Abstract
Background
Corneal lymphangiogenesis (LG) and hemangiogenesis (HG) accompany many diseases after inflammatory, infectious, traumatic or chemical insults. They also contribute to transplant rejection. It is known that corneal transplants in infants or children have a higher rejection rate than in adults. However, it has never been studied whether infant corneas differ from adult corneas in inflammatory LG, HG, or both, which is the focus of this study.
Methods and Results
Corneal inflammatory LG and HG were induced by a standard suture placement model in C57BL/6 mice of 3 weeks and 8 weeks of age, respectively. Corneal LG, HG, and macrophage infiltration were assessed by immunofluorescent microscopic studies using specific antibodies against CD31 (a panendothelial cell marker), LYVE-1 (a lymphatic marker), and F4/80 (a macrophage marker). Blood vessels were also examined by ophthalmic slit-lamp microscopic assays in vivo. Digital images were analyzed by NIH Image J software. It was found, for the first time, that infant corneas exhibited a higher level of LG, HG, and macrophage infiltration during inflammation. Infant lymphatic and blood vessels demonstrated greater density and invasion area but similar branching points. Additionally, infant lymphatic vessels were also of larger diameter.
Conclusions
Infant and adult corneas differ greatly in their inflammatory responses of LG, HG, and macrophage infiltration. These novel findings will shed some light on our understanding of the LG and HG processes, as well as the development of new therapeutic protocols for corneal diseases, particularly, in infants or children, where an early restoration of sight is critically important in preventing amblyopia or permanent vision loss.
Introduction
After centuries of negligence, lymphatic research has progressed rapidly in recent years, largely due to the advancement of modern technology and the discovery of several lymphatic–endothelium specific molecules, such as lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), vascular endothelial growth factor receptor-3, and Prox-1. Penetrating most tissues in the body, the lymphatic network is critically involved in many functions, including immune surveillance, tissue fluid homeostasis, and vitamin and fat absorption.1–8
Though not present in normal adult cornea, both lymphangiogenesis (LG; the development of new lymphatic vessels) and hemangiogenesis (HG; the development of new blood vessels) can be induced in this tissue after an inflammatory, infectious, or traumatic insult.3 Also due to its accessible location and transparent nature, the cornea provides an ideal site for lymphatic research. Since there are no pre-existing or background vessels, any vascular structures detected in the cornea are newly formed, which makes it exceptionally easy and straightforward to analyze neovascular responses in this tissue, whether it is LG or HG. Results from corneal studies also bear broad implications and can be readily applied to other research fields. Indeed, the use of this tissue for tumor angiogenesis research dates back to the 1970s.9–11
Since the lymphatic and blood vessel channels constitute the afferent and efferent arms of the immune reflex arc of corneal transplantation immunity, respectively,3,12 a number of studies have focused on interfering with these pathways to improve transplant survival in adult corneas and have achieved promising results.3,12–14 Most recently, it has also been demonstrated that LG plays a primary role in mediating corneal transplant rejection.15
It has long been known that corneal transplantation in infancy and early childhood has poorer prognosis than in adults.16–18 More recently, it was also reported that corneal grafts in infant rats were rejected at an accelerated rate.19 However, the underlying mechanisms for this phenomenon still remain largely unknown. In this study, we provide the first evidence that inflammatory LG, HG, and macrophage infiltration occur at much greater scale in infant than in adult cornea. Additionally, we have also identified the similarities and differences between the two vascular systems and age groups in response to the inflammatory stimulation. It is hopeful that these novel findings may shed some new light on our understanding and distinguishing of LG and HG processes, as well as the development of customized therapies for relevant ocular or nonocular disorders in infants and young children.
Materials and Methods
Animals
Three and 8-week-old male C57BL/6 mice (Taconic Farms, Germantown, NY) were used for the experiments. All mice were treated according to ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and all protocols were approved by the Animal Care and Use Committee, University of California, Berkeley. Mice were anesthetized using a mixture of ketamine, xylazine, and acepromazine (50 mg, 10 mg, and 1 mg/kg body weight, respectively) for each surgical procedure.
