Abstract
Asymmetry of inner and outer leaflet lipid composition is an important characteristic of eukaryotic plasma membranes. We previously described a technique in which methyl-β-cyclodextrin-induced lipid exchange is used to prepare biological membrane-like asymmetric small unilamellar vesicles (SUVs). Here, to mimic plasma membranes more closely, we used a lipid-exchange-based method to prepare asymmetric large unilamellar vesicles (LUVs), which have less membrane curvature than SUVs. Asymmetric LUVs in which sphingomyelin (SM) or SM + 1-palmitoyl-2-oleoyl-phosphatidylcholine was exchanged into the outer leaflet of vesicles composed of 1,2-dioleoyl-phosphatidylethanolamine (DOPE) and 1-palmitoyl-2-oleoyl-phosphatidylserine (POPS) were prepared with or without cholesterol. Approximately 80–100% replacement of outer leaflet DOPE and POPS was achieved. At room temperature, SM exchange into the outer leaflet increased the inner leaflet lipid order, suggesting significant interleaflet interaction. However, the SM-rich outer leaflet formed an ordered state, melting with a midpoint at ∼37°C. This was about the same value observed in pure SM vesicles, and was significantly higher than that observed in symmetric vesicles with the same SM content, which melted at ∼20°C. In other words, ordered state formation by outer-leaflet SM in asymmetric vesicles was not destabilized by an inner leaflet composed of DOPE and POPS. These properties suggest that the coupling between the physical states of the outer and inner leaflets in these asymmetric LUVs becomes very weak as the temperature approaches 37°C. Overall, the properties of asymmetric LUVs were very similar to those previously observed in asymmetric SUVs, indicating that they do not arise from the high membrane curvature of asymmetric SUVs.
Introduction
In mammalian plasma membranes, the aminophospholipids phosphatidylethanolamine (PE) and phosphatidylserine (PS) predominate in the inner leaflet, and phosphatidylcholine (PC) and sphingolipids predominate in the outer leaflet (1). The different lipid compositions in the inner and outer leaflets of the bilayer may affect various aspects of membrane structure and function, including the formation of lipid domains. Studies with symmetric model membranes have demonstrated that cholesterol molecules can tightly pack with lipids having saturated acyl chains (such as sphingolipids) to form detergent-resistant liquid-ordered (Lo) state domains. In cells, such domains may exist as lipid rafts that coexist with liquid-disordered (Ld) state domains rich in unsaturated phospholipids (2). Ordered domain formation can be detected in symmetric model membranes with lipid mixtures mimicking the outer leaflet (3), but not in membranes with lipid mixtures imitating the inner leaflet (4). Nevertheless, the recovery of PE (5) and inner-leaflet-associated proteins (6) in detergent-resistant membranes that may be derived from lipid rafts in cells seems to suggest the presence of lipid rafts in the inner leaflet of plasma membranes. To address the question of whether outer-leaflet ordered domains can form in asymmetric cell membranes and induce the formation of ordered domains in the inner leaflet of such membranes, it is necessary to develop model membranes with asymmetric inner- and outer-leaflet lipid compositions.
Increasing efforts are being made to prepare asymmetric model membranes (7–16) and use them to investigate ordered domain formation. Recent studies revealed that in asymmetric planar bilayers mimicking the lipid asymmetry of the plasma membrane, the formation of ordered domains in one leaflet can induce the occurrence of ordered domains in the other leaflet (10). By tuning the lipid composition of one leaflet, Collins and Keller (8) further showed that domains either induced or suppressed domain formation across asymmetric unsupported planar bilayers.
Nevertheless, for studies of domain formation and other membrane properties, it would be desirable to have more generally applicable methods to form lipid vesicles with highly controlled lipid asymmetry. In a recent work (7), we prepared asymmetric small unilamellar vesicles (SUVs) containing saturated phospholipids in the outer leaflet and unsaturated phospholipids in the inner leaflet using a methyl-β-cyclodextrin (MβCD)-induced lipid exchange method. In a second study (11), we prepared asymmetric giant unilamellar vesicles (GUVs) and used them to investigate coupling between inner- and outer-leaflet physical properties via microscopy methods. However, the most widely applicable model of membrane vesicles may be that of large unilamellar vesicles (LUVs), which can be used in a greater variety of spectroscopic and biochemical studies than GUVs and avoid the very high curvature of SUVs. In this report, a method of preparing asymmetric LUVs is described. The physical properties of asymmetric LUVs provide interesting insights into the consequences of asymmetry mimicking that found in mammalian plasma membranes.
Materials and Methods
Materials
Porcine brain SM, 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC), 1,2-dioleoylphosphatidylethanolamine (DOPE), 1-palmitoyl-2-oleoyl-phosphatidyl-L-serine (POPS), and cholesterol were purchased from Avanti Polar Lipids (Alabaster, AL). 1,6-Diphenyl-1,3,5-hexatriene (DPH), MβCD, and alamethicin were purchased from Sigma-Aldrich (St. Louis, MO). 1-(4-Trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene p-toluenesulfonate (TMADPH) was purchased from the Molecular Probes division of Invitrogen (Carlsbad, CA). The lipids were dissolved in chloroform and stored at –20°C. DPH and TMADPH were dissolved in ethanol. Concentrations were determined as described previously (7). pL4A18 peptide (acetyl-K2LA9LWLA9LK2-amide) was purchased from Anaspec (San Jose, CA) and purified via reverse-phase high-performance liquid chromatography (see below). Sephacryl S-200 was purchased from Amersham Biosciences (Piscataway, NJ). High-performance thin layer chromatography (HP-TLC) plates (Silica Gel 60) were purchased from VWR International (Batavia, IL).
