Abstract
Introduction
Long-lasting alterations in hormones, neurotransmitters and stress proteins after hyperthermia may be responsible for the impairment in motor performance during muscle fatigue.
Methods
Subjects (n = 25) performed a maximal intermittent fatigue task of elbow flexion after sitting in either 73 or 26 deg C to examine the effects of prior heat stress on fatigue mechanisms.
Results
The heat stress increased the tympanic and rectal temperatures by 2.3 and 0.82 deg C, respectively, but there was full recovery prior to the fatigue task. While prior heat stress had no effects on fatigue-related changes in volitional torque, EMG activity, torque relaxation rate, MEP size and SP duration, prior heat stress acutely increased the pre-fatigue relaxation rate and chronically prevented long-duration fatigue (p < 0.05).
Discussion
These findings indicate that prior passive heat stress alone does not alter voluntary activation during fatigue, but prior heat stress and exercise produce longer-term protection against long-duration fatigue.
Keywords: cortical inhibition, low frequency fatigue, central fatigue, peripheral fatigue, heat shock proteins, sex
INTRODUCTION
Neuromuscular fatigue is a multi-mechanistic phenomenon during repetitive use of skeletal muscle. Heat stress typically accompanies repetitive muscle use and plays a pivotal role in the fatigue process.1 However, little is known about the influence of prior heat stress on human muscle fatigue. Prior heat stress before physical muscle activity is particularly relevant to individuals with spinal cord injury (SCI), because they have an impaired capacity to sweat. 2 Our goal is to explore if prior passive whole body heat stress affects central and peripheral mechanisms of human skeletal muscle fatigue.
There is now strong evidence which indicates that some of the decline in motor performance during prolonged exercise is attributed to the central nervous system (CNS) not being able to generate or maintain optimal neuronal drive to the exercising muscles (central fatigue).3 Non-invasive stimulation of the primary motor cortex with transcranial magnetic stimulation (TMS) considerably enhanced our understanding in human motor control in various areas, including muscle fatigue. When TMS is applied while muscle is being fatigued, motor evoked potentials (MEPs) increased, 4–7 and the silent period (SP) duration increased.4,6,7 These results were interpreted to mean that muscle fatigue increases both excitatory and inhibitory circuits in the motor cortex, 7 although the relationship between these fatigue-related changes in CNS excitability and central fatigue is not fully understood.8
When there is an increase in core body temperature with exercise, the capacity to perform exercise is decreased even in young healthy adults.9–11 A recent study using TMS indicated that the primary motor cortex output becomes suboptimal with fatigue during heat stress compared to when the body temperature is not elevated.12
Electroencephalography recordings from the prefrontal cortex of exercising subjects also indicates that hyperthermia decreases subjects’ alertness or motivation.13 From these reports, some suggest that the brain is a primary site responsible for hyperthermia-induced central fatigue. 12–15 However, no previous study, to our knowledge, examined the influence of prior heat stress and recovery on the mechanisms of muscle fatigue.
The precise physiological mechanisms responsible for hyperthermia-induced central fatigue during exercise remain elusive. An increased ratio of central serotonin to dopamine levels is one of the proposed mechanisms.16 The concentration of prolactin (PRL) in circulating blood has often been used as an indirect marker of central serotonin and dopamine activity, since its release is stimulated by serotonergic activity and inhibited by dopaminergic activity.17,18 Interestingly, PRL stays elevated for over an hour after prolonged exercise19 and after passive heat stress,20 suggesting that central neurotransmitters may be impaired several hours after a prior dose of passive whole body heat stress.
It is reported that young females are more fatigue resistant than young males.21–24 Although some studies suggest that the majority of the sex difference can be explained by the intrinsic sex-related differences within skeletal muscle, 23,24 others suggest that impairment of voluntary activation (central fatigue) explains, at least in part, the sex differences during fatigue.21,22 Accordingly, if prior whole body heat stress triggers differential long-lasting central effects based on sex, then fatigue should be accentuated in males exposed to prior heat stress. In addition, the long term effects from two forms of stress (prior heat followed by fatigue task) may induce specific adaptations that are different from a fatigue task that is not preceded by heat stress.
Therefore, the primary purpose of this study was to determine whether prior whole body heat stress has long-lasting (~ 1 hour) effects on subsequent muscle fatigue, and secondarily, whether the effects vary in males and females. We hypothesized that prior whole body heat stress would have lasting adaptive effects on central fatigue, and males would be more susceptible to the central activation impairment after a bout of heat stress.
MATERIALS AND METHODS
Subjects
A total of 25 subjects (12 females) were recruited. Their age, height, and weight were 21.2 ± 2.1 (mean ± standard deviation, SD) and 23.2 ± 2.2 years old, 182.0 ± 11.2 and 167.8 ± 6.2 cm, and 77.2 ± 15.8 and 60.9 ± 6.7 kg for males and females, respectively. Both groups consisted of physically active college students with a similar perception and report of their physical activity level (for SF36 25 and Baecke Physical Activity 26). They had no known neurological or musculoskeletal diseases/disorders, and subjects who were actively involved in any passive heat stress method (hot tub, sauna) were excluded. Subjects were asked to refrain from exhaustive exercise and intake of caffeine 24 hours prior to participation in the study. Each subject participated in two sessions (heat and no heat session) separated by 7 to 10 days. Subjects were asked to log their dietary intake and were encouraged to maintain the same intake for the days of experiments. No differences were detected between days for dietary intake or studies performed on 7 or 10 days. These two sessions were carried out at a similar time of the day for each subject, and the order of participation in the sessions was counter balanced within sexes. The study was approved by the internal review board at the University of Iowa, and all subjects gave written informed consent prior to participation.
Instrumentation and Experimental Set-up
Whole Body Heat Stress Intervention
In order to induce whole body passive heat stress, we developed a custom designed heat stress chamber. Subjects sat in the heat chamber for 30 min. The temperature inside the chamber was set at 73 °C (relative humidity of 10%) at the face level. When subjects participated in the control session, no heat was turned on while they sat in the same heat chamber for 30 min. The temperature for the no heat session was about 26 °C. The heat intervention significantly increased the subjects’ core body temperature (infra-red tympanic sensor, ThermoScan IRT 4520 Braun, Germany) and heart rate (HR) (online Polar logging sensor) from 36.6 ± 0.35 to 38.9 ± 0.44 °C and from 83.9 ± 18.6 to 131.4 ± 22.4 beats per minute by the end of the heat session, respectively. The core body temperature was increased 0.82 °C from the baseline temperature of 37.7 °C as measured via a rectal thermister probe (B10014, MSR Electronics GmbH, Switzerland) inserted 10 cm beyond the anal sphincter. The temperature after leaving the heat stress chamber returned to baseline within 30 minutes, at which time we performed the fatigue testing. There were no differences in the temperature between pre-heat and 30 min post-heat in the heat stress group and between 30 min post-heat in the heat stress group and 30 min post-sitting in room temperature in the control group (p > 0.10 for both).
The hormonal and protein changes resulting from heat stress is the focus of a subsequent paper in preparation. Briefly, in order to confirm that the heat stress subjects actually induced changes in hormones, especially prolactin (an indirect marker of central fatigue 17,18), blood samples were collected before and after heat stress.