Induction of corneal inflammatory lymphangiogenesis and hemangiogenesis
The standard suture placement model was used to induce corneal inflammatory LG and HG as described previously.20–23 Briefly, three 11-0 nylon sutures (AROSurgical, Newport Beach, CA) were placed into central corneas at equal distance ratio (suture to limbus/corneal radius) in all mice. Seven or 14 days later, whole-mount flat corneas were collected for immunohistochemical studies on macrophages, and LG and HG, respectively. The experiments were repeated twice with a total of 10 mice in each group of study.
Immunofluorescent microscopic assay
The experiments were performed according to our standard protocol.21–24 Briefly, freshly excised corneas were fixed in acetone for immunofluorescent staining. Nonspecific staining was blocked with anti-Fc CD16/CD32 antibody (BD Biosciences, San Jose, CA) and donkey serum (Jackson ImmunoResearch Laboratories Inc. West Grove, PA). For vessel studies, samples were sequentially stained with FITC-conjugated rat anti-mouse CD31 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and purified rabbit anti-mouse LYVE-1 antibody (Abcam, Cambridge, MA), which was visualized by a cy3 conjugated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories Inc.). Vascular structures stained as CD31+LYVE-1- were identified as blood vessels while those stained as CD31+LYVE-1+ were defined as lymphatic vessels. For macrophage studies, samples were stained with purified rat anti-mouse F4/80 primary antibody (Abcam) and FITC-conjugated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories Inc.). Samples were covered with Vector Shield mounting medium (Vector Laboratories, Burlingame, CA) and examined and photographed by an epifluorescence deconvolution microscope (AxioImager M1, Carl Zeiss AG, Gottingen, Germany).
Vascular and macrophage quantification
Corneal vessels revealed by ex vivo immunofluorescent microscopic assays were graded and analyzed using the NIH Image J software as described previously with some modifications.21–25 Basically, vessels in each sample were evaluated using 4 parameters: invasion area, density, diameter, and branching points. The invasion area was presented in a percentage coverage score obtained by normalizing the vascular area to the total corneal area. The density was measured by normalizing the total vascular mass to the invasion area. Both diameter and branching points were quantified by averaging 5 randomly chosen areas in the sample. Macrophage infiltration was also evaluated according to the standard protocol.22,26,27 Briefly, total 10 areas (8 from the periphery and 2 from the center) of each sample were randomly picked and examined under the epifluorescence microscope. Total numbers of F4/80 positive cells were counted throughout the whole thickness of the picked area.
Bio-microscopic examination and quantification
Eyes were examined by an ophthalmic slit-lamp with an integrated digital camera system (SL-D4 and DC-3; Topcon Medical Systems, Tokyo, Japan). Corneal blood vessels were graded as described previously.14,23,28 Briefly, the quantification was based on 2 primary parameters: (1) the circumferential extent of 12 areas around the clock. A score of 1 was given to each area if the vessels were present in the sector, and (2) the centripetal extend of 10 circles toward the center of the cornea. A grade between 0 (at limbus) and 10 (at center) was given to each area if the longest vascular frond was present. Scores for each area were then summed to derive the final index (range, 0–120).
Statistical analysis
Data are expressed as the mean ± SEM. The statistical significance of the difference between each group (n = 10) was evaluated using Mann-Whitney test with GraphPad Prism® software (GraphPad Software, Inc., La Jolla, CA). P < 0.05 was considered significant.
Results
Infant cornea shows stronger HG response during inflammation
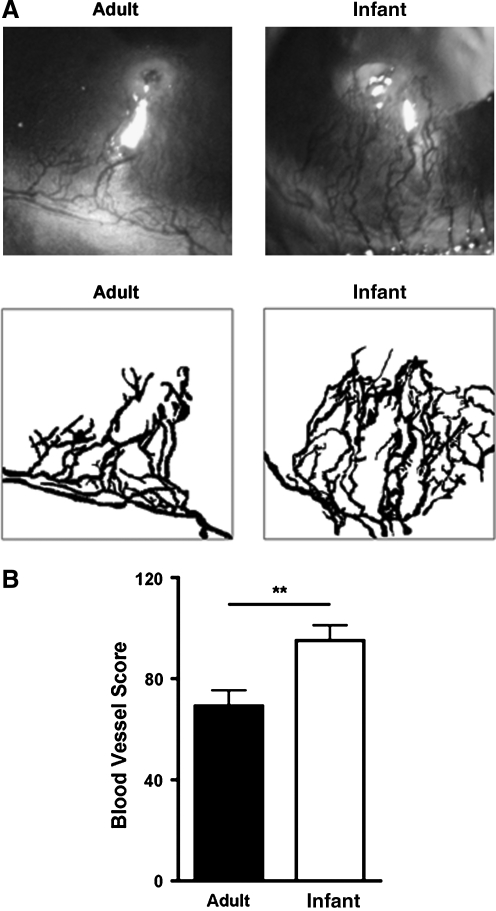
We first set out to investigate whether HG response differs in infant and adult mouse corneas during inflammation. As shown in Figure 1A by slit-lamp micrographs and digital images, our results from in vivo ophthalmic slit-lamp microscopic assays demonstrated that compared with those in adult corneas, the newly developed blood vessels in infant corneas occupied a significantly larger area. Results from repetitive experiments are presented in Figure 1B (**P < 0.01).
