Abstract
Background
There has been a dramatic rise in the consumption of alcohol mixed with energy drinks (AmED) in young people. AmEDs have been implicated in risky drinking practices and greater accidents and injuries have been associated with their consumption. Despite the increased popularity of these beverages (e.g., Red Bull and vodka), there is little laboratory research examining how the effects of AmED differ from alcohol alone. This experiment was designed to investigate if the consumption of AmED alters neurocognitive and subjective measures of intoxication compared with the consumption of alcohol alone.
Methods
Participants (n=56) attended one session where they were randomly assigned to receive one of four doses (0.65 g/kg alcohol, 3.57 ml/kg energy drink, AmED or a placebo beverage). Performance on a cued go/no-go task was used to measure the response of inhibitory and activational mechanisms of behavioral control following dose administration. Subjective ratings of stimulation, sedation, impairment and level of intoxication were recorded.
Results
Alcohol alone impaired both inhibitory and activational mechanisms of behavioral control, as evidenced by increased inhibitory failures and increased response times compared to baseline performance. Coadministration of the energy drink with alcohol counteracted some of the alcohol-induced impairment of response activation, but not response inhibition. For subjective effects, alcohol increased ratings of stimulation, feeling the drink, liking the drink, impairment and level of intoxication and alcohol decreased the rating of ability to drive. Coadministration of the energy drink with alcohol increased self-reported stimulation, but resulted in similar ratings of the other subjective effects as when alcohol was administered alone.
Conclusions
An energy drink appears to alter some of alcohol’s objective and subjective impairing effects, but not others. Thus AmEDs may contribute to a high risk scenario for the drinker. The mix of impaired behavioral inhibition and enhanced stimulation is a combination that may make AmED consumption riskier than alcohol consumption alone.
Keywords: alcohol, energy drink, behavioral control, reaction time, stimulation
Introduction
Underage and binge drinking are serious public health problems (Marczinski et al., 2009; Miller et al., 2007; SAMHSA, 2007). Despite substantial efforts to change this behavior, current levels of binge drinking in young people appear to be relatively unchanged from year 2000 levels (Fournier & Levy, 2006; SAMHSA, 2007). The constancy of underage and binge drinking in young people, despite increased attention to this crisis, begs the question of what unexamined factors may be contributing to the problem. One possible variable, which has received little research attention, is the shift in alcoholic drink preferences in high school and college students in the past decade. Young people have become enamored with the trend of mixing energy drinks with alcohol (e.g., Red Bull and vodka or other super-caffeinated cocktails like Jagerbombs, which are a mixture of the spirit Jagermeister with Red Bull) (Miller, 2008; O’Brien et al., 2008; Reissig et al., 2009). Despite the recent dramatic rise in the consumption of alcohol mixed with energy drinks (AmED), very little laboratory research has examined how these drinks alter objective and subjective measures of intoxication. It is plausible that consumption of AmED may be riskier than alcohol consumption alone. Mixing alcohol with another beverage with strong stimulant properties may alter perceptions of intoxication and lead individuals to think that they can drink more and for longer periods of time, thus escalating binge drinking activities.
Energy drinks (e.g., Red Bull, Monster, and Rockstar) are beverages marketed with claims of providing users with increased alertness and energy boosts (Miller, 2008). These new products contain a variety of compounds including plant-based stimulants (e.g., guarana), simple sugars (e.g., glucose, fructose), amino acids (e.g., taurine) and herbs (e.g., ginseng) (O’Brien et al., 2008). However, most researchers agree that the extremely high caffeine content (the principal active ingredient) of these beverages drive the stimulant properties that users often report after consumption (Ferreira et al., 2006; Reissig et al., 2009). For example, Coca-Cola Classic contains 2.9 mg of caffeine/fl oz., while the best-selling energy drink brand, Red Bull contains 9.6 mg of caffeine/fl oz. The U.S. Food and Drug Administration (FDA) does not regulate the caffeine content of energy drinks, and recent analyses have determined that the caffeine content of these beverages can contain 150–300% of the amount of caffeine that the FDA permits for cola beverages (Clauson et al., 2008; McCusker et al., 2006).
Survey data has revealed that the consumption of energy drinks, alone and in combination with alcohol, has become increasingly common among college students (Malinauskas et al., 2007; Marczinski, under review; Miller, 2008; O’Brien et al., 2008). For example, O’Brien et al. (2008) reported that ¼ of past 30-day alcohol drinkers consumed at least 1 AmED during the past month. Moreover, the students who reported AmED consumption reported significantly higher alcohol-related consequences, such as riding with an intoxicated driver, being physically hurt or injured, and requiring medical treatment, even after adjusting for the amount of alcohol consumed. Evidence from a recent field study further supports the notion that AmED may be riskier than alcohol alone. Thombs et al. (2010) asked college student patrons leaving local bars to report what they had drank, their intention whether or not to drive home and to provide a breath sample. The authors reported that patrons who had consumed AmEDs were at a 3-fold increased risk of leaving the bar highly intoxicated (i.e., BAC = or > .08 g%) and a 4-fold risk of intending to drive home, compared to other drinking patrons.
Why might the acute effects of AmED be riskier than the acute effects of alcohol alone in young social drinkers? The answer is unclear given that there have been few laboratory investigations of the objective and subjective reactions to the consumption of AmED in humans or animals to answer this question. One study with mice reported that the energy drink Red Bull increased locomotor activity in a dose-dependent manner and that alcohol-induced impairment of locomotor activity was antagonized by a high dose of the energy drink (Ferreira et al., 2004). Another study with human subjects suggested that there are important subjective response differences between alcohol and AmED (Ferreria et al., 2006). The investigators evaluated the acute effects of AmED (vodka and Red Bull) compared to alcohol or the energy drink alone. They reported that the acute effects of AmED were associated with reduced perception of headache, dry mouth, and weakness compared to alcohol alone. However, participants were similarly impaired by AmED and alcohol alone on two objective measures of motor coordination and visual reaction time These results are consistent with the larger literature on the findings of mixing caffeine with alcohol. Coadministration of caffeine with alcohol often reduces participant’s subjective perceptions of alcohol intoxication compared with the administration of alcohol alone. However, the evidence that the coadministration of caffeine can counteract the impairing effects of alcohol on a variety of behavioral and cognitive tasks is equivocal (for a review, see Fudin & Nicastro, 1988).
Impulse control is an important cognitive process to examine in the study of the acute effects of AmED. The acute effects of alcohol reduce impulse control, and much has been learned about the acute effects of alcohol on the specific neurocognitive mechanisms that regulate behavioral control by studying social drinkers in the laboratory (for a review, see Fillmore, 2003). Such research is based on theories that postulate that two distinct processes govern behavioral control; one that activates behavior and one that inhibits behavior (Fowles, 1987; Gray, 1976, 1977; Logan & Cowan, 1984; Patterson & Newman, 1993; Quay, 1977). These two processes have also been called the go and stop processes (Clay et al.,2008) or the hot and cold processes (Metcalfe & Mischel, 1999). It is thought that these two processes (e.g., activation and inhibition) act in opposition to one another and the relative strength of each is assumed to determine behavioral control. Deficient behavioral inhibition is inferred by observations of overactive, impulsive behavior (Logan et al., 1984) and is considered to be the primary mechanism by which alcohol and other drugs of abuse impair self-control (Fillmore, 2003; Jentsch & Taylor, 1999; Pernanen, 1993). Model-based assessments of behavioral control mechanisms (such as the cued go/no-go task) have been used to demonstrate that moderate doses of alcohol impair the ability to activate and inhibit responses (Marczinski & Fillmore, 2003a, 2003b, 2005a, 2005b, 2006), with particular susceptibility to response inhibition to the impairing effects of alcohol (Abroms et al., 2003; Fillmore et al., 2005). Deficient inhibition on the cued go/no-go task is measured by the proportion (p) of no-go targets in which a participant failed to inhibit a response. These p-inhibition failures have been shown to correlate with actual alcohol consumption levels (Weafer & Fillmore, 2008). Thus, it appears that the acute effects of alcohol decrease inhibition, resulting in an increase in impulsive behaviors including binge drinking.
