Abstract
Background
The human papillomavirus (HPV) Persistence and Progression Cohort is a natural history study of carcinogenic HPV positive women. Here, we present the HPV genotypes found in first ∼500 cases of cervical intraepithelial neoplasia grade 3 (CIN3) or more severe disease (CIN3+) diagnosed at the study baseline.
Methods
Women aged 30 and older were screened for cervical cancer using Pap smears and tested for carcinogenic HPV using Hybrid Capture 2 (HC2; Qiagen). We randomly selected women who tested HPV positive and were diagnosed with CIN3+ (n = 448) or without CIN3+ (<CIN3) (n = 830). Residual specimens were HPV genotyped using a MY09/11 L1-targeted PCR method.
Results
Among HC2-positive women, HPV16 (48.9%), HPV31 (9.2%), and HPV18 (8.5%) were the most common HPV genotypes in CIN3+. There was a decrease at older ages in the fraction of CIN3 (ptrend = 0.006), adenocarcinoma in situ (AIS) (ptrend = 0.08), and CIN3/AIS (ptrend = 0.002) associated with HPV16. Compared to the other carcinogenic HPV genotypes in aggregate, HPV18 was strongly associated with CIN3+ in women with a normal Pap (odds ratio ([OR] = 5.7, 95%CI = 1.2-26) but not in women with abnormal Pap (OR = 1.3, 95%CI = 0.74-2.3).
Conclusions
HPV16 is associated with cervical precancer diagnosed in younger women (vs. older women). HPV18 infections were linked to precancerous lesions that were missed by cytology.
Impact
The progression timeline of HPV16 differs from other carcinogenic HPV genotypes, which may impact the use of HPV16 detection in the management of HPV-positive women.
Keywords: Pap, cervical intraepithelial neoplasia (CIN), cervical cancer, human papillomavirus (HPV), atypical squamous cells of undetermined significance (ASC-US), hybrid capture 2 (HC2)
Introduction
Large cohort and case-control studies have clarified that human papillomavirus (HPV) is the requisite cause of cervical cancer and its immediate precursor (precancer) lesion, cervical intraepithelial neoplasia grade 3 (CIN3). The basic steps for cervical carcinogenesis are HPV acquisition, HPV persistence (vs. clearance), progression of a persistent carcinogenic HPV to CIN3, and invasion by the CIN3 lesion (1). Young women after sexual initiation are the most apt to acquire truly new HPV infections (2). Most HPV infections clear within a year or two (3-5). Carcinogenic HPV infections that do not clear within a year or two are strongly associated with and/or predict the development of CIN3 (5-9). Finally, there is an approximately 30% risk of large CIN3 found in older women becoming invasive if left untreated (10, 11).
Although these steps have been delineated, no single epidemiologic study has been sufficiently long enduring and large to study all these transitions in one population. The timeframe of developing cervical cancer from an incident HPV infection is approximately 25-30 years on average. While HPV infection is quite common, only a small fraction of women go on to the next steps in the pathway.
Previous cohort studies have been underpowered to study the transition from HPV infection to persistence to progression to CIN3 because despite large sample sizes, only a fraction of women are positive for carcinogenic HPV at any one-time point. For example, a random sample of 10,000 women living in Costa Rica yielded an HPV-positive sub-cohort of 1,000-1,500 women, and only a small proportion of the HPV-positive women were diagnosed with CIN3 over the next 5-7 years. Because of the small numbers of HPV infections and outcomes, studying these transitions for individual HPV genotypes is not possible except for perhaps HPV16, which is the most common carcinogenic HPV genotype, causing approximately half of all CIN3.
We have initiated a large cohort study to study the transition from acute HPV infection to CIN3. Using the carcinogenic HPV testing by Hybrid Capture 2 (HC2; Qiagen, Gaithersburg, MD) conducted as part of routine clinical practice, we have oversampled carcinogenic HPV-positive women (our sample of ∼60,000 women includes ∼50,000 HPV-positive women) (12, 13). A large cohort of HPV-positive women will permit us to study each carcinogenic HPV genotype individually rather than as a group.
