Abstract
Vegetation patterns and the presence of large numbers of nesting herons and egrets significantly altered the number of host-seeking Culex tarsalis Coquillett (Diptera: Culicidae) collected at dry ice-baited traps. The numbers of females collected per trap night at traps along the ecotone of Eucalyptus stands with and without a heron colony were always greater or equal to numbers collected at traps within or under canopy. No Cx. tarsalis were collected within or under Eucaplytus canopy during the peak heron nesting season, even though these birds frequently were infected with West Nile virus and large number of engorged females could be collected at resting boxes. These data indicate a diversion of host-seeking females from traps to nesting birds reducing sampling efficiency.
Keywords: Culex tarsalis, Anopheles freeborni, dry ice-baited traps, abundance, California
The abundance of host-seeking mosquitoes typically is estimated by counting the number of females of each species attracted to traps baited with CO2 subliming from dry ice or dispensed from a cylinder. Although considerable effort has been expended improving the design of these traps, less care has been devoted to where and how they are distributed over the landscape and to what factors may alter catch size. Previous studies have shown, for example, that questing females have species-specific flight paths in relation to terrain features (Bidlingmayer 1985) and that some species frequently exploit ecotonal boundaries (Bidlingmayer and Hem 1981, Lothrop and Reisen 2001). The Culex (Diptera: Culicidae) vectors of the North American encephalitides, including West Nile virus (family Flaviviridae, genus Flavivirus, WNV), frequently feed on birds (Reisen and Reeves 1990; Hamer et al. 2009; Kent et al. 2009; Molaei et al. 2008, 2010) and hunt both at ground level and in the canopy along vegetation ecotones (Reisen et al. 1990, Meyer et al. 1991, Anderson et al. 2006).
Concentrations of competent nesting birds, roosting birds, or both located near Culex vector populations present an excellent opportunity for increased abundance due to enhanced bloodmeal availability. Avian host concentrations also should enable efficient encephalitis virus enzootic amplification (Reisen et al. 2006, Diuk-Wasser et al. 2010). In Japan, for example, communal nesting colonies of herons in the family Ardeidae, including black-crowned night herons (Nycticorax nycticorax), were an important amplification focus for Japanese encephalitis virus near Tokyo, Japan (Scherer et al. 1959). However, our attempts to implicate nesting colonies of ardeids in California as amplification foci have produced mixed results, even though black-crowned night herons were abundant and are competent hosts of WNV (Reisen et al. 2005). When nesting over water, few nestlings were found infected despite considerable WNV activity at the surrounding wetlands, indicating that host-seeking female Culex tarsalis Coquillett would not fly short distances over water (Reisen et al. 2005). In contrast, when nesting in tree canopy over land, the same species of ardeids frequently were infected with WNV (Reisen et al. 2009). In addition, Cx. tarsalis females frequently were found to have bloodfed on ardeids (Thiemann 2011), supporting previous findings that this species would host seek within vegetation canopy (Meyer et al. 1991). However, despite frequent blood-feeding on these highly competent hosts, infection rates in host-seeking Culex mosquitoes collected at the edge of the farmstead containing the heronry were modest and were not different from those collected concurrently at nearby wetlands or within the town of Davis, CA (Nielsen et al. 2008). To address this quandary, in 2008 we attempted to trap females under trees supporting large numbers of nesting birds and within the canopy adjacent to active nests in an effort to determine whether higher Culex infection rates would be found nearer to the hosts. Unexpectedly, virtually no Culex were collected in these traps, whereas traps positioned at the edge of the property continued to collect large numbers of host-seeking females. Because thousands of engorged females were collected resting within the farmstead property (Thiemann 2011), we hypothesized that the large numbers of nesting ardeids outcompeted our traps for the host-seeking females. To test this hypothesis, in 2010 we compared the number of females collected in traps at ground level under the trees and within the canopy adjacent to active ardeid nests to counts at traps positioned along the heronry edge during periods when birds were present to the same sites in the fall after the birds had fledged and left the area. For comparison, counts at traps positioned at similar locations were measured concurrently at a similar farmstead without a nesting heron colony.