Ordinary (symmetric) vesicle preparation
Preparation of the vesicles and subsequent procedures were carried out at room temperature except when otherwise noted.
To prepare multilamellar vesicles (MLVs), lipid mixtures were mixed and dried under nitrogen followed by high vacuum for at least 1 h, dispersed at 70°C in phosphate-buffered saline (PBS; 1.8 mM KH2PO4, 10 mM Na2HPO4, 137 mM NaCl, and 2.7 mM KCl at pH 7.4), and vortexed in a multitube vortexer (VWR International, West Chester, PA) at 55°C for 15 min. In the case of SM MLVs (POPC MLV did not pellet well), to remove any small vesicles present before lipid exchange, the preparation was centrifuged at 11,000 × g for 5 min at room temperature. After the supernatant was discarded, the pellet obtained was resuspended to the original volume with PBS and then used for further experiments.
To prepare LUVs, MLVs containing 8 mM lipids were prepared in 1 ml of a solution containing 25% (w/v) sucrose dissolved in water. The MLVs were freeze/thawed for five cycles in (dry ice + acetone)/water and then passed through 100-nm or 200-nm polycarbonate filters (Avanti Polar Lipids) 11 times to obtain LUVs of uniform vesicle size. To wash away untrapped sucrose, the resulting LUV solutions were mixed with 3 ml PBS and subjected to ultracentrifugation at 190,000 × g for 45 min using a Beckman L8-55M ultracentrifuge. After the supernatant was discarded, the LUV pellet was resuspended to the original volume with PBS. LUV suspensions were diluted to the desired concentration (generally 4 mM) with PBS for further experiments.
Cholesterol-loaded MβCD preparation
Generally, 100 μmol of MβCD were dissolved in 600 μl of methanol and mixed with 30.8 μmol of cholesterol by vortexing at room temperature. The mixture was dried by nitrogen followed by high vacuum for at least 1 h and then dispersed in 2 ml of PBS. The resulting solution (which was turbid due to excess cholesterol) was sonicated in a bath sonicator (special ultrasonic cleaner, model G1112SP1; Laboratory Supplies, Hicksville, NY) for 3 min and then incubated in a shaker at 37°C overnight. The cholesterol-loaded MβCD (CLC)-containing solution was then filtered with a 0.22-μm-pore-size syringe filter, and the filtrate was used in subsequent experiments.
Asymmetric LUV preparation
First, 500 μl of the resuspended pellet from a 16 mM SM MLV preparation (see above) and 95 μl of 390 mM MβCD (1 ml water added to 825 mg MβCD) were mixed and vortexed in the multitube vortexer at 55°C for 2 h. Then 500 μl of 4 mM 2:1 DOPE/POPS LUVs (with entrapped 25 w/v% sucrose; see above) were added to the MLV-MβCD mixture. After the mixture was vortexed at 55°C for 30 min and then cooled for 10 min, it was overlaid onto 3 ml of 10 w/v % sucrose solution and subjected to ultracentrifugation at 190,000 × g for 45 min at room temperature. After the supernatant was removed, the resulting pellet was resuspended with 1 ml of 10% sucrose solution, overlaid onto a 3 ml of 10% sucrose solution, and again subjected to ultracentrifugation. The final pellet was resuspended in 1 ml PBS and then used for further experiments.
To prepare asymmetric LUVs with ∼20% CHOL (SMo/DOPE/POPSi/CHOL LUVs), 15 μl of CLC (see above) were added to 1 ml of the resuspended SMo/DOPE/POPSi LUVs and incubated for 30 min at 55°C. The mixture cooled to room temperature for 5 min. It was then chromatographed in PBS on a Sephacryl S-200 column (7 cm long, 1 cm in diameter), and 0.5-ml fractions were collected. Fractions 5–8 were combined and used for further experiments.
To prepare SM/POPC outside and DOPE/POPS inside LUVs (SM/POPCo/DOPE/POPSi LUVs), we prepared exchange vesicles using mixed MLVs. First, 250 μl of 16 mM SM MLVs were mixed with 47.5 μl of 390 mM MβCD, and 250 μl of 16 mM POPC MLVs were mixed with 47.5 μl of 390 mM MβCD. Each mixture was vortexed with the use of a multitube vortexer at 55°C for 2 h, and the two MLV-MβCD mixtures were then combined in one glass tube. Next, 500 μl of 4 mM 2:1 DOPE/POPS LUVs were added to the tube with the MLV-MβCD mixtures and vortexed at 55°C for 30 min. The exchanged LUVs were then isolated as described above.
Steady-state fluorescence anisotropy measurements
We measured fluorescence and fluorescence anisotropy on a SPEX FluoroLog 3 spectrofluorometer (Jobin-Yvon, Edison, NJ) with quartz semimicro cuvettes (excitation path length: 10 mm; emission: 4 mm) according to previously described protocols (7).