To validate this approach, we conducted a baseline case-control study of HPV genotypes in clinical specimens from HPV-positive women diagnosed with CIN3 or more severe and a random sample of HPV positives with <CIN3. The primary goal of this analysis was to evaluate the use of HC2 testing to create a carcinogenic HPV-positive cohort. Secondarily, we wanted to describe the HPV genotypes in women diagnosed with CIN3, adenocarcinoma in situ (AIS), squamous cell carcinoma (SCC), and adenocarcinoma (ADC)/adenosquamous carcinoma (ASC). There has been limited number of large studies of HPV genotypes in CIN3 or CIN3+ in the U.S. (14-16) and fewer still with appreciable numbers of AIS and adenocarcinoma (16-18).
Methods
At Kaiser Permanente Northern California (KPNC), women are tested by HC2 for carcinogenic HPV DNA to triage atypical squamous cells of undetermined significance (ASC-US) (since 2001) and as adjunct to Pap smears in women aged 30 and older (since 2003) in accordance with current screening guidelines (19, 20). The HPV Persistence and Progression Cohort (The PaP Cohort) was created by banking residual, waste cervical specimens, collected into specimen transport medium (STM; Qiagen), from women who tested HC2 positive in conjunction with routine cervical cancer screening. After specimens were used for HC2, the residual specimens were neutralized and archived (12, 13).
For this analysis, we selected specimens from all eligible cases of HC2-positive CIN3+ diagnosed between January, 2007 to October, 2008. Eligibility was defined as women 21 and older who had not opted out from having their specimen banked and tested for HPV-related biomarkers including HPV genotypes. HC2-negative CIN3 is not routinely available since the histologic diagnoses are made available weeks after the clinical HC2 is complete and the residual specimen is discarded if not selected for banking. HC2-positive women without CIN3+ (<CIN3) and HC2-negative women were selected at a ratio of 2:1 and 1:2, respectively, to the cases of CIN3+. We restricted this analysis to women 30 and older who are routinely cotested with HC2 and Pap. The final analytic group was 448 CIN3+ (one case of CIN3 did not have retrievable HC2 results) and 830 HC2 positives with <CIN3. More than 90% of women 30 and older undergoing cervical cancer screening elect to be screened by co-testing (vs. conventional Pap testing)(21).
HPV DNA testing
Hybrid Capture 2 (HC2; Qiagen Inc., Gaithersburg, MD, USA), a DNA test for a pool of 13 carcinogenic HPV genotypes (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), was conducted on the banked STM specimen per manufacturer's instruction as part of routine cervical cancer screening (triage of ASC-US Pap and HPV and Pap co-testing in women 30 and older) at KPNC.
The MY09/M11 L1 degenerate primer PCR (MY09/11 PCR) method used to test banked, residual STM specimens (after HC2 testing) for HPV genotypes has been described previously (22). Dot blot hybridization of the amplicons using HPV genotype-specific oligonucleotide probes was used to identify over 40 individual HPV genotypes (22). For some of the rarer non-carcinogenic HPV genotypes, small pools of probes were used (e.g., HPV26, 69, and 82) to probe the amplicons on the dot blots for a group of non-carcinogenic HPV gentoypes rather than single probes for individual non-carcinogenic HPV genotypes; because the focus of these analyses were HC2-targeted HPV genotypes, positive results for these pooled probe sets were simply classified as non-carcinogenic HPV. The HPV genotypes targeted by HC2 were considered the carcinogenic HPV genotypes (23), and all others were considered non-carcinogenic. We classified the HPV genotype detection hierarchically according to cancer risk (HPV risk group)(24): HPV16, else HPV16 negative but positive for HPV18, else HPV16 and HPV18 negative but positive for other carcinogenic HPV genotypes, else negative for all carcinogenic HPV genotypes but positive for non-carcinogenic HPV genotypes (including specimens that were genotype-negative but classified as PCR positive based on positive generic probe hybridization), else PCR negative. HPV genotypes were also grouped according to two main phylogenetic species with carcinogenic HPV genotypes (25): alpha-7 (HPV18, 39, 45, 59, 68, 70, 85, and 97) and alpha-9 (HPV16, 31, 33, 35, 52, 58, and 67).
PCR results were available for 445 CIN3+ (99.3%) and 819 <CIN3 (98.9%); there was no difference in the fraction of missing PCR results by study group (p = 0.6, Fisher's exact).
Specimens that tested positive for HPV but no HPV genotype was detected were re-hybridized and/or subjected to sequencing. Fifty-five CIN3+ and random sample of 69 PCR-negatives with <CIN3+ were retested to maximize HPV genotype detection.