It was currently unknown how the combined effect of alcohol and energy drinks impact the activation and inhibition of behavior differently than alcohol would alone. Our working hypothesis was that alcohol would impair both activation and inhibition response tendencies and that coadministration of an energy drink may counteract alcohol-induced impairment of activation without impacting alcohol-induced impairment of inhibition. Marczinski and Fillmore (2003a, 2006) examined the combined effects of caffeine with 0.65 g/kg alcohol and found that 4.0 mg/kg caffeine can counteract the impairing effects of alcohol on activation of responses. However, caffeine coadministration with alcohol does not counteract the impairing effects of a moderate dose of alcohol on inhibition (Marczinski & Fillmore, 2003a, 2006). In these past studies, when subjects were asked about their perceived impairment, caffeine coadministration reduced perceived impairment from alcohol (Marczinski & Fillmore, 2006). Thus, a worrisome scenario develops when individuals perceive themselves as feeling less intoxicated, even while impulse control remains significantly impaired (Marczinski & Fillmore, 2003a, 2006). In the real world, a drinker who can accurately assess his or her level of impairment is probably safer than a drinker who cannot.
In the present study, we examined if the effects of AmED alter objective and subjective responses to alcohol differently than if alcohol were administered alone. Participants (N = 56) were college student social drinkers who were randomly assigned to one of four dose conditions: 0.65 g/kg alcohol, 3.57 ml/kg energy drink, AmED or placebo. We examined the effects of these beverages on the cued go/no-go task performance and on subjective reactions to alcohol. We predicted that the coadministration of the energy drink with alcohol could counteract some of the impairing effects of alcohol, such as on response activation and subjective ratings. In addition, we predicted the energy drink would not counteract all of the impairing effects of alcohol, such as response disinhibition.
Materials and Methods
Participants
Fifty-six adults (28 men and 28 women) between the ages of 21 and 33 (mean age = 23.8 years, SD = 3.4) participated in this study. The self-reported racial-ethnic make-up of the sample included 5 African-Americans, 3 Asian-Americans and 48 Caucasian participants. Potential volunteers completed questionnaires that provided demographic information and physical and mental health status. Individuals with a self-reported psychiatric disorder, substance abuse disorder, diabetes, head trauma, or other injury of the central nervous system were excluded from the study. All participants were typical social drinking college students, on the basis of additional exclusion criteria that eliminated the extremely infrequent drinkers or drinkers with a potential risk of alcohol dependence. As such, any individual with a Short Michigan Alcoholism Screen Test (SMAST; Seltzer, Vinoker & Van Jooijen, 1975) score of 5 or higher or an Alcohol Use Disorders Identification Test (AUDIT; Barbor et al., 1989) score of 8 or higher were also excluded from study participation because of the risk for dependence (Barry & Fleming, 1993; Schmidt, Barry & Fleming, 1995). Furthermore, individuals who did not regularly drink alcohol (i.e., fewer than two standard drinks per month) were excluded because of ethical concerns of administering a 0.65 g/kg dose of alcohol to an individual unfamiliar with that amount of alcohol. Individuals must have consumed at least one energy drink in the past year, and have consumed at least one caffeinated beverage in the past two weeks (e.g., soft drink, tea, coffee, chocolate, and/or energy drink). All participants had normal or corrected-to-normal visual acuity and normal color vision.
Recent use of benzodiazepines, barbiturates, tetrahydrocannabinol, cocaine, amphetamines, and opiates was assessed by means of urinalysis. Any volunteer who tested positive for the presence of any of these drugs was excluded from the study. No female volunteers who were pregnant or breast-feeding participated in the research, as determined by self-report and urine gonadotrophin (HCG) levels. Participants were recruited through notices posted on community bulletin boards at the university. All volunteers provided informed consent before participating. The Northern Kentucky University Institutional Review Board approved this study, and volunteers received $30 for their participation.
Apparatus and Materials
Personal Drinking Habits Questionnaire (PDHQ: Vogel-Sprott, 1992)
The PDHQ measures an individual’s current, typical drinking habits including: (a) number of standard drinks (i.e., bottles of beer, glasses of wine, and shots of liquor) typically consumed during a single drinking occasion, (b) dose (grams of absolute alcohol per kilogram of body weight typically consumed during a single drinking occasion), (c) weekly frequency of drinking, and (d) hourly duration of a typical drinking occasion. The PDHQ also measures previous experience with alcohol in terms of the number of months that an individual has been drinking on a regular basis or customarily on social occasions. Using information gathered from the PDHQ, we also calculated the typical peak blood alcohol concentration (BAC) achieved. Calculations were based on the updated Widmark equation (Watson et al., 1981) where the amount of body weight capable of absorbing alcohol is estimated to be 75% for men and 66% for women.
Timeline Follow-Back (TLFB; Sobell & Sobell, 1992)
THE TLFB assesses daily patterns of alcohol consumption over the past 30 days and includes measures of: (a) maximum number of continuous days of drinking, (b) maximum number of continuous days of abstinence, (c) total number of drinking days in the past month, (d) total number of drinks consumed in the past month, (e) highest number of drinks consumed in 1 day, (f) total number of heavy drinking (five or more drinks) days in the past month, and (g) total number of “drunk” days in the past month.
Caffeine Use Questionnaire (CUQ)
This questionnaire provides a measure of a participant’s daily caffeine consumption in milligrams per kilogram of body weight. Estimates of the caffeine content in foods and beverages were taken from Barone and Roberts (1996) and manufacturer websites for newer products.
Questionnaire Measures of Impulsivity and Attention
Three questionnaires provided measures of self-reported impulsiveness and attention with higher scores indicating greater impulsivity or poorer attention. The Eysenck Impulsiveness Questionnaire (Eysenck et al., 1985) assesses impulsiveness by posing 19 yes-no questions. The Barratt Impulsiveness Scale-11 (BIS-11; Patton et al., 1995) assesses impulsiveness by asking participants to rate how typical 30 different statements are for them on a 4-point Likert scale ranging from Rarely/Never to Almost Always/Always. Finally, the ADD/H Adolescent Self-Report Scale – Short Form (Robin & Vandermay, 1996) assesses various problems related to attention (poor concentration, distraction) by having respondents endorse each of 11 items on a 4-point Likert-type scale from 0 (not at all) to 3 (very much).
Cued Go/No-Go Task
Response activation and inhibition were measured by a cued go/no-go task (Marczinski & Fillmore, 2003a,b) that was operated using E-Prime software (Schneider et al., 2002). A trial involved the following sequence of events: 1) a fixation point (+) for 800 ms, 2) a blank screen for 500 ms, 3) a cue (a horizontal or vertical white rectangle), displayed for one of five stimulus onset asynchronies (SOAs = 100, 200, 300, 400 and 500 ms), 4) a go or no-go target (green or blue rectangle), visible until a response occurs or 1000 ms elapses, and 5) an inter-trial interval of 700 ms.
The orientation of the cue (horizontal or vertical) correctly signaled the target 80% of the time. Participants were instructed to press the forward slash (/) key on the keyboard as soon as a go (green) target appeared and to inhibit this response if a no-go (blue) target appeared. Inhibitory and activational tendencies show rapid development of cue-dependence as cues come to elicit prepared processes for the inhibition or execution of behavior (Miller et al., 1991). For response inhibition, the go cue condition is of particular interest as it generates response prepotency yet subjects must overcome this response prepotency in order to inhibit the response when a no-go target is displayed. Similarly for response activation, the no-go cue condition is of particular interest because alcohol’s slowing effect on reaction time is most evident in this condition. A test consisted of 500 trials that presented the four possible cue-target combinations.
Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993)
Subjective ratings of stimulation and sedation were evaluated using this 14-adjective rating scale where 7 adjectives describe stimulation effects (e.g., stimulated, elated) while the remaining 7 describe sedation effects (e.g., sedated, sluggish). Participants rated each item on an 11-point Likert-type scale ranging from 0 (not at all) to 10 (extremely) and Stimulation and Sedation scores were summed separately (score subscale range = 0–70).
Subjective Effect Ratings
A 5-item 100 mm visual analogue scale was used to assess the subjective effects of the dose administered with end anchors of not at all and very much. Two items asked participants to rate the subjective effects of the drink in terms of how much they “feel the drink” (feel) and “like the effects” (like) (Fillmore, 2001). The other three items asked subjects to rate their overall level of impairment, mental fatigue, and ability to drive at the time of the rating (Beirness, 1987).
Intoxication Rating (Fillmore & Vogel-Sprott, 2000)
This scale asks subjects to report their perceived level of intoxication by reporting their perceived alcoholic content of the beverage administered in terms of bottles of beer containing 5% alcohol. The scale ranges from 0 to 10 bottles of beer, in 0.5 bottle increments.
