distinguish between fat mass and lean mass, particularly for men and the elderly.46 Individuals within the overweight category may be fit and muscular rather than having excess fat. Although this might explain the decreased mortality associated with overweight in our analyses, it does not explain the lack of association between obesity and mortality. In the elderly, where most deaths occur, misclassification by BMI may occur because muscle mass tends to be replaced with fat mass as we age.37 BMI has been demonstrated to misclassify ⩾50% individuals with excess fat as being within desirable or just inside overweight categories, whereas those with healthy fat levels may be classified as being overweight or obese.46, 47 This misclassification could attenuate the apparent impact of excess adiposity on mortality. Fitting continuous BMI in a single level model using two-term fractional polynomials identified the nadir of the relationship with all-cause mortality to be in the overweight region, with decreased hazard of death commencing at the upper end of the desirable weight category and continuing into the obese category. Despite these findings, and arguments, overweight has been associated with an increased risk of mortality by some researchers.48 Even if overweight is not linked to increased mortality it appears to be, along with obesity, associated with elevated morbidity.45, 49
A protective effect of an elevated BMI, known as the ‘obesity paradox', has been demonstrated in patients with chronic heart failure.50 Recently, a U-shaped relationship for this has been described with increased mortality among the underweight and those with a BMI>45 kg m−2.51 Being overweight has also been shown to be protective in those over 65 years of age.42, 52, 53, 54 There were no significant interactions between age and any of the anthropometric measurements in this study. In addition, our sensitivity analyses, where we investigated age stratification or excluded those over 64 years of age, did not alter estimates to any great extent. Importantly, this age restriction confined the sample to the age group common to all three surveys (that is 18–64 years). The consequent minimal change in estimates encouraged us to include the older age groups from the later surveys in our analyses to maximize power. However, it is recognized that we were unable to fully explore the possible influence of old age on the relationship between body size mortality because of the relative scarcity of individuals from the older age group. The stage at which overweight or obesity occurs in the life course is undoubtedly important. Men who were overweight or obese in late adolescence were more likely to have a premature death in one study.55 In another, it was demonstrated that an individual overweight or obese in midlife, combined with weight loss in later life, may have the greatest mortality risk in old age.56 Such complex dynamics might serve to mask the harmful effects of overweight and obesity in epidemiological studies.
The inability of BMI to distinguish between fat mass and lean mass may also explain the increased risk for all-cause mortality associated with the underweight in this study and others.2, 45 One hypothesis is that fat mass is positively associated with mortality, whereas fat-free mass follows a negative relationship; that is a lack of lean mass can be detrimental.57 An alternative explanation for the increased mortality associated with the underweight, and the attenuation of the impact of overweight or obesity on mortality, is that residual confounding (effect modification) has not been correctly accounted for.58 This residual confounding is principally described as relating to smoking and pre-existing (occult) illness (although unknown confounders could also be responsible). Using a novel approach it has recently been demonstrated that the increased mortality associated with a low BMI might well be due to residual confounding.59 The exclusion of all smokers, pre-existing illness and early death during follow-up has been suggested to account for this problem.60 However, the sensitivity analyses performed in our investigation, and by other researchers, would not always support these claims.5, 61 One difficulty is that the exclusion of so many cases results in the loss of precision and power. A growing number of studies have advised against the use of exclusions to account for residual confounding.62, 63, 64, 65 Instead of treating it as an artefact, a low BMI, even within the healthy spectrum, could be associated with increased mortality.66
This is not the only study to find that underweight, as defined by BMI, was associated primarily with non-cancer and non-CVD mortality.7 To a lesser extent, this was also true for the lowest WC category. Respiratory disease, both acute and chronic, would appear to be largely responsible for this finding, concurring with a number of other investigations.3, 5, 7 In the current analyses, there was an increased HR related to cancer mortality for the lowest category of all three measures, the remaining categories having no association. The relationships reported by previous studies have been inconsistent. This has included: a weak or no association with body size,7, 18 a stronger positive relationship for women and a relatively weak relationship for men24 and a positive association for women (BMI>35 kg m−2) and an increased risk for underweight and obese men.5 WHR was the sole identifier of the increased risk of adiposity with obesity-related cancer mortality. Previously, obesity-related cancer mortality had been predicted by a BMI⩾30 kg m−2 in one large sample.7
In developed countries, the relationship between socioeconomic status and obesity has been shown to have an inverse association among women and to be inconsistent for men.11 One Scottish study demonstrated this inverse relationship for both men and women.67 In our study, despite there being evidence of social inequalities in mortality, there was limited support for deaths associated with body size being explained to any great extent by such inequalities. This concurs with a previous study investigating social inequalities in mortality, which did not identify any differences in the influence of risk factors (that included BMI) at different levels of education.68 Others have suggested that some, but by no means all, of the socioeconomic differential in mortality can be explained by smoking and BMI.69
Direct measurements were used to create the anthropometric indices thus eliminating any bias associated with self-reporting, namely BMI is increasingly under reported as actual BMI increases.70 The thresholds for the indices used in this study are somewhat arbitrary in nature, chiefly being derived from informed opinion under the umbrella of the WHO.16 Therefore, they may not be the optimum for determining the recommendations for a healthy weight. Although not practical, in epidemiological surveys, body fat proportion can be directly measured, for example using dual-energy x-ray absorption. Additionally, cutoff selection is problematic because obesity is defined as excess body fat but when this continuum becomes detrimental is unclear. It is also hard to explain what the lowest categories for WC and WHR represent biologically.