Statistical Analysis
First, we calculated the prevalence of HPV risk groups with binomial 95% confidence intervals (95%CI) among HC2-positive women. Second, we calculated the prevalence of individual carcinogenic HPV genotypes, HPV risk groups, and alpha-9 and alpha-7 HPV genotypes for each histologic diagnosis, treating no histology as a separate category from negative histology, and for grouped histologic diagnoses, CIN3+ (CIN3, AIS, SCC, ADC, and ASC) vs. <CIN3. We calculated the risk ratio and 95% confidence intervals (95%CI) for CIN3+ to <CIN3 as a metric of carcinogenicity.
We were interested in whether there was a difference in the HPV16-positive fraction of cervical precancerous lesions, CIN3 and AIS, which is the primary target of a cervical cancer screening program. We therefore evaluated the fraction of HPV16 positivity in women with CIN3, AIS, and CIN3/AIS by age group (30-39, 40-49, 50-59, and ≥60) and as a reference, women with <CIN3. We tested for significant difference in the fraction of HPV16 positivity by age using a one-degree (Mantel-Haenzel) test of trend. We calculated the odds ratios and 95%CI as a measure of association of HPV16 with CIN3/AIS (vs. <CIN3) for each age group.
Finally, among those with retrievable Pap results (n = 1,237 of 1,278, 97%), we calculated the prevalence and age-adjusted OR (aOR) with 95%CI of HPV risk groups with CIN3+ (vs. <CIN3) in HC2-positive women 30 and older with and without concurrent cytologic abnormalities (normal vs. ≥ASC-US). We assessed the interaction between HPV risk group and cytologic status (normal vs. ≥ASC-US) for their association with CIN3+ (vs. <CIN3) using a likelihood ratio test.
Results
The prevalences of HPV genotypes and HPV risk groups for each histologic diagnosis among HC2-positive women are shown in Table 1. With increasing severity of diagnosis, there was a shift to the higher risk HPV risk group. Among women with a CIN3 diagnosis (n = 349), HPV16 (46.1%), HPV31 (10.9%), and HPV58 (8.3%) were the most common HPV genotypes. Among women with a SCC diagnosis (n = 35), HPV16 (71.4%), HPV52 (8.6%), and HPV18 (5.7%) were the most common HPV genotypes.
Table 1.
Distribution of carcinogenic HPV genotypes and hierarchical HPV risk groups (HPV16 > HPV18 > carcinogenic HPV excluding HPV16 and HPV18 > non-carcinogenic or PCR-) by severity of histologic diagnosis, as individual diagnoses and categorized as cervical intraepithelial neoplasia grade 3 (CIN3) or more severe (CIN3+) versus <CIN3. Women tested Hybrid Capture 2 positive. Risk ratios (CIN3+:<CIN3) with 95% confidence intervals (95%CI) are shown as a measure of the carcinogenicity of individual carcinogenic HPV. One case of CIN3+ was co-infected with HPV16 and HPV18, which was attributed to HPV16 in the hierarchical HPV risk groups. AIS: adenocarcinoma in situ; ADC: adenocarcinoma; ASC: adenosquamous carcinoma; SCC: squamous cell carcinoma
| Hybrid Capture 2 Positive | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Histology N= 625 |
Negative N = 70 |
CIN1 N = 106 |
CIN2 N = 18 |
CIN3 N = 349 |
AIS N = 43 |
ADC/ADSC N = 19 |
SCC N = 35 |
<CIN3 N = 819 |
CIN3+ N = 446 |