Procedure
Pre-laboratory Screening
Individuals who responded to the advertisements contacted the research assistant by e-mail to set up a time to participate in a telephone intake-screening interview conducted by a research assistant. During the telephone interview, volunteers were informed that the purpose of the experiment was to study the effects of alcohol and energy drinks on behavioral and mental functioning. Volunteers were told that they would be asked to perform computerized tasks and complete questionnaires. Moreover, they were informed that they would receive a beverage to consume, that could contain the maximum dose of alcohol found in 4 beers and the maximum dose of caffeine found in a cup of coffee or 2 cans of a soft drink. The research assistant determined if the participant met all eligibility requirements to participate. Eligible subjects then made an appointment for a treatment session. All sessions were conducted in the Psychology department laboratories at Northern Kentucky University and began between 10 a.m. and 6 p.m. Prior to the session, participants were required to fast for 2 hours, abstain from any form of caffeine for 8 hours and abstain from alcohol for 24 hours.
Baseline testing
Participants were tested individually by a research assistant. All testing was conducted in a small room that consisted of a chair and a desk with the computer that operated the cued go/no-go task. When participants arrived at the laboratory, they were asked to provide informed consent. Participants were weighed and completed a brief medical screening questionnaire to ensure that the participant was healthy, had followed fasting instructions and had not recently taken any medications. All subjects were then asked to provide a urine sample in a private bathroom. Urine samples were tested for the presence of drug metabolites for all participants and HCG for women only (Bioscreens Inc., Norfolk, VA). After urine drug/pregnancy testing, a zero blood alcohol concentration (BAC) was verified from participants, as determined from breath samples measured by an Intoxilyzer, Model 400 (CMI Inc., Owensboro, KY).
Participants then performed a baseline test on the cued go/no-go task. Participants were instructed to press the forward slash key (/) on the keyboard as quickly as possible whenever a green (i.e., go) target appeared and to suppress the response whenever a blue (i.e., no-go) target appeared. The computer displayed how fast a participant responded to each go target by presenting the milliseconds required from target onset until the key was pressed. Participants were encouraged to make fast responses (i.e., in the fewest milliseconds) while remaining accurate (i.e., not pressing the key when a no-go target appeared). Upon completion of the cued go/no-go task, participants completed the baseline measurements of BAES and mental fatigue ratings. Participants also completed the PDHQ, TLFB, CUQ, Eysenck, BIS-11 and the ADD/H questionnaires.
Dose Administration
Participants were randomly assigned to one of four dose conditions (alcohol, energy drink, alcohol+energy drink, or placebo) counterbalanced for gender. Dose administration was double-blind and doses were calculated on the basis of body weight. For the alcohol dose, a 0.65 g/kg dose of alcohol (using 40% alcohol/volume Smirnoff Red Label vodka, No. 21, Smirnoff Co., Norwalk, CT) was chosen as this dose produces an average peak blood alcohol concentration (BAC) of .08 g% which is the legal limit for driving. The 0.65 g/kg dose of alcohol was reduced to 87% for female subjects as women tend to achieve higher BACs than do men. The alcohol dose was mixed with a 3.57 ml/kg of Squirt, a decaffeinated soft drink (Dr. Pepper Snapple Group, Plano, TX) resulting in a 2:1 (soft drink:alcohol) ratio.
For the alcohol+energy drink condition, the 0.65 g/kg dose of alcohol was mixed with 3.57 ml/kg of Red Bull energy drink (Red Bull, Switzerland). This alcohol+energy drink mix was chosen because this 2:1 ratio (Red Bull:vodka) is the mixed drink typically served in bars. In the energy drink condition, subjects received 3.57 ml/kg Red Bull, and in the placebo condition, subjects received 3.57 ml/kg Squirt. In both the energy drink and placebo conditions, 10 ml of vodka was floated on the surface of the beverage to give the drink an alcohol scent, and previous research has demonstrated that individuals report that this beverage contains alcohol (Marczinski & Fillmore, 2006). The rationale for the choice of Red Bull as the energy drink beverage was that it is the most commonly purchased energy drink in the U.S. market and the most commonly used energy drink mixed with alcohol (Bryce & Dyer, 2007). A carbonated, lemon-flavored decaffeinated soda (Squirt) was chosen as the placebo beverage as it was found to be most similar in taste, carbonation and appearance to the energy drink. The 3.57 ml/kg energy drink dose resulted in the consumption of 91 mg of caffeine for the typical 76 kg participant. The energy drink and placebo beverages were approximately equivalent in calories and glucose content.
Following all baseline testing, participants were given their beverage in a plastic cup and were asked to consume the drink within 10 minutes. The exact contents of the beverages were never disclosed to participants in this study. Drinking was self-paced. After dose administration, participants relaxed and read magazines. BACs were measured at 30, 40, 70, 80, 90 min. after drinking. During the energy drink and placebo sessions, participants also provided breath samples at those times ostensibly to measure their BAC.
Testing battery
At 45 minutes after drinking began, participants’ cued go/no-go task performance was tested. Thus, the test occurred during the ascending to peak period when both alcohol and caffeine are most active. After the cued go/no-go test (70 minutes after drinking began), participants completed the BAES, all subjective effects ratings, and the subjective intoxication rating. These measures were typically completed within 10 minutes.
Detoxification period
Upon completion of the testing period at 90 min. post drinking, participants relaxed in a waiting room in the laboratory. Participants received a meal and remained at leisure to read magazines or watch DVDs until their BAC fell below .02 g%., at which time they were debriefed and released. Participants who had not received alcohol were immediately debriefed and released after the testing battery concluded.
Criterion Measures and Data Analyses
The two primary measures of interest from the cued go/no-go task were the participants’ change in speed of responding to go targets (response execution) from baseline to the post-drink test and participants’ change in failures to inhibit responses to no-go targets (failures of response inhibition) from baseline to the post-drink test.
Response execution
Response execution was measured by the mean reaction time (RT) to go targets in the go and no-go cue conditions for each test. Baseline scores for the different dose conditions were analyzed by separate one-way ANOVAs, separately for each cue condition. Dose effects were measured as the change from baseline. Change scores were calculated by subtracting the mean RT for the baseline test from the post-beverage mean RT for each subject and for each cue condition. Change scores for response execution were analyzed by a 2 (Alcohol Dose: 0.65 g/kg v. 0.0 g/kg) × 2 (Energy Drink Dose: 3.57 ml/kg v. 0.0 ml/kg) × 2 (Cue: valid go v. invalid no-go) mixed design ANOVA where Alcohol Dose and Energy Drink Dose were treated as between-subjects factors and Cue was treated as a within-subjects factor. One sample t-tests were used to indicate if change scores were significantly different from zero for each dose and cue condition. Omission errors were also recorded. These errors occurred when participants failed to respond to go targets. Omission errors were infrequent and occurred on less than 1% of go target trials (~2 trials per test).
Failures of response inhibition
Failures of response inhibition were measured as the proportion (p) of no-go targets in which a participant failed to inhibit a response in the go and no-go cue conditions for each test. Baseline scores for the different dose conditions were analyzed by one-way ANOVAs, separately for each cue condition. Dose effects were measured as the change from baseline. Change scores were calculated by subtracting the mean p-inhibition failure score for the baseline test from the post-beverage p-inhibition failure score for each subject and for each cue condition. Change scores for failures of response inhibition were analyzed by a 2 (Alcohol Dose: 0.65 g/kg v. 0.0 g/kg) × 2 (Energy Drink Dose: 3.57 ml/kg v. 0.0 ml/kg) × 2 (Cue: valid no-go v. invalid go) mixed design ANOVA where Alcohol Dose and Energy Drink Dose were treated as between-subjects factors and Cue was treated as a within-subjects factor. One sample t-tests were used to indicate if change scores were significantly different from zero for each dose and cue condition.
All analyses of change scores for mean RTs and p-inhibition failures were also supported by analyses of covariance (ANCOVAs) of observed scores that used the baseline scores as covariates. Given that change scores provide a direct indication of the response to the drug dose administered (energy drink and/or alcohol), all analyses and figures use these change scores to better illustrate the dose effects. The alpha level was set at .05 for all statistical tests and SPSS 17.0 was used to conduct all analyses.
Results
Demographic Characteristics, Self-reported Caffeine and Alcohol Use and Baseline Tests
Table 1 lists all demographic, questionnaire and baseline measures for participants in the 4 groups. Results of Chi-square tests showed that group assignment was independent of gender distribution and race/ethnicity, ps > .24. Results of one-way ANOVAs for each demographic, caffeine use, alcohol use, baseline subjective effects and baseline cued go/no-go task measures revealed no significant differences among the groups, ps > .10. The sample self-reported a mean (SD) typical alcohol dose of 0.94 g/kg (0.48) per occasion. This dose is equivalent to 4 standard bottles of beer for the average 75 kg participant in this study. The sample also reported a mean (SD) duration of drinking of 3.62 (1.54) hours with a mean (SD) weekly frequency of drinking of 1.49 days (1.17). Regarding self-reported caffeine use, the sample reported a mean (SD) daily caffeine use of 3.33 mg/kg (2.83). For our average 75 kg participant in this study, this caffeine dose would approximate 2 small cups of coffee or 1 grande Starbucks coffee (Barone & Roberts, 1984; McCusker et al., 2006).