Electromyography (EMG) Recordings
EMG was obtained using active bipolar surface electrodes (silver-silver chloride discs of 8 mm in diameter spaced 20 mm between centers) for short and long heads of biceps brachii (BBS and BBL, respectively), brachioradialis (BR), and triceps brachii (TB) of the left arm. Only 1 out of the 25 subjects was left handed. The locations of these electrodes were as follows: lateral (BBL) and medial (BBS) to the line between the medial acromion and the cubital fossa at 1/3 the distance from the cubital fossa, at about 2 cm distal to the elbow joint for BR, and at about 50% on the line between the acromion and the olecranon for TB. The skin at these locations was cleaned and mildly rubbed with an alcohol swab. A common ground electrode was placed on the dorsum of the tested hand. The signal was amplified (x 2k), band-pass filtered (20–4k Hz), and sampled at 4k Hz for all the muscles.
Torque Recording
Volitional torque around the elbow was measured using a torque transducer (model 45E15A-U760, JR3, Woodland, CA). Subjects sat on a custom designed chair with a padded seat and metal frame. The transducer was mounted on an adjustable metal bar extending from the metal frame. The transducer had an extended metal plate from its axis, and the axis of a subject’s elbow (the lateral epicondyle of the humerus) was aligned with the axis of the transducer. A cuff at the distal end of the metal plate held each subject’s wrist, keeping the forearm on the metal plate. Subjects were asked to pull their wrist toward them for elbow flexion or push the wrist away from them for extension. The shoulder was abducted 70° and horizontally flexed 45°; the elbow was flexed 70° (0°being full extension); and the forearm was midway between full supination and full pronation. Two non-elastic straps were used at the level of the chest and waist to minimize trunk movement. The analog signal from the transducer was sampled at 1k Hz.
TMS
Stimulation of the primary motor cortex was accomplished using a Magstim 2002 (Magstim Company Ltd., Whitland, Dyfed, UK) with a 7 cm diameter figure-of-eight coil. The coil was positioned tangential to the skull with the handle facing backward so that the current induced in the brain under the junction of the double coil was in the posterior-anterior direction during the rising phase of the monophasic pulse. The ideal position, where the largest MEP was observed in BBL, was found by moving the coil over the head. Once it had been found, the position was marked on a swim cap tightly fitted to the subject’s head. The coil was held manually to that position, and great care was taken to stimulate the same position of the head throughout the experiment. The active motor threshold (AMT) was determined as the minimum intensity required to elicit at least 4 of 8 MEPs whose amplitude was 100 micro-volts above the background volitional EMG (2% MVC). The stimulus intensity was then set at 1.8 times the AMT, and this intensity was used throughout the experiment. MEP responses were measured during muscle activation to better control for both cortical and motor neuron pool excitability, and therefore the MEP size becomes less variable.27 In addition, holding low background muscle activity ensures that changes in response to TMS are not simply a result of differences in subthreshold activation of neurons.28
Experimental Procedure
After subjects left the heat chamber, they dried thoroughly, and sat on the custom-designed chair. EMG recording and ground electrodes were placed on the tested arm, and the arm and the trunk were stabilized for torque measurements.
MVC Measurements
After some warm-up contractions, 3 MVCs into flexion followed by another 3 into extension were measured. Extension MVCs were performed only to normalize the antagonist EMG activity during the fatigue task. Subjects were given visual feedback of the exerted torque on a computer screen placed in front of them, and were verbally encouraged. Each MVC was ~5 sec, and a rest of about 1 min was given between each contraction. Two percent of the MVC torque in flexion was then displayed on the computer screen as a horizontal line. Pilot data showed that this approach maximized the sensitivity to TMS.
TMS Measurements
After finding the AMT and setting the stimulus intensity as described above, subjects were asked to maintain a static contraction at 2% of their MVC for ~20 s, during which 3 single pulses of TMS were delivered. Then subjects were asked to perform 2 – 3 flexion MVCs with a rest of about 1 min between. While they were exerting the maximal torque, one single pulse TMS was superimposed on each MVC. Subjects were asked to bring their torque back to the maximal level as quickly as possible after the superimposed TMS.
Fatigue Task
After all the pre-fatigue measurements described above had been obtained, subjects performed a 7-min fatigue task (Fig. 1A). The task consisted of thirty-five 7-s MVCs (epoch =5 MVCs in each minute, Fig. 1B). There was a rest of 3 s between contractions within each epoch. After the 5th contraction of each minute, subjects performed a target contraction (7 s) at pre-fatigue 2% MVC, at which time TMS was delivered. While subjects were performing the 3rd MVC of each epoch of 5 contractions, TMS was superimposed at the mid-point of the contraction. Subjects were verbally encouraged throughout the task. The computer display provided feedback for all force levels. Investigators also provided verbal cues to help subjects perform the task.
Figure 1.
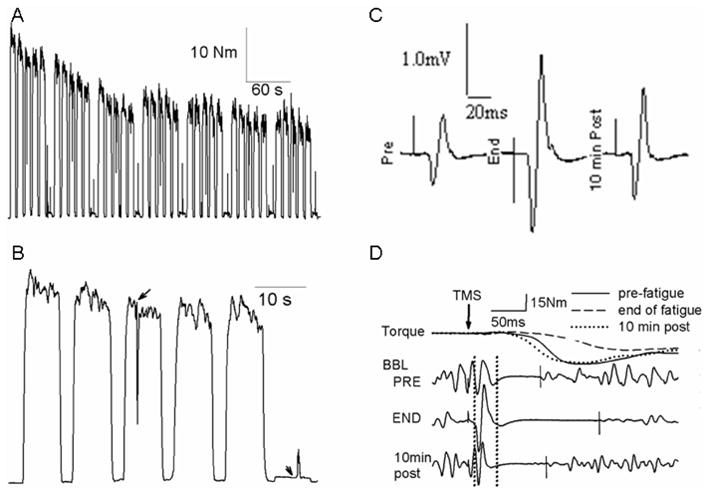
Representative examples of volitional torque during the 7-min fatigue task (A), an expanded signal of the torque showing only the 1st minute (B), MEPs (in BBL) recorded on the target 2% MVC (C) and torque and BBL EMG responses after TMS superimposed on MVC (D). Subjects performed five 7-s MVCs, followed by a 7-s target contraction at pre-fatigue 2% MVC for each minute. TMS was delivered on the 3rd MVCs and on the target contractions, which are indicated by arrows in B. In D, the torque immediately before TMS has been off-set for clarity purpose. The arrow and the two vertical dotted lines indicate the time of TMS delivery and the time window used to calculate the integrated amplitude for the MEP size, respectively. The short solid vertical lines on the EMG signals indicate the termination of the SP.
Post-fatigue Task
In order to examine recovery from the fatigue task, at 1 and 10 min post fatigue one 7-s MVC followed by a 7-s target contraction at 2% (3-s rest between) was performed with TMS superimposed on each contraction.