CIN3+ vs. <CIN3 | ||||||||||||
| HPV Genotypes | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | Risk Ratio | 95%CI |
| HPV16 | 81 | 12.9% | 10 | 14.3% | 9 | 8.5% | 2 | 11.1% | 161 | 46.1% | 24 | 55.8% | 9 | 47.4% | 25 | 71.4% | 102 | 12.4% | 219 | 49.1% | 3.9 | 3.2-4.8 |
| HPV18 | 31 | 5.0% | 2 | 2.9% | 14 | 13.2% | 1 | 5.6% | 17 | 4.9% | 13 | 30.2% | 6 | 31.6% | 2 | 5.7% | 48 | 5.9% | 38 | 8.5% | 1.5 | 0.97-2.2 |
| HPV31 | 50 | 8.0% | 5 | 7.1% | 12 | 11.3% | 3 | 16.7% | 38 | 10.9% | 3 | 7.0% | 0 | 0.0% | 1 | 2.9% | 70 | 8.5% | 42 | 9.4% | 1.1 | 0.77-1.6 |
| HPV33 | 15 | 2.4% | 2 | 2.9% | 3 | 2.8% | 1 | 5.6% | 13 | 3.7% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 21 | 2.6% | 13 | 2.9% | 1.1 | 0.57-1.2.2 |
| HPV35 | 37 | 5.9% | 2 | 2.9% | 4 | 3.8% | 2 | 11.1% | 18 | 5.2% | 0 | 0.0% | 0 | 0.0% | 1 | 2.9% | 45 | 5.5% | 19 | 4.3% | 0.78 | 0.64-1.3 |
| HPV39 | 23 | 3.7% | 1 | 1.4% | 7 | 6.6% | 0 | 0.0% | 8 | 2.3% | 1 | 2.3% | 0 | 0.0% | 0 | 0.0% | 31 | 3.8% | 9 | 2.0% | 0.53 | 0.26-1.1 |
| HPV45 | 11 | 1.8% | 4 | 5.7% | 3 | 2.8% | 1 | 5.6% | 8 | 2.3% | 6 | 14.0% | 3 | 15.8% | 1 | 2.9% | 19 | 2.3% | 18 | 4.0% | 1.7 | 0.92-3.3 |
| HPV51 | 35 | 5.6% | 3 | 4.3% | 13 | 12.3% | 2 | 11.1% | 16 | 4.6% | 0 | 0.0% | 0 | 0.0% | 1 | 2.9% | 53 | 6.5% | 17 | 3.8% | 0.59 | 0.35-1.0 |
| HPV52 | 44 | 7.2% | 5 | 7.1% | 6 | 5.7% | 1 | 5.6% | 28 | 8.0% | 0 | 0.0% | 0 | 0.0% | 3 | 8.6% | 56 | 6.4% | 31 | 7.0% | 1 | 0.67-1.6 |
| HPV56 | 27 | 4.3% | 4 | 5.7% | 6 | 5.7% | 0 | 0.0% | 5 | 1.4% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 37 | 4.5% | 5 | 1.1% | 0.25 | 0.10-0.63 |
| HPV58 | 29 | 4.6% | 3 | 4.3% | 6 | 5.7% | 3 | 16.7% | 29 | 8.3% | 2 | 4.7% | 0 | 0.0% | 0 | 0.0% | 41 | 5.0% | 31 | 7.0% | 1.4 | 0.88-2.2 |
| HPV59 | 10 | 1.6% | 1 | 1.4% | 4 | 3.8% | 0 | 0.0% | 1 | 0.3% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 15 | 1.8% | 1 | 0.2% | n/a | ---- |
| HPV68 | 55 | 8.8% | 5 | 7.1% | 7 | 6.6% | 0 | 0.0% | 9 | 2.6% | 0 | 0.0% | 1 | 5.3% | 0 | 0.0% | 67 | 8.2% | 10 | 2.2% | 0.27 | 0.14-0.53 |
| HPV Risk Group | ||||||||||||||||||||||
| HPV16 | 81 | 12.9% | 10 | 14.3% | 9 | 8.5% | 2 | 11.1% | 161 | 46.1% | 24 | 55.8% | 9 | 47.4% | 25 | 71.4% | 102 | 12.4% | 219 | 49.1% | 3.9 | 3.2-4.8 |
| HPV18 | 30 | 4.8% | 2 | 2.9% | 14 | 13.2% | 1 | 5.6% | 13 | 3.7% | 13 | 30.2% | 6 | 31.6% | 1 | 2.9% | 47 | 5.7% | 33 | 7.4% | 1.3 | 0.84-1.98 |
| Carcinogenic (exc. HPV16/18) | 296 | 47.3% | 31 | 44.3% | 60 | 56.6% | 12 | 66.7% | 139 | 39.8% | 5 | 11.6% | 3 | 15.8% | 6 | 17.1% | 399 | 48.7% | 153 | 34.3% | 0.7 | 0.61-0.82 |
| Non-Carcinogenic/PCR+ | 96 | 15.2% | 17 | 24.3% | 12 | 11.3% | 1 | 5.6% | 26 | 7.5% | 0 | 0.0% | 0 | 0.0% | 2 | 5.7% | 125 | 15.3% | 28 | 6.3% | 0.4 | 0.28-0.61 |
| PCR- | 123 | 19.7% | 10 | 14.3% | 11 | 10.4% | 2 | 11.1% | 10 | 2.9% | 1 | 2.3% | 1 | 5.3% | 1 | 2.9% | 146 | 17.8% | 13 | 2.9% | 0.16 | 0.09-0.28 |
Among women with an AIS diagnosis (n = 43), HPV16 (55.8%), HPV18 (30.2%), and HPV45 (14.0%) were the most common HPV genotypes. Similar to AIS, among women with an ADC/ASC diagnosis (n = 19), HPV16 (47.4%), HPV18 (31.6%), and HPV45 (15.8%) were the most common HPV genotypes.
