Abstract
5-HT plays a regulatory role in voluntary movements of the basal ganglia and has a major impact on disorders of the basal ganglia such as Parkinson's disease (PD). Clinical studies have suggested that 5-HT2 receptor antagonists may be useful in the treatment of the motor symptoms of PD. We hypothesized that 5-HT2A receptor antagonists may restore motor function by regulating glutamatergic activity in the striatum. Mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) exhibited decreased performance on the beam-walking apparatus. Peripheral administration of the 5-HT2A receptor antagonist M100907 improved performance of MPTP-treated mice on the beam-walking apparatus. In vivo microdialysis revealed an increase in striatal extracellular glutamate in MPTP-treated mice and local perfusion of M100907 into the dorsal striatum significantly decreased extracellular glutamate levels in saline and MPTP-treated mice. Our studies suggest that blockade of 5-HT2A receptors may represent a novel therapeutic target for the motor symptoms of PD.
Keywords: dopamine, glutamate, M100907, microdialysis, motor deficits, MPTP, parkinsonism, serotonin
Introduction
Serotonin (5-HT) projections from the dorsal raphe nucleus innervate all components of the basal ganglia circuitry (Lavoie and Parent, 1990). 5-HT has been shown to modulate not only dopamine (DA) neurotransmission in the striatum, but also GABA and glutamate transmission in the output regions of the basal ganglia via a number of 5-HT receptors (Nicholson and Brotchie, 2002). Thus, 5-HT may play a regulatory role in voluntary movements of the basal ganglia and have a major impact on disorders of the basal ganglia such as Parkinson's disease (PD; Di Matteo et al., 2008). Clinical studies have suggested that 5-HT2 receptor antagonists may be beneficial in the treatment of the motor symptoms of PD. For example, ritanserin, a 5-HT2A/C receptor antagonist, has been shown to reduce akinesia and improve gait in PD patients (Henderson et al., 1992) as well as ameliorate neuroleptic-induced parkinsonism (Bersani et al., 1990). It was reported that ritanserin reduces extrapyramidal side effects when added to typical antipsychotics (Miller et al., 1990, 1992). Whereas typical antipsychotic drugs (APD), which are potent DA D2 antagonists (such as haloperidol), cause parkinsonian side effects, atypical APD with relatively high 5-HT2A: DA D2 receptor affinities ratio (such as clozapine) are less prone to induce these extrapyramidal parkinsonian-like side effects (Altar et al., 1986; Meltzer et al., 1989; Matsubara et al., 1993; Nyberg et al., 1993). Furthermore, animal studies have shown that haloperidol-induced catalepsy is reduced by 5-HT2A receptor antagonists (Balsara et al., 1979; Neal-Beliveau et al., 1993; Bartoszyk et al., 1996; Lucas et al., 1997; Young et al., 1999). Lucas et al. (1997) showed in rats that the reduction of neuroleptic-induced catalepsy by ritanserin was independent of changes in DA dynamics suggesting that the anti-cataleptic effects of ritanserin may involve non-dopaminergic targets, possibly glutamatergic. Whereas 5-HT2A receptors are widely distributed in the brain (Pompeiano et al., 1994; Ward and Dorsa, 1996; Mijnster et al., 1997; Bubser et al., 2001), anatomical studies have suggested that cortico-striatal and pallidostriatal neurons are the major source of 5-HT2A receptor binding in the striatum (Bubser et al., 2001). As such, 5-HT2A-containing afferents to the striatum offer an anatomical substrate for the ability of 5-HT2A receptor antagonists to modulate basal ganglia circuitry that may be dysfunctional in PD. Recently, we have shown that the 5-HT2A receptor antagonist M100907 but not the selective 5-HT2C receptor antagonist SB 206553 improved motor impairments in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; Ferguson et al., 2010). A number of studies in the literature are consistent with the interpretation that the 5-HT2A receptor regulates glutamatergic transmission in the cortex (Aghajanian and Marek, 1997, 1999; Scruggs et al., 2000, 2003). Whether 5-HT2A receptor regulates glutamatergic transmission in the striatum has not been adequately studied. We hypothesized that 5-HT2A receptors localized on cortico-striatal axons regulate glutamatergic activity in the striatum and that 5-HT2A receptor antagonists may restore motor function by normalizing the overactive glutamatergic drive resulting from DA depletion.
We assessed the effect of the 5-HT2A receptor antagonist M100907 on motor impairments in MPTP-treated mice. Using in vivo microdialysis, we also determined whether intrastriatal M100907 administration will alter extracellular glutamate concentrations.