Data Analyses
All the analog signals were digitized and analyzed using Datapac 2K2 ver. 3.18 (Run Technologies Co., CA). Pre-fatigue and post-1 and 10 min MVC torque and EMG activity were determined by taking the mean and root mean square (RMS) amplitude of the torque and EMG signals, respectively, of a 2-s window where the torque was stable. For trials on which TMS was superimposed (post 1- and 10-min MVCs), 2 separate time windows were used before and after TMS, so TMS-induced extra torque (if any) and stimulus artifacts were not included. The higher value from the two windows was chosen to represent the maximal torque and EMG for that trial. During the fatigue task, volitional torque and EMG were calculated as mean and RMS amplitude of the middle 4 s for each 7-s MVC, and 5 values from each minute were averaged. Both peak-to-peak and integrated amplitude (between 10 ms and 40 ms after TMS, Fig. 1D) were used to quantify the MEP size, but only the peak-to-peak amplitude is reported in the results because these two measurements were highly correlated (R value = 0.93). The pre-stimulus EMG activity was determined by calculating the RMS amplitude of the 100-ms EMG signal immediately before TMS delivery, and this was used to normalize the MEP size. Although TMS was delivered both on MVCs and on the 2% MVC target contractions (Fig. 1B), SP duration and peak torque relaxation rate were quantified during the MVCs only. SP duration was determined as the time between the TMS delivery and the recurrence of continuous voluntary EMG activity after TMS using visual inspection. It has been recently reported 29 that the visual inspection approach to quantify the SP duration results in slightly lower between-visit variability than the mathematical approach described elsewhere.30 The peak relaxation rate was calculated as the maximal rate of decline in torque during SP.31 This was done by first calculating the slope of a regression line (25 ms) computed for successive moving intervals over the duration during which the voluntary torque was declining after TMS, and then the minimal slope (due to the torque decline) was chosen as the peak relaxation rate. Negative values of the rate were expressed as positive, so a decrease in the value indicates slowing of the rate of muscle relaxation. In order to account for the difference in rate with different torque levels, the peak relaxation rate was divided by the peak torque from which the torque started to decline. Whenever possible, values obtained during and after the fatigue task were expressed as a percentage of the corresponding value from the pre-fatigue measurements.
Statistical Analyses
In order to determine whether heat stress alone had any effects on the dependent variables, pre-fatigue measurements were compared using repeated measures 2 × 2 (sex as the between-subjects independent variable and heat/no heat as the within-subjects independent variable) analysis of variance (ANOVA). Although the heat intervention preceded the fatigue task only in the heat session, the level of agreement for each of the pre-fatigue variables was assessed across sessions using intraclass correlation (ICC) analyses. Dependent variables obtained during and after the fatigue task were analyzed using three-way ANOVA to determine whether time, sex and intervention had significant effects. A significance level of 0.05 was used for all statistical analyses. Results are reported as mean ± SD in the text and table, whereas figures show the means with standard error.
RESULTS
Prior whole body heat stress induced significant changes in blood plasma markers for heat stress proteins (HSP72), prolactin (PRL), and norepinephrine (NE) (Table 1). These findings confirm that our dose of passive heat stress produced changes similar to other reports.20 Importantly, the heat-induced increase in body temperature had returned to baseline before the fatigue task was performed (see Whole Body Heat Stress Intervention in Materials and Methods).
Table 1.
Absolute concentrations of stress protein and hormones before and after the heat stress.
| Protein and hormones | Before Heat | After Heat |
|---|---|---|
| HSP72 (ng/ml) | 2.82 ± 2.07 | 3.79 ± 2.29 * |
| PRL (ng/ml) | 7.60 ± 4.29 | 25.7 ± 11.8 * |
| NE (pg/ml) | 523.3 ± 237.2 | 768.9 ± 333.7 * |
HSP72 = heat stress protein 72. PRL = prolactin. NE = norepinephrine.
The asterisks indicate that there were significant (p < 0.05) increases after the prior passive heat stress. The whole body heat stress at 73 deg C for 30 min increased the HSP72, PRL and NE levels.
Pre-fatigue Measurements
Table 2 shows results from the measurements after either the no heat or heat intervention, but before performing any fatiguing muscle contractions. Heat did not affect (p > 0.05) the baseline MVC torque, AMT, MEP size on the target contraction or SP duration on the MVC. However, heat increased (p < 0.05) the maximal relaxation rate in both sexes. The MVC torque was lower (p < 0.01), and the relaxation rate was slower (p < 0.05) for females than those of males in both sessions. The ICC analyses performed on each of the pre-fatigue dependent variables across sessions ranged from 0.75 (SP duration of BBL) to 0.97 (MVC torque). These moderately high values indicate that the prior heat condition caused no acute affect on the pre-fatigue measurements.
Table 2.
Results (means ± SD) of measurements done after either the heat or no heat conditions, but before any fatiguing muscle contractions.
| Variables | Sex | No Heat | Heat | ICC |
|---|---|---|---|---|
| MVC torque (Nm) | Males | 63.6 ±13. 5 * | 65.9 ± 12.7 * | 0.9661 |
| Females | 33.7 ± 4.8 | 33.9 ± 5.8 | ||
| AMT (%MSO) | Males | 49.1 ± 5.8 * | 48.9 ± 6.4 * | 0.7735 |
| Females | 43.9 ± 6.2 | 43.5 ± 4.9 | ||
| MEP amplitude (mV) | Males | BBS 0.94 ± 0.66 | BBS 0.79 ± 0.61 | 0.9474 |
| BBL 1.06 ± 0.64 | BBL 0.97 ± 0.70 | 0.9516 | ||
| BR 0.36 ± 0.23 | BR 0.33 ± 0.29 | 0.7978 | ||
| Females | BBS 0.82 ± 0.77 | BBS 0.92 ± 0.84 | ||
| BBL 1.02 ± 0.64 | BBL 1.09 ± 0.58 | |||
| BR 0.51 ± 0.38 | BR 0.61 ± 0.69 | |||
| SP duration (ms) | Males | BBS 149.9± 56.7 | BBS 146.5 ± 48.4 | 0.7898 |
| BBL 147.3 ± 58.9 | BBL 142.3 ± 52.1 | 0.7542 | ||
| BR 165.0 ± 50.0 | BR 157.7 ± 48.3 | 0.8624 | ||
| Females | BBS 122.1 ± 49.2 | BBS 112.6 ± 34.2 | ||
| BBL 133.0 ± 51.5 | BBL 112.7 ± 37.0 | |||
| BR 136.8 ± 35.8 | BR 130.6 ± 39.4 | |||
| Relaxation rate (sec−1) | Males | 10.6 ± 1.4 *, † | 11.2 ± 1.3 * | 0.7827 |
| Females | 8.6 ± 1.3 † | 9.2 ± 1.2 |
No interaction (p > 0.05) of “sex” × “session” was found in any comparisons.
differences (p ≤ 0.05) between sexes within sessions.
differences (p < 0.05) between sessions within sexes. ICCs were calculated using data from both sexes.
MVC = maximal voluntary contraction. AMT = active motor threshold. MSO = maximal stimulator output. MEP = motor evoked potential (on the target 2% MVC). SP = silent period. ICC = intraclass correlation coefficient
Voluntary Torque and EMG Activation Levels
Voluntary torque decreased significantly in the first half of the 7-min fatigue task, and then gradually toward the end of the task (Fig. 2). The torque decrease with time was similar across sessions for both males and females. However, there were interactions (p < 0.01) of “sex” × “time” in both no heat and heat sessions. The torque of females, when expressed as a percentage of pre-fatigue values, was higher (p < 0.05) than that of males in both sessions after about 1/3 of the task. The quick recovery after a rest of 1 min was similar for all conditions.
Figure 2.