Women with CIN3/SCC were 79.4% positive for alpha-9 HPV genotypes and 13.8% for alpha-7 HPV genotypes. Women with AIS/ADC/ASC were 59.7% positive for alpha-9 HPV genotypes and 40.3% for alpha-7 HPV genotypes.
Dichotomizing diagnoses as CIN3+ and <CIN3, HPV16 (49.1%), HPV31 (9.4%), and HPV18 (8.5%) were the most common HPV genotypes in CIN3+. HPV161 was the most strongly associated with CIN3+ (vs. <CIN3) (Risk Ratio = 3.9, 95%CI = 3.2-4.8); HPV45 (Risk Ratio = 1.7, 95%CI = 0.92-3.3) no longer significant and HPV18 (Risk Ratio = 1.5, 95%CI = 0.97-2.2) no longer significant were the only other HPV genotypes positively associated with CIN3+. Conversely, HPV56 (Risk Ratio = 0.25), HPV68 (Risk Ratio = 0.27), and HPV39 (Risk ratio=0.53) were the most weakly associated with CIN3+. Stratification on whether single vs. multiple HPV genotypes were detected did not appreciably change these patterns although we only had 174 PCR-positive women with multiple HPV genotypes (16.4%) (data not shown).
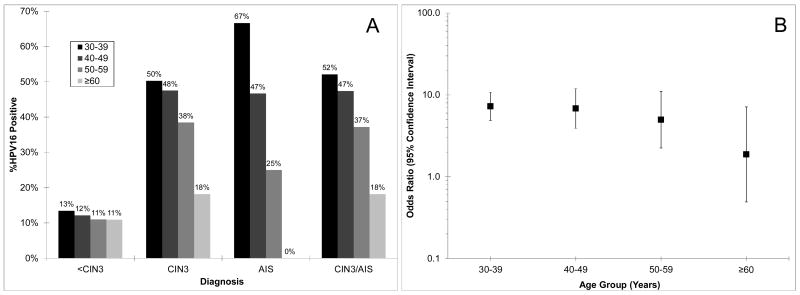
Figure 1 shows that the percentage of HPV16 positives in CIN3 (p = 0.006), AIS (p = 0.08), and CIN3/AIS (p = 0.002) decreased with older age groups; a similar trend for women with <CIN3 was not statistically significant (p = 0.4) (Panel A). Thus, the association of HPV16 with CIN3+ (vs. <CIN3) declined with age (Panel B).
Figure 1.
Distribution of HPV16 in women with a diagnosis of less than cervical intraepithelial neoplasia grade 3 (<CIN3), CIN3, adenocarcinoma in situ (AIS), and CIN3 or AIS (CIN3/AIS) (A) and the association (odds ratio with 95% confidence interval) of HPV16 with CIN3/AIS by age group (B).