Alamethicin experiments
For samples containing alamethicin, 2 μl of alamethicin (from a 250 μM in ethanol stock solution) were added into ∼100 μM lipid LUV suspension to yield a final alamethicin concentration of ∼0.5 μM. After a 15-min incubation at room temperature, blank fluorescence anisotropy was measured and then DPH or TMADPH was added and fluorescence anisotropy was measured as described previously (7).
Re-reconstitution experiments
The LUV suspension was divided into four tubes (250 μl/tube), and 750 μl of PBS were then added to each aliquot. Two aliquots were used to measure DPH and TMADPH fluorescence anisotropy, and the other two aliquots were first subjected to re-reconstitution. To that end, the samples were first dried by a nitrogen stream. Next, 500 μl of ethanol were added to each tube to dissolve the dried lipids. After the ethanol was dried by a nitrogen stream and then high vacuum for 1 h, 1 ml of 70°C prewarmed distilled water was added to rehydrate the dried lipids. The samples were mixed by vortexing for at least 15 min in a 55°C shaker, followed by freeze/thawing for five cycles. They were then allowed to reach room temperature, and DPH or TMADPH was added and fluorescence anisotropy measured as described previously (7). In control experiments, ordinary (symmetric) vesicles were dried and then re-reconstituted in the same manner.
HP-TLC
Asymmetric LUV samples were extracted with 2:2:1 (v/v) chloroform/methanol/(water + sample solution). After 5 min of low-speed centrifugation, the upper aqueous phase was discarded and the lower phase (containing the lipid extract) was dried with nitrogen. The dried lipid film was redissolved in 20 μl of 1:1 (v/v) chloroform/methanol, and 5 μl were spotted on an HP-TLC plate. Lipid standards were prepared and extracted by analogous procedures before loading on HP-TLC. The samples were then chromatographed on HP-TLC using a dual solvent system as described previously (7).
Peptide-vesicle interaction experiments
We purified pL4A18 peptide via reverse-phase high-performance liquid chromatography using a C18 column as described previously (17). The purified peptide was dried under a nitrogen stream, redissolved in 1:1 (v/v) water/2-propanol, and stored at 4°C. To examine the interaction between pL4A18 peptides and vesicles, we added 1 mol % (relative to lipid) of pL4A18 peptide to preformed symmetric or asymmetric LUVs and then measured Trp fluorescence after a 5-min incubation at room temperature. We obtained Trp fluorescence emission spectra measurements on a SPEX 2 FluoroLog spectrofluorometer with quartz semimicro cuvettes at room temperature as described previously (7).
Dynamic light scattering measurements
We determined the sizes of the LUVs by dynamic light scattering (DLS) using a ProteinSolution DynaPro instrument (Wyatt Technology, Santa Barbara, CA) at 20°C. To avoid interference from impurities in the buffer, we prepared 2.5–5 μM of symmetric LUVs using 0.22-μm-filtered PBS and 500- to 1000-fold diluted asymmetric LUVs using 0.22-μm-filtered PBS. Vesicle sizes were estimated with the use of the Dynamics V5.25.44 program supplied by Wyatt Technology. For samples containing alamethicin, vesicles were incubated with alamethicin for 15 min at room temperature before DLS measurements were made.
Results
Asymmetric LUV preparations
We prepared asymmetric LUVs with SM in the outer leaflet and 2:1 mol/mol DOPE/POPS in the inner leaflet (designated as SMo/2:1 DOPE/POPSi LUVs) by MβCD-induced lipid exchange using sucrose-loaded DOPE/POPS LUV and SM MLV. Because LUVs cannot be separated from MLVs by size, we altered the density of DOPE/POPS LUVs by trapping 25% sucrose inside them, and separated the exchanged donor and acceptor vesicle populations by ultracentrifugation to isolate an LUV containing pellet (see Materials and Methods, and Fig. S1 in the Supporting Material). As shown in Fig. S1 (lanes 3 and 4), SM MLVs were too light to form a pellet when the samples were overlaid onto a solution of 10% sucrose in water and subjected to ultracentrifugation. This was true in both the presence and absence of MβCD. DOPE/POPS LUVs, on the other hand, pelleted after centrifugation (lane 5). When we compared samples in which DOPE/POPS vesicles and SM vesicles were mixed with or without MβCD treatment (lanes 1 and 2), we only detected SM in the pellet when MβCD was present, indicating that the lipid exchange was MβCD-dependent. LUV size remained the same after exchange (Fig. S2), indicating that vesicle-vesicle fusion did not occur during asymmetric vesicle preparation. An HP-TLC analysis of the final pellet dispersed in 1 ml PBS showed that this method yielded 291 ± 174 μM (n = 8) or 715 ± 190 μM (n = 6) lipid using LUVs initially prepared with 100-nm- or 200-nm-pore-size filters, respectively.