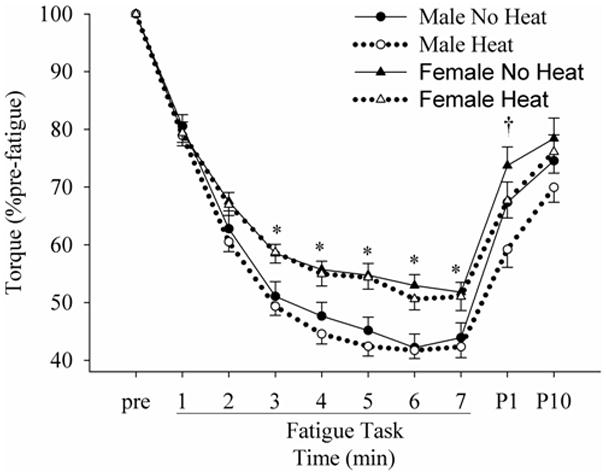
Changes in the normalized torque during and after the fatigue task in no heat and heat conditions. pre, P1 and P10 indicate pre-fatigue, post 1- and 10-min. * indicates differences (p < 0.05) between males and females. † indicates a difference (p < 0.05) between no heat and heat sessions when males and females were pooled.
During the fatigue task, the biceps brachii showed relatively constant EMG activity, whereas the BR showed a gradual decrease with time. The BR activity decreased significantly with time in all conditions during the fatigue task (Fig. 3C), but there was no main effect of “session” in either males or females. The decrease in BR activity was less in females than in males, resulting in significant differences between males and females at the end of the fatigue task in the no heat session. BBS activity of the males did not change significantly during the task with no session effect (Fig. 3A). In females, the BBS activity decreased only in the no heat session (significant main effect of “time” in no heat, but not in heat). The behavior of BBL was similar to that of BBS (Fig. 3B). The BBL activity decreased significantly with time only in males in both no heat and heat sessions (no significant main effect of “time” in females). No heat effect was found in either males or females in BBL, but there was a significant main effect of “sex” during the heat condition, resulting in significant differences between females and males at the 5th and 7th min. Although all EMG activity recovered with time after completion of the fatigue task, no differences were found between males and females or between no heat and heat sessions in any muscles. The antagonist, TB stayed consistent at 10% of its pre-fatigue MVC level throughout the fatigue task, and there were no main effects of “time”, ”sex” or ”session” (data not shown).
Figure 3.
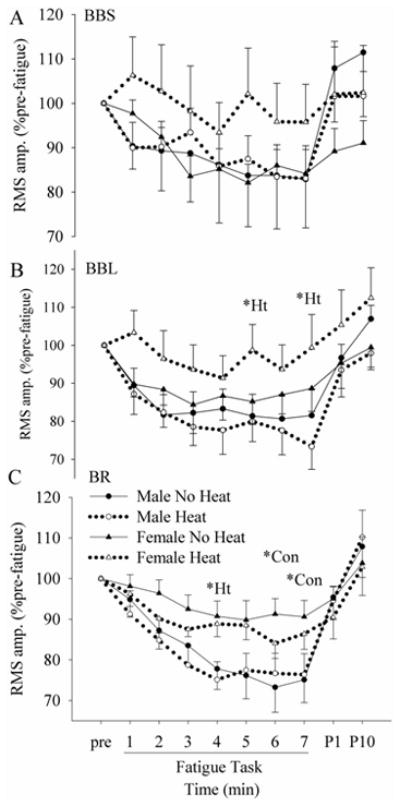
Changes in the EMG RMS amplitude of BBS (biceps brachii short head, A), BBL (biceps long head, B), and BR (brachioradialis, C) during and after the 7-min fatigue task in no heat and heat sessions. *Ht = differences between females and males in the heat session. *Con = differences between females and males in the no heat session
Central Cortical Excitability: Motor Evoked Potential (MEP) Size
The RMS amplitude of the EMG signal immediately before TMS on the target contractions stayed at ~ 1 – 2% MVC level throughout the task for all muscles (no main effect of time in any muscles), and there was no difference across groups or across sex. The size of MEPs recorded on the target 2% MVC contractions (Fig. 1C) increased with fatigue in all flexor muscles in all conditions (Fig. 4). However, the MEP size was not affected by either heat or sex. When the MEP size was normalized to the pre-stimulus EMG activity, the results did not change. The MEP size of flexor muscles during MVCs (Fig. 1D) was also analyzed, but results were similar to those elicited with the 2% target contractions. The MEP of the TB on flexion MVCs remained negligible throughout the fatigue task.
Figure 4.
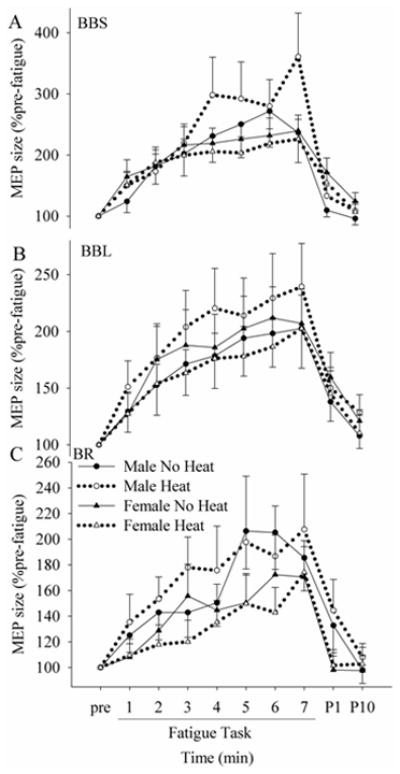
Changes in the MEP size on the target contraction in BBS (A), BBL (B) and BR (C) during and after the fatigue task in no heat and heat conditions. Although fatigue increased the MEP size, how it increased was similar across sessions and across sexes.
Central Cortical Inhibition: Silent Period (SP) Duration
Fatigue increased the duration of SP in both sessions (Figs. 1D and 5). During and after the fatigue task, SP durations of all the flexor muscles behaved similarly across sessions and across sexes (no “sex” or “session” effect with no interactions, p > 0.05). Although SP duration recovered with rest (post-1 and 10 min), there was no “sex” or “session” effect.
Figure 5.
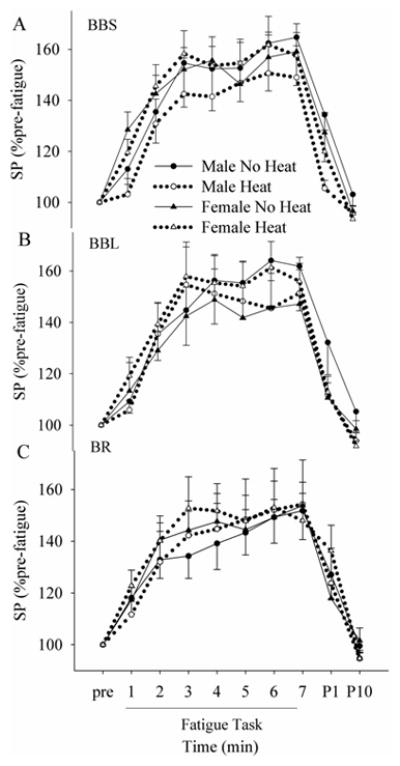
Changes in the SP duration in BBS (A), BBL (B) and BR (C) during and after the fatigue task in no heat and heat sessions. No (p > 0.05) “sex” or “session” effect was found in any of the muscles.
Peripheral Muscle Changes: Relaxation Rate
The peak relaxation rate was decreased with fatigue (Figs 1D and 6), but the absolute rate was higher for males than females at the start of the fatigue protocol (p < 0.01). At 2nd min, the relaxation rate in all conditions was similar (p > 0.05), and this was the case to the end of the task. At the post-10 min time, the rate of muscle relaxation was again higher for males (p = 0.01) than for females. The effect of heat was not significant (p > 0.05).
Figure 6.