Materials and Methods
Animals
Male C57BL/6J mice, 70–77 days of age at the start of experiments, were obtained from Jackson Labs (Bar Harbor, ME, USA). Animals were group housed in a temperature and humidity controlled room and maintained on a 12L:12D light–dark cycle (lights off at 1900 h). Food and water were provided ad libitum. All studies were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and under the oversight of the Meharry Medical College Animal Care and Use Committee.
Drug treatments
Mice were injected with 20 mg/kg (ip) MPTP (Sigma-Aldrich, St. Louis, MO, USA) or saline every 2 h for a total of four injections, resulting in a cumulative dose of 80 mg/kg. All experiments were done 3 weeks after MPTP administration. The selective 5-HT2A receptor antagonist, M100907 [(R-(+)-alpha-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenethyl)]-4-piperidinemethanol); Kehne et al., 1996], was dissolved in 0.1 M tartaric acid solution and adjusted to pH 6.5–7.0 with NaOH. All injection volumes were 10 ml/kg. M100903 was injected intraperitoneally 30 min before behavioral testing. All mice received a single dose of either vehicle or M100907. Thus, all comparisons were between subjects. The doses of M100907 (0.0001–0.01 mg/kg) used in the motor function test were based on the available literature as well as our previous studies (Ferguson et al., 2010) which showed that the selected doses did not alter general locomotor behavior.
Beam-walking apparatus
The apparatus used in this experiment was as described previously (Ferguson et al., 2010). It consisted of a 1 cm square stainless steel beam of 105 cm in length. The beam was suspended 49 cm above the floor of the test chamber. Mice were habituated to the goal box for 3 min, then placed a distance of 10 cm from goal box. They were allowed to traverse the beam to the goal box. Upon successful traversal of the beam to the goal box at the 10 cm distance, mice were placed at increasing distances of 30, 50, and 80 cm from the goal box and trained to traverse the beam for one trial at each distance for two consecutive days. Mice able to traverse the full 80 cm length of the beam to the goal box within 60 s were considered to reach criterion. Three weeks post-MPTP or saline treatment, mice were subjected to three consecutive trials on the beam during which time they were videotaped. The number of footslips off the beam in each trial, and the mean number of hindlimb footslips during a three-trial session, were recorded (Dluzen et al., 2001; Fernagut et al., 2004; Strome et al., 2006; Allbutt and Henderson, 2007; Quinn et al., 2007; Urakawa et al., 2007) by persons unaware of the treatment condition of the animals. A total of 104 mice were trained for studies using the beam-walking task. Separate groups of animals were used for assessment of baseline beam-walking performance of saline-treated (n = 31), MPTP-treated (n = 31) and MPTP-treated mice receiving three doses of M100907 (n = 14/dose). This approach reduces the possible confound of motor learning which can occur with multiple testing of the same animals.
Regional monoamine concentrations
Separate groups of animals were used to determine the effects of MPTP treatment on regional monoamine concentrations. Animals (saline, n = 15; MPTP, n = 18) were sacrificed 3 weeks after saline or MPTP administration, which is the same time point that the behavioral studies and in vivo microdialysis were performed. These animals did not receive pharmacological agents. The tissues harvested for monoamine determination included the striatum, nucleus accumbens, substantia nigra, and cerebellum. The tissues were dissected from 1.0 mm thick coronal slices (Deutch et al., 1985) and samples stored at −80°C until assayed. Regional concentrations of dopamine and serotonin were determined by HPLC-EC as previously described (Deutch and Cameron, 1992), with protein concentrations determined by the method of Lowry et al. (1951).