FIG. 1.
Inflammatory HG response is stronger in infant cornea. (A) Representative micrographs and digital images from in vivo ophthalmic slit-lamp microscopic assays showing that newly formed blood vessels in infant corneas occupied a larger percentage of corneal area 14 days after suture placement. Original magnification: X40. (B) Summarized data from repetitive experiments. **P < 0.01.
Infant cornea demonstrates stronger LG response during inflammation
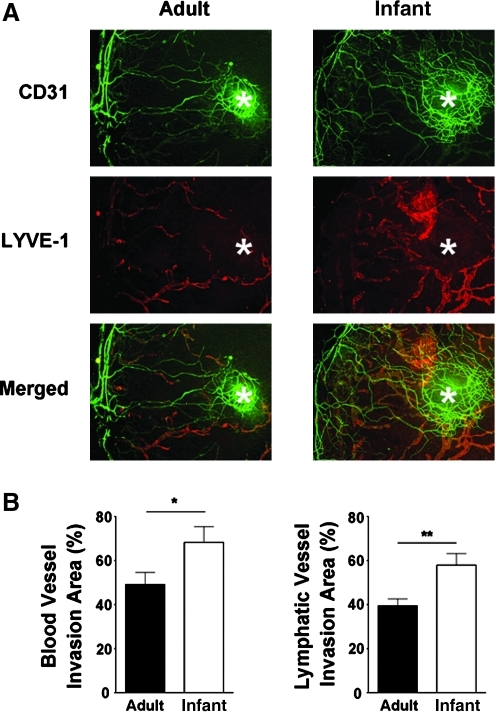
We next examined whether inflammatory LG also differs in infant and adult corneas and performed immunofluorescent microscopic assays using specific antibodies against CD31 and LYVE-1. As shown in Figure 2, it was revealed that similar to blood vessels, the newly formed lymphatic vessels in infant corneas also covered a larger area than those in the adult condition (*P < 0.05; **P < 0.01). The results on blood vessels from both slit-lamp and immunofluorescent microscopic assays were consistent with each other. Moreover, it was also observed that vascular response around the suture site (as indicated by asterisks) was stronger in infant corneas.
FIG. 2.
Inflammatory vascular response is stronger in infant cornea. (A) Representative micrographs from immunofluorescent microscopic assays showing more blood and lymphatic vessels in inflamed infant cornea. Green, CD31; red, LYVE-1; yellow, Merged. Asterisks: site of suture placement. Original magnification: X25. (B) Summarized data from repetitive experiments. *P < 0.05; **P < 0.01.
Differences between infant lymphatic and blood vessels
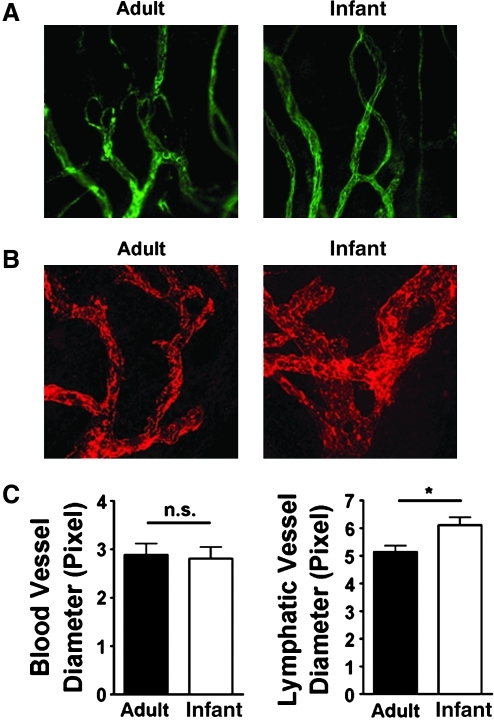
Interestingly, it was observed that in terms of vascular caliber, the difference was only present in lymphatic vessels, but not blood vessels. As shown in Figures 3A and 3C (left panels), no significant difference was found when comparing the diameter of infant and adult blood vessels. In contrast, infant lymphatic vessels exhibited significantly larger caliber, as demonstrated in Figure 3B and 3C (right panel; *P < 0.05).