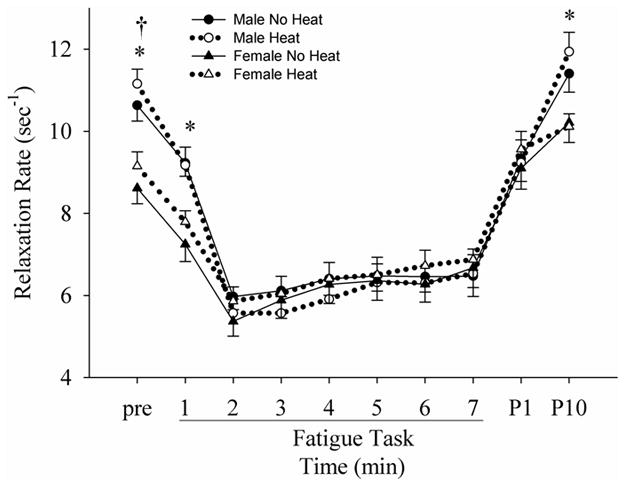
Changes in the normalized (to the torque before the torque decline) peak relaxation rate of the voluntary torque before, during and after the fatigue task in no heat and heat sessions. *: differences between males and females in both sessions. †: difference between no heat and heat sessions in both males and females.
Effect of Heat/Fatigue Test Order on Long Duration Muscle Fatigue
Half of the subjects participated in the no heat session prior to the fatigue test as their 1st session, and one week later they experienced the heat session and fatigue test. Conversely, the rest of the subjects participated in these sessions with a reversed order in that at the first session they experienced the heat and fatigue test and one week later they experienced only the fatigue test. Volitional torque recovered faster (p < 0.05) at 1- and 10-min in the group that experienced the heat and the fatigue test one week prior (1-min post fatigue (% pre-fatigue) = 75.7 ± 18.6, 64.7 ± 11.2, 62.5 ± 15.4 and 64.1 ± 17.4 and 10-min post-fatigue (% pre-fatigue) = 82.5 ± 19.9, 69.8 ± 16.9, 71.6 ± 16.4, 74.3 ±15.9 for fatigue test 1 week after heat/fatigue test, fatigue test day 1, heat/fatigue test day 1 and heat/fatigue test 1 week after fatigue test, respectively; Fig. 7A, B). This greater recovery from muscle fatigue was consistent in both the male and female cohorts (Fig. 7A, B).
Figure 7.
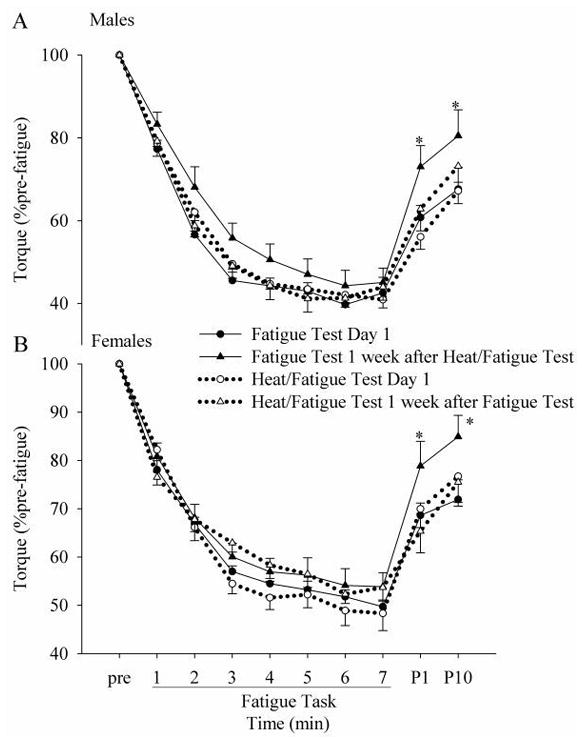
Changes in the normalized torque for each session (A for males and B for females). Half of the subjects (6 males and 6 females) participated in the no heat session first (Fatigue Test Day 1), followed by the heat session (Heat/Fatigue Test 1 week after Fatigue Test), while the rest of the subjects (7 males and 6 females) participated in these sessions with a reversed order (Heat/Fatigue Test Day 1 followed by Fatigue Test 1 week after Heat/Fatigue Test). * indicates differences in the torque in the Fatigue Test 1 week after Heat/Fatigue Test from that of other conditions.
DISCUSSION
The main purpose of this study was to investigate whether the previously reported hyperthermia-induced central fatigue 9–12 is long-lasting and independent of exercise. The second aim was to investigate whether males and females behave differently to fatigue after heat stress, since it has been shown that females are more fatigue resistant compared to males.21,22,32,33 Lastly, we aspired to probe into long term (1 week) adaptations when subjects were exposed to two versus one form of stress. The main findings of the study were: 1) males fatigued more than females but prior heat had no significant effect on torque decline, 2) the muscle contractile property (the peak relaxation rate) showed a greater fatigue-induced change in males than females, 3) the whole body heating did not influence the MEP size and SP duration before or after fatigue, and 4) a single bout of heat stress and fatiguing exercise delivered a week prior caused greater recovery in torque after muscle fatigue.
TMS Responses with Fatigue and with Heat
Although fatigue causes changes at multiple sites along the motor pathway from the cortex in the brain to the muscle sarcolemma, 3,34–36 there are several studies which indicate that the changes we observed in MEP size34,37 and SP duration7,38 are primarily of cortical origin.
In our study, whole body heating was applied before the fatigue task. Altered temperature with heating can change the properties of muscle fiber action potentials. The effect of temperature seems minimal, since our pilot study has shown that the skin temperature decreases rapidly after leaving the heat chamber, therefore, by the time the data collection started, skin temperature was similar for control and heat sessions. The reproducibility of the pre-fatigue dependent variables between groups in this study further indicates that conditions recovered quickly following heat stress.
Sex Difference in Muscle Fatigue
It has been shown that young females are more fatigue resistant than young males in certain fatigue tasks. Many of the proposed mechanisms for the sex difference in muscle fatigue lie within skeletal muscles.39–43 In this study, only peak relaxation rate provides information regarding muscle peripheral changes during fatigue. Males had a significantly higher muscle relaxation rate before fatigue, and this was negatively correlated with the extent of fatigue in the no heat session. Our previously reported 24 findings suggest that the contractile speed of a muscle alone can predict to some extent how much fatigue subjects are going to experience.
Previous studies have reported inconsistent results regarding whether there is a difference in the extent of central fatigue between males and females. In the ankle dorsiflexors 22 and knee extensors,21 males showed greater central fatigue than females, but no sex differences in central fatigue were found in the elbow flexors.23 In our study, although the relative decrease in torque was greater in males compared to females, neither MEP size nor SP duration showed differences between sexes during the fatigue task, indicating that the extent of fatigue-induced increases in intracortical inhibition and cortical excitability were similar for males and females, consistent with the finding of Hunter and colleagues.23
One of the proposed mechanisms for greater muscle fatigue in males compared to females is a sex difference in activity of group III and IV muscle afferents.22 The absence of differences in MEP size between sexes in our study indicates that the excitability of the motor pathway did not differentially change for males and females. Amann and colleagues have emphasized the critical role of somatosensory feedback from working muscles on centrally mediated determination of central motor drive.44 However, recently the notion that increased group III and IV muscle afferent activity reduces motoneuron excitability has been questioned in the elbow flexors,36,45 and therefore, it is not clear what role, if any, the small diameter muscle afferents have during fatigue.