Table 1.
Demographic Characteristics, Self-reported Alcohol and Caffeine Use and Baseline Measures
| Dose Condition | ||||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Energy Drink | Alcohol | AmED | |||||
| M | SD | M | SD | M | SD | M | SD | |
| Age | 23.93 | 3.71 | 23.36 | 2.95 | 23.14 | 2.98 | 24.86 | 4.07 |
| Gender (male:female) | 7:7 | 7:7 | 7:7 | 7:7 | ||||
| Weight (kg) | 75.88 | 19.25 | 73.45 | 13.88 | 76.98 | 11.37 | 76.40 | 17.80 |
| Body Mass Index | 25.15 | 4.27 | 23.66 | 4.24 | 26.02 | 2.84 | 25.00 | 5.34 |
| Daily caffeine use (mg/kg) | 3.08 | 2.66 | 4.43 | 2.77 | 2.31 | 2.33 | 3.51 | 3.35 |
| History (months) | 82.93 | 48.37 | 66.64 | 40.29 | 66.14 | 52.55 | 82.86 | 51.98 |
| Frequency (occasions/wk) | 1.37 | 1.57 | 1.86 | .79 | 1.26 | .98 | 1.47 | 1.21 |
| Drinks per occasion | 4.21 | 1.85 | 4.36 | 2.43 | 3.71 | 1.90 | 3.79 | 1.67 |
| Alcohol dose (g/kg) | .97 | .40 | 1.04 | .63 | .85 | .46 | .88 | .44 |
| Duration (hr) | 3.75 | 1.60 | 3.38 | 1.46 | 3.54 | 1.85 | 3.82 | 1.34 |
| Estimated BAC (mg/100 ml) | 51.31 | 38.84 | 59.30 | 61.49 | 44.49 | 39.59 | 44.01 | 41.46 |
| SMAST | .57 | .94 | .71 | .99 | .14 | .54 | .50 | 1.40 |
| AUDIT | 5.14 | 2.83 | 6.00 | 2.08 | 5.07 | 2.37 | 5.14 | 2.63 |
| TLFB: | ||||||||
| Continuous drinking days | 2.64 | 2.44 | 2.57 | 1.91 | 1.50 | .65 | 2.64 | 4.53 |
| Continuous abstinence days | 11.71 | 7.38 | 6.93 | 3.77 | 11.21 | 5.75 | 11.64 | 7.51 |
| Total no.drinking days | 6.21 | 5.58 | 9.07 | 6.28 | 4.86 | 2.60 | 6.21 | 6.54 |
| Total no.drinks | 24.00 | 26.72 | 40.57 | 35.76 | 18.61 | 13.26 | 23.57 | 28.92 |
| Highest no. drinks in 1 day | 6.57 | 4.67 | 8.00 | 4.33 | 6.57 | 4.11 | 5.29 | 3.83 |
| Heavy drinking days | 1.71 | 2.20 | 3.29 | 3.17 | 1.36 | 1.45 | 1.93 | 3.22 |
| Drunk days | .71 | .91 | 2.43 | 3.37 | 1.43 | 1.34 | .79 | 1.53 |
| Eysenck | 4.36 | 2.47 | 5.14 | 3.84 | 6.79 | 4.81 | 6.00 | 4.11 |
| BIS-11 | 54.07 | 5.54 | 54.43 | 8.03 | 54.71 | 11.77 | 50.57 | 11.53 |
| ADD/H | 11.93 | 5.55 | 10.43 | 6.54 | 11.93 | 8.22 | 10.07 | 6.02 |
| RT (ms) valid go cue | 287.55 | 16.64 | 283.76 | 26.87 | 290.18 | 21.12 | 291.71 | 28.80 |
| RT (ms) invalid no-go cue | 300.94 | 15.03 | 299.33 | 27.50 | 311.13 | 24.97 | 301.89 | 27.48 |
| p-inhibition failures valid no-go cue | .02 | .02 | .02 | .03 | .02 | .04 | .01 | .01 |
| p-inhibition failures invalid go cue | .04 | .03 | .05 | .05 | .06 | .08 | .04 | .03 |
| Sedation rating | 13.93 | 8.82 | 17.86 | 12.97 | 14.14 | 14.62 | 18.79 | 12.37 |
| Stimulation rating | 25.43 | 12.13 | 22.50 | 13.30 | 25.21 | 15.39 | 20.14 | 16.39 |
| Mental Fatigue rating | 27.29 | 21.30 | 43.50 | 29.22 | 28.07 | 22.36 | 42.79 | 27.52 |
BACs
No detectable BACS were observed under the placebo or energy drink conditions. Group and gender differences in BAC under the two active alcohol dose conditions were examined by a 2 (Group: alcohol v. AmED) × 2 (Gender) × 5 (Time) mixed design ANOVA. No main effects or interactions involving group or gender were observed, ps > .44. There was a main effect of time owing to the rise and fall of BAC over the course of the session, F(4,96) = 3.94, MSE = .001, p = .005 (see Table 2).
Table 2.
Breath alcohol concentrations (BACs) and subjective ratings of stimulation, sedation, mental fatigue, subjective intoxication, feel the drink, like the drink, impairment and willingness to drive under the 4 dose conditions. Participants gave the ratings at 70 min. after the onset of dose administration.
| Dose Condition | ||||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Energy Drink | Alcohol | AmED | |||||
| M | SD | M | SD | M | SD | M | SD | |
| BAC (g%) @ 30 min. | .072 | .005 | .080 | .008 | ||||
| BAC (g%) @ 40 min. | .089 | .007 | .081 | .005 | ||||
| BAC (g%) @ 70 min. | .083 | .004 | .077 | .005 | ||||
| BAC (g%) @ 80 min. | .078 | .003 | .070 | .004 | ||||
| BAC (g%) @ 90 min. | .077 | .004 | .070 | .004 | ||||
| Stimulation Rating | 20.21 | 15.27 | 23.36 | 13.77 | 32.29 | 19.46 | 34.57 | 16.45 |
| Sedation Rating | 17.14 | 16.30 | 15.64 | 12.76 | 21.07 | 13.98 | 19.21 | 15.78 |
| Mental Fatigue Rating | 28.86 | 28.93 | 44.64 | 34.34 | 40.21 | 28.48 | 28.43 | 27.42 |
| Subjective Intoxication | .39 | .56 | .43 | .87 | 3.61 | 1.62 | 3.32 | 1.60 |
| Feel Rating | 25.29 | 30.13 | 31.50 | 27.11 | 55.00 | 18.71 | 57.14 | 25.53 |