In vivo microdialysis
Two weeks after MPTP or saline treatment, mice (saline, n = 11; MPTP, n = 11) were implanted with a chronic indwelling guide cannula; 5–7 days later the mice were used in dialysis sessions examining the ability of the 5-HT2A antagonist M100907 to modify glutamate release in the striatum. One day prior to use, the efficiency of transmitter recovery by the probe was determined by collecting three 10-min samples (perfusing flow rate of 2.0 μL/min) after placing the probe in a solution of glutamate (200 pg/μL) in artificial cerebrospinal fluid (aCSF; 140 mM NaCl, 3.4 mM KCl, 1.5 mM CaCl2, 1.0 mM MgCl2, 1.4 mM NaH2PO4, and 4.85 mM NaHPO4, pH 7.4). Mice were anesthesized with isoflurane for stereotaxic surgery to place guide cannula (Plastics One; Roanoke, VA, USA) into the right striatum (anterior–posterior, +0.6 mm; dorso-ventral, −4.2 mm; and lateral, 2.0 mm relative to bregma; Franklin and Paxinos, 2008). A dual dental adhesive (Plastics One; Roanoke, VA, USA) was applied to the skull surface and base of the cannula, and then built up with a small amount of dental acrylic compound. Four to five days post-operatively, the dialysis probe (1.5 mm active exchange surface) was inserted and the animal was placed in a small Plexiglas chamber (9′ round) that is placed in the dialysis chamber. The swivel assembly and attached tubing was carefully counterbalanced to allow free movement of the mouse. The dialysis probe was perfused overnight at 0.2 μL/min with aCSF. The following morning, the flow rate was increased to 2.0 μL/min. Five 20-min baseline samples were collected, after which M100907 (100 nM) was administered through the dialysis probe and an additional seven fractions were collected. At the end of the experiment mice were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.), perfused intracardially with 4% paraformaldehyde and serial coronal sections (40 μm) were cut through the striatum and stained with cresyl violet. If the probe placement was not correct (i.e., outside the striatum), the data from that animal were discarded. The levels of amino acids in the dialysate were determined using reverse phase HPLC-EC and fluorescent detection. Aminobutyric acid was added to dialysis samples as an internal standard. Samples were derivatized using o-phthalaldehyde and loaded into an autosampler for injection onto a 1.5 micron C18 column (Alltech Associates; Deerfield, IL, USA). The mobile phase was 100 mM sodium phosphate buffer containing 10% methanol (pH 3.70) and flow rate was set at 1.2 ml/min with the column temperature maintained at 40°C. The glutamate derivatization products were detected with a RF-10Axl fluorescence detector (Shimadzu Corp; Kyoto, Japan) and an electrochemical detector (ESA; Chelmford, MA, USA) placed in series. Mean baseline levels of glutamate were calculated by averaging the concentrations of the five basal dialysate samples. If any baseline sample from an animal varies by more than 30% of the mean, it was eliminated; data from animals with less than three basal samples were not included in the analysis.
Data analysis
Data were expressed as means ± SEM and analyzed by one-way analysis of variance (ANOVA) or two-way ANOVA (microdialysis data). When appropriate, comparisons were carried out with Tukey's post hoc tests. Alpha levels were set at 0.05.
Results
Regional monoamine concentrations
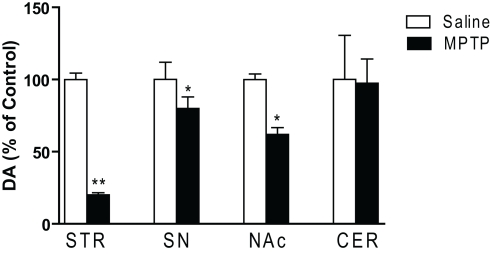
To evaluate the extent of dopaminergic lesion, the levels of DA in the striatum, substantia nigra, nucleus accumbens and cerebellum were measured by HPLC. MPTP treatment had significant main effect on DA in subcortical regions [F(7,107) = 4.542; p < 0.001; Figure 1]. Post hoc analysis revealed that DA was significantly decreased in the striatum (p < 0.01), substantia nigra and nucleus accumbens (p < 0.05) but not in the cerebellum. The 5-HT content in these brain regions of MPTP-treated mice remained generally unchanged except for a small but insignificant decrease in the substantia nigra (data not shown).
Figure 1.
Dopamine concentrations in subcortical regions of saline and MPTP-treated mice. Dopamine concentrations were determined 3 weeks after the last MPTP injection. In addition to changes in the striatal complex, a significant decrease in dopamine in the substantia nigra was seen (n = 10/group). Abbreviations: CER, cerebellar cortex; NAc, nucleus accumbens; SN, substantia nigra; STR, striatum. Data are expressed as percent of saline-injected control mice. Dopamine concentrations (mean ng/mg protein ± SEM) in control mice: STR: 144.7 ± 9.3; NAc: 14.1 ± 0.54; SN: 6.8 ± 0.5; CER: 0.17 ± 0.05. *p < 0.05, **p < 0.01; relative to saline-injected controls.
Effects of 5-HT2A receptor antagonist M100907 on performance on the beam-walking apparatus
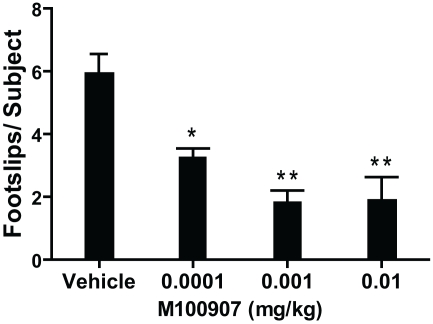
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine produced more than 10-fold increase in the number of footslips during beam traversal (number of footslips/subject: Control 0.42 ± 0.12; MPTP 5.97 ± 0.58; n = 31/group). We (Ferguson et al., 2010) and others (Fredriksson et al., 1990; Tillerson et al., 2002; Tillerson and Miller, 2003) have shown that levodopa can reverse the MPTP-induced motor deficits suggesting that the motor function test employed is sensitive to deficits in the nigrostriatal system, believed to underlie motor impairments in PD. There was significant main effect of M100907 on motor performance of MPTP-treated mice on the beam-walking apparatus [F(3,76) = 14.183; p < 0.0001; Figure 2]. Post hoc analysis showed that M100907 produced dose-dependent decreases (p < 0.001) in the number of footslips produced by MPTP treatment (Figure 2). The doses of M100907 used did not have any effects on the performance of beam-traversal task of control mice (Ferguson et al., 2010).