One of this study's strengths is that it used data from nationally representative surveys. Thus, the results may better reflect the general population than those from cohorts consisting of volunteers or those from select populations. It has been noted that estimated mortality risks from representative surveys have often been lower than those from such cohort studies.27, 62 The wide-ranging nature of the SHeSs permitted adjustment for a number of potentially confounding variables. Despite this, because of the observational nature of the study, we cannot exclude the possibility of residual confounding caused by unmeasured or imperfectly measured confounders. Error is also associated with the use of death certificates that might not reflect the actual cause, or the underlying diseases, that resulted in death. We did not adjust for physical activity or diet in this study as these could be considered to be on the causal pathway to obesity. Further, cholesterol and other biological markers were not adjusted for, as these are likely to be causal in CVD. Only individuals living in private households were surveyed, those living in communal establishments, such as residential care and prisons or those in the armed forces, will have been excluded. There is potential bias as a result of attrition from non-response to the original interview, non-consent to data linkage and refusal of the nurse visit. The declining response proportion in these surveys is of concern and brings into question how nationally representative they are; especially the most recent survey. We do believe the response proportion to the first two surveys to be relatively robust. Sensitivity analyses using survey weights that accounted for differential response did not alter estimates substantially. Unfortunately, it is not possible to assess differences between responders and non-responders using these data.
The largest component of missing data was for the anthropometric indices, particularly for the waist and hip measurements as they were conducted during the nurse visit. Complete data sets were created for use in analyses through multiple imputation thus avoiding potential bias that could arise through complete case analyses.71 We believe our exploratory analyses demonstrated a strong association between a range of demographic and health behaviour variables and missing data for BMI, WC and WHR. This suggests that our assumption that the missing mechanism for the anthropometric measures missing at random was reasonable. The estimates generated from the imputed data sets were similar to those in the complete case models, suggesting that the information lost by missing cases would not have had a major influence. There was no ability to assess changes in health behaviour either prior to or subsequent to the data capture, for example weight loss or weight gain. However, information was available from the data linkage concerning previous hospital admissions or cancer registrations. Individuals were assumed to be alive if they did not have a death record and this could lead to misclassification of émigrés who subsequently died. However, there is low emigration from Scotland and analyses limited to those on the CHI did not generate substantially different estimates.
It is important that as further follow-up becomes available from the SHeS-linked data that the associations between mortality and body size are re-analysed. The SHeS is being conducted on an annual basis from 2008 to 2011 and as data from these becomes available it should be possible to increase sample size and perhaps assess secular changes in the relationship between mortality and weight. Our results reaffirm that the mortality associated with obesity is predominately linked to CVD. Further analyses would be pertinent in light of the proposed influence on this relationship of recent improvements in medical care or changes in the prevalence of other risk factors. In conducting epidemiological studies investigating the impact of adiposity on health it would be prudent not to be completely reliant on BMI and to consider alternatives, which should include WC and WHR.