| Like Rating | 37.14 | 24.48 | 40.57 | 26.15 | 60.93 | 28.35 | 56.29 | 25.45 |
| Impairment Rating | 11.43 | 9.87 | 20.21 | 27.28 | 47.14 | 30.02 | 51.79 | 32.26 |
| Ability to Drive Rating | 84.93 | 26.88 | 92.71 | 13.36 | 38.07 | 39.08 | 35.64 | 39.80 |
Cued Go/No-go Task Performance
Response activation
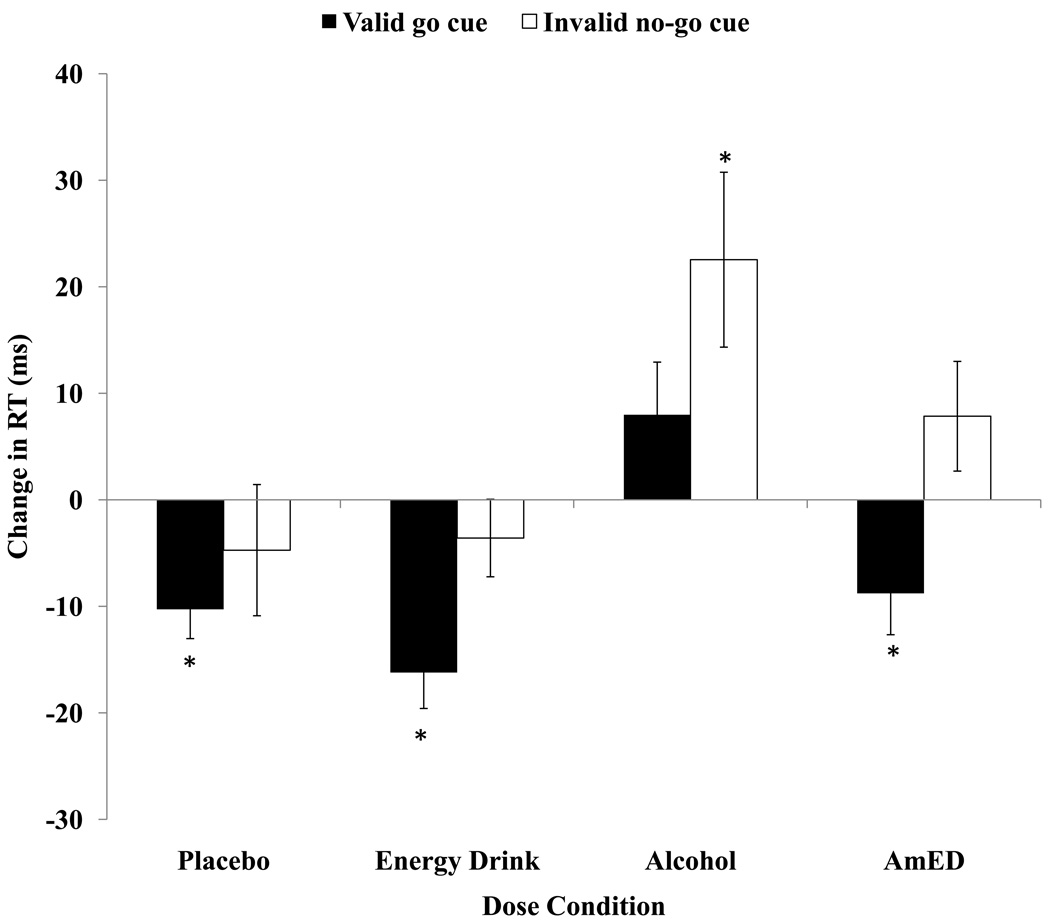
Change scores in mean reaction times (RTs) were submitted to a 2 (Alcohol Dose: 0.65 g/kg v. 0.0 g/kg) × 2 (Energy Drink Dose: 3.57 ml/kg v. 0.0 ml/kg) × 2 (Cue: valid go v. invalid no-go) mixed design ANOVA where Alcohol Dose and Energy Drink Dose were treated as between-subjects factors and Cue was treated as a within-subjects factor. The analysis revealed a significant main effects of Alcohol Dose, F(1,52) = 13.53, MSE = 7261.26, p = .001, Energy Drink Dose, F(1,52) = 4.29, MSE = 2301.88, p = .04, and Cue, F(1,52) = 4.29, MSE = 4264.83, p < .001. Figure 1 illustrates that RTs increased (i.e., were slowed) from baseline under the alcohol conditions compared to when no alcohol was administered. Moreover, RTs decreased from baseline under the energy drink conditions compared to when no energy drink was administered. Finally, RTs decreased from baseline for the valid go cue condition compared to the invalid no-go cue condition. There were no significant interactions for this analysis, ps > .13. Post-hoc one sample t-tests were used to indicate if change scores were significantly different from zero for each dose and cue condition. For the invalid no-go cue condition, change in mean RT was significantly slower when alcohol was administered alone, t(13) = 2.75, p = .02, but unchanged from baseline when the placebo, energy drink or AmED was administered, p > .15. For the valid go cue condition, change in mean RT was significantly faster when the placebo, energy drink or AmED was administered, ps < .05, but unchanged from baseline when alcohol was administered, p = .13.
Figure 1.
Mean difference scores representing the mean reaction time (ms) to the go target post-drink subtracted from the mean reaction time (ms) to the go target at baseline following valid (go) and invalid (no-go) cues for each dose condition. Positive change scores indicate impaired (i.e., slower) response activation compared with baseline. Standard errors are represented in the figure by the error bars attached to each column. An asterisk indicates a significant change from baseline (p < .05).
Failures of response inhibition
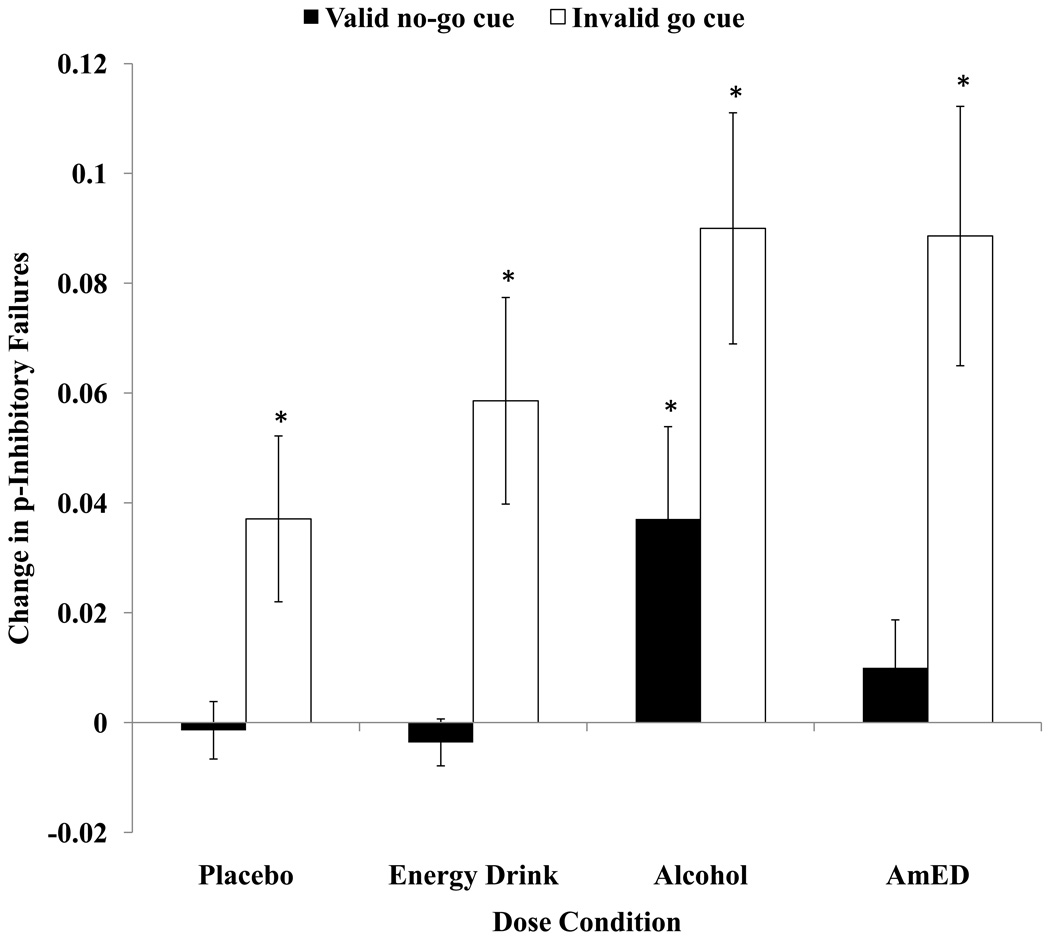
Change scores in p-inhibition failures were submitted to a 2 (Alcohol Dose: 0.65 g/kg v. 0.0 g/kg) × 2 (Energy Drink Dose: 3.57 ml/kg v. 0.0 ml/kg) × 2 (Cue: valid go v. invalid no-go) mixed design ANOVA. The analysis revealed significant main effects of Alcohol Dose, F(1,52) = 6.87, MSE = .032, p = .01, and Cue, F(1,52) = 40.92, MSE = .094, p < .001. Figure 2 illustrates that p-inhibition failures increased from baseline under the alcohol conditions compared to when no alcohol was administered. Moreover, p-inhibition failures increased from baseline in the invalid go condition compared to the valid no-go cue condition. There were no other significant main effects or interactions for this analysis, ps > .13. Post-hoc one sample t-tests were used to indicate if change scores were significantly different from zero for each dose and cue condition. For the invalid go cue condition, changes scores for p-inhibition failures were significantly increased under all dose conditions, ps > .03, indicating poorer inhibitory control. For the valid no-go cue condition, change scores for p-inhibition failures were significantly increased when alcohol was administered alone, t(13) = 2.21, p <.05, but unchanged from baseline when placebo, energy drink or AmED was administered, ps > .27.
Figure 2.
Mean difference scores representing the p-inhibition failures to the no-go target post-drink subtracted from the mean p-inhibition failures to the no-go target at baseline following valid (no-go) and invalid (go) cues for each dose condition. Positive change scores indicate impaired response inhibition compared with baseline. Standard errors are represented in the figure by the error bars attached to each column. An asterisk indicates a significant change from baseline (p < .05).