Figure 2.
M100907 relieved motor deficits in MPTP-treated mice. MPTP-treated mice received vehicle or various doses of M100907. Data are presented as mean ± SEM. M100907 dose-dependently reduced the number of footslips in MPTP-treated mice. *p < 0.05, **p < 0.01; relative to saline-injected controls; in one-way ANOVA with Tukey's post hoc comparison.
Effects of 5-HT2A receptor antagonist M100907 on extracellular glutamate in the striatum
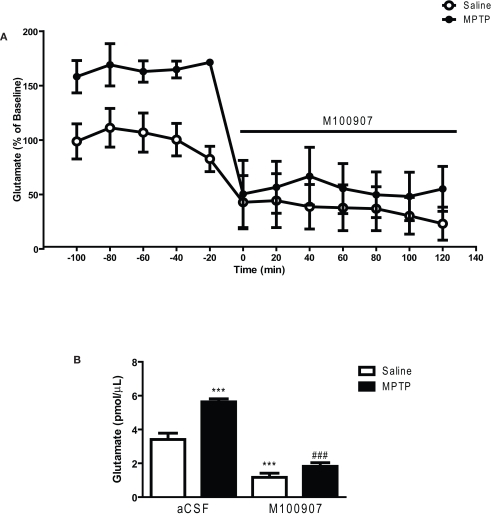
We hypothesized that 5-HT2A receptor antagonists may restore motor function by normalizing the overactive glutamatergic drive resulting from DA depletion. We have used in vivo microdialysis to determine whether extracellular striatal glutamate is increased in mice treated with MPTP and whether local administration of the 5-HT2A receptor antagonist M100907 will decrease striatal glutamate. The mean basal striatal extracellular glutamate levels in the dialysates obtained from saline-treated mice used in these studies were 3.41 ± .24 pmol/μL (mean ± SEM; n = 11; Figure 3). Local application of 1 μM tetrodotoxin resulted in a dramatic fall in basal glutamate output reaching 25% of baseline (data not shown). This suggests that a significant fraction of the resting level of striatal glutamate is of neuronal origin. Figure 3A depicts the time course of the effects of M100907 on basal glutamate levels (expressed as percentage of values of saline-injected controls) of saline and MPTP-treated mice. MPTP-treated mice exhibited increased basal extracellular glutamate levels compared to the saline-treated mice (Figure 3A). Local perfusion of 100 nM M100907 into the dorsal striatum decreased basal glutamate levels in saline- and MPTP-treated mice. Two-way ANOVA of the time course data revealed significant main effects for treatment [F(1,28) = 230.7; p < 0.0001, drug effect, F(1,28) = 305, p < 0.0001, and treatment × drug interaction F(1,28) = 65.67; p < 0.0001; Figure 3A]. The significant treatment x drug interaction suggests that despite elevated basal glutamate levels in the MPTP-treated mice, M100907 was capable of significantly suppressing glutamate output. Figure 3B compares the effects of M100907 on extracellular glutamate values of saline and MPTP-treated mice. The baseline data were obtained from the average of five time points and data for drug treatment were obtained from average of seven time points from Figure 3A. One-way ANOVA revealed significant main effects [F(3,28) = 49.20; p < 0.0001; Figure 3B]. Post hoc analysis using the Tukey's multiple comparison test revealed a significant increase (p < 0.001) in basal extracellular glutamate values in MPTP-treated mice (Figure 3B). Local perfusion of 100 nM M100907 into the dorsal striatum significantly decreased basal glutamate levels in saline (p < 0.001) and MPTP (p < 0.001)-treated mice (Figure 3B).
Figure 3.
M100907 decreased glutamate levels in the dorsal striatum in saline and MPTP-treated mice. Data are expressed as percentages of values in saline-injected control mice. Dialysis was carried out 3 weeks after MPTP or saline treatment and 7 days after the cannula implantation. (A) Five baseline samples were first collected and then a challenge dose of M100907 (100 nM) and an additional seven samples were collected. (B) The effects of M100907 on extracellular glutamate values of saline and MPTP-treated mice. ***p < 0.001 compared with saline-injected control mice; ###p < 0.001 when compared with MPTP-treated mice. The line segment on time course graphs indicate duration of drug administration.