We compared the distribution of HPV risk groups between CIN3+ and <CIN3 among HC2-positive women 30 and older with concurrent abnormal or normal Pap smears (Table 2). In general, we found similar prevalence of HPV risk groups in each subgroup although there tended to be more PCR negativity among Pap-negative (22.2%) than Pap-positive women (11.4%) who were HC2-positive with <CIN3. Compared to the other carcinogenic HPV types in aggregate, HPV16 was significantly and similarly associated with CIN3+ in women with (OR = 6.1, 95%CI = 4.0-9.2) and without (OR = 9.0, 95%CI = 3.0-23) concurrent abnormal Paps. Interestingly, HPV18 was not associated with CIN3+ in women with abnormal Paps (aOR = 1.3, 95%CI = 0.74-2.3) but was associated with CIN3+ in women with normal Paps (aOR = 5.7, 95%CI = 1.2-26) compared to the other carcinogenic HPV genotypes in aggregate. Overall, there was significant statistical interaction between HPV risk groups and cytologic status (p = 0.01, likelihood ratio test), driven it appears mainly by greater percentage of HPV16 and HPV18 in CIN3+ in women with normal cytology compared to women with abnormal cytology.
Table 2.
Distribution of hierarchical HPV risk groups (HPV16 > HPV18 > carcinogenic HPV > non-carcinogenic or PCR-) in Hybrid Capture 2-positive women with cervical intraepithelial neoplasia grade 3 (CIN3) or more severe (CIN3+) and without (<CIN3), stratified on concurrent cytology result. Age-adjusted odds ratios (aOR) with 95% confidence intervals (95%CI) as a measure of association of HPV risk groups with CIN3+ (vs. <CIN3) were calculated. ASCUS: atypical squamous cells of undetermined significance
| Normal Cytology | Abnormal Cytology (≥ASC-US) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <CIN3 | CIN3+ | aOR* | 95%CI | <CIN3 | CIN3+ | aOR* | 95%CI | |||||
| HPV Risk Group | N | % | N | % | N | % | N | % | ||||
| HPV16 | 58 | 12.7% | 15 | 65.2% | 11 | 3.7-31 | 38 | 11.7% | 204 | 48.2% | 6.0 | 4.0-9.1 |
| HPV18 | 18 | 3.9% | 3 | 13.0% | 5.9 | 1.3-27 | 26 | 8.0% | 30 | 7.1% | 1.3 | 0.73-2.3 |
| Carcinogenic (ref) | 216 | 47.4% | 5 | 21.7% | 1.0 | ---- | 165 | 50.9% | 148 | 35.0% | 1.0 | ---- |
| Non-Carcinogenic | 63 | 13.8% | 0 | 0.0% | n/a | n/a | 58 | 17.9% | 28 | 6.6% | 0.53 | 0.32-0.87 |
| PCR- | 101 | 22.1% | 0 | 0.0% | n/a | n/a | 37 | 11.4% | 13 | 3.1% | 0.38 | 0.20-0.74 |
| Total | 456 | 23 | 324 | 423 | ||||||||
Discussion
In this analysis, we made several observations. First, we demonstrated that we could successfully develop a natural history study of HPV based on banking waste cervical specimens in STM after the HC2 was completed. We had similar prevalences of carcinogenic HPV genotypes in CIN3, AIS, ADC/ADSC, and SCC as recently reported for the U.S. (16). For example, we observed the expected shift of testing positive for alpha-7 HPV genotypes, particularly HPV18, for glandular lesions compared to squamous lesions. We had a similar proportion of SCC positive for HPV16 and HPV18 as reported from a large systematic review (24) and a recent tissue-based worldwide study of cervical cancers (26) but greater than a tissue-based study of cervical cancers in New Mexico (16). This difference might be the result in differences in specimen type (cervical exfoliative cells vs. cervical tissues) and/or the population, or it might be due to random chance as we had only 35 SCC included this study. We also acknowledge that that there may be some misattribution due to multiple HPV-genotype infections (27), especially for HPV16 since we assume that any CIN3+ with multiple infections that included HPV16 are caused by it. Notably, we did not miss a significant proportion of carcinogenic HPV infections, as only 10 of 278 (3.6%, binomial 95%CI 1.7-6.5%) HC2-negative specimens (all ages) tested PCR positive for carcinogenic HPV (data not shown).
We noted one potential limitation to our approach, a slightly higher percentage of PCR negative results than expected. The cause is unknown but could be the result of the processing of the entire STM specimen for routine clinical HPV testing, prior to our PCR-based genotyping. This interpretation, if true, means that the problem is unavoidable.