Vesicle size and lipid content were then analyzed. DLS showed an average vesicle size of ∼120 nm diameter when LUVs were prepared with 100-nm-pore-size filters (Fig. S2). Assuming that a membrane bilayer is ∼4 nm thick (18), the calculated outer-leaflet area of the LUVs was ∼53% of total surface area in SMo/DOPE/POPSi LUVs for this vesicle size. HP-TLC analysis revealed that the average SM content in asymmetric LUV samples prepared with 100-nm filters was 56% ± 3% (n = 8) (Fig. S3), implying that nearly all of the outer-leaflet lipids were composed of SM. The final vesicle size of LUVs prepared with 200-nm filters was only ∼20% larger than that of LUVs obtained with 100-nm filters. This result suggests that the freeze-thawed vesicles were already <200 nm in diameter when they were subjected to extrusion. Lipid exchange levels were slightly lower with 200-nm filters compared with 100-nm filters (Fig. S3). (This may reflect residual multilamellarity, so there are internal DOPE/POPS vesicles not subject to exchange, and so the total percent of lipid exchanged is decreased. This is not a surprise, as 160 nm vesicles are smaller than the 200 nm filters used, suggesting the vesicles would not burst/reform during extrusion, a process that should reduce residual multilamellarity.)
Membrane order in asymmetric LUVs
To confirm that the outer leaflets of asymmetric vesicles were composed of SM, and to evaluate the difference between the physical states of the inner and outer leaflets, we used DPH and TMADPH as steady-state fluorescence anisotropy probes. DPH is a small, hydrophobic fluorescence probe that distributes throughout the bilayer. TMADPH contains a charged quaternary amino group, and thus is restricted to the outer leaflet when added to preformed vesicles. As shown in Table 1, pure SM LUVs, which exist in the ordered gel phase at room temperature, gave higher DPH and TMADPH anisotropy values than symmetric vesicles containing DOPE and POPS, which are in the Ld state, or symmetric SM/DOPE/POPS vesicles, in which ordered and Ld states coexist at room temperature (7). Table 1 also shows that the outer-leaflet TMADPH anisotropy in asymmetric LUVs was as high as that in pure SM vesicles, indicating that the outer leaflet of SMo/2:1 DOPE/POPSi LUVs was in the ordered state and probably was composed mainly of SM given that DOPE and POPS do not form ordered bilayers at room temperature. In contrast to TMADPH, in the asymmetric vesicles, DPH (which locates in both leaflets) exhibited an anisotropy significantly lower than that of pure SM, indicating that the inner leaflet is less ordered than the outer leaflet. The difference between the inner and outer leaflets reflects the presence of an asymmetrical lipid distribution across the membrane bilayer in SMo/2:1 DOPE/POPSi LUVs. This anisotropy-detected difference between the level of order in the inner and outer leaflets was not observed in symmetric SM/DOPE/POPS vesicles with an overall lipid composition almost identical to that of the asymmetric vesicles (Table 1).
Table 1.
Fluorescence anisotropy in symmetric and asymmetric LUVs at room temperature
| Samples | Anisotropy (A) |
% ordered |
||
|---|---|---|---|---|
| DPH | TMADPH | DPH | TMADPH | |
| SM | 0.32 ± 0.01 (n = 5) | 0.33 ± 0.03 (n = 5) | ≡ 100 | ≡ 100 |
| 2:1 PE/PS | 0.11 ± 0.01 (n = 8) | 0.23 ± 0.01 (n = 5) | ≡ 0 | ≡ 0 |
| SMo/PE/PSi | 0.26 ± 0.01 (n = 7) | 0.33 ± 0.01 (n = 7) | 71 | 104 |
| 3:2:1 SM/PE/PS | 0.19 ± 0.01 (n = 5) | 0.27 ± 0.02 (n = 5) | 38 | 34 |
Average anisotropy and SD from five to eight preparations (number shown in parentheses) of LUVs prepared with a 100-nm-pore-size filter are shown. DPH or TMADPH (0.1 μM) was added to samples containing symmetric LUVs (100 μM) or asymmetric SMo/2:1 DOPE/POPSi LUVs (50–135 μM). The percent ordered state bilayer (from DPH anisotropy) or ordered state outer leaflet (from TMADPH anisotropy) was estimated from anisotropy (A) by the following equation: percent ordered = (Asample –A100% Ld)/(A100% ordered – A100% Ld). A100% ordered is that in SM LUVs, and A100% Ld is that in 2:1 DOPE/POPS LUVs (shown as 2:1 PE/PS in table). SMo/PE/PSi represents SMo/2:1 DOPE/POPSi LUVs, and 3:2:1 SM/PE/PS represents 3:2:1 SM/DOPE/POPS LUVs.
In addition, Table 1 shows that DPH anisotropy was higher in SMo/2:1 DOPE/POPSi LUVs than in the corresponding symmetric vesicles, indicating that the overall level of order is much higher in asymmetric vesicles than in symmetric vesicles of the same composition. Assuming that DPH anisotropy reflects the order in both leaflets, and that TMADPH reflects only the outer-leaflet order, we can calculate the percentage of order in the inner leaflet. Correcting for the fact that in LUVs ∼53% of total surface area of the vesicle is in the outer leaflet, 38% of the inner-leaflet lipid in asymmetric LUVs can be crudely estimated to be in an ordered state as calculated from the following equation: overall % order in both leaflets = outer leaflet % ordered × 0.53 + inner leaflet % ordered × 0.47, where the overall % order in the bilayer was derived from the DPH anisotropy value, and the outer-leaflet % order was derived from the TMADPH anisotropy (see Table 1). This level of order in the inner leaflet implies the existence of a significant level of interleaflet interaction (see Discussion).