Discussion
5-HT may play a role in voluntary movements regulated by the basal ganglia and have a major impact on disorders of the basal ganglia such as PD (Di Matteo et al., 2008). Clinical studies have suggested that 5-HT2 receptor antagonists may be useful in the treatment of the motor symptoms of PD (Bersani et al., 1990; Henderson et al., 1992). We hypothesized that 5-HT2A receptor antagonists may restore motor function by regulating glutamatergic activity in the striatum. We have shown that the 5-HT2A receptor antagonist M100907 improved performance of MPTP-treated mice on the beam-walking apparatus. In vivo microdialysis studies revealed an increase in striatal extracellular glutamate in MPTP-treated mice and local perfusion of M100907 into the dorsal striatum significantly decreased basal glutamate levels in saline and MPTP-treated mice.
Recently, it has been shown that ACP-103 (pimavancerin), a potent 5-HT2A receptor inverse agonist that blocks 5-HT agonists at 5-HT2A receptors (Vanover et al., 2006) can suppress tacrine-induced tremulous jaw movements in rats (Vanover et al., 2008). Mianserin, a 5-HT2A/C receptor antagonist produced similar anti-tremor effects in rats (Carlson et al., 2003). These data and our studies point to the potential beneficial effects of blockade to 5-HT2A receptors in motor symptoms of PD.
A possible mechanism of regulation of striatal function by 5-HT2A receptors comes from studies showing that following DA depletion, the hyperactive locomotor behaviors induced by intrastriatal infusion of the D1 agonist SKF82958 can be reduced by the 5-HT2A receptor antagonist M100907 (Bishop et al., 2005). It appears the direct striatal output pathway plays a major role in these effects of 5-HT2A receptors (Gresch and Walker, 1999). A more plausible mechanism to explain our findings is an indirect action occurring through regulation of glutamate release. There are a number of studies that support our hypothesis. For example, in situ hybridization and immunohistochemical studies have revealed widespread distribution of 5-HT2A receptors in the striatum (Pompeiano et al., 1994; Ward and Dorsa, 1996; Mijnster et al., 1997; Bubser et al., 2001), however the major source of 5-HT2A receptors appears to be the heteroceptors located on the terminals of the cortico-striatal glutamatergic axons (Bubser et al., 2001). It has been postulated that the loss of striatal DA removes a tonic inhibitory constraint on cortico-striatal glutamtergic axons (DeLong, 1990). Thus, anatomical studies in the 6-hydroxydopamine (6-OHDA)-lesioned rat, MPTP-treated primates, and PD patients reveal adaptive changes at cortico-striatal synapses suggestive of hyperactivity at these glutamatergic synapses (Anglade et al., 1996; Meshul et al., 1999, 2002; Raju et al., 2008). Consistent with the anatomical studies, we (this study) and others (Robinson et al., 2003) using in vivo microdialysis studies and proton magnetic resonance spectroscopy (Chassain et al., 2008) have demonstrate increased glutamate concentrations in the striatum of MPTP-treated mice. Activation of 5-HT2A heteroceptors in several brain areas has been shown to evoke glutamate release (Aghajanian and Marek, 1997; Scruggs et al., 2000, 2003). Thus, it is conceivable that 5-HT2A receptor antagonists may restore motor function by normalizing the overactive glutamatergic drive resulting from DA depletion. Indeed we have shown that local perfusion of M100907 into the dorsal striatum significantly decreased basal glutamate levels in MPTP-treated mice. A more direct approach will be to demonstrate improvement in motor performance following local application of M100907 into the dorsal striatum. These experiments will be the subject of future studies.
In conclusion we have shown that the antiparkinsonian effects of 5-HT2A receptor antagonists may be mediated by regulation of striatal glutamate and that antagonism of striatal 5-HT2A receptors may offer non-dopaminergic therapeutic target for the motor symptoms of PD.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to Dr. Elaine Sanders-Bush, Vanderbilt University for the generous gift of M100907. This work was supported by U54 NS041071.