Second, albeit based on small numbers, we found that HPV18 was more strongly associated with CIN3+ among women with normal Pap than with abnormal Pap (n.b., HC2-positive, Pap-negative women would not normally be referred to colposcopy unless there was a positive screening result on the previous visit). One obvious implication is that HPV testing may identify HPV-positive, especially HPV18-positive, precancerous lesions that would be otherwise missed by Pap. Perhaps not surprisingly, there were more glandular lesions among HC2-positive, Pap-negative women diagnosed with CIN3+ than among the Pap-positive women (p = 0.02, Fisher's exact) (data not shown); the three CIN3+ cases found among HPV18-positive, Pap-negative women included two AIS and one adenocarcinoma. The rise in the incidence of adenocarcinoma juxtaposed with the decline of squamous cell carcinoma suggests that screening with Pap alone does not effectively detect precursors, e.g., AIS, of invasive adenocarcinoma in a timely fashion (28, 29). Recent reports from ongoing randomized trials evaluating HPV testing have found a non-significantly greater proportion of AIS in the HPV study arm compared to the conventional Pap (30-32), providing further evidence that HPV testing improves the detection of glandular precancerous disease.
Finally, we also found evidence in this screening population that HPV16-related precancerous lesions, CIN3 or AIS, occur at younger ages than those caused by other carcinogenic HPV genotypes. This is consistent with several previous reports indicating that HPV16-related CIN3 occurred at a younger age than CIN3 caused by other HPV genotypes (14, 16, 33) and HPV16-related cervical cancers (and HPV18-related cancers) (n.b., using tissue-based HPV genotyping) occurred at a younger age than cervical cancers caused by other HPV genotypes (16, 26). These data indicate that HPV16-induced (and perhaps HPV18-induced) cervical carcinogenesis proceeds more rapidly than that induced by other carcinogenic HPV genotypes. Our data extend the finding to precancerous glandular lesions, and highlight the unique natural history and carcinogenicity of HPV16. We surmise that in populations HPV vaccinated before becoming sexually active, with HPV16 removed, the peak age of precancerous lesions will shift to older ages and it would be beneficial to delay initiation of cervical cancer screening in the U.S to the age of 25 or even 30 years of age to increase the programmatic specificity. In addition, the removal of HPV16 and HPV18 from the general population should dramatic decrease the predictive value of a positive screening test, HPV or cytology, resulting in ambiguity about the best method of screening, the frequency of screening, and the appropriate management of screen positives (ref). It has been suggested that a switch to HPV-based screening might avoid some of the difficulties of trying to maintain a cytology-based program in a very low-risk population in which nearly all cytologic readings will be normal, resulting in reader “fatigue”(34).
The different natural history of HPV16 may also have clinical implications for using HPV16 detection as a triage for HPV-positive, Pap-negative women (20, 35): a smaller fraction of precancerous lesions will be attributable to HPV16 in older women versus younger women. Thus, clinical sensitivity of using HPV16 as a triage for HPV-positive, Pap-negative will decrease with age, leaving more women with undetected CIN3 to one-year follow-up rather than immediate colposcopy. We hope to address this and other questions in our large HPV-positive cohort study.
Footnotes
Acknowledgements: This research was supported by the Intramural Research Program of the NIH, NCI and by Kaiser Permanente Northern California (KPNC).
Risk ratio as used in this paper is the prevalence of a HPV genotype in the CIN3+ group divided by the prevalence in the <CIN3 group. Note that there is no external reference group as there is for odds ratios.