Thermal stability of ordered domains in asymmetric LUVs
To further analyze the properties of ordered domains in asymmetric LUVs, we determined the thermal stability of ordered domains. To that end, we defined the temperature at which ordered domains melt (Tm) via the change in DPH anisotropy versus temperature. As shown in Fig. 1, sigmoidal melting curves are detected in mixtures containing SM, and Tm can be defined as the midpoint of these curves (19,20). As shown in Fig. 1 A, at low temperature, anisotropy was highest in pure SM vesicles, as expected, whereas asymmetric SMo/2:1 DOPE/POPSi LUVs had an intermediate DPH anisotropy falling between that of SM LUVs and 2:1 DOPE/POPS LUVs. As noted above, this indicates that in the asymmetric vesicles, part of the bilayer (i.e., the outer leaflet) is in an ordered state and the remainder of the bilayer (i.e., the inner leaflet) is in a more disordered state. Nevertheless, the Tm of asymmetric SMo/2:1 DOPE/POPSi LUVs was as high as that of pure SM LUVs (Fig. 1, A and B), indicating that the ordered state in the asymmetric LUVs was as thermally stable as in pure SM. This means that in the asymmetric vesicles, the presence of the largely disordered DOPE/POPS inner leaflet did not decrease the stability of the ordered state formed by SM in the outer leaflet. This has important implications for interleaflet coupling (see Discussion).
Figure 1.
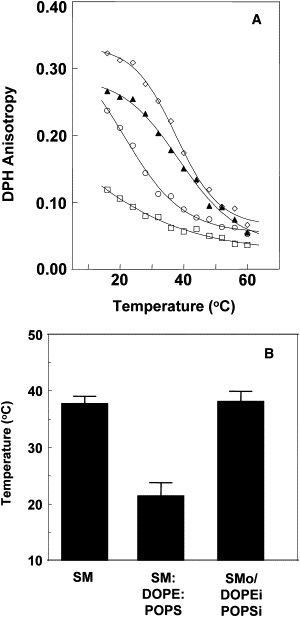
Thermal stability of ordered domains in symmetric and asymmetric LUVs prepared with 100-nm-pore-size filters. (A) Temperature dependence of DPH anisotropy in symmetric and asymmetric LUVs. Symbols: (▴) SMo/2:1 DOPE/POPSi LUV; (◊) SM LUVs; (○) 3:2:1 SM/DOPE/POPS LUVs; (□) 2:1 DOPE/POPS LUVs. A representative result from n ≥ 4 experiments is shown. (B) Average Tm-values and standard deviation (SD) derived from n ≥ 4 experiments are shown. Tm-values were derived from the midpoint of a sigmoidal fit of the data (7).
Additional methods confirming lipid asymmetry
We previously demonstrated that the change in vesicle properties that occurs after asymmetric SUVs are dissolved in solvent, and lipids are re-reconstituted into symmetric SUVs can be used to confirm lipid asymmetry (7). It has been reported that the peptide alamethicin can induce transverse movements of lipid molecules in the bilayer (21). Because this method does not require vesicle destruction, we used alamethicin to examine the asymmetry of the SMo/2:1 DOPE/POPSi LUVs (Fig. 2). When alamethicin was added to the vesicles, TMADPH anisotropy and Tm in asymmetric vesicles (gray bars) decreased from values almost identical to those of SM vesicles (black bars) to values close to those observed in ordinary (symmetric) 3:2:1 SM/DOPE/POPS LUVs (white bars), indicating a loss of asymmetry. This result rules out the hypothesis that the difference between the properties found in ordinary vesicles and asymmetric LUVs is due mainly to a difference in overall lipid composition rather than to the asymmetrical lipid distribution in the latter vesicles. A significant difference in lipid composition would have resulted in a large difference between Tm and anisotropy in ordinary vesicles and asymmetric vesicles to which alamethicin was added. (A change in the properties of asymmetric vesicles similar to that observed after alamethicin addition was also observed when asymmetry was destroyed by solubilization in ethanol followed by re-reconstitution to form symmetric LUVs in aqueous solution; data not shown.) Vesicle sizes, as measured by DLS, were very similar in the presence or absence of alamethicin (data not shown), indicating that alamethicin did not cause vesicle disruption or fusion. This eliminates the possibility that the physical properties of the SMo/2:1 DOPE/POPSi LUV preparation were the result of it consisting of a mixture of symmetric SM LUVs and 2:1 DOPE/POPS LUVs that fuse when alamethicin is added.
Figure 2.