Prior Whole Body Heating and Muscle Fatigue
Whether prior whole body heating has any long-lasting effects on human muscle fatigue was a central thesis of this study. The rationale for this study was grounded in the construct that prolactin, an indirect marker of central fatigue, has been shown to stay elevated (~ 60 min) after stress.19,20 We confirmed that the current heat stress intervention caused a nearly 4-fold increase in prolactin. Taken together, these previous findings raised the possibility that central fatigue could “carry over” after selective changes in CNS chemistry (prolactin is an indirect indicator of neurotransmitters) in the absence of exercise.
Previous studies have found that whole body heat stress exaggerates the decrease in torque during fatiguing contractions compared to when no heat stress is applied.11,12,46 However, most of these studies examined fatigue during heat stress and not after a prior bout of heat stress and recovery. One of the studies that investigated fatigue during heat stress 12 suggests that the heat-induced central fatigue was due to the inability of the central nervous system to maintain the firing frequencies necessary to generate fully fused contractions.
Increased brain temperature during exercise has been reported to be a clear perturbation in performing exercise, 1,11 but an increase in contractile speeds with heat would create an even more challenging situation for the CNS to maintain maximal voluntary contractions. We did not observe significant differences in the relaxation rate between sessions during the fatigue task (Fig. 6), and therefore, prior heat-induced peripheral changes in muscle (contractile speeds) did not contribute to the development of central fatigue in this study. It has been suggested that hyperthermia-induced central fatigue functions to prevent a further increase in body temperature, so that any dangerous conditions such as heat stroke can be avoided.14,47–49 If this is true, the heat-induced detrimental effect on muscle fatigue should recover quickly as the body temperature returns to a normal, pre-heated level. This suggests that the long-lasting changes in neurotransmitters (serotonergic inhibitors) do not directly cause heat-induced central fatigue, as has often been postulated. It is possible that long-lasting changes in neurotransmitters act in combination with other mechanisms that become saturated during an intense fatigue task as performed in this study. It may be that by allowing the body to cool down, acute primary signaling to the hypothalamus recovers,50 and the longer term changes in neurotransmitters (i.e. serotonin-dopamine) alone does not preferentially affect central fatigue during an isolated fatigue task.
We did not find any differences in EMG responses to TMS between heat and no heat sessions. This is consistent with the only previous study that used TMS during a fatigue task under hyperthermic conditions.12 This prompted the authors to conclude that the site responsible for the heat-induced central fatigue is “upstream” of the primary motor cortex. Interestingly, rest after muscle fatigue is associated with recovery from any EMG changes related to repetitive activation of the motor pathway (changes in MEP size and SP duration) even when the fatigued muscle was kept ischemic (and therefore increased activity of group III and IV muscle afferents).8 Conversely, supraspinal fatigue (increased interpolated twitch) did not improve until blood flow to the muscle was restored.8 These findings suggest that small diameter muscle afferents can reduce voluntary activation without impairing the excitability of the motor pathway from the primary motor cortex to the muscle fibers. Accordingly, the mechanisms responsible for central fatigue are likely multi-mechanistic and involve “online” peripheral inputs as well as central components. Hence, in this study, we induced central systemic changes, but once the fatigue protocol ensued, peripheral signaling may have become more prominent. It may be that systemic changes as a result of passive heat stress will have a greater impact on “central fatigue” during aerobic whole body exercise rather than on anaerobic isolated muscle fatigue as performed in this study.
Long-lasting Effect of Heat Stress and Exercise on Recovery from Muscle Fatigue
The extent of the torque recovery after fatigue was greater in the 2nd no heat session compared to that seen in any other conditions. It does not seem that heat stress had any acute (~ 1 h) effect on muscle fatigue since the torque decline and recovery was similar in the heat and no heat sessions. Similarly, fatiguing exercise performed a week in advance without prior heat stress had no significant effect on the torque recovery. Therefore, our data indicates that two stressful events (heat stress and fatiguing exercise) combined caused a “protection” that enabled the torque to recover in both males and females.
This significant recovery was unexpected and raised several possibilities to consider. First, no significant differences existed in baseline torque measures between days. Moreover, all dependent variables changed similarly during fatigue. Thus, we suggest this effect was a protective adaptation associated with the dose of stress delivered. One of the protective responses triggered with stress is the induction of stress proteins known as heat shock proteins (HSPs). When cells are exposed to sub-lethal stress, inducible forms of HSPs are up-regulated, resulting in the acquisition of greater stress resistance.51 In fact, the HSP level has been reported to be increased markedly 2–7 days after a single bout of non-damaging, aerobic exercise in exercised skeletal muscles.52,53 Because HSPs are known to be up-regulated by various forms of stress,51 it is possible that the heat stress applied before the fatiguing exercise in the first heat session may have enhanced the induction of HSPs in the muscle compared to the fatiguing exercise alone. The torque in the 2nd no heat session differed from that of other sessions only in the recovery from muscle fatigue. This may be due to a protective role of HSPs on low-frequency fatigue (a preferential decrease in the force elicited with electrical stimulation at a low frequency, compared to a high frequency).54,55 After the strenuous repetitive MVCs, it is possible that the elbow flexors experienced low-frequency fatigue to some extent in all conditions. Low frequency fatigue has been attributed to compromise of excitation-contraction coupling,55 and it has been suggested that HSPs may be involved in modulation of the excitation-contraction coupling process.56 Further study is underway to determine if a dose of passive heat stress plus exercise translates into a strategy to offset long duration fatigue.
Clinical Implications
Individuals with paralysis have a limited capacity to generate repetitive muscle force after SCI.57–61 Moreover, individuals with SCI lose the ability to sweat and shiver, which causes rapid shifts in core body temperature based on the environment. 2 Consequently, an elevated body temperature in a warm climate may shift below normal when a cooler environment is entered (e.g. air conditioning). The ability for individuals with SCI to use their intact upper extremities after an acute dose of heat stress is unknown. This study suggests that a dose of heat stress minimally influences central drive and peripheral muscle fatigue in those with intact central nervous systems, however the effect on those with impaired nervous systems remains unknown. Finally, isolated repetitive exercise after an increase in core body temperature may render the upper extremity muscles of those with paraplegia resistant to prolonged fatigue.
Low-frequency fatigue may also contribute to injuries in sporting events, 62,63 and it has been known that heat shock protein induced in skeletal muscle attenuates the extent of low-frequency fatigue.56 If a combination of passive heat stress and fatiguing contractions is a viable method to induce Hsp greater than fatiguing exercise alone, this could be used as a preventive procedure for human performance-related injuries. Future studies need to investigate this possibility, and associated physiological mechanisms.
Summary and Conclusions
Whole body heat stress had a minor influence on human muscle fatigue after the increased body temperature had returned to baseline levels. Although females fatigued less compared to males, the decrease in torque was similar before and after heat for either sex. However, torque recovery from muscle fatigue was improved if heat stress and fatiguing exercise were performed a week before, suggesting that these stressful events led to a long-lasting recovery effect on muscle fatigue. The precise mechanisms contributing to this effect require further study.
Acknowledgments
This work was supported in part by the National Institutes of Health (NIH). We thank the assistance with data collection from Andrew E. Littmann PT, MA, Shuo-Hsiu Chang PhD, PT, Lydia A. Wester, MA, Melissa E. Boerman, DPT, Jill E. Esdohr, DPT, Jessica L. Hintze, DPT, and Andrew J. Phillips DPT.