References
- Aghajanian G. K., Marek G. J. (1997). Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 36, 589–599 10.1016/S0028-3908(97)00051-8 [DOI] [PubMed] [Google Scholar]
- Aghajanian G. K., Marek G. J. (1999). Serotonin and hallucinogens. Neuropsychopharmacology 21, 16S–23S [DOI] [PubMed] [Google Scholar]
- Allbutt H. N., Henderson J. M. (2007). Use of the narrow beam test in the rat, 6-hydroxydopamine model of Parkinson's disease. J. Neurosci. Methods 159, 195–202 [DOI] [PubMed] [Google Scholar]
- Altar C. A., Wasley A. M., Neale R. F., Stone G. A. (1986). Typical and atypical antipsychotic occupancy of D2 and S2 receptors: an autoradiographic analysis in rat brain. Brain Res. Bull. 16, 517–525 [DOI] [PubMed] [Google Scholar]
- Anglade P., Mouatt-Prigent A., Agid Y., Hirsch E. (1996). Synaptic plasticity in the caudate nucleus of patients with Parkinson's disease. Neurodegeneration 5, 121–128 10.1006/neur.1996.0018 [DOI] [PubMed] [Google Scholar]
- Balsara J. J., Jadhav J. H., Chandorkar A. G. (1979). Effect of drugs influencing central serotonergic mechanisms on haloperidol-induced catalepsy. Psychopharmacology (Berl.) 62, 67–69 [DOI] [PubMed] [Google Scholar]
- Bartoszyk G. D., Roos C., Ziegler H. (1996). 5-HT1A receptors are not involved in clozapine's lack of cataleptogenic potential. Neuropharmacology 35, 1645–1646 10.1016/S0028-3908(96)00110-4 [DOI] [PubMed] [Google Scholar]
- Bersani G., Grispini A., Marini S., Pasini A., Valducci M., Ciani N. (1990). 5-HT2 antagonist ritanserin in neuroleptic-induced Parkinsonism: a double-blind comparison with orphenadrine and placebo. Clin. Neuropharmacol. 13, 500–506 10.1097/00002826-199012000-00003 [DOI] [PubMed] [Google Scholar]
- Bishop C., Daut G. S., Walker P. D. (2005). Serotonin 5-HT2A but not 5-HT2C receptor antagonism reduces hyperlocomotor activity induced in dopamine-depleted rats by striatal administration of the D1 agonist SKF 82958. Neuropharmacology 49, 350–358 10.1016/j.neuropharm.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Bubser M., Backstrom J. R., Sanders-Bush E., Roth B. L., Deutch A. Y. (2001). Distribution of serotonin 5-HT(2A) receptors in afferents of the rat striatum. Synapse 39, 297–304 [DOI] [PubMed] [Google Scholar]
- Carlson B. B., Wisniecki A., Salamone J. D. (2003). Local injections of the 5-hydroxytryptamine antagonist mianserin into substantia nigra pars reticulata block tremulous jaw movements in rats: studies with a putative model of Parkinsonian tremor. Psychopharmacology 165, 229–237 [DOI] [PubMed] [Google Scholar]
- Chassain C., Bielicki G., Durand E., Lolignier S., Essafi F., Traore A., Durif F. (2008). Metabolic changes detected by proton magnetic resonance spectroscopy in vivo and in vitro in a murin model of Parkinson's disease, the MPTP-intoxicated mouse. J. Neurochem. 105, 874–882 10.1111/j.1471-4159.2007.05185.x [DOI] [PubMed] [Google Scholar]
- DeLong M. R. (1990). Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285 10.1016/0166-2236(90)90110-V [DOI] [PubMed] [Google Scholar]
- Deutch A. Y., Cameron D. S. (1992). Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience 46, 49–56 10.1016/0306-4522(92)90007-O [DOI] [PubMed] [Google Scholar]
- Deutch A. Y., Tam S. Y., Roth R. H. (1985). Footshock and conditioned stress increase 3,4-dihydroxyphenylacetic acid (DOPAC) in the ventral tegmental area but not substantia nigra. Brain Res. 333, 143–146 10.1016/0006-8993(85)90134-9 [DOI] [PubMed] [Google Scholar]
- Di Matteo V., Pierucci M., Esposito E., Crescimanno G., Benigno A., Di Giovanni G. (2008). Serotonin modulation of the basal ganglia circuitry: therapeutic implication for Parkinson's disease and other motor disorders. Prog. Brain Res. 172, 423–463 10.1016/S0079-6123(08)00921-7 [DOI] [PubMed] [Google Scholar]
- Dluzen D. E., Gao X., Story G. M., Anderson L. I., Kucera J., Walro J. M. (2001). Evaluation of nigrostriatal dopaminergic function in adult+/+ and +/− BDNF mutant mice. Exp. Neurol. 170, 121–128 [DOI] [PubMed] [Google Scholar]