Acknowledgments
The Social and Public Health Sciences Unit is jointly funded by the Medical Research Council and the Chief Scientist Office of the Scottish Government Health Directorate. This research was funded by the Chief Scientist Office as part of the ‘Measuring health, variations in health and determinants of health' programme, wbs U.1300.00.001.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary Material
References
- Banegas JR, Lopez-Garcia E, Gutierrez-Fisac JL, Guallar-Castillon P, Rodriguez-Artalejo F. A simple estimate of mortality attributable to excess weight in the European Union. Eur J Clin Nutr. 2003;57:201–208. doi: 10.1038/sj.ejcn.1601538. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BJ, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- Prospective Studies Collaboration Body-mass index and cause-specific mortality in 9 00 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmarzyk PT, Craig CL, Bouchard C. Underweight, overweight and obesity: relationships with mortality in the 13-year follow-up of the Canada fitness survey. J Clin Epidemiol. 2001;54:916–920. doi: 10.1016/s0895-4356(01)00356-0. [DOI] [PubMed] [Google Scholar]
- Klenk J, Nagel G, Ulmer H, Strasak A, Concin H, Diem G, et al. Body mass index and mortality: results of a cohort of 1 84 697 adults in Austria. Eur J Epidemiol. 2009;24:83–91. doi: 10.1007/s10654-009-9312-4. [DOI] [PubMed] [Google Scholar]
- Stevens J, McClain JE, Truesdale KP. Selection of measures in epidemiologic studies of the consequences of obesity. Int J Obes. 2008;32:S60–S66. doi: 10.1038/ijo.2008.88. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BJ, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- Bromley C, Bradshaw P, Given L.(eds)The Scottish Health Survey 2008 The Scottish Government: Edinburgh; 2009 , http://www.scotland.gov.uk/Publications/2009/09/28102003/0 , accessed 9 July 2010. [Google Scholar]
- Hanlon P, Lawder R, Elders A, Clark D, Walsh D, Whyte B, et al. An analysis of the link between behavioural, biological and social risk factors and subsequent hospital admission in Scotland. J Public Health-UK. 2007;29:405–412. doi: 10.1093/pubmed/fdm062. [DOI] [PubMed] [Google Scholar]
- Mackenbach JP, Stirbu I, Roskam A-JR, Schaap MM, Menvielle G, Leinsalu M, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358:2468–2481. doi: 10.1056/NEJMsa0707519. [DOI] [PubMed] [Google Scholar]
- McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- Dong W, Erens B.(eds)Scottish Health Survey 1995 The Stationery Office: Edinburgh; 1997 , http://www.archive.official-documents.co.uk/document/scottish/shealth/shhm.htm , accessed 9 July 2010. [Google Scholar]
- Shaw A, McMunn A, Field J.(eds).The Scottish Health Survey 1998 The Stationery Office: Edinburgh; 2000 , http://www.sehd.scot.nhs.uk/scottishhealthsurvey/sh8-00.html , accessed 9 July 2010. [Google Scholar]
- Bromley C, Sproston K, Shelton N.(eds).The Scottish Health Survey 2003 The Stationery Office: Edinburgh; 2005 , http://www.scotland.gov.uk/Publications/2005/12/02160336/03367 , accessed 9 July 2010. [Google Scholar]
- Gray L, Batty GD, Craig P, Stewart C, Whyte B, Finlayson A, et al. Cohort profile: the Scottish health surveys cohort: linkage of study participants to routinely collected records for mortality, hospital discharge, cancer and offspring birth characteristics in three nationwide studies. Int J Epidemiol. 2010;39:345–350. doi: 10.1093/ije/dyp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Obesity: Preventing and Managing the Global EpidemicWHO Technical Report Series 8942000 , http://whqlibdoc.who.int/trs/WHO_TRS_894.pdf , accessed 9 July 2010. [PubMed]
- Lean MEJ, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. Br Med J. 1995;311:158–161. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JA, MacInnis RJ, Peeters A, Hopper JL, Giles GG, English DR. A comparison of adiposity measures as predictors of all-cause mortality: the Melbourne Collaborative Cohort Study. Obesity. 2007;15:994–1003. doi: 10.1038/oby.2007.622. [DOI] [PubMed] [Google Scholar]
- McLoone P.Carstairs Scores for Scottish Postcode Sectors from the 2001 Census Medical Research Council Social & Public Health Sciences Unit: Glasgow; 2004 , http://www.sphsu.mrc.ac.uk/files/File/library/other%20reports/Carstairs_report.pdf , accessed 9 July 2010. [Google Scholar]
- Anderson RN, Minino AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2001;49:1–32. [PubMed] [Google Scholar]
- Rubin D. Multiple Imputation for Nonresponse in Surveys. Wiley: New York; 1987. [Google Scholar]
- Rasbash J, Browne WJ, Healy M, Cameron B. MLwiN Version 2.1. Centre for Multilevel Modelling. University of Bristol: Bristol; 2009. [Google Scholar]
- Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body mass index and mortality in a prospective cohort of US adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- Bender R, Trautner C, Spraul M, Berger M. Assessment of excess mortality in obesity. Am J Epidemiol. 1998;147:42–48. doi: 10.1093/oxfordjournals.aje.a009365. [DOI] [PubMed] [Google Scholar]
- Lahmann PH, Lissner L, Gullberg B, Berglund G. A prospective study of adiposity and all-cause mortality: the Malmo diet and cancer study. Obes Res. 2002;10:361–369. doi: 10.1038/oby.2002.50. [DOI] [PubMed] [Google Scholar]
- Orpana HM, Berthelot J-M, Kaplan MS, Feeny DH, McFarland B, Ross NA. BMI and mortality: results from a national longitudinal study of Canadian adults. Obesity. 2010;18:214–218. doi: 10.1038/oby.2009.191. [DOI] [PubMed] [Google Scholar]
- Welborn TA, Dhaliwal SS. Preferred clinical measures of central obesity for predicting mortality. Eur J Clin Nutr. 2007;61:1373–1379. doi: 10.1038/sj.ejcn.1602656. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Cheng YLJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- Leyland AH, Dundas R, McLoone P, Boddy FA.Inequalities in Mortality in Scotland Medical Research Council Social & Public Health Sciences Unit: Glasgow; 2007. Report no.: 16, http://www.sphsu.mrc.ac.uk/files/File/reports/OP016.pdf , accessed 9 July 2010. [Google Scholar]
- Calle EE, Teras LR, Thun MJ. Obesity and mortality. N Engl J Med. 2005;353:2197–2199. doi: 10.1056/NEJM200511173532020. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail M. Weight associated deaths in the United States. J Womens Health. 2007;16:1368–1370. doi: 10.1089/jwh.2007.0547. [DOI] [PubMed] [Google Scholar]
- Sjostrom LV. Mortality of severely obese subjects. Am J Clin Nutr. 1992;55:516S–523C. doi: 10.1093/ajcn/55.2.516s. [DOI] [PubMed] [Google Scholar]
- Dyer AR, Stamler J, Garside DB, Greenland P. Long-term consequences of body mass index for cardiovascular mortality: the Chicago Heart Association Detection Project in Industry study. Ann Epidemiol. 2004;14:101–108. doi: 10.1016/S1047-2797(03)00121-2. [DOI] [PubMed] [Google Scholar]
- Pouliot M-C, Despres J-P, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- Lemieux S, Prud'homme D, Bouchard C, Tremblay A, Despres J-P. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64:685–693. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- Snijder MB, Van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them. Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Zhu S, Heymsfield SB, Toyoshima H, Wang Z, Pietrobelli A, Heshka S. Race-ethnicity-specific waist circumference cutoffs for identifying cardiovascular disease risk factors. Am J Clin Nutr. 2005;81:409–415. doi: 10.1093/ajcn.81.2.409. [DOI] [PubMed] [Google Scholar]
- de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28:850–856. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- Reis JP, Macera CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL. Comparison of overall obesity and body fat distribution in predicting risk mortality. Obesity. 2009;17:1232–1239. doi: 10.1038/oby.2008.664. [DOI] [PubMed] [Google Scholar]
- Bigaard J, Frederiksen K, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL, et al. Waist and hip circumferences and all-cause mortality: usefulness of the waist-to-hip ratio. Int J Obes. 2004;28:741–747. doi: 10.1038/sj.ijo.0802635. [DOI] [PubMed] [Google Scholar]
- Pischon T, Boeing H, Hoffman K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- Ringback-Weitoft G, Eliasson M, Rosen M. Underweight, overweight and obesity as risk factors for mortality and hospitalization. Scand J Public Health. 2008;36:169–176. doi: 10.1177/1403494807085080. [DOI] [PubMed] [Google Scholar]
- Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazon-Clavell ML, Korinek J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959–996. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes Relat Metab Disord. 2010;34:791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–788. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham L, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9 doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- Kapoor JR, Heidenreich PA. Obesity and survival in patients with heart failure and preserved systolic function: a U-shaped relationship. Am Heart J. 2010;159:75–80. doi: 10.1016/j.ahj.2009.10.026. [DOI] [PubMed] [Google Scholar]
- Auyeung TW, Lee JSW, Leung J, Kwok T, Leung PC, Woo J. Survival in older men may benefit from being slightly overweight and centrally obese—a 5-year follow-up study in 4000 older adults using DXA. J Gerontol A Biol Sci Med Sci. 2010;65A:99–104. doi: 10.1093/gerona/glp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R, Jockel KH, Trautner C, Spraul M, Berger M. Effect of age on excess mortality in obesity. JAMA. 1999;281:1498–1504. doi: 10.1001/jama.281.16.1498. [DOI] [PubMed] [Google Scholar]