Subjective Ratings
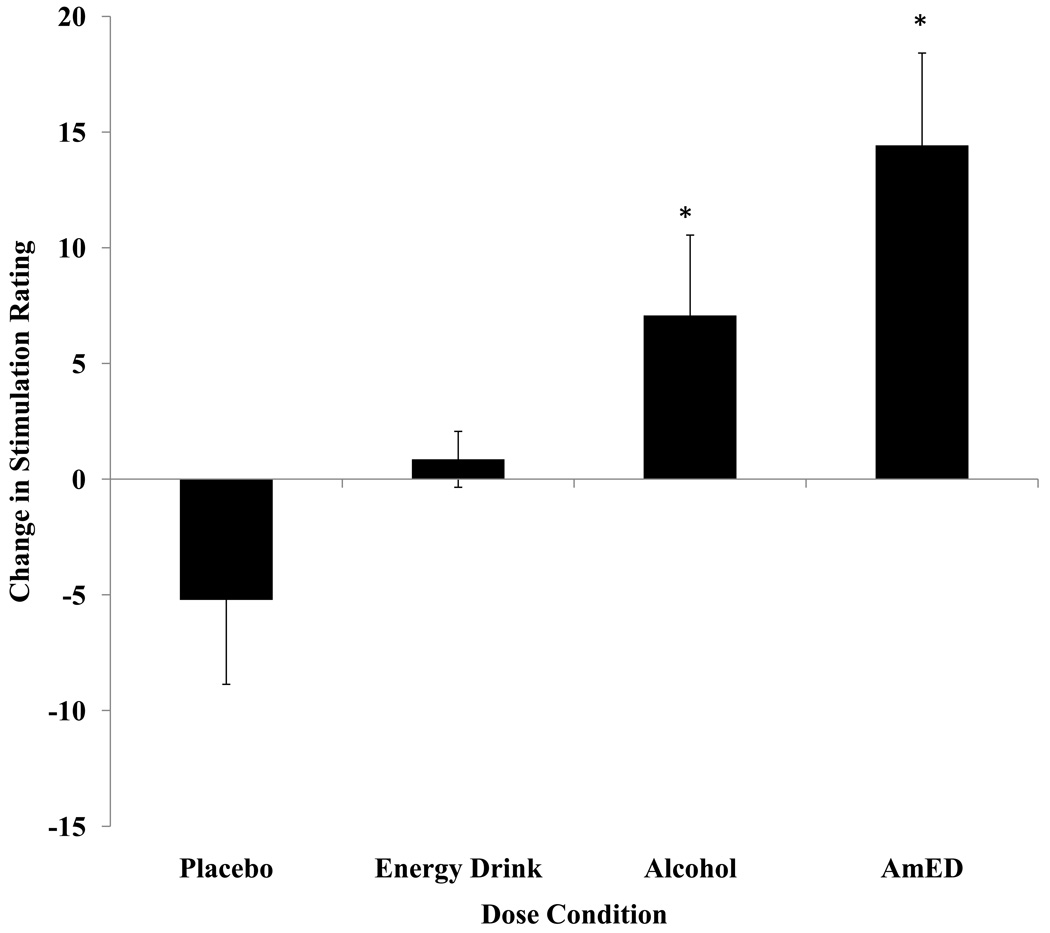
Table 2 illustrates the mean stimulation, sedation, mental fatigue, subjective intoxication, feel the drink, like the drink, impairment and ability to drive ratings that were administered 70 min. after the onset of dose administration. Subjective ratings (change scores or post-dose ratings) were analyzed by separate 2 (Alcohol Dose: 0.65 g/kg v. 0.0 g/kg) × 2 (Energy Drink Dose: 3.57 ml/kg v. 0.0 ml/kg) ANOVAs. For the change in stimulation ratings, significant main effects of Alcohol Dose, F(1,52) = 15.63, MSE = 2340.07, p < .001, and Energy Drink Dose, F(1,52) = 4.22, MSE = 631.14, p = .045, were obtained. Figure 3 illustrates that stimulation ratings increased from baseline under the alcohol conditions compared to when no alcohol was administered. Stimulation ratings also increased from baseline under the energy drink conditions compared to when no energy drink was administered. There was no significant interaction for the stimulation ratings, p = .85. Post-hoc one sample t-tests were used to indicate if change scores were significantly different from zero for each dose condition. Stimulation ratings were increased from baseline under the alcohol and AmED conditions, ps < .05, but unchanged from baseline in the placebo and energy drink conditions, ps > .18.
Figure 3.
Mean difference scores representing the mean stimulation rating post-drink subtracted from the mean stimulation rating at baseline for each dose condition. Positive change scores indicate greater stimulation compared with baseline. Standard errors are represented in the figure by the error bars attached to each column. An asterisk indicates a significant change from baseline (p < .05).
For the change in sedation ratings, there were no significant main effects (ps > .09) or interaction (p = .88). However, there was a nonsignificant trend for a main effect of the Energy Drink Dose, F(1,52) = 2.99, MSE = 498.02, p = .09, as the sedation ratings decreased from baseline under the energy drink conditions compared to when no energy drink was administered. For the change in mental fatigue ratings, there were no significant main effects (ps > .09) or interaction (p = .10). However, there was a nonsignificant trend for a main effect of the Energy Drink Dose, F(1,52) = 2.92, MSE = 2538.02, p = .09, as the mental fatigue ratings decreased from baseline under the energy drink conditions compared to when no energy drink was administered.
Analyses of subjective intoxication, feel, like, impairment, and ability to drive ratings showed only significant main effects of alcohol (ps < .01). Table 2 shows that under alcohol and AmED conditions, ratings of subjective intoxication, feel, like, and impairment were greatest and ratings of the ability to drive were lowest. There were no other main effects or interactions for any of these ratings (ps > .35).
Discussion
This research examined if alcohol mixed with energy drinks (AmED) alter objective and subjective responses differently compared to when alcohol is administered alone. We used the cued go/no-go RT task to examine the separate and combined effects of alcohol and energy drinks on aspects of behavioral control. The results showed that alcohol impaired both response execution and response inhibition. The energy drink antagonized alcohol-induced impairment of response execution, as measured by change in RT, but did not antagonize the alcohol-induced impairment of response inhibition. Participants’ subjective ratings also revealed reliable effects of alcohol. The energy drink escalated reported levels of stimulation, but did not alter the alcohol effects observed for the other ratings, including level of intoxication and ability to drive.
The observed dissociations in the energy drink antagonism of alcohol-induced impairment of behavioral control in this study are not surprising given prior studies that examined caffeine antagonism of alcohol-induced impairment of performance. Some studies have shown that the coadministration of caffeine can reduce the impairing effects of alcohol (Burns & Moskowitz, 1990; Fillmore & Vogel-Sprott, 1999). However, other studies have failed to demonstrate counteracting effects of caffeine (Fillmore & Vogel-Sprott, 1995; Liguori & Robinson, 2001). These discrepancies with respect to alcohol-caffeine interactions made been documented in research reviews that concluded that the evidence for a caffeine antagonism is equivocal (Fudin & Nicastro, 1988). Previously, we suggested that tasks which rely on activational aspects of behavioral control might be more likely to show caffeine antagonism of alcohol-induced impairment compared with tasks that rely on inhibitory aspects of control (Marczinski & Fillmore, 2003a). The pattern of results obtained in the current study is consistent with this idea, as we observed that the energy drink antagonized the alcohol-induced impairment of response execution but not the alcohol-induced impairment of response inhibition.
Many researchers have argued that despite the multitude of ingredients found in energy drinks, the high caffeine content is the principal active ingredient driving the stimulant properties that users report after consumption (Ferreira et al., 2006; Marczinski & Fillmore, 2006; Reissig et al., 2009). Given this stance in the literature and the relative newness of energy drink products to the market, it is unsurprisingly that task forces convened to examine the risks of mixing energy drinks and alcohol have relied on findings from published studies that mixed caffeine and alcohol to determine the safety risks of premixed alcohol energy drink products, such as Four Loko (FDA, 2010). However, the results from the current study suggest that energy drinks result in greater effects than would be predicted based on their caffeine content alone. For the behavioral control task used in the current study, previous work demonstrated that a 4.0 mg/kg dose of caffeine was needed to antagonize some of the impairing effects of alcohol on RT, and that 2.0 m/kg caffeine was insufficient to antagonize alcohol impairment (Marczinski & Fillmore, 2003a, 2006). However, the caffeine dose contained in the Red Bull drink in the current study was rather low, only 1.14 mg/kg. Yet, the energy drink significantly antagonized alcohol effects. Thus, the assumption in the literature that it is just the ‘high caffeine content’ in energy drinks that drives the stimulant properties that users often report after consumption of a drink is probably not quite correct. The other ingredients/properties (such as taurine, glucose, ginseng, and level of carbonation) seem to matter and warrant further investigation. Given that social drinkers have become enamored with mixing energy drinks and alcohol, these trendy new drinks are probably not declining in popularity any time soon. As such, laboratory research that specifically examines the acute and chronic effects of alcohol mixed with energy drinks is needed rather than assuming that the field can extrapolate from the prior caffeine alcohol literature to answer its questions regarding safety and abuse potential.