- Ferguson M. C., Nayyar T., Deutch A. Y., Ansah T. A. (2010). 5-HT2A receptor antagonists improve motor impairments in the MPTP mouse model of Parkinson's disease. Neuropharmacology 59, 31–36 10.1016/j.neuropharm.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernagut P. O., Diguet E., Bioulac B., Tison F. (2004). MPTP potentiates 3-nitropropionic acid-induced striatal damage in mice: reference to striatonigral degeneration. Exp. Neurol. 185, 47–62 [DOI] [PubMed] [Google Scholar]
- Franklin K. B. J., Paxinos G. (2008). The Mouse Brain in Stereotaxic Coordinates, 3rd Edn San Diego: Academic Press [Google Scholar]
- Fredriksson A., Plaznik A., Sundstrom E., Jonsson G., Archer T. (1990). MPTP-induced hypoactivity in mice: reversal by L-dopa. Pharmacol. Toxicol. 67, 295–301 [DOI] [PubMed] [Google Scholar]
- Gresch P. J., Walker P. D. (1999). Synergistic interaction between serotonin-2 receptor and dopamine D1 receptor stimulation on striatal preprotachykinin mRNA expression in the 6-hydroxydopamine lesioned rat. Brain Res. Mol. Brain Res. 70, 125–134 10.1016/S0169-328X(99)00138-2 [DOI] [PubMed] [Google Scholar]
- Henderson J., Yiannikas C., Graham J. S. (1992). Effect of ritanserin, a highly selective 5-HT2 receptor antagonist, on Parkinson's disease. Clin. Exp. Neurol. 29, 277–282 [PubMed] [Google Scholar]
- Kehne J. H., Baron B. M., Carr A. A., Chaney S. F., Elands J., Feldman D. J., Frank R. A., van Giersbergen P. L., McCloskey T. C., Johnson M. P., McCarty D. R., Poirot M., Senyah Y., Siegel B. W., Widmaier C. (1996). Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J. Pharmacol. Exp. Ther. 277, 968–981 [PubMed] [Google Scholar]
- Lavoie B., Parent A. (1990). Immunohistochemical study of the serotoninergic innervation of the basal ganglia in the squirrel monkey. J. Comp. Neurol. 299, 1–16 [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- Lucas G., Bonhomme N., De Deurwaerdere P., Le Moal M., Spampinato U. (1997). 8-OH-DPAT, a 5-HT1A agonist and ritanserin, a 5-HT2A/C antagonist, reverse haloperidol-induced catalepsy in rats independently of striatal dopamine release. Psychopharmacology (Berl.) 131, 57–63 [DOI] [PubMed] [Google Scholar]
- Matsubara S., Matsubara R., Kusumi I., Koyama T., Yamashita I. (1993). Dopamine D1, D2 and serotonin2 receptor occupation by typical and atypical antipsychotic drugs in vivo. J. Pharmacol. Exp. Ther. 265, 498–508 [PubMed] [Google Scholar]
- Meltzer H. Y., Matsubara S., Lee J. C. (1989). The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol. Bull. 25, 390–392 [PubMed] [Google Scholar]
- Meshul C. K., Emre N., Nakamura C. M., Allen C., Donohue M. K., Buckman J. F. (1999). Time-dependent changes in striatal glutamate synapses following a 6-hydroxydopamine lesion. Neuroscience 88, 1–16 10.1016/S0306-4522(98)00189-4 [DOI] [PubMed] [Google Scholar]
- Meshul C. K., Kamel D., Moore C., Kay T. S., Krentz L. (2002). Nicotine alters striatal glutamate function and decreases the apomorphine-induced contralateral rotations in 6-OHDA-lesioned rats. Exp. Neurol. 175, 257–274 [DOI] [PubMed] [Google Scholar]
- Mijnster M. J., Raimundo A. G., Koskuba K., Klop H., Docter G. J., Groenewegen H. J., Voorn P. (1997). Regional and cellular distribution of serotonin 5-hydroxytryptamine2a receptor mRNA in the nucleus accumbens, olfactory tubercle, and caudate putamen of the rat. J. Comp. Neurol. 389, 1–11 [PubMed] [Google Scholar]
- Miller C. H., Fleischhacker W. W., Ehrmann H., Kane J. M. (1990). Treatment of neuroleptic induced akathisia with the 5-HT2 antagonist ritanserin. Psychopharmacol. Bull. 26, 373–376 [PubMed] [Google Scholar]
- Miller C. H., Hummer M., Pycha R., Fleischhacker W. W. (1992). The effect of ritanserin on treatment-resistant neuroleptic induced akathisia: case reports. Prog. Neuropsychopharmacol. Biol. Psychiatry 16, 247–251 [DOI] [PubMed] [Google Scholar]
- Neal-Beliveau B. S., Joyce J. N., Lucki I. (1993). Serotonergic involvement in haloperidol-induced catalepsy. J. Pharmacol. Exp. Ther. 265, 207–217 [PubMed] [Google Scholar]