Materials and Methods
Study Area
Approximately 10,000 birds in the family Ardeidae, including black-crowned night herons, great egrets (Ardea alba), snowy egrets (Egretta thula), and cattle egrets (Bubulcus ibis), communally nest from May to August in a stand of Eucalyptus trees within a farmstead ≈4 km NE of Davis (38.603 N, −121.711 W). The farmstead was ≈3 km W of the Yolo bypass of the Sacramento River, a large rice farming area that produced most of the mosquitoes in this area. A second farmstead also supporting a large stand of Eucalyptus, but without nesting ardeids, was located 5 km NW of Davis and used for comparison as a no-bird or negative control. This site was surrounded by a poorly managed slough that probably produced many of the mosquitoes collected in our study.
Sampling
Mosquitoes were collected by CDC-style traps (Sudia and Chamberlain 1962) operated without lights and baited with ≈1 kg of dry ice (Newhouse et al. 1966) in an adjacent Styrofoam holder, as described and evaluated previously (Reisen et al. 2000). Three replicate traps each were hung from T-poles at 1.5-m elevation at the farmstead edge or under the canopy within the Eucalyptus stand. Replicate traps within the canopy were hung at 4–6-m elevation from a rope pulley system adjacent to active ardeid nests at the heronry or within representative trees at the comparison site. Traps were operated at the heronry and comparison locations from before dusk until early morning for three consecutive nights during summer (June–July) when adults and nestlings were on the nest and during early fall (September) after the birds had fledged and departed. Mosquitoes from each collection were anesthetized with triethylamine, enumerated to species, pooled into lots of ≤50 females each and then frozen at −80°C until tested for WNV by using real-time quantitative reverse transcription-polymerase chain reaction assay (Shi and Kramer 2003).
Statistics
Counts were transformed by ln(y + 1) before analysis of variance (ANOVA) to control the variance and normalize the distribution (Reisen and Lothrop 1999). In a preliminary two-way repeated measures ANOVA (Hintze 1998) with nights within seasons as the repeated factor, and trap locations (edge, under, and canopy) and seasons (birds present versus absent) as main effects, there was significant interaction between trap locations and seasons. Therefore, trap locations were compared separately within seasons by ANOVA, with location means compared by a posteriori Tukey–Kramer test (Hintze 1998). Back-transformed or geometric means were used for data presentation. In addition, to emphasize the impact of the heron presence on trap counts, we standardized the mean counts for replicate traps at sites under and within canopy locations by dividing these by the mean counts at edge traps, and then we compared these ratios by two-way ANOVA with bird presence or absence and trap locations as main effects.
Results
In total, 10,650 female mosquitoes representing seven species were collected during 142 trap nights during 2008 and 2010 (Table 1). Cx. tarsalis was most abundant species, making up 91% of the catch, followed by Anopheles freeborni Aitken, making up 7%. Subsequent statistical analyses were confined to these species that presented an interesting contrast, because Cx. tarsalis at the heronry was predominantly a bird feeder (Thiemann 2011), whereas An. freeborni typically feed on large mammals (Washino and Tempelis 1967). Only a single pool of Cx. tarsalis from an edge trap at the heronry during summer was positive for WNV RNA during 2010, precluding planned comparisons of infection rates among trap locations.
Table 1.