Abbreviations
- AMT
active motor threshold
- ANOVA
analysis of variance
- BBL
biceps brachii long head
- BBS
biceps brachii short head
- BR
brachioradialis
- CNS
central nervous system
- EMG
electromyography
- HR
heart rate
- HSP
heat shock protein
- ICC
intraclass correlation
- MEP
motor evoked potential
- MVC
maximal voluntary contraction
- PRL
prolactin
- RMS
root mean square
- SCI
spinal cord injury
- SD
standard deviation
- SP
silent period
- TB
triceps brachii
- TMS
transcranial magnetic stimulation
Footnotes
Disclosures
The authors have declared that no competing interests exist.
References
- 1.Nybo L. Exercise and heat stress: cerebral challenges and consequences. Prog Brain Res. 2007;162:29–43. doi: 10.1016/S0079-6123(06)62003-7. [DOI] [PubMed] [Google Scholar]
- 2.Price MJ. Thermoregulation during exercise in individuals with spinal cord injuries. Sports Med. 2006;36(10):863–879. doi: 10.2165/00007256-200636100-00005. [DOI] [PubMed] [Google Scholar]
- 3.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 4.Benwell NM, Mastaglia FL, Thickbroom GW. Differential changes in long-interval intracortical inhibition and silent period duration during fatiguing hand exercise. Exp Brain Res. 2007;179(2):255–262. doi: 10.1007/s00221-006-0790-2. [DOI] [PubMed] [Google Scholar]
- 5.McKay WB, Stokic DS, Sherwood AM, Vrbova G, Dimitrijevic MR. Effect of fatiguing maximal voluntary contraction on excitatory and inhibitory responses elicited by transcranial magnetic motor cortex stimulation. Muscle Nerve. 1996;19(8):1017–1024. doi: 10.1002/mus.880190803. [DOI] [PubMed] [Google Scholar]
- 6.Sacco P, Thickbroom GW, Thompson ML, Mastaglia FL. Changes in corticomotor excitation and inhibition during prolonged submaximal muscle contractions. Muscle Nerve. 1997;20(9):1158–1166. doi: 10.1002/(sici)1097-4598(199709)20:9<1158::aid-mus11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol. 1996;490 (Pt 2):519–528. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor JL, Todd G, Gandevia SC. Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol. 2006;33(4):400–405. doi: 10.1111/j.1440-1681.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- 9.Galloway SD, Maughan RJ. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc. 1997;29(9):1240–1249. doi: 10.1097/00005768-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Parkin JM, Carey MF, Zhao S, Febbraio MA. Effect of ambient temperature on human skeletal muscle metabolism during fatiguing submaximal exercise. J Appl Physiol. 1999;86(3):902–908. doi: 10.1152/jappl.1999.86.3.902. [DOI] [PubMed] [Google Scholar]
- 11.Thomas MM, Cheung SS, Elder GC, Sleivert GG. Voluntary muscle activation is impaired by core temperature rather than local muscle temperature. J Appl Physiol. 2006;100(4):1361–1369. doi: 10.1152/japplphysiol.00945.2005. [DOI] [PubMed] [Google Scholar]
- 12.Todd G, Butler JE, Taylor JL, Gandevia SC. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol. 2005;563(Pt 2):621–631. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen B, Hyldig T, Bidstrup F, Gonzalez-Alonso J, Christoffersen GR. Brain activity and fatigue during prolonged exercise in the heat. Pflugers Arch. 2001;442(1):41–48. doi: 10.1007/s004240100515. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol. 1993;460:467–485. doi: 10.1113/jphysiol.1993.sp019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruck K, Olschewski H. Body temperature related factors diminishing the drive to exercise. Can J Physiol Pharmacol. 1987;65(6):1274–1280. doi: 10.1139/y87-203. [DOI] [PubMed] [Google Scholar]
- 16.Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc. 1997;29(1):45–57. doi: 10.1097/00005768-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Bridge MW, Weller AS, Rayson M, Jones DA. Responses to exercise in the heat related to measures of hypothalamic serotonergic and dopaminergic function. Eur J Appl Physiol. 2003;89(5):451–459. doi: 10.1007/s00421-003-0800-z. [DOI] [PubMed] [Google Scholar]
- 18.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 19.Daly W, Seegers CA, Rubin DA, Dobridge JD, Hackney AC. Relationship between stress hormones and testosterone with prolonged endurance exercise. Eur J Appl Physiol. 2005;93(4):375–380. doi: 10.1007/s00421-004-1223-1. [DOI] [PubMed] [Google Scholar]
- 20.Laatikainen T, Salminen K, Kohvakka A, Pettersson J. Response of plasma endorphins, prolactin and catecholamines in women to intense heat in a sauna. Eur J Appl Physiol Occup Physiol. 1988;57(1):98–102. doi: 10.1007/BF00691246. [DOI] [PubMed] [Google Scholar]
- 21.Martin PG, Rattey J. Central fatigue explains sex differences in muscle fatigue and contralateral cross-over effects of maximal contractions. Pflugers Arch. 2007;454(6):957–969. doi: 10.1007/s00424-007-0243-1. [DOI] [PubMed] [Google Scholar]
- 22.Russ DW, Kent-Braun JA. Sex differences in human skeletal muscle fatigue are eliminated under ischemic conditions. J Appl Physiol. 2003;94(6):2414–2422. doi: 10.1152/japplphysiol.01145.2002. [DOI] [PubMed] [Google Scholar]
- 23.Hunter SK, Butler JE, Todd G, Gandevia SC, Taylor JL. Supraspinal fatigue does not explain the sex difference in muscle fatigue of maximal contractions. J Appl Physiol. 2006;101(4):1036–1044. doi: 10.1152/japplphysiol.00103.2006. [DOI] [PubMed] [Google Scholar]
- 24.Wust RC, Morse CI, de Haan A, Jones DA, Degens H. Sex differences in contractile properties and fatigue resistance of human skeletal muscle. Exp Physiol. 2008;93(7):843–850. doi: 10.1113/expphysiol.2007.041764. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: QualityMetric Inc; 1993, 2000. [Google Scholar]
- 26.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 27.Darling WG, Wolf SL, Butler AJ. Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Exp Brain Res. 2006;174(2):376–385. doi: 10.1007/s00221-006-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridding MC, Rothwell JC. Reorganisation in human motor cortex. Can J Physiol Pharmacol. 1995;73(2):218–222. doi: 10.1139/y95-032. [DOI] [PubMed] [Google Scholar]
- 29.Damron LA, Dearth DJ, Hoffman RL, Clark BC. Quantification of the corticospinal silent period evoked via transcranial magnetic stimulation. J Neurosci Methods. 2008;173(1):121–128. doi: 10.1016/j.jneumeth.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Daskalakis ZJ, Molnar GF, Christensen BK, Sailer A, Fitzgerald PB, Chen R. An automated method to determine the transcranial magnetic stimulation-induced contralateral silent period. Clin Neurophysiol. 2003;114(5):938–944. doi: 10.1016/s1388-2457(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 31.Todd G, Taylor JL, Butler JE, Martin PG, Gorman RB, Gandevia SC. Use of motor cortex stimulation to measure simultaneously the changes in dynamic muscle properties and voluntary activation in human muscles. J Appl Physiol. 2007;102(5):1756–1766. doi: 10.1152/japplphysiol.00962.2006. [DOI] [PubMed] [Google Scholar]