- Nicholson S. L., Brotchie J. M. (2002). 5-hydroxytryptamine (5-HT, serotonin) and Parkinson's disease – opportunities for novel therapeutics to reduce the problems of levodopa therapy. Eur. J. Neurol. 9(Suppl. 3), 1–6 10.1046/j.1468-1331.9.s3.1.x [DOI] [PubMed] [Google Scholar]
- Nyberg S., Farde L., Eriksson L., Halldin C., Eriksson B. (1993). 5-HT2 and D2 dopamine receptor occupancy in the living human brain. A PET study with risperidone. Psychopharmacology (Berl.) 110, 265–272 [DOI] [PubMed] [Google Scholar]
- Pompeiano M., Palacios J. M., Mengod G. (1994). Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res. Mol. Brain Res. 23, 163–178 10.1016/0169-328X(94)90223-2 [DOI] [PubMed] [Google Scholar]
- Quinn L. P., Perren M. J., Brackenborough K. T., Woodhams P. L., Vidgeon-Hart M., Chapman H., Pangalos M. N., Upton N., Virley D. J. (2007). A beam-walking apparatus to assess behavioural impairments in MPTP-treated mice: pharmacological validation with R-(-)-deprenyl. J. Neurosci. Methods 164, 43–49 [DOI] [PubMed] [Google Scholar]
- Raju D. V., Ahern T. H., Shah D. J., Wright T. M., Standaert D. G., Hall R. A., Smith Y. (2008). Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of Parkinsonism. Eur. J. Neurosci. 27, 1647–1658 [DOI] [PubMed] [Google Scholar]
- Robinson S., Freeman P., Moore C., Touchon J. C., Krentz L., Meshul C. K. (2003). Acute and subchronic MPTP administration differentially affects striatal glutamate synaptic function. Exp. Neurol. 180, 74–87 [DOI] [PubMed] [Google Scholar]
- Scruggs J. L., Patel S., Bubser M., Deutch A. Y. (2000). DOI-Induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J. Neurosci. 20, 8846–8852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs J. L., Schmidt D., Deutch A. Y. (2003). The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci. Lett. 346, 137–140 [DOI] [PubMed] [Google Scholar]
- Strome E. M., Cepeda I. L., Sossi V., Doudet D. J. (2006). Evaluation of the integrity of the dopamine system in a rodent model of Parkinson's disease: small animal positron emission tomography compared to behavioral assessment and autoradiography. Mol. Imaging Biol. 8, 292–299 10.1007/s11307-006-0051-6 [DOI] [PubMed] [Google Scholar]
- Tillerson J. L., Caudle W. M., Reveron M. E., Miller G. W. (2002). Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Exp. Neurol. 178, 80–90 [DOI] [PubMed] [Google Scholar]
- Tillerson J. L., Miller G. W. (2003). Grid performance test to measure behavioral impairment in the MPTP-treated-mouse model of Parkinsonism. J. Neurosci. Methods 123, 189–200 [DOI] [PubMed] [Google Scholar]
- Urakawa S., Hida H., Masuda T., Misumi S., Kim T. S., Nishino H. (2007). Environmental enrichment brings a beneficial effect on beam walking and enhances the migration of doublecortin-positive cells following striatal lesions in rats. Neuroscience 144, 920–933 10.1016/j.neuroscience.2006.10.038 [DOI] [PubMed] [Google Scholar]
- Vanover K. E., Betz A. J., Weber S. M., Bibbiani F., Kielaite A., Weiner D. M., Davis R. E., Chase T. N., Salamone J. D. (2008). A 5-HT2A receptor inverse agonist, ACP-103, reduces tremor in a rat model and levodopa-induced dyskinesias in a monkey model. Pharmacol. Biochem. Behav. 90, 540–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover K. E., Weiner D. M., Makhay M., Veinbergs I., Gardell L. R., Lameh J., Del Tredici A. L., Piu F., Schiffer H. H., Ott T. R., Burstein E. S., Uldam A. K., Thygesen M. B., Schlienger N., Andersson C. M., Son T. Y., Harvey S. C., Powell S. B., Geyer M. A., Tolf B. R., Brann M. R., Davis R. E. (2006). Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropylo xy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J. Pharmacol. Exp. Ther. 317, 910–918 [DOI] [PubMed] [Google Scholar]
- Ward R. P., Dorsa D. M. (1996). Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C, and 5-HT6 with neuropeptides in rat striatum. J. Comp. Neurol. 370, 405–414 [DOI] [PubMed] [Google Scholar]
- Young C. D., Bubser M., Meltzer H. Y., Deutch A. Y. (1999). Clozapine pretreatment modifies haloperidol-elicited forebrain Fos induction: a regionally-specific double dissociation. Psychopharmacology (Berl.) 144, 255–263 [DOI] [PubMed] [Google Scholar]