FIG. 3.
Difference between inflammatory infant lymphatic and blood vessels. (A) Representative micrographs from immunofluorescent microscopic assays showing no significant difference between infant and adult blood vessels in terms of diameter measurement. Green, CD31+LYVE-1- vessels. Original magnification: X100. (B) Representative micrographs from immunofluorescent microscopic assays showing infant lymphatic vessels were of larger diameter. Red, LYVE-1. Original magnification: X100. (C) Summarized data from repetitive experiments. *P < 0.05; n.s. no significant difference.
Similarities between infant and adult lymphatic or blood vessels
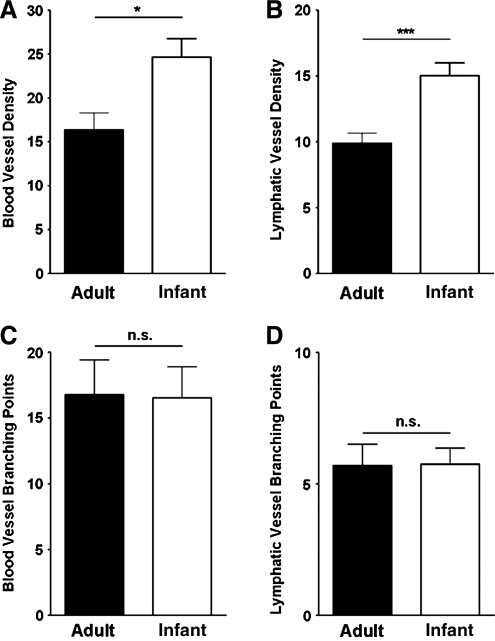
Vascular branching point is an indicator of the complexity of the vessels. Our additional examination on this parameter for both lymphatic and blood vessels, however, did not reveal any significant differences between infant and adult conditions, as summarized in Figure 4A. However, it was found that both vessel types exhibited greater density in infant corneas (Fig. 4B; *P < 0.05; ***P < 0.001).
FIG. 4.
Similarities between inflammatory infant and adult lymphatic or blood vessels. Summarized data from repetitive experiments showing that both blood and lymphatic vessels demonstrated greater density (A and B) but similar branching points (C and D) in infant corneas. *P < 0.05; ***P < 0.001; n.s. no significant difference.
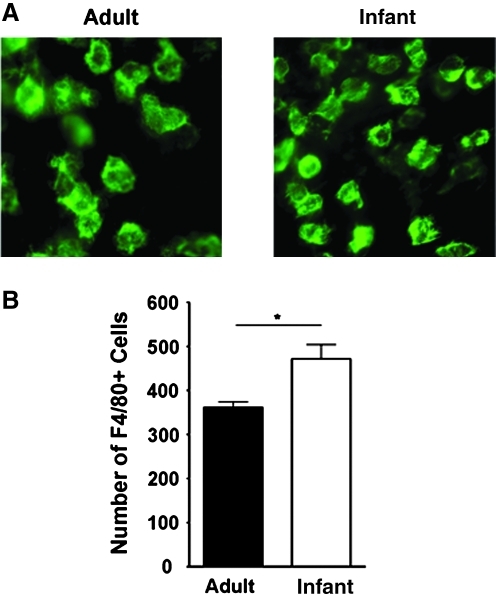
Infant cornea exhibits enhanced macrophage infiltration
It is known that macrophages contribute to corneal LG in adult tissues.22,26,29 We next investigated whether macrophage infiltration also differs in infant and adult corneas. To approach this, we compared the number of macrophages in infant and adult corneas 1 week after suture placement using the standard protocol as described previously.22,26,27 Our results from this assay revealed that the number of macrophages was significantly higher in infant than in adult corneas, as shown in Figure 5 (*P < 0.05).