- Janssen I, Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev. 2007;8:41–59. doi: 10.1111/j.1467-789X.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Neovius M, Sundstrom J, Rasmussen F. Combined effects of overweight and smoking in late adolescence on subsequent mortality: nationwide cohort study. Br Med J. 2009;338:b496. doi: 10.1136/bmj.b496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg TE, Strandberg AY, Salomaa VV, Pitkala KH, Tilvis RS, Sirola J, et al. Explaining the obesity paradox: cardiovascular risk, weight change, and mortality during long-term follow-up in men. Eur Heart J. 2009;30:1720–1727. doi: 10.1093/eurheartj/ehp162. [DOI] [PubMed] [Google Scholar]
- Allison DB, Zhu S, Plankey M, Faith MS, Heo M. Differential associations of body mass index and adiposity with all-cause mortality among men in the first and second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) follow-up studies. Int J Obes. 2002;26:410–416. doi: 10.1038/sj.ijo.0801925. [DOI] [PubMed] [Google Scholar]
- Manson JE, Bassuk SS, Hu FB, Stampfer MJ, Colditz GA, Willett WC. Estimating the number of deaths due to obesity: can the divergent findings be reconciled. J Womens Health. 2007;16:168–176. doi: 10.1089/jwh.2006.0080. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, Sterne JAC, Fraser A, Tynelius P, Lawlor DA, Rasmussen F. The association between BMI and mortality using offspring BMI as an indicator of own BMI: large intergenerational mortality study. Br Med J. 2009;339:b5043. doi: 10.1136/bmj.b5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Stampfer MJ, Hennekens CH, Willett WC. Body weight and longevity: a reassessment. JAMA. 1987;257:353–358. [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Impact of smoking and preexisting illness on estimates of the fractions of deaths associated with underweight, overweight, and obesity in the US population. Am J Epidemiol. 2007;166:975–982. doi: 10.1093/aje/kwm152. [DOI] [PubMed] [Google Scholar]
- Cooper RS. Which factors confound or modify the relationship between body weight and mortality. Int J Obes. 2008;32:S47–S51. doi: 10.1038/ijo.2008.85. [DOI] [PubMed] [Google Scholar]
- Diverse Populations Collaborative Group Effect of smoking on the body mass index-mortality relation: empirical evidence from 15 studies. Am J Epidemiol. 1999;150:1297–1308. doi: 10.1093/oxfordjournals.aje.a009961. [DOI] [PubMed] [Google Scholar]
- Allison DB, Heo M, Flanders DW, Faith MS, Carpenter KM, Williamson DF. Simulation study of the effects of excluding early deaths on risk factor-mortality analyses in the presence of confounding due to occult disease: the example of body mass index. Ann Epidemiol. 1999;9:132–142. doi: 10.1016/s1047-2797(98)00039-8. [DOI] [PubMed] [Google Scholar]
- Sempos CT, Durazo-Arvisu RA, McGee DL, Cooper RS, Prewitt TE. The influence of cigarette smoking on the association between body weight and mortality. The Framingham heart study revisited. Ann Epidemiol. 1998;8:289–300. doi: 10.1016/s1047-2797(97)00233-0. [DOI] [PubMed] [Google Scholar]
- Lopez-Jimenez F. Speakable and unspeakable facts about BMI and mortality. Lancet. 2009;373:1055–1056. doi: 10.1016/S0140-6736(09)60628-0. [DOI] [PubMed] [Google Scholar]
- Chen R, Tunstall-Pedoe H. Socioeconomic deprivation and waist circumference in men and women: the Scottish MONICA surveys 1989-1995. Eur J Epidemiol. 2005;20:141–147. doi: 10.1007/s10654-004-4498-y. [DOI] [PubMed] [Google Scholar]
- Schnohr C, Hojbjerre L, Riegels M, Ledet L, Larsen T, Schultz-Larsen K, et al. Does educational level influence the effects of smoking, alcohol, physical activity, and obesity on mortality? A prospective population study. Scand J Public Health. 2004;32:250–256. doi: 10.1080/14034940310019489. [DOI] [PubMed] [Google Scholar]
- McFadden E, Luben R, Wareham N, Bingham S, Khaw KT. Occupational social class, educational level, smoking and body mass index, and cause-specific mortality in men and women: a prospective study in the European Prospective Investigation of Cancer and Nutrition in Norfolk (EPIC-Norfolk) cohort. Eur J Epidemiol. 2008;23:511–522. doi: 10.1007/s10654-008-9267-x. [DOI] [PubMed] [Google Scholar]
- Niedhammer I, Bugel I, Bonenfant S, Goldberg M, Leclerc A. Validity of self-reported weight and height in French GAZEL cohort. Int J Obes. 2000;24:1111–1118. doi: 10.1038/sj.ijo.0801375. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Br Med J. 2009;339:157–160. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.