Total numbers of mosquitoes collected in CO2 traps at the heronry and control locations during 2008 and 2010
| Canopy | ingfe | Under | Total | |
|---|---|---|---|---|
| Control 2010 | ||||
| Cx. tarsalis | 745 | 517 | 905 | 2,167 |
| Cx. pipiens | 36 | 35 | 33 | 104 |
| Cx. erythrothorax | 0 | 0 | 0 | 0 |
| An. freeborni | 6 | 169 | 205 | 380 |
| Ae. melanimon | 0 | 1 | 4 | 5 |
| Cs. inornata | 0 | 0 | 0 | 0 |
| Cs. incidens | 0 | 0 | 1 | 1 |
| Heronry 2008 | ||||
| Cx. tarsalis | 1,857 | 2,446 | 2,193 | 6,496 |
| Cx. pipiens | 9 | 2 | 2 | 13 |
| Cx. erythrothorax | 0 | 1 | 0 | 1 |
| An. freeborni | 4 | 11 | 46 | 61 |
| Ae. melanimon | 0 | 3 | 3 | 6 |
| Cs. inornata | 0 | 0 | 10 | 10 |
| Cs. incidens | 0 | 0 | 0 | 0 |
| Heronry 2010 | ||||
| Cx. tarsalis | 60 | 942 | 73 | 1,075 |
| Cx. pipiens | 1 | 1 | 0 | 2 |
| Cx. erythrothorax | 0 | 0 | 0 | 0 |
| An. freeborni | 5 | 284 | 37 | 326 |
| Ae. melanimon | 0 | 2 | 1 | 3 |
| Cs. inornata | 0 | 0 | 0 | 0 |
| Cs. incidens | 0 | 0 | 0 | 0 |
| Total | 2,723 | 4,414 | 3,513 | 10,650 |
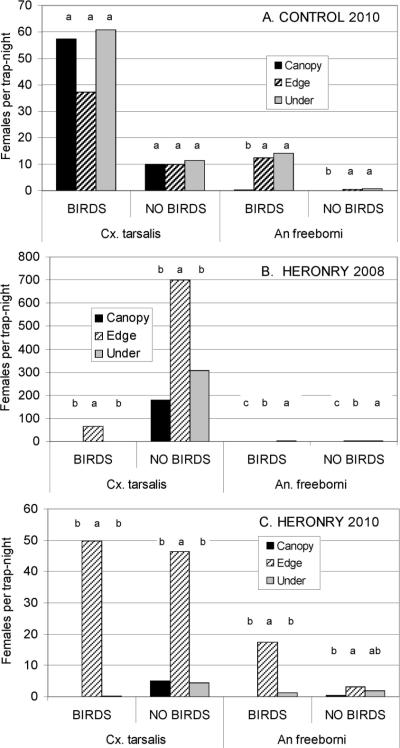
At the control farmstead without the presence of a large colony of nesting ardeid birds, there were no significant differences (ANOVA: P>0.05) among trap locations (i.e., canopy, edge, or under) for Cx. tarsalis during summer (bird nesting period at the heronry) or fall (Fig. 1A). In contrast, significantly fewer An. freeborni were collected in canopy traps (P<0.05; Tukey–Kramer test) than in either edge or under traps at 1.5-m height; numbers collected at traps at 1.5-m height were not significantly different (P>0.05; Tukey–Kramer test). These data indicated that without large numbers of nesting ardeids present, both mosquito species were collected seeking bloodmeals at the edge and under a grove of Eucalyptus trees; comparable numbers of Cx. tarsalis also were collected in the canopy.
Fig. 1.
Geometric mean number of host-seeking Cx. tarsalis and An. freeborni females collected in three CO2 traps positioned at the edge, under, and within Eucalyptus tree canopy and operated during three nights during months with (July) and without (September) nesting ardeids. (A) Control farmstead during 2010. (B) Heronry during 2008. (C) Heronry during 2010. Groups of columns under similar letters were not significantly different by a Tukey–Kramer posteriori test (P > 0.05).
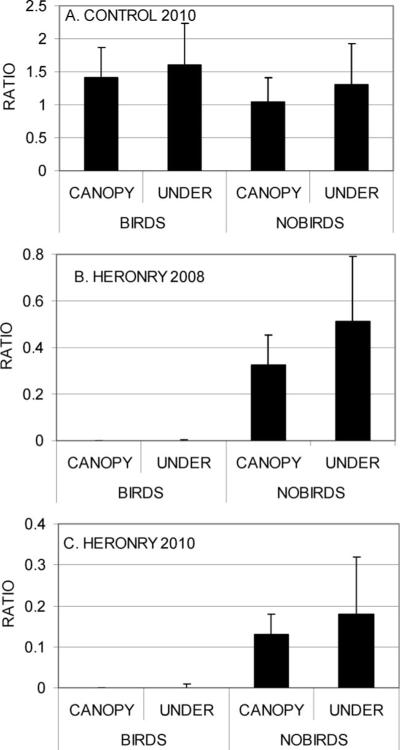
In marked contrast, significantly more Cx. tarsalis females were collected at the heronry during both 2008 (Fig. 1B) and 2010 (Fig. 1C) at traps along the edge of the farmstead (P < 0.05; Tukey–Kramer test) than in the Eucalyptus grove, either within or under the canopy whether or not the ardeids birds were present. However, when the mean numbers of Cx. tarsalis collected per trap per night in canopy and under traps were standardized by dividing by the mean number collected by edge traps, the resulting ratios increased significantly (ANOVA: P<0.01) from <0.01 when the ardeids were present to >0.39 after they departed during 2008 (Fig. 2B) and to >0.15 after they departed in 2010 (Fig. 2C). There were no significant differences in the ratios between the under and canopy traps during both years (P > 0.05). There also were no significant differences (P > 0.05) between ratios during summer and fall or between canopy and under traps at the control site (Fig. 2A).