The results of the present research offer a new perspective for interpreting the findings of previous research suggesting that the coadministration of an energy drink with alcohol increases alcohol ingestion and binge drinking in young people (Arria et al., 2010; Price et al., 2010). For example, Price et al. (2010) surveyed college students and used the timeline follow-back (TLFB) procedure to assess recent drinking patterns. They reported that relative to alcohol drinking sessions in which energy drinks were not used, the participants reported drinking significantly more alcohol when it was co-administered with energy drinks. The results from our study suggest that consumption of AmED increases the stimulation experienced by individuals compared to the consumption of the same amount of alcohol administered alone. Increasing levels of stimulation with an energy drink may increase the rewarding aspects of drinking alcohol, leading to greater consumption especially when inhibitory control remains impaired by the alcohol.
In this study, we tested participants on the rising to peak section of the blood alcohol curve. Future research is needed to determine the effects of AmEDs on feelings of stimulation and sedation for all portions of the blood alcohol curve. Typically, individuals receiving a moderate dose of alcohol report stimulation on the rising limb and sedation on the declining limb (Martin et al., 1993). It is possible that an energy drink could ameliorate some of the sedation experienced on the declining limb, thus encouraging an individual to drink more and for longer periods of time. Moreover, the majority of decisions to drive are made on the descending limb of the blood alcohol curve (Jones, 1990; Levine & Smialek, 2000; Shore et al., 1988). Interoceptive cues concerning one’s level of intoxication likely play a role in decisions to drive. In the current study, we asked participants to rate their ability to drive at the peak of the BAC curve and the ratings were similar for the alcohol and AmED dose conditions. In future, it would be important to ask this same question on the declining limb. Given that a recent field study reported that AmED users were more likely to consider driving home compared to alcohol users (Thombs et al. 2010), it is important to determine the effects of AmED on willingness to drive ratings while closely monitoring BACs in a controlled laboratory setting.
This study raises some important questions, some of which are due to limitations of the current study design. Only one type of energy drink (Red Bull) and one dose level for the alcohol and the energy drink was used for this study. However, the constituent components of energy drinks can differ dramatically among brands. We chose Red Bull for this study since the brand grosses the highest sales in the energy drink market in the U.S. (65% of market share in 2005), and the company that owns the product has been very effective at marketing the use of this energy drink with alcohol (Bryce & Dyer, 2007). However, young people are mixing a variety of different types of energy drinks (e.g., Monster, Rockstar, etc.) with different kinds of alcohol (e.g., vodka, Jagermeister, etc.). Future studies should examine the variety of different energy drinks to determine the importance of caffeine, taurine, glucose and the other ingredients in the effects observed in participants. Moreover, we chose to administer a 0.65 g/kg dose of alcohol to have participants reach a peak BAC of .08 g%, which has real world relevance for impaired driving. However, the comparisons of the effects of AmED versus alcohol alone for doses above and below the level used in the current study are needed, especially since inferences about potential pharmacological mechanisms of AmEDs would require dose-response curves. Another aspect of our study included the fact that the participants were blind to what drink they were receiving and they consumed their drinks while alone in a lab testing room. This was critical as an initial test as we needed to understand that pharmacological effects of AmED versus alcohol. However, expectation is known to play a critical role in how participants display behavioral improvement or impairment in response to alcohol and caffeine (Fillmore et al., 1994; Fillmore et al., 2002; Fillmore & Vogel-Sprott, 1992). Therefore, future studies should examine the role of expectation in response to AmED, especially since energy drinks are marketed as beverages that will increase energy and allay fatigue. Moreover, college students typically drink in social settings. Thus, the ecological validity of this study is limited as the drinkers were alone while drinking and when tested. Future studies need to incorporate the variety of social factors that may play important roles in the selection of these drinks and the effects they produce. Finally, it is important to recognize that we used a relatively small sample size which restricted our ability to examine a variety of individual difference variables that may be of great importance. For example, previous studies have demonstrated that binge or heavy drinkers are more disinhibited by alcohol and feel less sedated by alcohol than their more moderate social drinking peers (Holdstock et al., 2000; Marczinski et al., 2007, 2008). Future studies are needed to examine what other factors exacerbate the differences between the effects of alcohol and AmEDs.
In summary, the results of the present study indicate that the acute effects of AmEDs may differ in important ways from the effects of alcohol alone. Given the dramatic escalation in the popularity of AmEDs among young people, more controlled laboratory studies are needed to determine if AmEDs are escalating risky drinking practices in a demographic group with high levels of binge drinking. Given that the FDA does not regulate energy drinks, a closer examination of the effects of these drinks, especially when combined with alcohol, is warranted.
Acknowledgments
The project described was supported by Award Number R15AA019795 from the National Institute on Alcohol Abuse and Alcoholism and a Faculty Fellowship awarded by the Kentucky Biomedical Research Infrastructure Network (NIH grant P20RR016481), both to C.A. Marczinski. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health.
References
- Abroms BD, Fillmore MT, Marczinski CA. Alcohol-induced impairment of behavioral control: Effects on the alternation and suppression of prepotent responses. J Stud Alcohol. 2003;64:687–695. doi: 10.15288/jsa.2003.64.687. [DOI] [PubMed] [Google Scholar]
- Arria AM, Caldeira KM, Kasperski SJ, O’Grady KE, Vincent KB, Griffiths RR, Wish ED. Increased alcohol consumption, nonmedical prescription drug use, and illict drug use are associated with energy drink consumption among college students. J Addict Med. 2010;4:74–80. doi: 10.1097/ADM.0b013e3181aa8dd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbor TF, de la Fuente JR, Saunders J, Grant M. WHO/MNH/DAT 89.4. Geneva, Switzerland: World Health Organization; 1989. AUDIT The alcohol use disorders identification test: Guidelines for use in primary health care. [Google Scholar]
- Barone JJ, Roberts HR. In: Human consumption of caffeine, in Caffeine: Perspectives from Recent Research. Dews PB, editor. Berlin: Springer-Verlag; 1984. pp. 59–73. [Google Scholar]
- Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- Barry KL, Fleming MF. The Alcohol Use Disorders Identification Test (AUDIT) and the SMAST-13: predictive validity in a rural primary care sample. Alcohol Alcohol. 1993;23:33–42. [PubMed] [Google Scholar]
- Beirness DJ. Self-estimates of blood alcohol concentration in drinking-driving context. Drug Alcohol Depend. 1987;19:79–90. doi: 10.1016/0376-8716(87)90089-5. [DOI] [PubMed] [Google Scholar]
- Bryce DJ, Dyer JH. Strategies to crack well-guarded markets. Harvard Bus Rev. 2007;85:84–92. [PubMed] [Google Scholar]
- Burns M, Moskowitz H. Two experiments on alcohol-caffeine interaction. Clin Pharmacol Therapeutics. 1990;32:98–106. [Google Scholar]
- Clauson KA, Shields KM, McQueen CE, Persad N. Safety issues associated with commercially available energy drinks. J Am Pharm Ass. 2008;48:e55–e63. doi: 10.1331/JAPhA.2008.07055. [DOI] [PubMed] [Google Scholar]
- Clay SW, Allen J, Parran T. A review of addiction. Postgrad Med. 2008;120:E01–E07. doi: 10.3810/pgm.2008.07.1802. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Pearson PR, Easting G, Allsop JF. Age norms for impulsiveness, venturesomeness and empathy in adults. Pers Ind Diff. 1985;6:613–619. [Google Scholar]
- FDA U.S. Food and Drug Administration. [FDA website] FDA Warning Letters issued to four makers of caffeinated alcoholic beverages. [Accessed Nov. 21, 2010];2010 Nov. 17 Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm234109.htm.