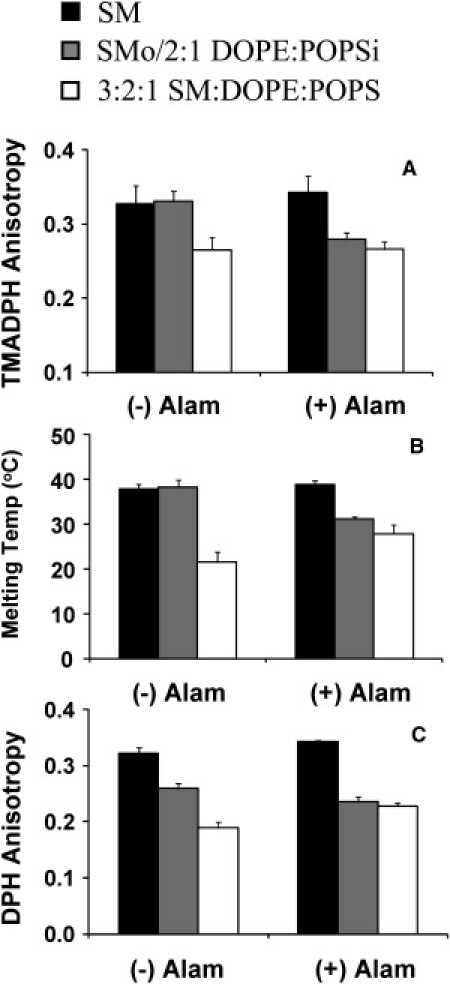
Effect of destroying asymmetry with alamethicin on the level and thermal stability of ordered domains in symmetric and asymmetric LUVs. (A) TMADPH anisotropy at room temperature. (B) Tm of ordered domains. (C) DPH anisotropy at room temperature. Samples contained vesicles composed of (black bars) SM, (gray bars) SMo/2:1 DOPE/POPSi, or (white bars) 3:2:1 SM/DOPE/POPS. (−) Alam, no alamethicin added to samples; (+) Alam, 0.5 μM of alamethicin was added to samples. LUVs were prepared with 100-nm-pore-size filters. Average results from four to seven preparations and the SD are shown.
Cationic peptides bind more strongly to vesicles with an anionic surface than to vesicles with a zwitterionic surface. Taking advantage of this finding, we further assayed the extent of lipid asymmetry (Table 2) using a Lys-flanked peptide of moderate hydrophobicity, pL4A18 (acetyl-K2LA9LWLA9LK2-amide). Because the pL4A18 peptide contains a Trp residue in the middle of its sequence, its association with membranes can be monitored by Trp fluorescence emission. The wavelength of maximum emission, λmax, is more blue-shifted (i.e., shifted to a lower wavelength) in the membrane-bound state than in the nonmembrane-bound state. The λmax of the pL4A18 peptide was much more blue-shifted in the presence of DOPE/POPS vesicles than in the presence of SM vesicles. This reflects the stronger binding of cationic peptides to vesicles with an anionic surface charge, as well as the difference in binding to ordered and disordered bilayers (17). A strong blue shift in fluorescence was also observed in symmetric SM/DOPE/POPS LUVs, and when SM LUVs were mixed with DOPE/POPS LUVs, reflecting the presence of anionic lipid (POPS) on the outer surface of vesicles in both of these cases. However, a strong blue shift was not observed in asymmetric SMo/2:1DOPE/POPSi LUVs, consistent with an asymmetry in which the POPS was not exposed on the exterior of the vesicles (i.e., the outer leaflet was composed of SM). A comparison of the λmax of the asymmetric vesicles with a standard curve of λmax versus % DOPE+POPS (Fig. S6) indicates that the outer leaflet is ∼90% SM.
Table 2.
Verification of lipid asymmetry of asymmetric SMo/2:1 DOPE/POPSi LUV by measurement of pL4A18 peptide binding
| Lipid composition | λmax |
|---|---|
| PBS | 358 ± 1 |
| SM LUV | 350 ± 2 |
| 3:2:1 SM/DOPE/POPS LUV | 333 ± 1 |
| 2:1 DOPE/POPS LUV | 332 ± 1 |
| SM LUV and 2:1 DOPE/POPS LUV mix (1:1 mol/mol ratio) | 333 ± 2 |
| SMo/2:1 DOPE/POPSi LUV | 346 ± 6 |
λmax of pL4A18 in symmetric and asymmetric vesicles. Average λmax-values and SD were obtained from three different preparations, except for the sample containing an SM LUV and 2:1 DOPE/POPS LUV mix, for which the range from two different preparations is shown. The LUVs used in this experiment were prepared using 100-nm-pore-size filters. Peptide was added after the vesicles were prepared.
Extension of the method to other lipid mixtures
An important question is whether the protocol used to prepare asymmetric LUVs can be extended to other lipids. We investigated two additional examples. In the first case, asymmetric LUVs were prepared with a 1:1 mol/mol mixture of SM/POPC outside in place of SM (Fig. S4). The observed level of exchange indicated that SM and POPC were introduced into the LUV at a 1:1 ratio and a total level consistent with replacement of almost all of the outer-leaflet DOPE/POPS. The Tm-value after exchange was consistent with an outer leaflet with ordered domains having a lower thermal stability than in SMo outer-leaflet LUVs, as expected due to the presence of POPC. Asymmetry was confirmed by the decrease in thermal stability upon re-reconstitution. In the second case, asymmetric SMo/DOPE/POPSi vesicles containing cholesterol were prepared. This involved the introduction of ∼20 mol % cholesterol in a second exchange step (Fig. S5). Asymmetry was again confirmed by the decrease in ordered domain thermal stability upon re-reconstitution.