Fig. 2.
Ratio (+SD) of arithmetic mean number of Cx. tarsalis females collected in traps within and under Eucalyptus canopy divided by mean number collected at edge traps during months with (July) and without (September) nesting ardeids. (A) Control farmstead during 2010. (B) Heronry during 2008. (C) Heronry during 2010.
Although relatively few An. freeborni were collected at the heronry during 2008, significantly more females were collected at ground level under the canopy than at the edge or in the canopy when birds were present or absent (Fig. 1B). The number of An. freeborni increased during 2010, especially during summer, and significantly more females were collected at the edge than under the canopy; few were collected within the canopy when birds were present or absent.
Discussion
Trap placement in relation to vegetation and the presence of a large number of avian hosts clearly altered estimates of Cx. tarsalis abundance in the current study. At the control farmstead with a large Eucalyptus stand without an ardeid nesting colony, catch of females was similar at CO2 traps positioned along the edge, under, and within the Eucalyptus canopy during summer and fall. In marked contrast at the heronry site, although females were abundant at CO2 traps along the edge of the farmstead and engorged females collected at a resting site under the canopy frequently had fed upon ardeid hosts (Thiemann 2011), almost no females were collected within the Eucalyptus stand when birds were nesting, even when traps were placed within the canopy adjacent to active nests. High WNV infection and antibody rates in ardeid nestlings (Reisen et al. 2009) supported the idea that these birds were fed upon frequently by infectious Cx. tarsalis females. Collectively, these data indicated that although females approaching the farmstead were readily intercepted by and attracted to CO2 traps at the ecotone, once they entered the Eucalyptus grove the large numbers of ardieds seemed to divert virtually all the questing females from our traps placed under or within the canopy. Similarly, our previous studies at urban sites in Los Angeles, CA, have shown that CO2 traps are far less efficient for collecting Culex quinquefasciatus Say females than gravid female traps baited with hay infusion (Reisen et al. 1990, Kwan et al. 2010), perhaps indicating that similar competition from bloodmeal hosts may have contributed to low CO2 trap sensitivity.
Our previous studies in desert (Lothrop et al. 2001, Lothrop and Reisen 2001) and foothill (Meyer et al. 1991) habitats in California indicated that Cx. tarsalis seemed to hunt at vegetation ecotones and were markedly less abundant at CO2-baited traps or unbaited suction traps placed at 1–2-m height under vegetation canopy. This pattern was similar to that shown at the heronry, because more females were collected at traps placed at the edge rather than under or within the canopy, even when the birds departed in early fall. This pattern was not seen at the control location, where traps within or under the canopy collected similar numbers of females as those at the edge of the grove. Although tree density seemed greater than at the control site, the trees were planted in rows, perhaps providing flight corridors for access. In addition, many of the females collected at the control location probably had emerged from the surrounding slough and initially entered the Eucalyptus grove to rest before initiating appetitive behaviors. Perhaps in the absence of competition from large numbers of blood-meal hosts, these females already resting within the grove were readily attracted to nearby traps placed under and within the canopy.
In general, An. freeborni were collected readily at traps placed at the edge or under, but not within the Eucalyptus canopy. This difference in host-seeking behavior would facilitate locating mammals such as rabbits or cows, their most frequently selected hosts (Washino and Tempelis 1967), which normally inhabit open pasture or mixed savannah habitats interspersed with groves of trees. These data clearly show how differences in foraging behavior and appetitive flight patterns align with host preferences (Chaves et al. 2010) and may combine to affect trapping success using dry ice-baited traps.