- 32.Hicks AL, Kent-Braun J, Ditor DS. Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev. 2001;29(3):109–112. doi: 10.1097/00003677-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Hunter SK, Critchlow A, Shin IS, Enoka RM. Men are more fatigable than strength-matched women when performing intermittent submaximal contractions. J Appl Physiol. 2004;96(6):2125–2132. doi: 10.1152/japplphysiol.01342.2003. [DOI] [PubMed] [Google Scholar]
- 34.Taylor JL, Butler JE, Gandevia SC. Altered responses of human elbow flexors to peripheral-nerve and cortical stimulation during a sustained maximal voluntary contraction. Exp Brain Res. 1999;127(1):108–115. doi: 10.1007/s002210050779. [DOI] [PubMed] [Google Scholar]
- 35.Garland SJ. Role of small diameter afferents in reflex inhibition during human muscle fatigue. J Physiol. 1991;435:547–558. doi: 10.1113/jphysiol.1991.sp018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler JE, Taylor JL, Gandevia SC. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci. 2003;23(32):10224–10230. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benwell NM, Sacco P, Hammond GR, Byrnes ML, Mastaglia FL, Thickbroom GW. Short-interval cortical inhibition and corticomotor excitability with fatiguing hand exercise: a central adaptation to fatigue? Exp Brain Res. 2006;170(2):191–198. doi: 10.1007/s00221-005-0195-7. [DOI] [PubMed] [Google Scholar]
- 38.Brasil-Neto JP, Cammarota A, Valls-Sole J, Pascual-Leone A, Hallett M, Cohen LG. Role of intracortical mechanisms in the late part of the silent period to transcranial stimulation of the human motor cortex. Acta Neurol Scand. 1995;92(5):383–386. doi: 10.1111/j.1600-0404.1995.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 39.Russ DW, Lanza IR, Rothman D, Kent-Braun JA. Sex differences in glycolysis during brief, intense isometric contractions. Muscle Nerve. 2005;32(5):647–655. doi: 10.1002/mus.20396. [DOI] [PubMed] [Google Scholar]
- 40.Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol. 1989;257(4 Pt 1):E567–572. doi: 10.1152/ajpendo.1989.257.4.E567. [DOI] [PubMed] [Google Scholar]
- 41.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97(1):393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- 42.Huang A, Sun D, Koller A, Kaley G. Gender difference in flow-induced dilation and regulation of shear stress: role of estrogen and nitric oxide. Am J Physiol. 1998;275(5 Pt 2):R1571–1577. doi: 10.1152/ajpregu.1998.275.5.R1571. [DOI] [PubMed] [Google Scholar]
- 43.Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol. 1996;80(1):245–251. doi: 10.1152/jappl.1996.80.1.245. [DOI] [PubMed] [Google Scholar]
- 44.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587(Pt 1):271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin PG, Weerakkody N, Gandevia SC, Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol. 2008;586(5):1277–1289. doi: 10.1113/jphysiol.2007.140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrison S, Sleivert GG, Cheung SS. Passive hyperthermia reduces voluntary activation and isometric force production. Eur J Appl Physiol. 2004;91(5–6):729–736. doi: 10.1007/s00421-004-1063-z. [DOI] [PubMed] [Google Scholar]
- 47.Gregson WA, Drust B, Batterham A, Cable NT. The effects of pre-warming on the metabolic and thermoregulatory responses to prolonged submaximal exercise in moderate ambient temperatures. Eur J Appl Physiol. 2002;86(6):526–533. doi: 10.1007/s00421-002-0580-x. [DOI] [PubMed] [Google Scholar]
- 48.Cheung SS, McLellan TM. Heat acclimation, aerobic fitness, and hydration effects on tolerance during uncompensable heat stress. J Appl Physiol. 1998;84(5):1731–1739. doi: 10.1152/jappl.1998.84.5.1731. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol. 1999;86(3):1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- 50.Nybo L. Hyperthermia and fatigue. J Appl Physiol. 2008;104(3):871–878. doi: 10.1152/japplphysiol.00910.2007. [DOI] [PubMed] [Google Scholar]
- 51.Kopecek P, Altmannova K, Weigl E. Stress proteins: nomenclature, division and functions. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2001;145(2):39–47. doi: 10.5507/bp.2001.010. [DOI] [PubMed] [Google Scholar]
- 52.Khassaf M, Child RB, McArdle A, Brodie DA, Esanu C, Jackson MJ. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J Appl Physiol. 2001;90(3):1031–1035. doi: 10.1152/jappl.2001.90.3.1031. [DOI] [PubMed] [Google Scholar]
- 53.Morton JP, MacLaren DP, Cable NT, Bongers T, Griffiths RD, Campbell IT, Evans L, Kayani A, McArdle A, Drust B. Time course and differential responses of the major heat shock protein families in human skeletal muscle following acute nondamaging treadmill exercise. J Appl Physiol. 2006;101(1):176–182. doi: 10.1152/japplphysiol.00046.2006. [DOI] [PubMed] [Google Scholar]
- 54.Iguchi M, Baldwin K, Boeyink C, Engle C, Kehoe M, Ganju A, Messaros AJ, Shields RK. Low frequency fatigue in human quadriceps is fatigue dependent and not task dependent. J Electromyogr Kinesiol. 2008;18(2):308–316. doi: 10.1016/j.jelekin.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones DA. High-and low-frequency fatigue revisited. Acta Physiol Scand. 1996;156(3):265–270. doi: 10.1046/j.1365-201X.1996.192000.x. [DOI] [PubMed] [Google Scholar]
- 56.Nosek TM, Brotto MA, Essig DA, Mestril R, Conover RC, Dillmann WH, Kolbeck RC. Functional properties of skeletal muscle from transgenic animals with upregulated heat shock protein 70. Physiol Genomics. 2000;4(1):25–33. doi: 10.1152/physiolgenomics.2000.4.1.25. [DOI] [PubMed] [Google Scholar]
- 57.Dudley-Javoroski S, Littmann AE, Iguchi M, Shields RK. Doublet stimulation protocol to minimize musculoskeletal stress during paralyzed quadriceps muscle testing. J Appl Physiol. 2008;104(6):1574–1582. doi: 10.1152/japplphysiol.00892.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dudley-Javoroski S, Shields RK. Muscle and bone plasticity after spinal cord injury: review of adaptations to disuse and to electrical muscle stimulation. J Rehabil Res Dev. 2008;45(2):283–296. doi: 10.1682/jrrd.2007.02.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shields RK, Dudley-Javoroski S. Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J Neurophysiol. 2006;95(4):2380–2390. doi: 10.1152/jn.01181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shields RK, Dudley-Javoroski S. Musculoskeletal adaptations in chronic spinal cord injury: effects of long-term soleus electrical stimulation training. Neurorehabil Neural Repair. 2007;21(2):169–179. doi: 10.1177/1545968306293447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shields RK, Dudley-Javoroski S, Littmann AE. Postfatigue potentiation of the paralyzed soleus muscle: evidence for adaptation with long-term electrical stimulation training. J Appl Physiol. 2006;101(2):556–565. doi: 10.1152/japplphysiol.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahnama N, Reilly T, Lees A. Injury risk associated with playing actions during competitive soccer. Br J Sports Med. 2002;36(5):354–359. doi: 10.1136/bjsm.36.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Small K, McNaughton L, Greig M, Lovell R. The effects of multidirectional soccer-specific fatigue on markers of hamstring injury risk. J Sci Med Sport. 2008 doi: 10.1016/j.jsams.2008.08.005. [DOI] [PubMed] [Google Scholar]