Discussion
Preparation of asymmetric LUVs
The results of this study show that asymmetric LUVs with different lipid compositions can be prepared. Several methods confirmed that the vesicles had an asymmetric lipid distribution. The amount of SM exchanged into the vesicles was a little more than 50% as judged by both TLC and the similarity of the properties of the exchanged vesicles after asymmetry was destroyed in comparison with ordinary vesicles with 50% SM. Combined with the observation that the outer leaflet is almost all SM as judged by TMADPH anisotropy, and ∼90% SM as judged by peptide binding, this leaves little, if any, SM exchanged into the vesicles that is unaccounted by the amount of SM in the outer leaflet, and thus there cannot be much, if any, SM in the inner leaflet. This is not surprising, because MβCD should have no access to the inner leaflet. Furthermore, the lack of an increase in vesicle size after exchange and the fact that trapped sucrose is retained after exchange (as shown by the ability to pellet vesicles after exchange) rule out transient leakage or vesicle opening and fusion, both of which might allow MβCD to access the vesicle interior during exchange. Nevertheless, we cannot rule out the possibility that a small amount of SM somehow reached the interior of the vesicles. In this regard, it is important to note that we were able to eliminate the differences in physical properties between exchange vesicles and symmetric vesicles by scrambling the lipids with alamethicin, or by redissolving the lipids and reforming the vesicles. This means that these differences reflect asymmetry. If there were an incomplete degree of asymmetry after exchange because some SM was in the inner leaflet, and the outer leaflet was not pure SM, this would mean that in fully asymmetric vesicles the differences between exchange and symmetric vesicles would be even larger than we observed.
Interleaflet coupling in asymmetric LUVs
Of interest, the asymmetric LUVs were more highly ordered than symmetric LUVs with the same lipid composition. In addition, the presence a highly ordered outer leaflet increased the degree of inner-leaflet order, indicating a significant degree of coupling between the inner- and outer-leaflet physical states. This observation agrees with the coupling between ordered domain formation in the inner and outer leaflets found by other groups using asymmetric planar bilayers (8,10,22,23). It also agrees with our previous observation in asymmetric GUVs that outer- and inner-leaflet lateral diffusion can be coupled when SM is selectively introduced into the outer leaflet (11).
How strong is this coupling? Fig. 3 shows schematically what would be expected for very weak, intermediate, and very strong interleaflet coupling. Very weak or no coupling would just give anisotropy values for the outer and inner leaflets that correspond to those for pure SM (fully ordered) and pure DOPE/POPS vesicles (fully disordered) at lower temperatures, respectively. Instead, considerable order is detected in the inner leaflet. Very strong coupling would predict that the lipid bilayer acts as a single-unit solid (gel) at low temperature, is liquid disordered at high temperature, and has a Tm-value intermediate between those of SM and DOPE/POPS (certainly much lower than that of pure SM). However, we do not see an intermediate Tm-value; instead, the Tm in asymmetric vesicles is close to that of SM. Thus, coupling cannot be very strong or very weak. Therefore, there must be an intermediate level of coupling that disappears as the temperature increases to values close to the Tm of SM. The fact that the inner-leaflet anisotropy is not as high as that of the outer leaflet also supports the idea of an intermediate level of coupling. (It should be pointed out that the lack of interleaflet coupling at 37°C cannot be generalized to interleaflet coupling in vivo, due to the difference between lipid composition in our vesicles and cell membranes, and a lack of membrane proteins in our asymmetric vesicles.)
Figure 3.
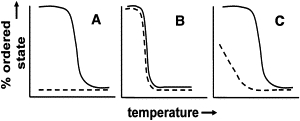
Schematic illustration of consequences of different interleaflet coupling strengths in vesicles containing high Tm lipids in the outer leaflet and low Tm lipids (with a Tm below the experimental temperature range) in the inner leaflet. (A) Very weak coupling. The outer leaflet (solid line) and inner leaflet (dashed line) melt independently and at similar temperatures to those made of pure high Tm or low Tm lipid, respectively. (B) Very strong coupling. Outer and inner leaflet melt simultaneously at an intermediate Tm. (C) Intermediate coupling. At lower temperatures, coupling that increases inner leaflet lipid Tm is present at lower temperatures, but this coupling is lost as the temperature approaches the Tm of the outer leaflet, whereas outer leaflet Tm is similar to that in vesicles composed of pure high Tm lipid.
Curvature and asymmetric vesicle properties
The properties of the asymmetric LUVs were very similar to those we previously observed for asymmetric SUVs (7). However, in our previous study we were unable to interpret the implications of the physical properties of asymmetric SUVs because their properties can be influenced by the high curvature of the SUVs. The ratio of lipids in the outer and inner leaflets is ∼2:1 in such vesicles. This factor by itself might increase the influence of outer-leaflet lipids (i.e., SM) on those in the inner leaflet. In addition, curvature could affect the nature of the steric contacts between lipids in the inner and outer leaflets. In the asymmetric LUVs used here, the outer-/inner-leaflet lipid ratio is much lower, ∼1.13:1. Thus, the observation of similar properties with regard to membrane order and interleaflet coupling in asymmetric SUVs and asymmetric LUVs implies that the severe membrane curvature of SUVs does not greatly influence interleaflet coupling.