Reference List
- 1.Wright TC, Jr, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med. 2003;348(6):489–90. doi: 10.1056/NEJMp020178. [DOI] [PubMed] [Google Scholar]
- 2.Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157(3):218–26. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 3.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195(11):1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 4.Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12(6):485–90. [PubMed] [Google Scholar]
- 5.Rodriguez AC, Schiffman M, Herrero R, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100(7):513–7. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castle PE, Rodriguez AC, Burk RD, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;339:b2569. doi: 10.1136/bmj.b2569. b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koshiol J, Lindsay L, Pimenta JM, et al. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol. 2008;168(2):123–37. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ. 2002;325(7364):572. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2001;286(24):3106–14. doi: 10.1001/jama.286.24.3106. [DOI] [PubMed] [Google Scholar]
- 10.Schiffman M, Rodriguez AC. Heterogeneity in CIN3 diagnosis. Lancet Oncol. 2008;9(5):404–6. doi: 10.1016/S1470-2045(08)70110-4. [DOI] [PubMed] [Google Scholar]
- 11.McCredie MR, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–34. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 12.LaMere BJ, Kornegay J, Fetterman B, et al. Human papillomavirus genotyping after denaturation of specimens for Hybrid Capture 2 testing: feasibility study for the HPV persistence and progression cohort. J Virol Methods. 2007;146(1-2):80–5. doi: 10.1016/j.jviromet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaMere BJ, Howell R, Fetterman B, Shieh J, Castle PE. Impact of 6-month frozen storage of cervical specimens in alkaline buffer conditions on human papillomavirus genotyping. J Virol Methods. 2008;151(2):298–300. doi: 10.1016/j.jviromet.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castle PE, Schiffman M, Wheeler CM, Wentzensen N, Gravitt PE. Human papillomavirus genotypes in cervical intraepithelial neoplasia grade 3. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1675–81. doi: 10.1158/1055-9965.EPI-10-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wentzensen N, Schiffman M, Dunn ST, et al. Grading the severity of cervical neoplasia based on combined histopathology, cytopathology, and HPV genotype distribution among 1,700 women referred to colposcopy in Oklahoma. Int J Cancer. 2009;124(4):964–9. doi: 10.1002/ijc.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler CM, Hunt WC, Joste NE, et al. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Natl Cancer Inst. 2009;101(7):475–87. doi: 10.1093/jnci/djn510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altekruse SF, Lacey JV, Jr, Brinton LA, et al. Comparison of human papillomavirus genotypes, sexual, and reproductive risk factors of cervical adenocarcinoma and squamous cell carcinoma: Northeastern United States. Am J Obstet Gynecol. 2003;188(3):657–63. doi: 10.1067/mob.2003.132. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz SM, Daling JR, Shera KA, et al. Human papillomavirus and prognosis of invasive cervical cancer: a population-based study. J Clin Oncol. 2001;19(7):1906–15. doi: 10.1200/JCO.2001.19.7.1906. [DOI] [PubMed] [Google Scholar]
- 19.Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103(2):304–9. doi: 10.1097/01.AOG.0000109426.82624.f8. [DOI] [PubMed] [Google Scholar]
- 20.Wright TC, Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;11(4):201–22. doi: 10.1097/LGT.0b013e3181585870. [DOI] [PubMed] [Google Scholar]
- 21.Castle PE, Fetterman B, Poitras N, et al. Five-year experience of human papillomavirus DNA and papanicolaou test cotesting. Obstet Gynecol. 2009;113(3):595–600. doi: 10.1097/AOG.0b013e3181996ffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castle PE, Schiffman M, Gravitt PE, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002;68(3):417–23. doi: 10.1002/jmv.10220. [DOI] [PubMed] [Google Scholar]
- 23.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10(4):321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 24.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121(3):621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 25.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur HH. Classification of papillomaviruses. Virology. 2004;324(1):17–27. doi: 10.1016/j.virol.2004.03.033. 20. [DOI] [PubMed] [Google Scholar]
- 26.de S S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 27.Wentzensen N, Schiffman M, Dunn T, et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer. 2009;125(9):2151–8. doi: 10.1002/ijc.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang SS, Sherman ME, Hildesheim A, Lacey JV, Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976-2000. Cancer. 2004;100(5):1035–44. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- 29.Bray F, Carstensen B, Moller H, et al. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2191–9. doi: 10.1158/1055-9965.EPI-05-0231. [DOI] [PubMed] [Google Scholar]
- 30.Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370(9601):1764–72. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 31.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010 doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 32.Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ. 2010;340:c1804. doi: 10.1136/bmj.c1804. c1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porras C, Rodriguez AC, Hildesheim A, et al. Human papillomavirus types by age in cervical cancer precursors: predominance of human papillomavirus 16 in young women. Cancer Epidemiol Biomarkers Prev. 2009;18(3):863–5. doi: 10.1158/1055-9965.EPI-08-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franco EL, Mahmud SM, Tota J, Ferenczy A, Coutlee F. The expected impact of HPV vaccination on the accuracy of cervical cancer screening: the need for a paradigm change. Arch Med Res. 2009;40(6):478–85. doi: 10.1016/j.arcmed.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97(14):1072–9. doi: 10.1093/jnci/dji187. 20. [DOI] [PubMed] [Google Scholar]