In summary, landscape, vegetation, and the presence of large numbers of attractive bloodmeal hosts altered the number of mosquitoes collected and there-fore estimates of risk that use numbers caught in CO2 traps, a surrogate for host seeking abundance or bites per host per night (Kramer 2009). The current study indicated that host-seeking Cx. tarsalis were best sampled by placing CO2 traps along vegetation ecotones thereby precluding interference by both vegetation and competing avian hosts.
Acknowledgments
This research was funded, in part, by grant AI55607-A02 from the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases; by using American Recovery and Reinvestment Act support, the Research and Policy in Infectious Disease Dynamics Program, Fogarty Center, NIH, and Department of Homeland Security; and a grant from the California Mosquito and Vector Control Association Research Foundation.
References Cited
- Anderson JF, Andreadis TG, Main AJ, Ferrandino FJ, Vossbrinck CR. West Nile virus from female and male mosquitoes (Diptera: Culicidae) in subterranean, ground, and canopy habitats in Connecticut. J. Med. Entomol. 2006;43:1010–1019. doi: 10.1603/0022-2585(2006)43[1010:wnvffa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bidlingmayer WL. The measurement of adult mosquito population changes—some considerations. J. Am. Mosq. Control Assoc. 1985;1:328–348. [PubMed] [Google Scholar]
- Bidlingmayer WL, Hem DG. Mosquito flight paths in relation to the environment. Effect of the forest edge upon trap catches in the field. Mosq. News. 1981;41:55–59. [Google Scholar]
- Chaves LF, Harrington LC, Keogh CL, Nguyen AM, Kitron UD. Blood feeding patterns of mosquitoes: random or structured? Front. Zool. 2010;7:3. doi: 10.1186/1742-9994-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Molaei G, Simpson JE, Folsom-O'Keefe CM, Armstrong PM, Andreadis TG. Avian communal roosts as amplification foci for West Nile virus in urban areas in northeastern United States. Am. J. Trop. Med. Hyg. 2010;82:337–343. doi: 10.4269/ajtmh.2010.09-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am. J. Trop. Med. Hyg. 2009;80:268–278. [PubMed] [Google Scholar]
- Hintze J. NCSS statistical software. NCSS; Kaysville, UT: 1998. [Google Scholar]
- Kent R, Juliusson L, Weissmann M, Evans S, Komar N. Seasonal blood-feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J. Med. Entomol. 2009;46:380–390. doi: 10.1603/033.046.0226. [DOI] [PubMed] [Google Scholar]
- Kramer VL. California State mosquito-borne virus surveillance and response plan. 2009 doi: 10.4269/ajtmh.2003.68.508. http://westnile.ca.gov/resources.php. [DOI] [PubMed]
- Kwan JL, Kluh S, Madon MB, Reisen WK. West Nile virus emergence and persistence in Los Angeles, California, 2003–2008. Am. J. Trop. Med. Hyg. 2010;83:400–412. doi: 10.4269/ajtmh.2010.10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothrop HD, Reisen WK. Landscape affects the host-seeking patterns of Culex tarsalis (Diptera: Culicidae) in the Coachella Valley of California. J. Med. Entomol. 2001;38:325–332. doi: 10.1603/0022-2585-38.2.325. [DOI] [PubMed] [Google Scholar]
- Lothrop HD, Lothrop B, Reisen WK. Use of non-attractant traps to determine patterns of mosquito abundance in habitats in the Coachella Valley, California. Proc. Mosq. Vector Control Assoc. Calif. 2001;69:60–62. [Google Scholar]