- Ferreira SE, de Mello MT, Pompeia S, de Souza-Formigoni MLO. Effects of energy drink ingestion on alcohol intoxication. Alcohol Clin Exp Res. 2006;30:598–605. doi: 10.1111/j.1530-0277.2006.00070.x. [DOI] [PubMed] [Google Scholar]
- Ferreira SE, Hartmann Quadros IM, Trindade AA, Takahashi S, Koyama RG, Souza-Formigoni MLO. Can energy drinks reduce the depressor effect of ethanol? An experimental study with mice. Physiol Beh. 2004;82:841–847. doi: 10.1016/j.physbeh.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Cognitive preoccupation with alcohol and binge drinking in college students: Alcohol-induced priming of the motivation to drink. Psychol Addict Beh. 2001;15:325–332. [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: Current approaches and findings. Beh Cogn Neurosci Rev. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol. 2005;66:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Mulvihill LE, Vogel-Sprott M. The expected drug and its expected effect interact to determine placebo responses to alcohol and caffeine. Psychopharmacol. 1994;115:383–388. doi: 10.1007/BF02245081. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Roach EL, Rice JT. Does caffeine counteract alcohol-induced impairment? The ironic effects of expectancy. J Stud Alcohol. 2002;63:745–754. doi: 10.15288/jsa.2002.63.745. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Expected effect of caffeine on motor performance predicts the type of response to placebo. Psychopharmacol. 1992;106:209–214. doi: 10.1007/BF02801974. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Behavioral effects of combining alcohol and caffeine: The contribution of drug-related expectancies. Exp Clin Psychopharm. 1995;3:33–38. [Google Scholar]
- Fillmore MT, Vogel-Sprott M. An alcohol model of impaired inhibitory control and its treatment in humans. Exp Clin Psychopharm. 1999;7:49–55. doi: 10.1037//1064-1297.7.1.49. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Response inhibition under alcohol: Effects of cognitive and motivational conflict. J Stud Alcohol. 2000;61:239–246. doi: 10.15288/jsa.2000.61.239. [DOI] [PubMed] [Google Scholar]
- Fournier ME, Levy S. Recent trends in adolescent substance use, primary care screening, and updates in treatment options. Curr Opin Pediatr. 2006;18:352–358. doi: 10.1097/01.mop.0000236381.33907.9d. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Application of a behavioral theory of motivation to the concepts of anxiety and impulsivity. J Res Personality. 1987;21:417–435. [Google Scholar]
- Fudin R, Nicastro R. Can caffeine antagonize alcohol-induced performance decrements in humans? Percep Motor Skills. 1988;67:375–391. doi: 10.2466/pms.1988.67.2.375. [DOI] [PubMed] [Google Scholar]
- Gray JA. The behavioral inhibition system: A possible substrate for anxiety, in Theoretical and Experimental Bases of Behavior Therapies. In: Feldman MP, Broadhurst A, editors. London: Wiley; pp. 3–41. [Google Scholar]
- Gray JA. Drug effects of fear and frustration. Possible limbic site of action of minor tranquilizers. In: Iverson LL, Iverson SD, Snyder SH, editors. Handbook of Psychopharmacology. New York: Plenum; pp. 433–529. [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24:789–794. [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from fronto-striatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacol. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jones AW. Status of alcohol absorption among drinking drivers. J Analyt Toxicol. 1990;14:198–200. doi: 10.1093/jat/14.3.198. [DOI] [PubMed] [Google Scholar]
- Levine B, Smialek JE. Status of alcohol absorption in drinking drivers killed in traffic accidents. J Forensic Sci. 2000;45:3–6. [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: A model and a method. J Exp Psychol: Human Perc Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Malinauskas BM, Aeby VG, Overton RF, Carpenter-Aeby T, Barber-Heidal K. A survey of energy drink consumption patterns among college students. Nutrition J. 2007;6:35. doi: 10.1186/1475-2891-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, Fillmore MT. Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychol Addict Beh. 2007;21:346–354. doi: 10.1037/0893-164X.21.3.346. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Dissociative antagonistic effects of caffeine on alcohol-induced impairment of behavioral control. Exp Clin Psychopharmacol. 2003a;11:228–236. doi: 10.1037/1064-1297.11.3.228. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Exp Clin Psychopharmacol. 2003b;11:110–117. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Alcohol increases reliance on cues that signal acts of control. Exp Clin Psychopharmacol. 2005a;13:15–24. doi: 10.1037/1064-1297.13.1.15. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Compensating for alcohol-induced impairment of control: Effects on inhibition and activation of behavior. Psychopharmacol. 2005b;181:337–346. doi: 10.1007/s00213-005-2269-4. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Clubgoers and their trendy cocktails: Implications of mixing caffeine into alcohol on information processing and subjective reports of intoxication. Exp Clin Psychopharmacol. 2006;14:450–458. doi: 10.1037/1064-1297.14.4.450. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Grant EC, Grant VJ. Nova Science. New York: 2009. Binge Drinking in Adolescents and College Students. [Google Scholar]
- Marczinski CA, Harrison ELR, Fillmore MT. Effects of alcohol on simulated driving and perceived driving impairment in binge drinkers. Alcohol Clin Exp Research. 2008;32:1329–1337. doi: 10.1111/j.1530-0277.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McCusker RR, Goldberger BA, Cone EJ. Caffeine content of energy drinks, carbonated sodas, and other beverages. J Anal Toxicol. 2006;30:112–114. doi: 10.1093/jat/30.2.112. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychol Rev. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatr. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- Miller J, Schaffer R, Hackley SA. Effects of preliminary information in a go versus no-go task. Acta Psychol. 1991;76:241–292. doi: 10.1016/0001-6918(91)90022-r. [DOI] [PubMed] [Google Scholar]
- Miller KE. Energy drinks, race, and problem behaviors among college students. J Adolesc Health. 2008;43:490–497. doi: 10.1016/j.jadohealth.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien MC, McCoy TP, Rhodes SD, Wagoner A, Wolfson M. Caffeinated cocktails: Energy drink consumption, high-risk drinking, and alcohol-related consequences among college students. Acad Emerg Med. 2008;15:453–460. doi: 10.1111/j.1553-2712.2008.00085.x. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Newman JP. Reflectivity and learning from aversive events: Toward a psychological mechanism for the syndromes of disinhibition. Psychol Rev. 1993;100:716–736. doi: 10.1037/0033-295x.100.4.716. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pernanen K. Research approaches in the study of alcohol-related violence. Alcohol Health Res World. 1993;17:101–107. [Google Scholar]
- Price SR, Hilchey CA, Darredeau C, Fulton HG, Barrett SP. Energy drink coadministration is associated with increased reported alcohol ingestion. Drug Alcohol Rev. 2010;29:331–333. doi: 10.1111/j.1465-3362.2009.00163.x. [DOI] [PubMed] [Google Scholar]
- Quay HC. Inhibition and attention deficit hyperactivity disorder. J Abnorm Child Psychol. 1997;25:7–13. doi: 10.1023/a:1025799122529. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks—a growing problem. Drug Alcohol Depend. 2009;99:1–10. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin AL, Vandermay SJ. Validation of a measure for adolescent self-report of attention deficit disorder symptoms. J Dev Behav Pediatrics. 1996;17:211–215. [PubMed] [Google Scholar]
- Schmidt A, Barry KL, Fleming MF. Detection of problem drinkers: the alcohol use disorders identification test (AUDIT) South Med J. 1995;88:52–59. [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user’s guide. Pittsburgh, PA: Psychology Software Tools; 2002. [Google Scholar]
- Seltzer ML, Vinokur A, Van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Shore ER, McCoy ML, Toonen LA, Kuntz EJ. Arrests of women for driving under the influence. J Stud Alcohol. 1988;49:7–10. doi: 10.15288/jsa.1988.49.7. [DOI] [PubMed] [Google Scholar]
- Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40:1243–1255. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back: A technique for assessing self-reported alcohol consumption, in Measuring Alcohol Consumption. In: Litten R, Allen J, editors. Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; pp. 41–72. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2006 National Survey on Drug Use and Health: National Findings. Rockville, MD: SAMHSA Office of Applied Statistics; 2007
- Thombs DL, O’Mara RJ, Tsukamoto M, Rossheim ME, Weiler RM, Merves ML, Goldberger BA. Event-level analyses of energy drink consumption and alcohol intoxication in bar patrons. Addict Beh. 2010;35:325–330. doi: 10.1016/j.addbeh.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M. New York, NY: Guilford; 1992. Alcohol Tolerance and Social Drinking: Learning the Consequences. [Google Scholar]
- Watson PE, Watson ID, Batt RD. Prediction of blood alcohol concentrations in human subjects: Updating the Widmark equation. J Stud Alcohol. 1981;42:547–556. doi: 10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacol. 2008;201:315–324. doi: 10.1007/s00213-008-1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]