FIG. 5.
Macrophage infiltration is enhanced in inflamed infant cornea. (A) Representative micrographs of immunofluorescent microscopic assays showing the number of F4/80+ macrophages was significantly higher in the inflamed infant cornea. Green, F4/80. Original magnification: X400. (B) Summarized data from repetitive experiments. *P < 0.05.
Conclusions
In this study, we provide the first analysis on infant inflammatory LG and HG in the cornea, which is important for our understanding and management of vascular diseases in infants and young children. At least three important conclusions can be drawn from our data: 1) inflammatory LG and HG occur at a greater scale in infant than in adult corneas; 2) while sharing some similarities, infant LG and HG also differ from each other; and 3) inflammatory macrophage infiltration is also significantly enhanced in infant tissues.
There are several implications for this study. First, it is indicated that LG and HG exhibit a greater scale in infant corneas, which may partially explain the higher rejection rate of corneal grafts in infants or young children. Our finding on the age difference of LG between infant and adult corneas is consistent or complementary with our previous report showing that lymphatic vessels are present in neonatal or immature murine corneas, which undergo spontaneous regression as the cornea matures.21 Taken together, it seems that the corneas of early ages are more prone to LG, whether it is a physiological or pathological event. Further investigation on this interesting phenomenon may reveal new mechanisms underlying LG processes. Second, this study also provides new evidence supporting that blood and lymphatic vessels can behave differentially under certain circumstances. Our data showing that the diameter disparity only exists between infant and adult lymphatic vessels, but not blood vessels, indicate that these two vascular systems do not always accompany each other and may undergo different anatomical or physiological events when facing the same challenges. Third and last, it is suggested that more potent anti-LG and anti-HG therapeutic regimens should be considered to treat relevant diseases in infants and young children, such as inflammation, transplant rejection, and cancer metastasis. Corneal transplantation is a major sight saving strategy for various congenital and non-congenital diseases of corneal opacity in infants and young children. If left untreated or past the critical age window of the treatment, these patients may suffer from permanent vision loss or amblyopia for the rest of their lives. It is therefore particularly important to treat patients in these age groups with an early and effective intervention. Such intervention should include a successful transplantation surgery as well as a potent pharmaceutical regimen. Unfortunately, the pharmacotherapy of corneal transplants has changed little over the past several decades, despite the fact that corticosteroids are only variably effective and fraught with serious side-effects, such as glaucoma, cataracts, and opportunistic infections. It is hopeful that results from this study will offer new insights and therapeutic guidelines for transplant rejection and possibly other LG- and HG-related diseases in infants and young children.
Footnotes
This study is supported in part by research grants from National Institutes of Health, Department of Defense, and the University of California at Berkeley.
Acknowledgments
The authors thank Jeffrey LeDue (University of California Berkeley) for his excellent technical assistance with the immunofluorescent microscopic studies.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Tammela T. Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 2.Rockson SG. Lymphatic research: Past, present, and future. Lymphat Res Biol. 2009;7:183–187. doi: 10.1089/lrb.2009.7402. [DOI] [PubMed] [Google Scholar]
- 3.Chen L. Ocular lymphatics: State-of-the-art review. Lymphology. 2009;42:66–76. [PMC free article] [PubMed] [Google Scholar]
- 4.Brown P. Lymphatic system: Unlocking the drains. Nature. 2005;436:456–458. doi: 10.1038/436456a. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Kaipainen A. Genes tell lymphatics to sprout or not. Nat Immunol. 2004;5:11–12. doi: 10.1038/ni0104-11. [DOI] [PubMed] [Google Scholar]
- 6.Oliver G. Detmar M. The rediscovery of the lymphatic system: Old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 7.Banerji S. Ni J. Wang SX. Clasper S. Su J. Tammi R. Jones M. Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockson SG. The broad spectrum of lymphatic health and disease. Lymphat Res Biol. 2010;8:101. doi: 10.1089/lrb.2010.8203. [DOI] [PubMed] [Google Scholar]