Conclusions
In conclusion, this study shows that asymmetric membranes have significantly different properties compared with symmetric membranes. The preparation of asymmetric LUVs with lipid compositions that mimic those of plasma membranes should find numerous applications in studies of membrane structure and function.
Acknowledgments
This work was supported by the National Institutes of Health (grant GM-48596).
Supporting Material
References
- 1.Verkleij A.J., Zwaal R.F., van Deenen L.L. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim. Biophys. Acta. 1973;323:178–193. doi: 10.1016/0005-2736(73)90143-0. [DOI] [PubMed] [Google Scholar]
- 2.Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed S.N., Brown D.A., London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- 4.Wang T.Y., Silvius J.R. Cholesterol does not induce segregation of liquid-ordered domains in bilayers modeling the inner leaflet of the plasma membrane. Biophys. J. 2001;81:2762–2773. doi: 10.1016/S0006-3495(01)75919-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown D.A., Rose J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 6.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H.T., Megha, London E. Preparation and properties of asymmetric vesicles that mimic cell membranes: effect upon lipid raft formation and transmembrane helix orientation. J. Biol. Chem. 2009;284:6079–6092. doi: 10.1074/jbc.M806077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins M.D., Keller S.L. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc. Natl. Acad. Sci. USA. 2008;105:124–128. doi: 10.1073/pnas.0702970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hope M.J., Redelmeier T.E., Cullis P.R. Phospholipid asymmetry in large unilamellar vesicles induced by transmembrane pH gradients. Biochemistry. 1989;28:4181–4187. doi: 10.1021/bi00436a009. [DOI] [PubMed] [Google Scholar]
- 10.Kiessling V., Crane J.M., Tamm L.K. Transbilayer effects of raft-like lipid domains in asymmetric planar bilayers measured by single molecule tracking. Biophys. J. 2006;91:3313–3326. doi: 10.1529/biophysj.106.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiantia S., Schwille P., London E. Asymmetric GUVs prepared by MβCD-mediated lipid exchange: an FCS study. Biophys. J. 2011;100:L1–L3. doi: 10.1016/j.bpj.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eastman S.J., Hope M.J., Cullis P.R. Transbilayer transport of phosphatidic acid in response to transmembrane pH gradients. Biochemistry. 1991;30:1740–1745. doi: 10.1021/bi00221a002. [DOI] [PubMed] [Google Scholar]
- 13.Everett J., Zlotnick A., Holloway P.W. Fluorescence quenching of cytochrome b5 in vesicles with an asymmetric transbilayer distribution of brominated phosphatidylcholine. J. Biol. Chem. 1986;261:6725–6729. [PubMed] [Google Scholar]
- 14.Malewicz B., Valiyaveettil J.T., Brown R.E. The 3-hydroxy group and 4,5-trans double bond of sphingomyelin are essential for modulation of galactosylceramide transmembrane asymmetry. Biophys. J. 2005;88:2670–2680. doi: 10.1529/biophysj.104.057059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanhuanpää K., Somerharju P. γ-Cyclodextrins greatly enhance translocation of hydrophobic fluorescent phospholipids from vesicles to cells in culture. Importance of molecular hydrophobicity in phospholipid trafficking studies. J. Biol. Chem. 1999;274:35359–35366. doi: 10.1074/jbc.274.50.35359. [DOI] [PubMed] [Google Scholar]
- 16.Hamada T., Miura Y., Takagi M. Construction of asymmetric cell-sized lipid vesicles from lipid-coated water-in-oil microdroplets. J. Phys. Chem. B. 2008;112:14678–14681. doi: 10.1021/jp807784j. [DOI] [PubMed] [Google Scholar]
- 17.Shahidullah K., London E. Effect of lipid composition on the topography of membrane-associated hydrophobic helices: stabilization of transmembrane topography by anionic lipids. J. Mol. Biol. 2008;379:704–718. doi: 10.1016/j.jmb.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagle J.F., Tristram-Nagle S. Structure of lipid bilayers. Biochim. Biophys. Acta. 2000;1469:159–195. doi: 10.1016/s0304-4157(00)00016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lentz B.R., Barenholz Y., Thompson T.E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 2 Two-component phosphatidylcholine liposomes. Biochemistry. 1976;15:4529–4537. doi: 10.1021/bi00665a030. [DOI] [PubMed] [Google Scholar]
- 20.Wenz J.J., Barrantes F.J. Steroid structural requirements for stabilizing or disrupting lipid domains. Biochemistry. 2003;42:14267–14276. doi: 10.1021/bi035759c. [DOI] [PubMed] [Google Scholar]
- 21.Wimley W.C., White S.H. Determining the membrane topology of peptides by fluorescence quenching. Biochemistry. 2000;39:161–170. doi: 10.1021/bi991836l. [DOI] [PubMed] [Google Scholar]
- 22.Kiessling V., Wan C., Tamm L.K. Domain coupling in asymmetric lipid bilayers. Biochim. Biophys. Acta. 2009;1788:64–71. doi: 10.1016/j.bbamem.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan C., Kiessling V., Tamm L.K. Coupling of cholesterol-rich lipid phases in asymmetric bilayers. Biochemistry. 2008;47:2190–2198. doi: 10.1021/bi7021552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