- Meyer RP, Reisen WK, Milby MM. Influence of vegetation on CO2 trap effectiveness for sampling mosquitoes in the Sierra Nevada foothills of Kern County, California. J. Am. Mosq. Control Assoc. 1991;7:471–475. [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Diuk-Wasser M. Host-feeding patterns of potential mosquito vectors in Connecticut, U.S.A.: molecular analysis of bloodmeals from 23 species of Aedes, Anopheles, Culex, Coquillettidia, Psorophora, and Uranotaenia. J. Med. Entomol. 2008;45:1143–1151. doi: 10.1603/0022-2585(2008)45[1143:hpopmv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Molaei G, Cummings RF, Su T, Armstrong PM, Williams GA, Cheng ML, Webb JP, Andreadis TG. Vector-host interactions governing epidemiology of West Nile virus in southern California. Am. J. Trop. Med. Hyg. 2010;83:1269–1282. doi: 10.4269/ajtmh.2010.10-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse VF, Chamberlain RW, Johnston JG, Jr., Sudia WD. Use of dry ice to increase mosquito catches of the CDC miniature light trap. Mosq. News. 1966;26:30–35. [Google Scholar]
- Nielsen CF, Armijos MV, Wheeler S, Carpenter TE, Boyce WM, Kelley K, Brown D, Scott TW, Reisen WK. Risk factors associated with human infection during the 2006 West Nile virus outbreak in Davis, a residential community in Northern California. Am. J. Trop. Med. Hyg. 2008;78:53–62. [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD. Effects of sampling design on the estimation of adult mosquito abundance. J. Am. Mosq. Control Assoc. 1999;15:104–114. [PubMed] [Google Scholar]
- Reisen WK, Reeves WC. Bionomics and ecology of Culex tarsalis and other potential mosquito vector species. In: Reeves WC, editor. Epidemiology and control of mosquito-borne arboviruses in California, 1943–1987. California Mosquito and Vector Control Association; Sacramento, CA: 1990. pp. 254–329. [Google Scholar]
- Reisen WK, Meyer RP, Tempelis CH, Spoehel JJ. Mosquito abundance and bionomics in residential communities in Orange and Los Angeles counties, California. J. Med. Entomol. 1990;27:356–367. doi: 10.1093/jmedent/27.3.356. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Meyer RP, Cummings RF, Delgado O. Effects of trap design and CO2 presentation on the measurement of adult mosquito abundance using CDC style miniature light traps. J. Am. Mosq. Control Assoc. 2000;16:13–18. [PubMed] [Google Scholar]
- Reisen WK, Wheeler SS, Yamamoto S, Fang Y, Garcia S. Nesting ardeid colonies are not a focus of elevated West Nile virus activity in southern California. Vector Borne Zoonotic Dis. 2005;5:258–266. doi: 10.1089/vbz.2005.5.258. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Barker CM, Carney R, Lothrop HD, Wheeler SS, Wilson JL, Madon MB, Takahashi R, Carroll B, Garcia S, et al. Role of corvids in epidemiology of West Nile virus in southern California. J. Med. Entomol. 2006;43:356–367. doi: 10.1603/0022-2585(2006)043[0356:rocieo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Wheeler S, Armijos MV, Fang Y, Garcia S, Kelley K, Wright S. Role of communally nesting ardeid birds in the epidemiology of West Nile virus revisited. Vector Borne. Zoonotic Dis. 2009;9:275–280. doi: 10.1089/vbz.2008.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer WF, Buescher EL, McClure HE. Ecologic studies of Japanese encephalitis virus in Japan. V. Avian factors. Am. J. Trop. Med. Hyg. 1959;8:689–697. doi: 10.4269/ajtmh.1959.8.689. [DOI] [PubMed] [Google Scholar]
- Shi PY, Kramer LD. Molecular detection of West Nile virus RNA. Expert Rev. Mol. Diagn. 2003;3:357–366. doi: 10.1586/14737159.3.3.357. [DOI] [PubMed] [Google Scholar]
- Sudia WD, Chamberlain RW. Battery-operated light trap, an improved model. Mosq. News. 1962;22:126–129. [PubMed] [Google Scholar]
- Thiemann T. Ph.D. Dissertation. University of California, Davis; 2011. Bloodfeeding patterns of Culex tarsalis and the Culex pipiens complex in California. [Google Scholar]
- Washino RK, Tempelis CH. Host-feeding patterns of Anopheles freeborni in the Sacramento Valley, California. J. Med. Entomol. 1967;4:311–314. doi: 10.1093/jmedent/4.3.311. [DOI] [PubMed] [Google Scholar]