- 9.Muthukkaruppan V. Auerbach R. Angiogenesis in the mouse cornea. Science. 1979;205:1416–1418. doi: 10.1126/science.472760. [DOI] [PubMed] [Google Scholar]
- 10.Gimbrone MA., Jr. Cotran RS. Leapman SB. Folkman J. Tumor growth and neovascularization: An experimental model using the rabbit cornea. J Natl Cancer Inst. 1974;52:413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- 11.Chen L. Hann B. Wu L. Experimental models to study lymphatic and blood vascular metastasis. J Surg Oncol. 2011;103:475–483. doi: 10.1002/jso.21794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cursiefen C. Chen L. Dana MR. Streilein JW. Corneal lymphangiogenesis: Evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22:273–281. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Chong EM. Dana MR. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008;28:209–222. doi: 10.1007/s10792-007-9099-9. [DOI] [PubMed] [Google Scholar]
- 14.Chen L. Huq S. Gardner H. de Fougerolles AR. Barabino S. Dana MR. Very late antigen 1 blockade markedly promotes survival of corneal allografts. Arch. Ophthal. 2007;125:783–788. doi: 10.1001/archopht.125.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich T. Bock F. Yuen D. Hos D. Bachmann BO. Zahn G. Wiegand S. Chen L. Cursiefen C. Cutting edge: Lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535–539. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanathi M. Panda A. Vengayil S. Chaudhuri Z. Dada T. Pediatric keratoplasty. Surv Ophthalmol. 2009;54:245–271. doi: 10.1016/j.survophthal.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Al-Ghamdi A. Al-Rajhi A. Wagoner MD. Primary pediatric keratoplasty: Indications, graft survival, and visual outcome. J AAPOS. 2007;11:41–47. doi: 10.1016/j.jaapos.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Dana MR. Moyes AL. Gomes JA. Rosheim KM. Schaumberg DA. Laibson PR. Holland EJ. Sugar A. Sugar J. The indications for and outcome in pediatric keratoplasty. A multicenter study. Ophthalmology. 1995;102:1129–1138. doi: 10.1016/s0161-6420(95)30900-1. [DOI] [PubMed] [Google Scholar]
- 19.Schwartzkopff J. Berger M. Birnbaum F. Bohringer D. Reinhard T. Accelerated corneal graft rejection in baby rats. Br J Ophthalmol. 2010;94:1062–1066. doi: 10.1136/bjo.2008.154435. [DOI] [PubMed] [Google Scholar]
- 20.Chen L. Hamrah P. Cursiefen C. Zhang Q. Pytowski B. Streilein JW. Dana MR. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H. Hu X. Tse J. Tilahun F. Qiu M. Chen L. Spontaneous lymphatic vessel formation and regression in the murine cornea. Invest Ophthalmol Vis Sci. 2011;52:334–338. doi: 10.1167/iovs.10-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimaldo S. Yuen D. Ecoiffier T. Chen L. Very late antigen-1 mediates corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2011 Mar 2; doi: 10.1167/iovs.10-6580. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuen D. Pytowski B. Chen L. Combined blockade of VEGFR-2 and VEGFR-3 inhibits inflammatory lymphangiogenesis in early and middle stages. Invest Ophthalmol Vis Sci. 2011;52:2593–2597. doi: 10.1167/iovs.10-6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ecoiffier T. Yuen D. Chen L. Differential distribution of blood and lymphatic vessels in the murine cornea. Invest Ophthalmol Vis Sci. 2010;51:2436–2440. doi: 10.1167/iovs.09-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dastjerdi MH. Al-Arfaj KM. Nallasamy N. Hamrah P. Jurkunas UV. Pineda R. Pavan-Langston D. Dana R. Topical bevacizumab in the treatment of corneal neovascularization: Results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009;127:381–389. doi: 10.1001/archophthalmol.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung ES. Chauhan SK. Jin Y. Nakao S. Hafezi-Moghadam A. van Rooijen N. Zhang Q. Chen L. Dana R. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am J Pathol. 2009;175:1984–1992. doi: 10.2353/ajpath.2009.080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rashid S. Jin Y. Ecoiffier T. Barabino S. Schaumberg DA. Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–225. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 28.Dana MR. Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996;37:2485–2494. [PubMed] [Google Scholar]
- 29.Cursiefen C. Chen L. Borges LP. Jackson D. Cao J. Radziejewski C. D'Amore PA. Dana MR. Wiegand SJ. Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]